Abstract
This study describes a patient who experienced hepatobiliary Mycobacterium avium infection associated with neutralizing anti–interferon gamma (IFN-γ) autoantibodies during treatment for disseminated M. avium disease. Hepatobiliary M. avium infection should be considered in jaundiced patients with neutralizing anti–IFN-γ autoantibodies, including those receiving antimycobacterial therapy for disseminated M. avium disease.
Keywords: Anti–interferon gamma (IFN-γ) autoantibody, disseminated Mycobacterium aviumcomplex infection, hepatobiliary infection, jaundice, Mycobacterium aviumcomplex (MAC)
Neutralizing anti–interferon gamma (IFN-γ) autoantibodies have been reported to cause adult-onset immunodeficiency, resulting in severe opportunistic infections due to intracellular pathogens such as fungi and mycobacteria [1], [2], [3]. Disseminated infections caused by nontuberculous mycobacteria (NTM) involve various organs, primarily the lungs, lymph nodes and skin [1], [2], [4]. To our knowledge, however, hepatobiliary NTM infection with obstructive jaundice has never been reported.
A 74-year-old Japanese man sought care at a previous hospital with a 2-month history of general fatigue and lymphadenopathy. The findings at physical examination were unremarkable except for cervical and axillary lymphadenopathy. A chest computed tomography revealed right supraclavicular, mandibular and bilateral axillary lymphadenopathy and bilateral psoas abscess. Culture of biopsy samples of his right supraclavicular and axillary lymph nodes was positive for Mycobacterium avium, indicating disseminated M. avium disease. He was treated by antimycobacterial therapy with rifampicin (450 mg per day), ethambutol (750 mg per day) and clarithromycin (800 mg per day). Standard antimycobacterial therapy for over 2 years improved his clinical symptoms and lesions.
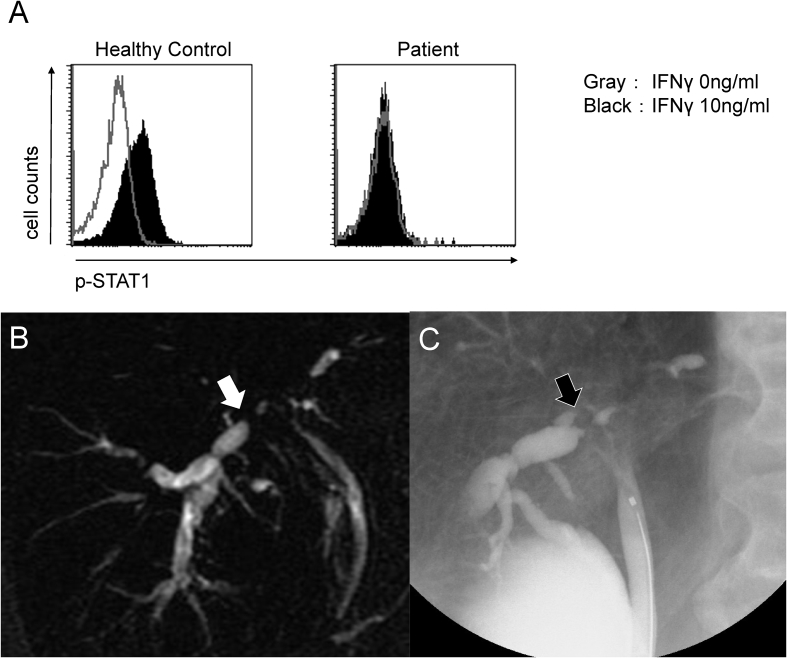
One month later, he was admitted to our hospital with portal hepatic and right axillary lymphadenopathy, which were positive for M. avium. Total bilirubin level was 6.1 mg/dL, and direct-reacting bilirubin level was 3.9 mg/dL. Immunoglobulin levels were within the normal range (IgG, 1555 mg/dL; IgM, 126 mg/dL; IgA, 348 mg/dL). HIV antibody testing was negative, and his CD4/CD8 lymphocyte counts were within normal ranges. Epstein-Barr virus nuclear antigen, viral capsid antigen IgG 2.6 and viral capsid antigen IgM 0.9 were detected by the enzyme immunoassay method. The positive control for QuantiFERON-TB Gold (QFT-3G) was undetectable. We detected a high titre of serum neutralizing anti–IFN-γ autoantibodies by enzyme-linked immunosorbent assay (patient, 50.85 EU; control, 25.49 EU). These antibodies are also potent inhibitors of IFN-γ–stimulated signal transducer and activator of transcription 1 (STAT1) phosphorylation in leukocytes using a previously described method [2] (Fig. 1(A)). Briefly, recombinant human IFN-γ was added to Jurkat cells inoculated with serum. After the cells were permeabilized, anti–phospho-STAT1 antibodies were conjugated, and the cells were subsequently analysed by flow cytometry. According to the results of this assay, anti–IFN-γ autoantibodies were proved to have a neutralizing effect in this patient.
Fig. 1.
(A) Analysis of IFN-γ–induced STAT1 phosphorylation in leukocytes by flow cytometry. IFN-γ (10 ng/mL) increased p-STAT1 in healthy controls but did not increase p-STAT1 in patient. Recombinant human IFN-γ was added to Jurkat cells inoculated with serum. After cells were permeabilized, anti-pSTAT1 antibodies were conjugated, and cells were subsequently analysed by flow cytometry, revealing that anti–IFN-γ autoantibodies worked as neutralizing antibodies in this patient. (B) MRCP showing intrahepatic bile duct dilation (white arrow). (C) Endoscopic retrograde cholangiopancreatography showing hilar bile duct stricture (black arrow). IFN-γ, interferon gamma; MRCP, magnetic resonance cholangiopancreatography; p-STAT1, phosphorylated signal transducer and activator of transcription 1; STAT1, signal transducer and activator of transcription 1.
After the detection of anti–IFN-γ autoantibodies, we continued antimycobacterial chemotherapy. After 1 year of therapy, he sought care for jaundice and systemic pruritus. Contrast-enhanced abdominal computed tomography and magnetic resonance cholangiopancreatography (Fig. 1(B)) and hilar bile duct stricture by endoscopic retrograde cholangiopancreatography (ERCP) (Fig. 1(C)) revealed intrahepatic bile duct dilation. PCR and culture from bile was positive for M. avium, whereas sputum, blood and bone marrow results were negative, resulting in a diagnosis of hepatobiliary M. avium infection. In addition to continuing the same antimycobacterial regimen, a stent was placed in the bile duct after insertion of an endoscopic nasobiliary drainage tube. His symptoms and laboratory findings improved, with no relapse observed during 2 years of treatment.
HIV-associated cholangiopathy due to biliary obstruction resulting from a benign stricture of the biliary tract has been reported [5]. Patients with HIV-associated cholangiopathy showed near-normal bilirubin concentrations, with only a few patients requiring a procedure to resolve the biliary obstruction [6], suggesting the pathogenesis of cholangiopathy in our patient was likely not HIV associated, while neutralizing anti–IFN-γ autoantibodies could cause similar immunodeficiency in advanced HIV infection.
To our knowledge, non–HIV-associated hepatobiliary NTM infection has never been reported. Although adjunct therapy with rituximab was considered in our patient [7], stent placement during ERCP had successfully improved patient outcome without disease relapse. Hepatobiliary M. avium infection should be considered in the differential diagnosis of jaundiced patients with neutralizing anti–IFN-γ autoantibodies, even those receiving antimycobacterial therapy for disseminated M. avium disease [8]. In addition to antimycobacterial therapy, biliary decompression by stent placement using the ERCP procedure may be an effective treatment option.
Acknowledgement
Supported in part by the Japan Agency for Medical Research and Development (project 17fk0108116h0401).
Conflict of interest
None declared.
References
- 1.Browne S.K., Burbelo P.D., Chetchotisakd P., Suputtamongkol Y., Kiertiburanakul S., Shaw P.A. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki A., Sakagami T., Yoshizawa K., Shima K., Toyama M., Tanabe Y. Clinical significance of interferon-gamma neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clin Infect Dis. 2018;66:1239–1245. doi: 10.1093/cid/cix996. [DOI] [PubMed] [Google Scholar]
- 3.Asakura T., Namkoong H., Sakagami T., Hasegawa N., Ohkusu K., Nakamura A. Disseminated Mycobacterium genavense infection in patient with adult-onset immunodeficiency. Emerg Infect Dis. 2017;23:1208–1210. doi: 10.3201/eid2307.161677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi C.Y., Lin C.H., Ho M.W., Ding J.Y., Huang W.C., Shih H.P. Clinical manifestations, course, and outcome of patients with neutralizing anti–interferon-gamma autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003927. e3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margulis S.J., Honig C.L., Soave R., Govoni A.F., Mouradian J.A., Jacobson I.M. Biliary tract obstruction in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;105:207–210. doi: 10.7326/0003-4819-105-2-207. [DOI] [PubMed] [Google Scholar]
- 6.Tonolini M., Bianco R. HIV-related/AIDS cholangiopathy: pictorial review with emphasis on MRCP findings and differential diagnosis. Clin Imaging. 2013;37:219–226. doi: 10.1016/j.clinimag.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Browne S.K., Zaman R., Sampaio E.P., Jutivorakool K., Rosen L.B., Ding L. Anti-CD20 (rituximab) therapy for anti–IFN-gamma autoantibody–associated nontuberculous mycobacterial infection. Blood. 2012;119:3933–3939. doi: 10.1182/blood-2011-12-395707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valour F., Perpoint T., Sénéchal A., Kong X.F., Bustamante J., Ferry T. Interferon-γ autoantibodies as predisposing factor for nontuberculous mycobacterial infection. Emerg Infect Dis. 2016;22:1124–1126. doi: 10.3201/eid2206.151860. [DOI] [PMC free article] [PubMed] [Google Scholar]