Abstract
Aim: CCL22, mainly synthesized by monocyte-derived alternative (M2) macrophages, belongs to the CC family of chemokines and is involved in monocyte migration and recruitment. We have previously investigated CCL22 and histamine in atherosclerosis. Here, we investigated the hypothesis that CCL22 is involved in atherosclerosis, which is influenced by the differentiation of macrophage phenotypes via histamine.
Methods: CCL22 expression was investigated in human carotid arteries and coronary arteries with bare metal stents. Ligated carotid arteries of wild-type (C57BL/6J) and apolipoprotein E-deficient mice were also used as atherosclerotic models. The localization and expression of CCL22 and classical (M1)-like and M2-like macrophages in various human and mouse atherosclerotic lesions were investigated by immunohistochemical examination and quantitative real-time polymerase chain reaction. Histamine is expressed in atherosclerosis, and it induces inflammation and immunity. Human- and mice-derived monocytes and macrophages were used to examine the role of histamine in macrophage differentiation and CCL22-expression. Macrophages derived from histamine receptor 1 (H1R)- and 2 (H2R)-knockout (KO) mice were also examined.
Results: Atherosclerotic lesions showed a distribution of heterogeneous macrophage phenotypes with M1-like and M2-like macrophage dominant sites. CCL22 was distributed in sparse areas of vascular smooth muscle cells (VSMCs) and associated with M2-like macrophages. Moreover, H2R stimulation was associated with CCL22 expression via M2-like macrophage dominant differentiation.
Conclusion: The expression of M1- or M2-like macrophages in atherosclerosis were observed to be dependent on the distribution of VSMCs owing to differences in causal stimuli and the switching of histamine receptors via Th1 or Th2 cytokines. These results suggest that CCL22 may control atherosclerosis.
Keywords: CCL22, Histamine, Atherosclerosis, Macrophage, Chemokine
Introduction
Of the various cytokines involved in inflammation, chemotactic peptides known as chemokines that are induced in response to inflammatory and immunological stimuli are the most significant in terms of leukocyte recruitment. More than 50 known human-chemokines are grouped into four families on the basis of conserved cysteine residues near their amino termini and are designated as C, CC, CXC, and CX3C subfamilies. CCL22 is a member of the CC subfamily1, 2).
Atherosclerosis is a progressive, inflammatory disease of the arterial wall modulated by immune responses. The initial step in atherosclerosis involves the infiltration of monocytes into the arterial wall3). After monocytes have adhered to the endothelium, they migrate into the intima, where they can differentiate into macrophages and phagocytose lipids to become foam cells4, 5). Macrophages are a predominant source of cytokines, growth factors, and chemokines, including CCL22 that contribute to the recruitment of monocytes in the subendothelial space6). In addition to macrophages, dendritic cells are also present in the atherosclerotic lesions and produce more CCL22 than monocytes1, 6–9).
Macrophages are present in various regions of plaque, and are exposed to different environmental stimuli that modulate their activation and polarization. Even though the process of differentiation of monocytes into macrophages is irreversible, macrophage-polarization appears reversible10, 11). Monocyte-derived macrophages are the principal mediators of tissue homeostasis and repair along with the response to pathogenesis and inflammation in organs12). Macrophages can be divided into classically Th1 polarized M1-like and Th2 polarized M2-like macrophage phenotypes13, 14). In addition to M1-like macrophages, M2-like macrophages are present in the atherosclerotic lesions15) and contribute to the pathogenesis of atherosclerosis16). Furthermore, M2-like macrophages express CCL2217).
Histamine is one of the principal mediators of allergic reactions, inflammation, and cardiovascular hemodynamics. Histamine is a classical and immunological mediator, mainly produced by mast cells and basophils. However, in addition to these cells, monocytes and macrophages also produce histamine that is gradually secreted in atherosclerosis18).
Macrophages express CCL22 in response to histamine stimulation via the H2 receptor19, 20). In this study, we investigated the localization of CCL22 and the various macrophage phenotypes in atherosclerotic lesions, and the relationship between macrophage phenotypes and histamine receptors.
Materials and Methods
Human Arteries
To investigate human common carotid arteries and the effect of coronary bare metal stents on human coronary arteries by histological and immunohistochemical examinations, 22 samples each that did not show aortitis syndrome and autoimmune disease were obtained at autopsy.
Resected tissues were fixed in 10% formalin and embedded in paraffin. The sections (3 µm) were stained with hematoxylin and eosin (H&E) and used for immunohistochemical examinations by the EnVision method (Dako Japan, Kyoto, Japan). Monoclonal anti-human CD68 (clone KP1, 1:100) and αSMA (clone 1A/4, 1:150) antibodies were obtained from Dako Japan, rabbit polyclonal antibodies to CCL22 (1:100) and monocyte chemoattractant protein (MCP)-1 (1:200) were obtained from Abcam (Tokyo, Japan), and monoclonal anti-human CD4 antibody (clone 4B12, undiluted) was obtained from Nichirei (Tokyo, Japan).
This investigation adhered to all the principles prescribed in the Declaration of Helsinki. The Ethics Committee of Experimentation, University of Occupational and Environmental Health, Japan, approved all procedures of the present study.
Animals
We used male apoE knockout (KO) mice (Jackson Laboratory, Bar Harbor, ME) that were maintained for 30 weeks on a normal chow diet. Another group of male wild-type (WT) C57BL/6J mice (Charles River, Yokohama, Japan), weaned at 8 weeks of age, was used to produce ligation-induced vascular injury models. We ligated the left common carotid artery with a 7-0 silk suture for 2 weeks at a site proximal to the carotid bifurcation.
After the animals were euthanized, carotid arteries were removed, fixed in 10% formalin, and embedded in paraffin for histological and immunohistochemical examination as described previously21). Thin sections (3 µm) were cut and stained with H&E and used for immunohistochemical detection. Immunohistochemical staining was carried out (EnVision kit, Dako Japan) using antibodies against CCL22 (rabbit polyclonal, 1:50; GeneTex, Irvine, CA), Mac-3 (clone M3/84, 1:50; BD Bioscience Pharmingen, Tokyo, Japan), and αSMA (1:150; Dako, as above).
Immunofluorescence Staining
Immunofluorescence assays were performed on thin sections (4 µm) of human coronary artery, with and without coronary stenting. Experiments were performed using anti-CCL22 (as described above) and anti-CD163 (1:200; Leica Biosystems, Newcastle, UK) primary antibodies to detect M2-like macrophages, and anti-MCP-1 primary antibody (as above) to detect M1-like macrophages. In addition, antibodies to αSMA (as above), CD4 (as above), CCR4 (goat polyclonal, 1:100: Abcam), and interleukin (IL)-4 (rabbit polyclonal, 1:2000; Abcam) were used. Rhodamine-conjugated goat anti-mouse IgG (H+L; 1:200) and donkey anti-rabbit IgG (1:200; Merck, Tokyo, Japan), and FITC-conjugated goat anti-rabbit IgG (H+L) (1:200), donkey anti-mouse IgG (H+L) (1:200) and donkey anti-goat IgG (H+L; 1:200; Merck) were used as secondary antibodies, respectively, and incubated for 90 min at 25°C. After washing with phosphate buffered saline (PBS), specimens were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; GeneTex, Hsinchu City, Taiwan) and mounted. Immunostained tissues were evaluated with a Nikon ECLIPSE E600 inverted fluorescence microscope (Nikon, Tokyo, Japan). Image analysis was completed with LuminaVision software (version 2.2.2; Mitani Corporation, Tokyo, Japan).
Cell Cultures
The murine macrophage-like cell line, J774A.1, was obtained from the Health Science Research Resources Bank (Tokyo, Japan) and cultured in Dulbecco's minimal essential medium (DMEM) with 10% fetal bovine serum (FCS). In addition, these cells were treated with 100 ng/mL of lipopolysaccharide (LPS; Wako, Tokyo, Japan) and 20 ng/mL of interferon (IFN) γ (Wako) for M1-like macrophages or 20 ng/mL of IL-4 (R&D Systems, Minneapolis, MN) for M2-like macrophages.
A human macrophage cell line was obtained from PromoCell GmbH (Heidelberg, Germany) and cultured in Monocyte Attachment Medium (PromoCell GmbH). In addition, for their differentiation into M1- and M2-like macrophages, cells were cultured in M1-Macrophage Generation Medium DXF (Promo-Cell GmbH) and M2-Macrophage Generation Medium DXF (PromoCell GmbH), respectively.
Preparation of Peritoneal Macrophages
Peritoneal macrophages from 8-week-old WT, H1R-KO, and H2R-KO mice fed a normal chow diet were isolated by peritoneal lavage with ice-cold PBS, 3 days after the intraperitoneal injection of 2.5 mL of 4.05% (wt/vol) sterile thioglycollate medium (Becton Dickinson, Franklin Lakes, NJ) as described in our previous studies20, 21).
Real-Time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (qPCR) was carried out using a TaqMan assay and a 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). The conditions for PCR were 95°C for 10 min, followed by 40 cycles at 95 °C for 15 sec (denaturation) and 60°C for 1 min (annealing-extension). The primers and fluorogenic probes for CCL22 and IL-4 were from a pre-made primer/probe set (assay ID, Mm00436439_ml and Mm00445259_ml; Applied Biosystems Japan, Ltd., Tokyo, Japan). Primers for the H1 and H2 receptors, inducible nitric oxide synthase (iNOS), interleukin (IL)-6, and tumor necrosis factor (TNF)-α have been listed in Table 1. The levels of mRNA expression were normalized with those of the expression of 18s ribosomal RNA in the same samples.
Table 1. List of Primers and Probes for Real-Time PCR Analysis.
| Gene | Forward | Reverse | Probe |
|---|---|---|---|
| H1R | CTTTAGTGTCTTCATCCTGTGTATTGATC | GTAGCTGAAGCACGGGTCTTG | TGTCCAGCAACCCCTCCGGTACCT |
| H2R | CCTTCTCTGCCATTTACCAGTTG | CATCACATCCAGGCTGGTGTAG | AGTGGAGGTTTGGCCAGGTCTTCTGC |
| Il6 | TTACACATGTTCTCTGGGAAATCG | TTGGTAGCATCCATCATTTCTTTG | TGAGAAAAGAGTTGTGCAATGGCAATTCTGAT |
| iNOS | GCAGTGGAGAGATTTTGCATGAC | ATGGACCCCAAGCAAGACTTG | CACCACAAGGCCACATCGGATTTCAC |
| TNF-α | CCCAGACCCTCACACTCAGATC | TGCTCCTCCACTTGGTGGTT | ATTCGAGTGACAAGCCTGTAGCCCACG |
Statistical Analysis
Analysis of variance (ANOVA) was used to determine statistically significant differences. Data were expressed as mean ± standard deviation (SD), and a probability (P) value of less than 0.05 was taken to be significant.
Results
Expression and Localization of CCL22 in Human Common Carotid Arteries
To investigate the expression of CCL22 and its localization in human common carotid arteries, we performed immunohistochemical staining using antibodies against human CCL22, CD68 (a macrophage marker), and αSMA (a smooth muscle cell marker). Although the intimal thickness of the common carotid artery looked relatively concentric at a glance (Fig. 1A), the distribution of αSMA in VSMCs in the intima of common carotid arteries were uneven (Fig. 1B). The density of VSMCs, classified into three levels (α, β, and γ), was examined in more detail. Regions with a moderate density of VSMCs (α) showed foamy, CD68-positive macrophages and were also positive for CCL22 (Fig. 1C-α H&E, CD68, CCL22). At sites with a high density of VSMCs (β), CD68-positive macrophages were dispersed but were negative for CCL22 (Fig. 1C-β αSMA, CD68, CCL22). Vascular smooth muscle cells were not found in regions of concentrated cholesterol clefts (γ), while CCL22-staining was strongly positive, and CD68-staining was weakly positive (Fig. 1C-γ αSMA, CD68, CCL22). In summary, the expression of CCL22 in atherosclerosis appeared to inversely correlate with the density of VSMCs, with the absence of VSMCs associated with high CCL22 staining (Supplemental Fig. 2A).
Fig. 1.
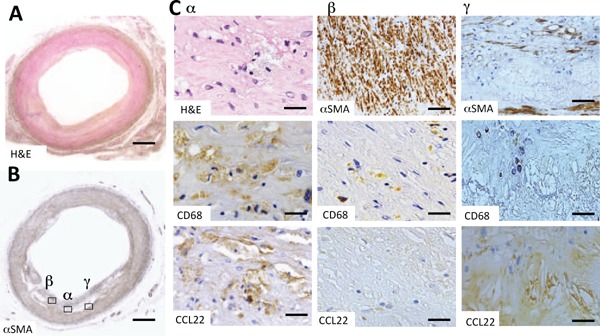
Expression and localization of CCL22 in a human common carotid artery
Fig. A and Fig. B are low-power magnification images of H&E (H & E) and α-smooth muscle actin (αSMA) stains. In Fig. B, α signifies an area of intermediate vascular smooth muscle (VSMC) cell density and the accumulation of foamy macrophages, β signifies an area with a high degree of VSMC density and γ signifies an area with virtually no VSMC. Figures C-α are high-power magnified images showing H & E and immunohistochemical stains for CD68 and CCL22, respectively, of the α-area in Fig. B. Fig. C-β shows immunohistochemical staining for αSMA, CD68, and CCL22, respectively, of the β-area in Fig. B. Fig. C-γ shows immunohistochemical staining for αSMA, CD68, and CCL22 of the γ-area in Fig. B. At first glance, the distribution of VSMCs appeared heterogeneous, even in atherosclerotic lesions that were concentric (A, B).
CD68 positive foamy macrophages were also positive for CCL22 (C-α). Macrophages in close proximity to VSMC densities were CCL22 negative (C-β). VSMCs were not present around cholesterol clefts; however, positive CCL22 staining was present (C-γ). Bars indicate 1000 µm (A and B), 10 µm (C-α), 100 µm (C-β αSMA and C-γ αSMA), and 50 µm (C-β and C-γ, CD68 and CCL22). The negative control staining of Fig. C-γ CCL22 was shown in Supplemental Fig. 1A.
Supplemental Fig. 1.
Negative control staining of CCL22 in lipid clefts
Fig. A shows negative control staining of CCL22 in Fig. 1C-γ, and B is a negative control for the CCL22 stain in Fig. 5A.
Expression and Localization of CCL22 in Human Coronary Arteries with Stents
Next, we investigated how arteries artificially induced the heterogeneous stimulation of VSMCs. Coronary arteries with bare metal stents were used. Staining for αSMA revealed the strong appearance of VSMCs at sites where stent-struts were in direct contact with the arterial wall (Fig. 2A, 2B). Macrophages (CD68-positive cells) at sites where VSMCs were dense were CCL22 negative (Fig. 2C H&E, CD68, αSMA, CCL22). Thus, where VSMCs proliferated, as in the intima, the expression of CCL22 was significantly suppressed as previously found with common carotid arteries (Supplemental Fig. 2B).
Fig. 2.
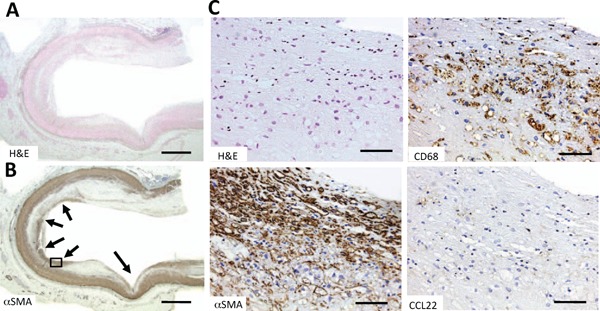
Histochemical staining of CCL22 in a human coronary artery with a bare metal stent
Fig. A and Fig. B are low-power magnified images of H&E (H & E) and α-smooth muscle actin (αSMA) stains, respectively. Arrows in Fig. B indicate the former positions of stent-struts. Fig. C shows high-power magnified images of H & E, αSMA, CD68, and CCL22 stains, respectively, of the area in the box in Fig. B. Densities of VSMCs were high in the intima of the former contact sites of the stent-struts (Fig. C αSMA) while macrophages at these sites were CCL22-negative (Fig. C CCL22).
Bars indicate 1000 µm (Fig. A and B) and 100 µm (Fig. C).
Supplemental Fig. 2.
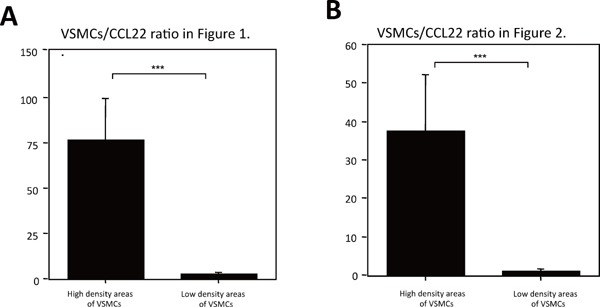
Expression ratios of VSMCs to CCL22
The expression levels of VSMCs and CCL22 were quantified as positive areas by Photoshop CS (Adobe Systems Incorporated, San Jose, CA). A. and B. show the positive area ratios of VSMCs/CCL22 of high and low VSMC density areas in a human common carotid artery (Fig. 1) and human coronary artery with a bare metal stent (Fig. 2). The measurements were performed independently by three pathologists. A significant negative correlation between the expression levels of VSMCs and CCL22 was found. Values represent the mean ± SD of triplicate measurements from three independent experiments (***P < 0.001).
Cells Surrounding Macrophages in the Intima
MCP-1 is known to be one of the cytokines predominantly produced by M1-like macrophages. CCL22-negative macrophages were found to express MCP-1 (Fig. 3A MCP-1 and Supplemental Fig. 3). Macrophages were positive for MCP-1 at sites where VSMCs were dense (Fig. 3A αSMA and MCP-1). Several Th2 cells (CD4, CCR4, and IL-4 positive cells) were found surrounding CCL22 positive macrophages (Fig. 3B CD4, CCL22, and CD4, CCR4, IL-4).
Fig. 3.
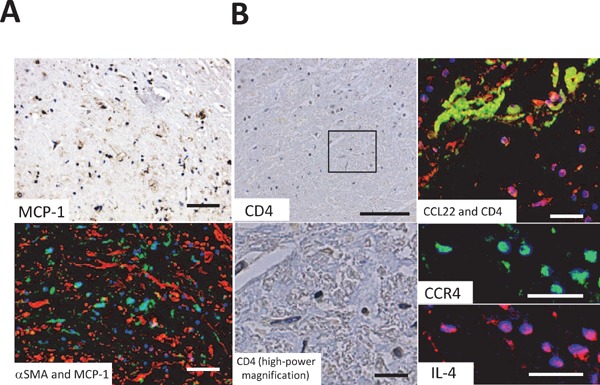
Cells surrounding macrophages in the intima
Fig. A is the same area as Fig. 1B-β and Fig. B is the same area as 1B-γ. Fig. A MCP-1 shows monocyte chemoattractant protein (MCP)-1 immunohistochemical staining (green) and Fig. A MCP-1 and α-smooth muscle actin (αSMA) shows immunofluorescence staining of both MCP-1 (green) and αSMA (red). MCP-1-positive cells and smooth muscle cells were present in close proximity. Fig. B CD4 and CD4 high-power magnification (high-power magnified image of the box in Fig. B CD4) show the immunohistochemical staining of CD4. Fig. B CCL22 and CD4 show immunofluorescence staining of both CCL22 (green) and CD4 (red). Fig. B CCR4 and interleukin (IL)-4 (high-power magnified image of Fig. B CCL22 and CD4) shows immunofluorescence staining of CCR4 (CCL22 receptor; green) and IL-4 (red). CD4-positive cells appeared around CCL22-positive cells whereas CD4-positive cells showed CCR4-staining and expressed IL-4. Blue staining (DAPI) indicates the nuclei of cells.
Bars indicate 50 µm (Fig. A), 100 µm (Fig. B CD4) and 30 µm (Fig. B CD4 [high-power magnification], CCL22 and CD4, CCR4, and IL-4).
Supplemental Fig. 3.
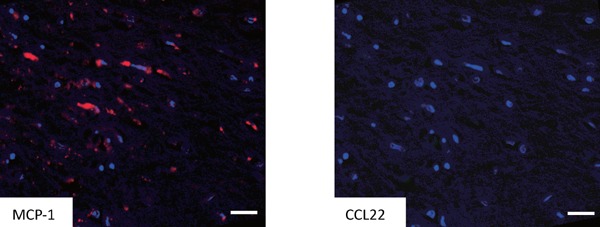
Immunofluorescence staining of MCP-1 and CCL22 in an area with a high degree of VSMC density.
Fig. depict the same area as Fig. 1B-β. Immunofluorescence staining showed monocyte chemoattractant protein (MCP)-1 (clone 2.2-4H5-01A11, 10 µg/ml PeproTech Inc., Rocky Hill, NJ; red) positive cells were negative for CCL22 (green). Bars indicate 50 µm.
Comparison of Expression of CCL22 and CD163 in Atherosclerosis
CD163 is a marker used to assess M2-macrophage distribution; this was compared with the localization of CCL22 using immunofluorescence staining of coronary arteries containing bare metal stents. Where the artery contacted the stent, CD163 and CCL22 were not expressed (Fig. 4A, H&E, CCL22, and CD163). In contrast, the expression of a large amount of both CD163 and CCL22 was observed in intima that did not contact the stent (Fig. 4B, H&E, CCL22, and CD163). Double staining of CD163 and CCL22 was shown in almost the same cells (Fig. 4B, CCL22 [high-power magnification], CD163 [high-power magnification], and CCL22 and CD163). Therefore, in atherosclerosis, CCL22 appears to be expressed in M2-like macrophages.
Fig. 4.
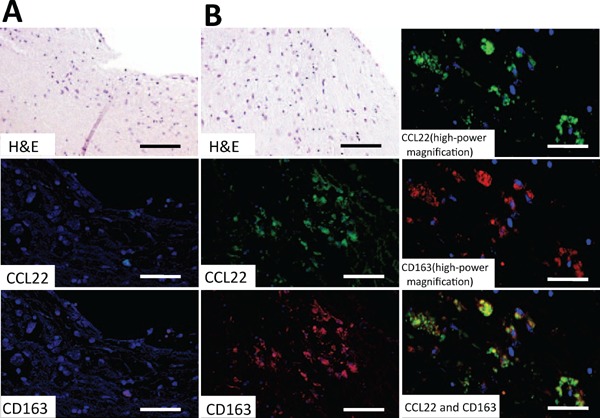
Immunofluorescence staining of CCL22 and CD163 in a human coronary artery with a bare metal stent
Fig. A shows the contact site of the stent-strut and Fig. B shows the lesion between struts. Fig. B CCL22 and CD163 (high-power magnification) are high-power magnified immunofluorescence images of Fig. B H&E. Fig. A and B CCL22 show immunofluorescence staining for CCL22 (green), and Fig. A and B CD163 show immunofluorescence staining for CD163 (red). Fig. B CCL22 and CD163 images show double immunofluorescence staining of CCL22 and CD163. Almost all of the CCL22-positive cells expressed CD163. Blue staining (DAPI) indicates the nuclei of cells. Bars indicate 200 µm (Fig. A, B, H&E, CCL22, and CD163) and 100 µm (Fig. B CCL22, CD163 [high-power magnification], and CCL22, and CD163).
Expression and Localization of CCL22 in Atherosclerosis in Carotid Arteries of ApoE-KO Mice and Ligation-Induced Neointimal Hyperplasia in Carotid Arteries of Wild-Type Mice
To investigate the difference in localization of VSMCs and CCL22 expression due to differences in the causes of atherosclerosis, we compared common carotid arteries of apoE-KO mice and ligated carotid arteries of WT-mice. We performed immunohistochemical staining using antibodies against mouse CCL22, αSMA, and Mac-3 (a macrophage marker). In the atheroma of an apoE-KO mouse (Fig. 5A), mononuclear cells infiltrating the atheroma were strongly positive for Mac-3 and faintly positive for CCL22; a positive αSMA stain surrounded the atheroma. Lipid clefts in the core of the atheroma were strongly positive for CCL22 (Fig. 5A). Such results are similar to findings relating to atherosclerotic lesions observed in the aortas of humans and apoE-KO mice19, 20). M2 macrophages were considered to dominate in this lesion.
Fig. 5.
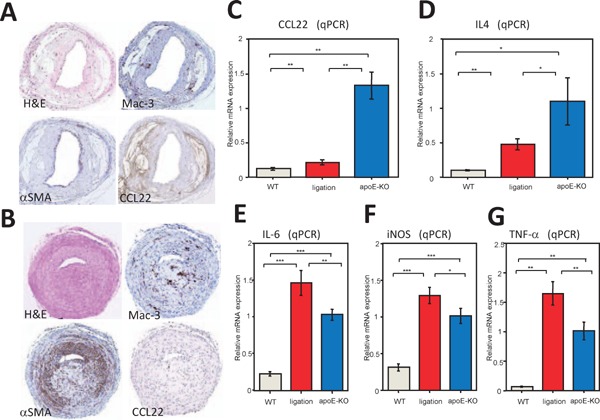
Expression of CCL22 and cytokine mRNAs in lesions of atherosclerosis mouse models.
Fig. A shows the common carotid artery of an apolipoprotein E (apoE-KO) mouse and Fig. B shows the ligated common carotid artery of a WT mouse. Fig. A and B show H&E (H & E), α-smooth muscle actin (αSMA), Mac-3, and CCL22 stains. The atherosclerotic lesion of an apoE-KO mouse showed few VSMCs and expressed a large amount of CCL22 (Fig. A). In contrast, the intima of a ligated common carotid artery of a WT-mouse displayed abundant VSMCs and expressed large amounts of Mac-3, while CCL22 was barely expressed (Fig. B). Realtime (q) PCR of mRNA from common carotid arteries of WT (n = 9), ligated WT (n = 9), and apoE-KO (n = 9) mice was performed (C and D). Fig. C and D show the expression of CCL22 and interleukin (IL)-4 mRNAs, respectively. The arteries in apoE-KO mice predominantly expressed CCL22 and IL-4 mRNA in comparison with those of WT-mice or ligated arteries of WT-mice. Values represent the mean ± SD of triplicate measurements from three independent experiments. (*P < 0.05, **P < 0.01). qPCR of interleukin (IL)-6 (Fig. E), inducible nitric oxide synthase (iNOS) (Fig. F), and TNF-α (Fig. G) mRNAs from atherosclerotic lesions in common carotid arteries of WT (n = 9), ligated WT (n = 9), and apoE-KO (n = 9) mice were examined. Such cytokines were predominantly expressed in atherosclerotic lesions from ligated common carotid arteries of WT-mice compared with those of apoE-KO mice. Values represent the mean ± SD of triplicate measurements from three independent experiments. (*P < 0.05, **P < 0.01, and ***P < 0.001). The negative control staining of Fig. A CCL22 was shown in Supplemental Fig. 1B.
In ligation-induced neointimal hyperplasia (Fig. 5B), the intima differed from that of apoE-KO mice. VSMCs were observed to be widely distributed in the thickened intima (αSMA). Mac-3-positive macrophages were also widely distributed. However, only a few CCL22-positive cells were present. In contrast to the lesions of apoE-KO mice, M1-like macrophages were considered to make up a minor component of the lesion.
Expression of CCL22 and IL-4 mRNAs in Carotid Artery
qPCR of various mRNAs from the carotid arteries of WT-mice (control), ligated carotid arteries of WT-mice and apoE-KO mice was performed. The relative CCL22-mRNA level was significantly higher in the carotid arteries of apoE-KO mice than that of the ligated carotid arteries of WT-mice (Fig. 5C; P < 0.01). IL-4 is an important factor for inducing M2-macrophages and showed the same expression pattern as CCL22 (Fig. 5D; P < 0.05). Thus, it appears that the expression of CCL22 in apoE-KO mice correlates with IL-4 expression.
Expression of M1 Macrophage Markers in Carotid Arteries
IL-6, iNOS, and TNF-α are representative inflammatory cytokines secreted by M1-macrophages. The carotid arteries of WT-mice were used as controls to which the ligated carotid arteries of WT-mice and of apoE-KO mice were compared. Compared with controls, each atherosclerosis model showed an increase in these cytokines. Further, ligated carotid arteries in WT-mice showed significantly higher expression of IL-6, iNOS, and TNF-α mRNAs than carotid arteries in apoE-KO mice (Fig. 5E, F, and G; P < 0.01, P < 0.05, and P < 0.01, respectively).
Role of Histamine Receptors in the Expression of CCL22 and M1-Associated Cytokine mRNAs in Mouse Macrophages
The influence of histamine on the expression of CCL22- and M1-associated IL-6, iNOS, and TNF-α cytokines was examined in macrophages of histamine receptor KO mice. Previous studies have shown that CCL22 is regulated through the H2 receptor19). In this study, CCL22-mRNA was significantly decreased in the macrophages of H2 receptor KO (H2R-KO) mice compared to H1R-KO mice (Fig. 6A (ⅰ); P < 0.001), despite the serum histamine concentration of H2R-KO mice being significantly higher than that of WT or H1R-KO mice (Supplemental Fig. 4; P < 0.05 for both). In comparison, IL-6, iNOS, and TNF-α mRNAs were significantly decreased in the macrophages of H1R-KO mice compared to H2R-KO mice (Fig. 6A (ⅱ), A (ⅲ), and A (ⅳ); P < 0.001, P < 0.01, and P < 0.001, respectively). Thus, these results suggest that cytokines are regulated through the expression ratio of histamine receptors in macrophages in addition to the switching of histamine receptors in monocyte/macrophages in atherosclerosis18, 22).
Fig. 6.
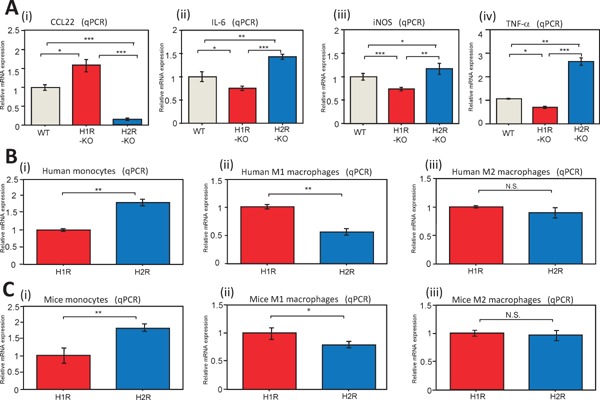
Regulation of CCL22 and cytokine mRNAs via histamine receptors in macrophages
Fig. A shows real-time (q) PCR of CCL22 (A-ⅰ), interleukin (IL)-6 (A-ⅱ), inducible nitric oxide synthase iNOS (A-ⅲ), and TNF-α (A-ⅳ) mRNAs in peritoneal macrophages from WT; n = 10), histamine receptor 1 knockout (H1R-KO; n = 10) and histamine receptor 2 knockout (H2R-KO; n = 10) mice. CCL22 was regulated by H2R. In comparison, IL-6, iNOS, and TNF-α were regulated by H1R. Furthermore, we examined the relationship between macrophage phenotypes and histamine receptors. Fig. B and C relate to human and mouse monocytes and macrophages, respectively. Human (PromoCell) monocytes (B-ⅰ) expressed predominantly H2R. In contrast, human M1-like macrophages (B-ⅱ) expressed predominantly H1R. Human M2-like macrophages (B-ⅲ) exhibited intermediate expression of H1R and H2R relative to monocytes and M1-like macrophages, without a significant difference in the expression of H1R and H2R being apparent. Mouse monocytes and macrophages displayed the same trends as for human monocytes and macrophages (C-ⅰ, -ⅱ, and -ⅲ). Values represent the mean ± SD of triplicate measurements from three independent experiments.
(*P < 0.05, **P < 0.01, ***P < 0.001; N.S., not significant).
Supplemental Fig. 4.
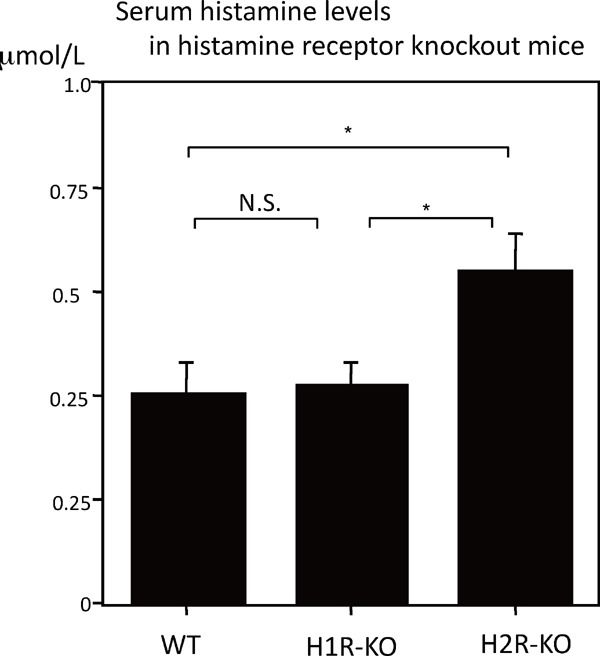
The concentration of histamine in sera of histamine receptor knockout mice.
An ELISA (R&D Systems and Immunotech, Marseille, France) showed that histamine receptor knockout mice (H2R-KO) mice (n = 8) had higher serum levels of histamine than WT (n = 8), and H1R-KO mice (n = 8) that were maintained for 11 weeks on a normal chow diet. Values represent the mean ± SD of triplicate measurements from three independent experiments (*P < 0.05, N.S: not significant).
Expression of Histamine Receptors in Mouse Macrophages and a Human Macrophage Cell Line
The relationship between M1- and M2-like macrophage differentiation, and the expression of histamine receptors was examined in human and mouse monocytes, and in M1- and M2-like macrophages. Human and mouse monocytes were found to express predominantly H2 compared to H1 receptors, as found in previous studies (Fig. 6B (ⅰ) and C (ⅰ); P < 0.01 for both). Human and mouse M1-like macrophages displayed predominantly H1 compared with H2 receptors (Fig. 6B (ⅱ) and C (ⅱ); P < 0.01 and P < 0.05, respectively). In contrast, for M2-like macrophages, a significant difference in expression of H1 and H2 receptors was not found [Fig. 6B (ⅲ) and C (ⅲ)]. Thus, these results suggested that histamine tended to enhance inflammation via M1-like macrophages and tended to increase the expression of CCL22 via M2-like macrophages.
Discussion
This study is a continuation of our work on the expression of CCL22 in atherosclerotic lesions19, 20). We previously found that the expression of CCL22 increased with the maturation of macrophages and the stabilization of lesions, but differences in the cellular localization of CCL22 were not investigated19, 20). The migration and proliferation of VSMCs are processes that occur during atherosclerosis23, 24), with the non-uniform distribution of VSMCs in the atheroma of the intima one cause for the asymmetric thickenings observed in this tissue. In this study, we examined the relationship between VSMCs and the localization of macrophage subtypes.
M1-like macrophages were mainly present at sites where VSMCs were observed in high density, while M2 -like macrophages were present where VSMCs were sparse. Similar results were observed in coronary arteries in which the distribution of VSMCs was artificially induced by stent placement. VSMCs express IFNγ, macrophage colony-stimulated factor (M-CSF), and MCP-125–27), which allow the migration of macrophages and their differentiation into M1-like macrophages. Furthermore, contact between monocytes, activated via M-CSF, and VSMCs, as well as the expression of TNF-α leads to apoptosis in VSMCs28, 29). The proximity of M1-like macrophages and VSMCs may be one of the factors that create the sparsity of VSMCs observed in areas of atherosclerotic lesions.
We then carried out a comparative study with respect to two different models of atherosclerosis in mice, and with reference to our previous studies20, 21, 30, 31). In ligation-induced neointimal hyperplasia, intimal hyperplasia was observed mainly in the smooth muscle cell layer. In this model, CCL22-negative macrophages were the dominant macrophages found in atherosclerotic lesions. In the common carotid artery atherosclerosis of apoE-KO mice, lesions were observed with a reduced increase in VSMCs and mainly contained foamy macrophages and cholesterol. In comparing these two experimental models, lesions with mainly smooth muscle cell hyperplasia, similar to human atherosclerosis, showed little expression of CCL22, particularly, in many cases, at sites with few VSMCs.
The expression of CCL22 is generally one of the features of M2 macrophages. We found that the localization of M2 macrophages in atherosclerosis is inversely proportional to the distribution of VSMCs. Conversely, it was thought that M1 macrophages migrated to a site with many VSMCs, and that the latter cells then increased at that site. VSMCs express IFNγ26), which is thought to promote the differentiation of M1-macrophages and to further increase VSMCs. CCR4 is a receptor that is expressed mainly in Th2 cells that produce IL-432). In this study, the expression of IL-4 in the atherosclerotic lesion was observed more abundantly in the carotid arteries of apoE-KO mice than in the ligated carotid arteries of WT-mice.
The regulation of expression of CCL22 has been shown to be related to the H2 receptor using H1R/H2R agonists and antagonists on human peripheral blood monocytes, human and mouse monocytes/macrophages19, 20), and to macrophage phenotypes (Supplemental Fig. 5). The relationship between the expression of histamine receptors and macrophage subtypes revealed that the H1 receptor was dominant in M1- like macrophages, whereas in M2-like macrophages a significant difference in expression of H1 and H2 receptors was not noted.
Supplemental Fig. 5.
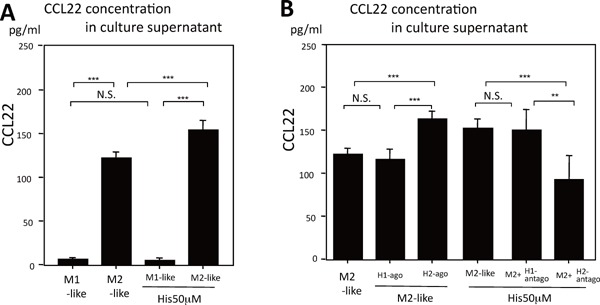
CCL22 expression in response to histamine and effects of histamine agonist and antagonist in J774A.1 cells
A) Culture supernatants of J774A.1 cells (1 × 106 cells/mL) containing CCL22 differentiated into M1-/M2-like cells in 24 h culture with/without 50 µM histamine (Wako) were measured by enzyme-linked immunoassay (ELISA; R&D Systems, Minneapolis, MN, USA). Histamine increased the concentration of CCL22 in the culture supernatant of M2-like macrophages. In contrast, M1-like macrophages were hardly affected by histamine, and did not significantly express CCL22 protein compared with M2-like macrophages.
B) Expression of CCL22 in J774A.1 cells incubated for 24 h with 50 µM histamine, 50 µM H1 or 50 µM H2 receptor agonist (2-pyridylethylamine dihydrochloride and dimaprit dihydrochloride, respectively from Wako), or 50 µM histamine added with 10 µM pyrilamine (H1 receptor blocker from Sigma) or 50 µM histamine added with 10 µM cimetidine (H2 receptor blocker from Sigma).
CCL22 levels increased significantly when the histamine H2 agonist was added to the culture, but were not influenced by the histamine H1 agonist. The increase of CCL22 from M2-macrophages by 50 µM histamine stimulation was significantly reduced by the addition of pyrilamine, but not cimetidine.
H1-ago = 2-pyridylethylamine dihydrochloride, H2-ago = dimaprit dihydrochloride, H1-antago = pyrilamine, H2-antago = cimetidine
The values are the mean ± SD of triplicate measurements from 3 independent experiments (**P < 0.01, ***P < 0.001, N.S: not significant).
Our group has also been studying the causal relationship between histamine and atherosclerosis. In in vitro experiments of atherosclerosis and in the carotid artery-ligated model-mice, the H1 receptor predominantly induced the development of atherosclerosis21, 33); however, in apoE-KO mice, the H2 receptor played a major role in the development of atherosclerosis32). In addition, it has also been shown that H1 antagonists, but not H1 agonists, increased the progression of atherosclerosis34). IL-4 has long been considered as an anti-atherosclerotic substance and, in fact, is also known to promote atherosclerosis35). The presence of CCL22 was considered to be one reason for such paradoxical phenomena in atherosclerosis.
In conclusion, the expression of H1 and H2 receptors, and the migration of Th1 and Th2 cells influenced the expression balance in M1- and M2-like macrophages and may be involved in the processes and progression of atherosclerosis (Fig. 7). Although the cellular microenvironment and cell–cell interactions have recently been gaining increasing attention in studies on the pathology of cancer36), it may be that a similar pathology exists in atherosclerosis.
Fig. 7.
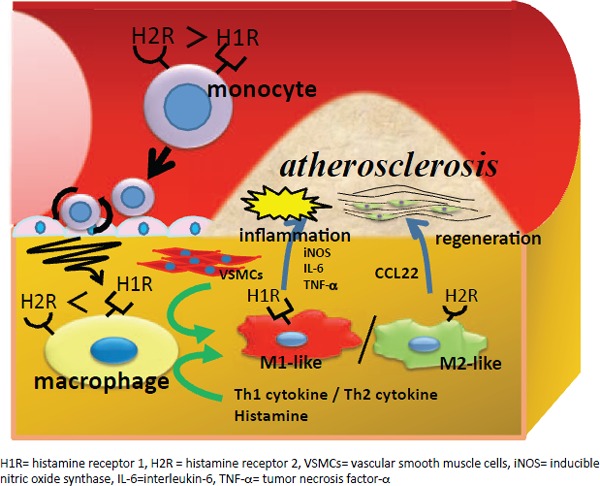
Diagram of proposed mechanisms for atherosclerosis
Both M1- and M2-like macrophages are present in atherosclerotic lesions. Their balance is associated with the distribution of VSMC and is regulated by histamine and cytokines that affect atherosclerosis.
Acknowledgments
The authors would like to thank Hana Nishimura, Rie Soeda, Yumiko Igawa, Fusa Murayama, Sayumi Kimura, and Miyo Kimura for their expert technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest associated with the manuscript.
References
- 1). Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997; 185: 1595-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arteriovascular Thromb Vasc Biol. 2008; 28: 1928-1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Libby P, Hansson GK. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991; 64: 5-15 [PubMed] [Google Scholar]
- 4). Tjurmin A. V., Ananyeva NM, Smith EP, Gao Y, Hong MK, Leon MB, Haudenschild CC. Studies on the histogenesis of myxomatous tissue of human coronary lesions. Arterioscler Thromb Vasc Biol. 1999; 19: 83-97 [DOI] [PubMed] [Google Scholar]
- 5). Cao W, Bobryshev YV, Lord RS, Oakley RE, Lee SH, Lu J. Dendritic cells in the arterial wall express C1q: Potential significance in atherogenesis. Cardiovasc Res. 2003; 60: 175-186 [DOI] [PubMed] [Google Scholar]
- 6). Greaves DR, Häkkinen T, Lucas AD, Liddiard K, Jones E, Quinn CM, Senaratne J, Green FR, Tyson K, Boyle J, Shanahan C, Weissberg PL, Gordon S, Ylä-Hertualla S: Linked chromosome 16q13 chemokines, macrophage-derived chemokine, fractalkine, and thymus- and activation-regulated chemokine, are expressed in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001; 21: 923-929 [DOI] [PubMed] [Google Scholar]
- 7). Hashimoto S, Suzuki T, Dong HY, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocytes and macrophages. Blood. 1999; 94: 837-844 [PubMed] [Google Scholar]
- 8). Hashimoto S, Suzuki T, Dong HY, Nagai S, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocyte-derived dendritic cells. Blood. 1999; 94: 845-852 [PubMed] [Google Scholar]
- 9). Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, Wick G. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler Thromb Vasc Biol. 2001; 21: 503-508 [DOI] [PubMed] [Google Scholar]
- 10). Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005; 142: 481-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). De Paoli F, Staels B, Chinetti-Gbaguidi G. Macrophage phenotypes and their modulation in atherosclerosis. Circ J. 2014; 78: 1775-1781 [DOI] [PubMed] [Google Scholar]
- 12). Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016; 44: 450-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009; 29: 1419-1423 [DOI] [PubMed] [Google Scholar]
- 14). Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010; 32: 593-604 [DOI] [PubMed] [Google Scholar]
- 15). Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007; 6: 137-143 [DOI] [PubMed] [Google Scholar]
- 16). Komohara Y, Fujiwara Y, Ohnishi K, Shiraishi D, Takeya M. Contribution of Macrophage Polarization to Metabolic Diseases. J Atheroscler Thromb. 2016; 23: 10-17 [DOI] [PubMed] [Google Scholar]
- 17). Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004; 25: 677-686 [DOI] [PubMed] [Google Scholar]
- 18). Sasaguri Y, Tanimoto A. Role of macrophage-derived histamine in atherosclerosis-- chronic participation in the inflammatory response --. J Atheroscler Thromb. 2004; 11: 122-130 [DOI] [PubMed] [Google Scholar]
- 19). Kimura S, Tanimoto A, Wang KY, Shimajiri S, Guo X, Tasaki T, Yamada S, Sasaguri Y. Expression of macrophage-derived chemokine (CCL22) in atherosclerosis and regulation by histamine via the H2 receptor. Pathol Int. 2012; 62: 675-683 [DOI] [PubMed] [Google Scholar]
- 20). Kimura S, Wang KY, Yamada S, Guo X, Nabeshima A, Noguchi H, Watanabe T, Harada M, Sasaguri Y. CCL22/Macrophage-derived chemokine expression in apolipoprotein E-deficient mice and effects of histamine in the setting of atherosclerosis. J Atheroscler Thromb. 2015; 22: 599-609 [DOI] [PubMed] [Google Scholar]
- 21). Yamada S, Wang KY, Tanimoto A, Guo X, Nabeshima A, Watanabe T, Sasaguri Y. Histamine receptors expressed in circulating progenitor cells have reciprocal actions in ligation-induced arteriosclerosis. Pathol Int. 2013: 435-447 [DOI] [PubMed] [Google Scholar]
- 22). Wang KY, Arima N, Higuchi S, Shimajiri S, Tanimoto A, Murata Y, Hamada T, Sasaguri Y. Switch of histamine receptor expression from H2 to H1 during differentiation of monocytes into macrophages. FEBS Lett. 2000; 473: 345-348 [DOI] [PubMed] [Google Scholar]
- 23). Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993; 362: 801-809 [DOI] [PubMed] [Google Scholar]
- 24). Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nat Med. 2001; 7: 382-383 [DOI] [PubMed] [Google Scholar]
- 25). Leon ML, Zuckerman SH. Gamma interferon: a central mediator in atherosclerosis. Inflamm Res. 2005; 54: 395-411 [DOI] [PubMed] [Google Scholar]
- 26). Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008; 28: 812-819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Filonzi EL, Zoellner H, Stanton H, Hamilton JA. Cytokine regulation of granulocyte-macrophage colony stimulating factor and macrophage-colony stimulating factor production in human arterial smooth muscle cells. Atherosclerosis. 1993; 99: 241-252 [DOI] [PubMed] [Google Scholar]
- 28). Seshiah PN, Kereiakes DJ, Vasudevan SS, Lopes N, Su BY, Flavahan NA, Goldschmidt-Clermont PJ. Activated monocytes induce smooth muscle cell death: role of macrophage colony-stimulating factor and cell contact. Circulation. 2002; 105: 174-180 [DOI] [PubMed] [Google Scholar]
- 29). Stoneman VE, Bennett MR. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond). 2004; 107: 343-354 [DOI] [PubMed] [Google Scholar]
- 30). Sasaguri Y, Wang KY, Tanimoto A, Tsutsui M, Ueno H, Murata Y, Kohno Y, Yamada S, Ohtsu H. Role of histamine produced by bone marrow-derived vascular cells in pathogenesis of atherosclerosis. Circ Res. 2005; 96: 974-981 [DOI] [PubMed] [Google Scholar]
- 31). Yamada S, Wang KY, Tanimoto A, Sasaguri Y. Novel function of histamine signaling in hyperlipidemia-induced atherosclerosis: Histamine H1 receptors protect and H2 receptors accelerate atherosclerosis. Pathol Int. 2015; 65: 67-80 [DOI] [PubMed] [Google Scholar]
- 32). Cronshaw DG, Kouroumalis A, Parry R, Webb A, Brown Z, Ward SG. Evidence that phospholipase-C-dependent, calcium-independent mechanisms are required for directional migration of T-lymphocytes in response to the CCR4 ligands CCL17 and CCL22. J Leukoc Biol. 2006; 79: 1369-1380 [DOI] [PubMed] [Google Scholar]
- 33). Kimura S, Wang KY, Tanimoto A, Murata Y, Nakashima Y, Sasaguri Y. Acute inflammatory reactions caused by histamine via monocytes/macrophages chronically participate in the initiation and progression of atherosclerosis. Pathol Int. 2004; 54: 465-474 [DOI] [PubMed] [Google Scholar]
- 34). Raveendran VV, Smith DD, Tan X, Sweeney ME, Reed GA, Flynn CA, Tawfik OW, Milne G, Dileepan KN. Chronic ingestion of H1-antihistamines increase progression of atherosclerosis in apolipoprotein E-/- mice. PLoS One. 2014; 9: e102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003; 163: 1117-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. J Pathol. 2017; 241: 313-315 [DOI] [PubMed] [Google Scholar]


