Abstract
Vulvar carcinoma is the fourth most common gynecological malignancy. Two separate carcinogenic pathways are suggested, where one is associated with the human papillomavirus (HPV) and HPV16 the most common genotype.
The aim of this study was to evaluate HPV-markers in a set of primary tumors, metastases and recurrent lesions of vulvar squamous cell carcinomas (VSCC). Ten HPV16-positive VSCC with metastatic regional lymph nodes, distant lymphoid/hematogenous metastases or local recurrent lesions were investigated for HPV genotype, HPV16 variant, HPV16 viral load, HPV16 integration and HPV16 E2BS3 and 4 methylation.
In all 10 analyzed case series, the same HPV genotype (HPV16), HPV16 variant and level of viral load were detected in all lesions within a patient case. Primary tumors with a high E2/E6 ratio were found to have fewer vulvar recurrences and/or metastases after diagnosis and treatment. Also, a significantly lower viral load was evident in regional lymph nodes compared to primary tumors.
The data presented strengthens the evidence for a clonal HPV-induced pathway for vulvar carcinoma
Keywords: Vulvar carcinoma, Human papillomavirus, Recurrences, Metastases, Integration, Viral load
1. Introduction
Human papillomavirus (HPV) infection is an important etiological factor of cervical cancer and this virus has also been strongly associated with other ano-genital malignancies. In vulvar carcinoma, two separate carcinogenic pathways are suggested, one being HPV-associated [1], [2] with HPV16 as the most common genotype [3], [4], [5], [6], [7]. This the fourth most common invasive gynecological cancer affects mostly older women and the incidence rates are highest in the western countries [8]. Prognostic factors include lymph node status as well as tumor size and tumor recurrences [9], [10], [11]. Relapse (vulvar, LN metastases or hematogenous metastases) occurs in up to 40% of all patients [10], [12], but no single factor has been found to be prognostically relevant [13]. The prognostic role of HPV in vulvar cancer is debated, where some studies have been able to show a better survival for women with HPV-positive vulvar tumors [14], [15], others have not [4], [16], [17]. Even less in known about viral spread and impact on metastasis.
The multi-step process of metastasis is often the cause of mortality in cancer [18], [19]. The metastasis cascade describes events necessary for cell dislocation from primary site, vascular transport, adhesion to a new site, and reinitiating of proliferation. Besides the necessary genetic and epigenetic alterations, signals are also preprocessed by surrounding cells and by diverse transcription factors [20], [21], [22]. Vulvar carcinomas metastasize via the lymphatic system to inguinal or femoral, but also to pelvic and distant lymph nodes. However, hematogenous spread is unusual [9], [23].
In cervical cancer, HPV in lymph nodes has been found to be a predictive factor for recurrences [24], [25], but not for patient prognosis [26], [27]. Fewer studies have been conducted in vulvar carcinomas. Lindell et al. [14] reported that HPV-positive primary tumors also had HPV-positive metastatic lymph nodes, but the presence of HPV in lymph nodes did not affect patient prognosis. Pinto et al. could confirm a 100% HPV presence in metastatic lymph nodes from HPV-positive primary tumors [17]. In an earlier study [28] the concordance of HPV in metastatic lymph nodes to primary tumor was 82% (9/11). HPV genotypes found in lymph nodes have been found to be identical to genotypes found in primary tumors [14], [17], [28].
The capacities of the viral oncoproteins E6 and E7, such as generating cell genomic instability and prolonged cell proliferation [29] might also be important in a metastatic scenario, where a high renewal capacity is important. In addition, E6 and E7 have been found to initiate epithelial-mesenchymal transition (EMT) [30], an example of how the virus promotes dislocation of cells and metastatic spread. Viral load and other viral markers affecting oncoproteins could theoretically affect metastatic abilities. Integration of viral genes has been shown to increase levels of E6 and E7 [29] when the regulatory E2 protein no longer binds to conserved E2-binding sites in the long control region (LCR) of the virus. Methylation of the same E2-binding sites provides another mechanism whereby regulation is disturbed. This epigenetic process likewise prohibits the regulatory E2 binding resulting in high levels of viral oncoproteins [31], potentially influencing cancer cell invasiveness.
The aim of this study was to evaluate HPV-markers in a set of primary tumors, metastases and vulvar recurrent lesions of vulvar SCC to achieve knowledge of tumor biology and viral occurrence.
2. Material and method
2.1. Material
Ten HPV16-positive vulvar squamous cell carcinomas (VSCC) with metastatic regional lymph nodes, distant lymphoid/hematogenous metastases or local recurrent lesions were investigated for viral characteristics, Table 1. Vulvar carcinomas were a subset from a consecutive series of 133 VSCC collected between 1983 and 2008 [4] from which there were follow-up material. Metastatic regional lymph nodes were either present at diagnosis (n = 5) or after disease free intervals at a minimum of 28 months (n = 2). Distant metastases (lymphoid and hematogenous) were detected after a minimum of disease free intervals of 9 months. Vulvar recurrences were defined as a new vulvar lesion after patient treatment (minimum of two months).
Table 1.
Characteristics of ten VSCC with metastatic regional lymph nodes, distant metastases or recurrent lesions included in the study. LN= lymph node. Histology classification (histopathologic type of squamous cell carcinoma: basaloid, mixed, keratinizing) performed by pathologist (MK). Stage at diagnosis. Viral load = copies of HPV16 E6 in 20 ng of total DNA. Viral load per cell = viral copies/(HBB copies/2). E1/E6 and E2/E6 = ratios of viral copy numbers of E1 and E2 compared to E6. E2BS3 and 4 = mean of methylation levels in E6 positions 37, 43, 52 and 58. Distant met. = distant metastasis.
|
Patients |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Primary VSCC | Stage IV | Stage III | Stage III | Stage IV | Stage II | Stage II | Stage IV | Stage II | Stage III | Stage II | |
| Mixed | Basaloid | Keratinizing | Keratinizing | Mixed | Basaloid | Basaloid | Mixed | Basaloid | Basaloid | ||
| Regional LN | At diagnosis | At diagnosis | At diagnosis | After disease free interval | At diagnosis | After disease free interval | At diagnosis | ||||
| Distant met. | Lymphoid | Hematogenous | |||||||||
| First Recurrence | Mixed | Basaloid | Keratinizing | Mixed | Basaloid | ||||||
| Last Recurrence | Basaloid | ||||||||||
| HPV type | Primary VSCC | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| Regional LN | 16 | 16 | 16 | 16 | 16 | 16 | 16 | ||||
| Distant met. | 16 | 16 | |||||||||
| Recurrence | 16 | 16 | 16 | 16 | 16 | ||||||
| Recurrence | 16 | ||||||||||
| HPV16 variant | Primary VSCC | E-p | E-G350G | E-p | E-C109G | AA/NA1 | E-p | E-G131T | E-p | E-G350G | E-G350G |
| Regional LN | E-p | E-G350G | E-p | E-C109G | E-G131T | E-p | E-G350G | ||||
| Distant met. | E-G350G | E-G350G | |||||||||
| Recurrence | AA/NA1 | E-p | E-G131T | E-p | E-G350G | ||||||
| Recurrence | E-p | ||||||||||
| Viral load | Primary VSCC | 5396 | 59.3 | 21.1 | 2236 | 573 | 16.1 | 2637 | 11520 | 8.47 | 17010 |
| Regional LN | 1283 | 16.1 | 40.7 | 116 | 1318 | 8960 | 0.79 | ||||
| Distant met. | 17 | 2488 | |||||||||
| Recurrence | 674 | 23 | 981 | 28270 | 16550 | ||||||
| Recurrence | 16.2 | ||||||||||
| Viral load per cell | Primary VSCC | 105.8 | 1.85 | 5.17 | 62.3 | 32.1 | 0.59 | 320 | 1477 | 0.275 | 565 |
| Regional LN | 22.7 | 7.16 | 20.6 | 245 | 31.9 | 586 | 0.003 | ||||
| Distant met. | 0.33 | 363 | |||||||||
| Recurrence | 27.7 | 2.5 | 50 | 1303 | 470 | ||||||
| Recurrence | 1.71 | ||||||||||
| E1/E6 | Primary VSCC | 4.78 | 0.54 | 0.74 | 0.68 | 0.03 | 0.78 | 0.65 | 0.73 | 0.87 | 1.04 |
| Regional LN | 4.29 | 0.52 | 0.61 | 0.51 | 0.75 | 0.65 | 0.46 | ||||
| Distant met. | 0.90 | 0.85 | |||||||||
| Recurrence | 0.11 | 0.74 | 0.64 | 0.72 | 0.89 | ||||||
| Recurrence | 0.73 | ||||||||||
| E2/E6 | Primary VSCC | 6.34 | No result | 4.29 | 1.14 | 0.82 | 0.02 | 0.92 | 0.05 | 1.37 | 2.46 |
| Regional LN | 9.10 | No result | 2.22 | 1.08 | 1.09 | 0.05 | 0.98 | ||||
| Distant met. | 1.78 | 1.69 | |||||||||
| Recurrence | 1.13 | No result | 0.84 | 0.03 | 1.19 | ||||||
| Recurrence | No result | ||||||||||
| E2BS3 and 4 methylation (%) | Primary VSCC | 4.7 | 3.2 | 3.6 | 2.0 | 1.6 | 3.0 | 10 | 91.6 | No result | 73.9 |
| Regional LN | 4.9 | 2.3 | 2.5 | 1.1 | 12 | 90.4 | 1.5 | ||||
| Distant met. | No result | 55.1 | |||||||||
| Recurrence | 2.1 | 1.5 | 5.9 | 86.2 | 50.6 | ||||||
| Recurrence | 2.6 | ||||||||||
From the consecutive series of 133 VSCC, two HPV-16 positive LN without histological evidence of metastases were available for analysis of potential HPV micro metastases. Lymph nodes were handled according to diagnostic routine and sectioned with two non-serial sections for each node followed by eosin staining.
All samples were formalin fixed and paraffin embedded (FFPE) and collected for diagnostic purposes. The 12 patients originated from Örebro University Hospital, and the central hospitals in Falun and Eskilstuna. Clinical data were gathered from patient records at the Department of Oncology, Örebro University Hospital. Dates for diagnosis and follow-up were collected from the laboratory data system and referred to date of report from the pathologist. The study was approved by the regional ethics committee board in Uppsala (Dnr 2008/294).
2.2. DNA extraction
DNA was extracted with Qiamp DNA Minikit (Qiagen, Hilden, Germany) according to instructions by the manufacturer, either using 1 mm biopsy punches (Miltex GmbH, Germany) or sections of tissue blocks (2–5, 10 µm cuttings). To select relevant areas for DNA extraction, stained sections were evaluated by a pathologist (MK, Örebro cases). A histological classification of primary VSCC, vulvar recurrences and hematogenous metastasis was performed (MK).
2.3. HPV genotyping with real time PCR
Anyplex II HPV28 (Seegene, Seoul, Korea) was used for HPV detection and genotyping. This PCR assay detects 28 HPV genotypes (HPV6, 11, 16, 18, 26, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 60, 61, 66, 68, 69, 70, 73 and 82) in 2 reactions, also including the human control gene beta-globulin. Results are semi-quantitative, with melting curve analysis after 30, 40 or 50 cycles of PCR. The method has been found useful for FFPE material and is described elsewhere [32].
2.4. HPV16 variant determination with pyrosequencing
Seven positions in the E6-gene (nt 109, 131, 132, 143, 145, 178 and 350, reference sequence NC_001526) were investigated with PCR and pyrosequencing as previously described [4]. PCR products covering all seven positions were sequenced using PSQ 96 MA system (Qiagen, Hilden, Germany). Peaks were compared to a reference genome holding the European variant E-p and variants were designated according to Swan et al. [33]. Variant data for the primary VSCC has been published elsewhere [4].
2.5. HPV16 viral load using droplet digital PCR (ddPCR)
HPV16 and human control gene HBB were assessed in the same reaction. As previously described [34], absolute quantities of PCR-amplified fragments are obtained from nano-liter sized droplets, where molecules are encapsulated in water-in-oil partitions. Briefly, samples (20 ng of DNA in 5 µl) were combined with ddPCR Supermix for Probes (no dUTP, BIO-RAD Hercules, CA USA), primers and probes (Applied biosystems, Hilden, Germany) targeting the E6 gene of HPV 16 and HBB. Primers and probes according to Lillsunde Larsson et al. [32], [34]. Droplet reader software results provide copies/µl of each target and results are presented both as HPV16 copies in 20 ng of DNA as well as copy number per cell (viral copies/(HBB copies/2)). Viral load data has been presented for primary VSCC [34].
2.6. HPV16 integration using droplet digital PCR (ddPCR)
HPV16 genes E2 and E1 were combined with viral E6 in duplex reactions (E1 + E6 and E2 + E6) using ddPCR. Primers and probe sequences for E2 region was used according to Peitsaro et al. [35] while primers and probe for E6 region from Lillsunde Larsson et al. [34]. For E1 region, design of ddPCR amplicon was made using Primer3Plus, covering a 122 bp stretch of the gene (nt1259–1380, Genbank ID: KO2718), F: GCGGGTATGGCAATACTGAA, R: ACTGTACTGACTGCAACCAC and probe: TCAGCAGATGTTACAGGTAGAAGGGCGCCA. For signal detection, black hole quencher was used in combination with reporters FAM (E6) and VIC (E1 and E2).
ddPCR mastermix included 1x ddPCR Supermix for Probes (no dUTP, BIO-RAD), 0.9 µM primer and 0.25 µM probe (Applied Biosystems) together with 5 µl of cleaved sample DNA (restriction enzyme BamH1; Sigma-Aldrich, Schnelldorf, Germany). Droplet generation was performed in QX200 Droplet Generator™ (BIO-RAD). Transferred droplets were amplified in the Veriti 96 well thermal cycler (Applied Biosystems) with a ramp rate at 2 °/s. Primarily, enzyme was activated 10 min at +95 °C where after 40 cycles followed of +94 °C for 30 s and +62 °C for one minute. Deactivation of enzyme was done at +98 °C. The QX200 Droplet reader™ (BIO-RAD) and Quantasoft Version 1.6.6 was used to analyze ddPCR data. Negative controls (NTC=non-template control) were included to verify the absence of contamination between samples and to situate threshold. Ratios of E1/E6 and E2/E6 viral load were calculated. A ratio of 1.0 indicated equal amounts of E1, E2 and E6 and ratios below 1.0 a loss of E1 and E2 copies compared to E6 copies.
Triplicate results from five samples were used for intraassay calculation and for interassay calculations; three different runs of same samples were compared. For E1/E6 combination, interassay CV was 3.2–11.9% for E1 and 2.0–4.9% for E6, respectively, while intraassay CV was 0.9–8.7% (E1) and 1.0–5.3% (E6). For E2/E6 combination, interassay CV was 0.9–6.1% for E2 and 0.6–13.1 for E6, while intraassay CV was 2.2–6.6% (E2) and 1.4–8.1% (E6), respectively.
2.7. Methylation of viral E2BS3 and 4 with bisulphite treatment, PCR and pyrosequencing
DNA was treated with bisulphite according to the manufacturer using EZ DNA Methylation-Gold (Zymo research, Irvine, USA). PCR for bisulphite treated DNA of the region has been described elsewhere [36]. Briefly, nucleotide positions 37, 43, 52 and 58, (Genbank ID: KO2718) were identified in one PCR reaction using duplicate reactions. Single-strand sequencing template was sequenced on the PSQ 96 MA system using PyroMark TM Gold Q96 Reagents (Qiagen). Peak results were analyzed in the Pyro Q-CpG TM Software using mean of sample results for calculation. E2BS3 and 4 methylation data for primary VSCC has been published elsewhere [36].
2.8. Statistics
Descriptive statistics was performed using IBM SPSS Statistics version 22 (IBM, New York, USA).
Mann–Whitney U-test was used to compare viral characteristics of the primary tumor, 1: with or without recurrences/metastasis after diagnosis, 2: with or without distant metastasis, and 3: with or without vulvar recurrences.
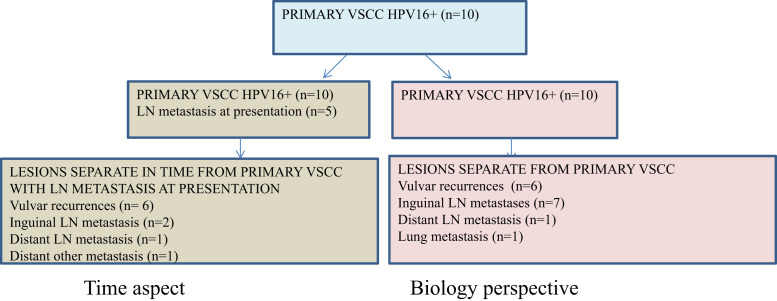
Mann–Whitney U-test was also used to investigate differences in viral characteristics between lesions at presentation compared to lesions detected after various disease-free intervals. In addition, it was used to compare viral characteristics between primary tumor and all other lesions in a biological approach, Fig. 1.
Fig. 1.
Groups for evaluation of viral characteristics in relation to time or biology. Two different approaches were used to investigate differences in viral characteristics between lesions at presentation compared to lesions detected after disease-free intervals (left) as well as to compare viral characteristics between primary VSCC and all other lesions (right) in a biological approach.
Fisher's exact test was used for comparison of proportions of viral characteristics between stage and histological classification. Groups were defined as above or below the mean value for each variable. The related-samples Wilcoxon signed rank test was used to compare median of differences between primary viral data and regional lymph nodes within cases.
3. Results
3.1. Clinical characteristics
Tumor stage distribution for this cohort was stage II in four patients, stage III in three patients and stage IV in three patients. Histological type of the primary tumors was basaloid squamous carcinoma (n = 5), keratinizing squamous carcinoma (n = 2) and mixed squamous carcinoma (n = 3). For vulvar recurrences, the same histological subtype as in primary tumor was found for all patients, except in one patient where the basaloid primary type was changed to keratinizing histology in the vulvar recurrent lesion, Table 1.
Primary surgery was performed for all 10 patients. Five women (50%) had a partial vulvectomy, four (40%) a local excision and one woman a radical vulvectomy (10%). All women received additional radiotherapy. The cancer-specific 5-year survival rate was 47%.
3.2. HPV characteristics
3.2.1. HPV genotype and HPV16 variant
All analyzed primary VSCC, regional/distant metastases and local recurrent lesions were positive for HPV16. Variant of HPV16 was also consistent in the patient series, Table 1. For the two cases with cancer-free inguinal lymph nodes, the primary VSCC was positive for HPV16 but lymph node was negative for HPV.
3.2.2. Viral characteristics in primary VSCC
Primary VSCC tumors were assessed for difference in viral characteristics in relation to: [1] recurrences or metastases after diagnosis, [2] distant metastases and [3] vulvar recurrences.
When comparing the viral characteristics in primary VSCC, there was no difference in viral load, viral load per cell, E1/E6 ratio or E2BS3 and 4 methylation between primary VSCC with local recurrences and metastases after diagnosis (n = 7) compared to primary VSCC without local recurrences and metastases after diagnosis (n = 3). However, for the E2/E6 ratio there was a borderline significant difference. Fewer recurrences were found in patients where primary tumors showed a high E2/E6 integration ratio (Mann–Whitney U-test; p = 0.056). In 2/3 of primary VSCC without vulvar recurrences and metastases after diagnosis, the E2/E6 ratio was very high (4.29 and 6.34).
There was no significant difference of any of the viral characteristics (viral load, viral load per cell, E1/E6, E2/E6 ratio or E2BS3 and 4 methylation) in primary VSCC when comparing patients that later had distant (lymphoid/ hematogenous) metastases (n = 2) compared to those that had no distant (lymphoid/ hematogenous) metastases (n = 8) nor between primary VSCC with (n = 5) or without (n = 5) local vulvar recurrences.
None of the investigated viral characteristics in primary VSCC were related to stage or histological classification.
3.2.3. Viral characteristics in relation to time or to biology
There was no difference in viral load, viral load per cell, E1/E6 ratio, E2/E6 ratio or E2BS3 and 4 methylation between lesions at presentation (primary VSCC and lymph node metastasis at presentation, n = 15) compared to lesions detected after disease-free intervals (local recurrences, regional and distant metastases, n = 10) nor between primary VSCC (n = 10) and all other lesions (biological approach, n = 15), Table 2.
Table 2.
Viral characteristics in relation to time or to biology. In the time approach, comparison is made between primary diagnosis samples (primary tumors and regional lymph node at diagnosis, n = 15) and lesions after disease-free intervals (n = 10). In the biology approach, primary tumors (n = 10) are compared to all other lesions (n = 15).
| Primary VSCC and LN metastasis at diagnosis | Lesions after disease-free intervals | Mann–Whitney U-test | |||||||
| Time approach | Range | Mean | Median | SD | Range | Mean | Median | SD | |
| Viral load | 0.79–17,010 | 2809 | 573 | 4989 | 16.2–28,270 | 5810 | 828 | 9566 | p = 0.461 |
| Viral load/cell | 0–1477 | 177 | 22.7 | 392 | 0.33–1303 | 283 | 38.9 | 421 | p = 0.461 |
| E1/E6 | 0.03–4.78 | 1.16 | 0.73 | 1.39 | 0.11–0.19 | 0.67 | 0.73 | 0.23 | p = 0.849 |
| E2/E6 | 0–9.10 | 2.05 | 1.09 | 2.62 | 0–1.78 | 0.78 | 0.96 | 0.71 | p = 0.374 |
| E2BS3 and 4 methylation | 1.6–91.6 | 19.3 | 4.15 | 30.4 | 1.10–90.4 | 29.7 | 4.25 | 37.2 | p = 0.709 |
| Primary VSCC at diagnosis | Lesions other than primary VSCC | Mann–Whitney U-test | |||||||
| Biology approach | Range | Mean | Median | SD | Range | Mean | Median | SD | |
| Viral load | 8.47–17,010 | 3948 | 1404 | 5846 | 0.79–28,270 | 4050 | 674 | 8099 | p = 0.723 |
| Viral load/cell | 0.27–1477 | 257 | 47.2 | 467 | 0–1303 | 194 | 24.5 | 362 | p = 0.765 |
| E1/E6 | 0.03–4.78 | 1.08 | 0.73 | 1.33 | 0.11–4.29 | 0.89 | 0.72 | 0.96 | p = 0.495 |
| E2/E6 | 0–6.34 | 1.74 | 1.03 | 2.08 | 0–9.10 | 1.41 | 1.08 | 2.24 | p = 0.683 |
| E2BS3 and 4 methylation | 1.6–91.6 | 21.5 | 3.60 | 35.1 | 1.10–90.4 | 24.9 | 4.90 | 32.9 | p = 0.861 |
3.2.4. Patient specific results
3.2.4.1. Viral load
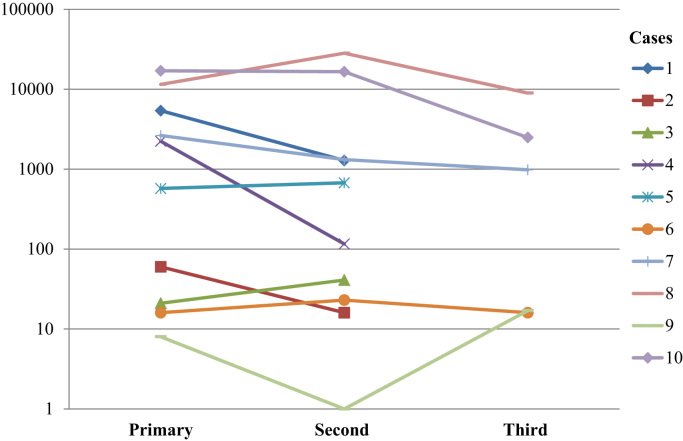
The level of viral load and viral load per cell varied between patients and the range of viral load was between 0.79 and 28,270 copies of HPV16 E6 in 20 ng of total DNA. The range of viral load per cell was between 0.003 and 1477 (Table 1). The viral load was found to be relatively consistent among lesions from the same patient (Fig. 2).
Fig. 2.
Distribution of viral load among lesions in patients 1–10. Logarithmic scale. Viral load presented as HPV16 E6 copies in 20 ng of total DNA. Time points refer to the lesions in the order they were diagnosed.
6/7 patients had a lower viral load in the regional LN metastases compared to the primary vulvar SCC and this difference was statistically significant (Related-samples Wilcoxon signed rank test; p = 0.043). For viral load per cell, 4/7 patients had lower viral load per cell in the regional LN metastases than in the primary vulvar SCC while 3/7 patients had more viral load per cell in the regional LN metastases than in the primary vulvar SCC (p = 0.499), Table 1.
3.2.4.2. E1/E6 and E2/E6
The range for the ratio of E1/E6 was between 0.03 and 4.78. All primary vulvar SCC, regional and distant metastases and recurrent lesions had some component of E1 present. E1/E6 ratios were generally equivalent through each patient series. 1/10 patient presented with abundant amount of E1 copies compared to E6 copies resulting in a high E1/E6 ratio.
The range for the ratio of E2/E6 was between 0.02 and 9.10. 1/10 patients had no PCR E2 result for either primary vulvar SCC or regional lymph node. 1/10 patients had low viral load for E2 compared to E6 in the primary VSCC which resulted a ratio of 0.02. For the same patient, there was no PCR E2 result in both recurrent vulvar samples.
1/10 patients had ratios above 1.1 for both E1/E6 and E2/E6. 5/10 patients had E2/E6 ratios above 1.1 but E1/E6 ratios below 1.1, Table 1.
3.2.4.3. Methylation of E2BS3 and 4
The range for E2BS3 and 4 methylation was between 1.1% and 91.6%. 6/10 patients had a lower E2BS3 and 4 methylation in the regional LN metastases, distal metastases or recurrent lesions compared to in the primary vulvar SCC. A 10% or lower E2BS3 and 4 methylation was found in 8/10 patients (in all lesions) and 2/10 patients had a 50% or higher E2BS3 and 4 methylation (in all lesions). 1/10 patients had no PCR result for E2BS3 and 4 in the primary VSCC or in the distant metastases. However, for the same patient, PCR was successful in the patient's regional lymph node, Table 1.
3.2.4.4. Histology
Vulvar recurrent lesions were also evaluated for histopathological type. 4/5 patients had same histological type in vulvar SCC and vulvar recurrent lesion. In 1/10 patients, a change was seen from the primary tumor to the recurrent lesion (basaloid to keratinizing histology), Table 1.
4. Discussion
The aim of this study was to evaluate HPV markers in a set of primary tumors, regional and distant metastases and vulvar recurrent lesions of vulvar SCC. The major findings in this study were: [1] The concordance of HPV positivity and HPV16 variants in all lesions from each patient, [2] A high E2/E6 ratio observed in the primary vulvar SCC of patients that did not have any vulvar recurrences or metastases after diagnosis, [3] A significantly lower viral load in regional lymph nodes compared to primary tumors. Thus, the data presented here strengthens the evidence for a clonal HPV-induced pathway for vulvar carcinoma.
There was a complete concordance of HPV within the 10 HPV16- positive VSCC with regional or distant metastases or vulvar recurrent lesions. Others have also shown high concordance of HPV in metastatic lymph nodes from HPV-positive vulvar carcinomas [14], [17], [28], but the presence of HPV-positive micro metastatic disease is more uncommon [14] and sparsely investigated. In this study, HPV could not be detected in the two HPV16-positive VSCC with cancer-free lymph nodes. Lymph nodes were worked-up according to clinical diagnostic procedures using only two sections for staining and not the entire lymph node. A thorough serial section might have revealed other findings and is advisable when planning for prospective studies.
Previous studies have also reported findings of the same HPV genotype in primary lesion and in lymph node and this study support these findings. The current study is, as far as we know, the first one investigating both recurrent vulvar lesions as well as regional and distant metastatic lesions for different HPV16 viral characteristics. We have shown that for HPV16-positive VSCC, the virus was included in cell spread from the original tumor site and was even detected in lesions far away from primary site. Distant spread in vulvar carcinoma is unusual [37] but in a few published case reports cardiac [38], kidney [39], liver and lung metastasis [40] are reported.
A substantial variation in all investigated viral characteristics (viral load, E1/E6, E2/E6 and viral methylation) was noted between patients. When evaluating viral characteristics in primary VSCC in relation to recurrences or metastases, only the ratio of E2/E6 showed borderline significance. Fewer recurrences were found in patients where primary tumors showed a high E2/E6 integration ratio. E1 and E2 are both early viral proteins involved in the initiation of viral transcription. E2 is also involved in regulation of viral transcription [41], [42]. A high ratio (E1/E6 or E2/E6) reflects an excess of E1 or E2 copies compared to E6 copies and argues for a viral genome in episomal state. In episomal cells the E1 and E2 genes are intact and without the disruption that is often seen upon integration in the human genome. Viral integration is often a rationale for dysregulation of the viral oncoproteins E6 and E7. There are however studies on HPV-positive cervical- and vulvar cancers that have shown tumors without viral integration. In that case, other mechanisms favor a malignant transformation [36], [43]. An amplification of viral E1 and E2 has been shown to enhance viral replication time in cells with stable episomes in productive infections [44], but viral amplification is uncommon in cancerous lesions [45]. In the patient series where ratios of both E1/E6 and E2/E6 where high, one explanation could be a continued viral amplification. Five patients had high ratios of E2/E6 but not of E1/E6. An excessive E2 protein can affect regulation of the E6 and E7 proteins because of its repressive function. This supports the result of a high E2/E6 ratio associated with patients not developing recurrences or metastases after diagnosis. High ratios may provide functional E2 protein regulating oncoproteins expression.
A complete loss of E2 (no PCR result) was present in two cases, while for E1, some proportion of the gene was consistent in all patient samples. The assays for integration (ddPCR) were used to achieve exact quantitation. Normally, standard curves are used to quantify samples when using real time PCR. With ddPCR this is no longer needed since the method itself provides exact quantities of the examined amplicon. The E2 assay was developed with primers from Peitsaro et al. [35] and the E1 amplicon in an area known as being a site of disruption if the virus becomes integrated [46], [47]. Besides the E2/E6 ratio, no other viral marker was associated with recurrences or metastasis after diagnosis. Other potential viral influencers could probably not be identified in a sparse material like this and this argues for larger studies in order to provide solid data for the clinical management of the disease.
No significant differences in any of the HPV16 viral characteristics were shown between lesions at presentation compared to lesions detected after disease-free intervals nor between primary VSCC and all other lesions. Hence, time elapsed and new milieu seem not to have affected viral load, integration or methylation in a specific direction when including all type of lesions. Despite these findings above, viral load in regional lymph nodes were significantly lower compared with viral load in the primary vulvar samples. In other studies, the HPV viral load in lymph node metastases were found to be equal to [48] or lower [49] than in the primary tumor. An association was not found for viral load per cell and may be caused by the different cell compositions. The excessive lymphoid cells in the lymph node can cause a dilution effect that is not as evident in a potentially intact tumor area.
Although viral load and viral load per cell varied somewhat between time points, the log viral load chart shows that patient results are relatively constant as either high or low. There is clearly variation between patients on how much virus is presented in their respective tumors, metastases and vulvar recurrences. For tumors with a lower viral load, other mutational pressure might be present instead.
There were differences in terms of ratios (integration) of E1/E6 and E2/E6 between the sample series. Six of seven regional lymph nodes had lower E1/E6 ratio compared with the primary tumor. Both higher and lower ratios were found for E2/E6. In four of the five cases with vulvar recurrences, E1/E6 and E2/E6 ratios were lower in the recurrent lesions compared with the primary VSCC. One tumor harbored fully integrated virus (loss of E2) and partially integration of E1 and this finding was consistent in both regional lymph node and in the primary tumor. In another patient, very few copies of E2 were found in the primary VSCC, and they were later lost altogether in both investigated vulvar recurrences. A decrease in integration ratio indicates a higher proportion of integrated cells compared with episomal cells and this is an example of how new HPV-related conditions may interact with tumor cells.
Eight of the ten patients had a high E2BS3 and 4 methylation while eight were more modestly methylated. A somewhat higher level of E2BS3 and 4 methylation was generally found in primary VSCC compared to lesions at other sites. Methylation has been suggested to be an alternate mechanism (besides integration) for dysregulation of oncoproteins E6 and E7 [31] and has been shown to alter during the neoplastic process. High levels of promotor methylation are described in cancer samples [50], [51] especially in cells with episomal viral genome [36], [43]. Viral methylation is carried out by human methyltransferases [52] and could therefore be subject of change in new settings.
Histological classification was reevaluated in this series of primary VSCC and the vulvar recurrences and the histological type was changed in one patient (patient 3). The basaloid primary type was changed to keratinizing histology in the recurrent lesion. Molecular factors involved in EMT transition and spread of vulvar cancer has been investigated. This includes transcription regulators Snail and Slug [53] in relation to expression of E-cadherin and β-catenin [54]. HPV oncoproteins have themselves also been found to be a trigger of transition [30], and further studies on cell adhesion protein expression may shed more light upon specific markers for spread in relation to HPV-positivity.
Funding
This work was supported by the Örebro County Council Research Committee.
References
- 1.Del Pino M., Rodriguez-Carunchio L., Ordi J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology. 2013;62(1):161–175. doi: 10.1111/his.12034. [DOI] [PubMed] [Google Scholar]
- 2.McCluggage W.G. Recent developments in vulvovaginal pathology. Histopathology. 2009;54(2):156–173. doi: 10.1111/j.1365-2559.2008.03098.x. [DOI] [PubMed] [Google Scholar]
- 3.De Vuyst H., Clifford G.M., Nascimento M.C., Madeleine M.M., Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J. Cancer. 2009;124(7):1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 4.Larsson G.L., Helenius G., Andersson S., Elgh F., Sorbe B., Karlsson M.G. Human papillomavirus (HPV) and HPV 16-variant distribution in vulvar squamous cell carcinoma in Sweden. Int J. Gynecol. Cancer. 2012;22(8):1413–1419. doi: 10.1097/IGC.0b013e31826a0471. [DOI] [PubMed] [Google Scholar]
- 5.Skamperle M., Kocjan B.J., Maver P.J., Seme K., Poljak M. Human papillomavirus (HPV) prevalence and HPV type distribution in cervical, vulvar, and anal cancers in central and eastern Europe. Acta Dermatovenerol. Alp. Pannonica Adriat. 2013;22(1):1–5. [PubMed] [Google Scholar]
- 6.Smith J.S., Backes D.M., Hoots B.E., Kurman R.J., Pimenta J.M. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet. Gynecol. 2009;113(4):917–924. doi: 10.1097/AOG.0b013e31819bd6e0. [DOI] [PubMed] [Google Scholar]
- 7.de Sanjose S., Alemany L., Ordi J., Tous S., Alejo M., Bigby S.M. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur. J. Cancer. 2013;49(16):3450–3461. doi: 10.1016/j.ejca.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 8.IARC: Cancer Incidence in 5 Continents. [Internet], 2012 [cited 7/2/2018]. Available from: 〈http://ci5iarcfr/CI5i-ix/ci5i-ix.htm〉.
- 9.Beller U., Quinn M.A., Benedet J.L., Creasman W.T., Ngan H.Y., Maisonneuve P. Carcinoma of the vulva. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J. Gynaecol. Obstet. 2006;95(Suppl 1):S7–S27. doi: 10.1016/S0020-7292(06)60028-3. [DOI] [PubMed] [Google Scholar]
- 10.Gadducci A., Cionini L., Romanini A., Fanucchi A., Genazzani A.R. Old and new perspectives in the management of high-risk, locally advanced or recurrent, and metastatic vulvar cancer. Crit. Rev. Oncol. Hematol. 2006;60(3):227–241. doi: 10.1016/j.critrevonc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J. Gynaecol. Obstet. 2009;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Te Grootenhuis N.C., van der Zee A.G., van Doorn H.C., van der Velden J., Vergote I., Zanagnolo V. Sentinel nodes in vulvar cancer: long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol. Oncol. 2016;140(1):8–14. doi: 10.1016/j.ygyno.2015.09.077. [DOI] [PubMed] [Google Scholar]
- 13.Te Grootenhuis N.C., Pouwer A.W., de Bock G.H., Hollema H., Bulten J., van der Zee A.G.J. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: a systematic review. Gynecol. Oncol. 2017 doi: 10.1016/j.ygyno.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Lindell G., Nasman A., Jonsson C., Ehrsson R.J., Jacobsson H., Danielsson K.G. Presence of human papillomavirus (HPV) in vulvar squamous cell carcinoma (VSCC) and sentinel node. Gynecol. Oncol. 2010;117(2):312–316. doi: 10.1016/j.ygyno.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Monk B.J., Burger R.A., Lin F., Parham G., Vasilev S.A., Wilczynski S.P. Prognostic significance of human papillomavirus DNA in vulvar carcinoma. Obstet. Gynecol. 1995;85(5 Pt 1):709–715. doi: 10.1016/0029-7844(95)00045-s. [DOI] [PubMed] [Google Scholar]
- 16.Alonso I., Fuste V., del Pino M., Castillo P., Torne A., Fuste P. Does human papillomavirus infection imply a different prognosis in vulvar squamous cell carcinoma? Gynecol. Oncol. 2011;122(3):509–514. doi: 10.1016/j.ygyno.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Pinto A.P., Schlecht N.F., Pintos J., Kaiano J., Franco E.L., Crum C.P. Prognostic significance of lymph node variables and human papillomavirus DNA in invasive vulvar carcinoma. Gynecol. Oncol. 2004;92(3):856–865. doi: 10.1016/j.ygyno.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Mehlen P., Puisieux A. Metastasis: a question of life or death. Nat. Rev. Cancer. 2006;6(6):449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 19.Steeg P.S. Targeting metastasis. Nat. Rev. Cancer. 2016;16(4):201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.K. Jin, T. Li, H. van Dam, F. Zhou, L. Zhang, M. Mermod, et al. Molecular Insights Into Tumour Metastasis: Tracing The Dominant Events, LID, 〈 10.1002/path.4871〉. [DOI] [PubMed]
- 21.Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. EMT: 2016. Cell. 2016;166(1):21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Alkatout I., Schubert M., Garbrecht N., Weigel M.T., Jonat W., Mundhenke C. Vulvar cancer: epidemiology, clinical presentation, and management options. Int. J. Women's Health. 2015;7:305–313. doi: 10.2147/IJWH.S68979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durst M., Hoyer H., Altgassen C., Greinke C., Hafner N., Fishta A. Prognostic value of HPV-mRNA in sentinel lymph nodes of cervical cancer patients with pN0-status. Oncotarget. 2015;6(26):23015–23025. doi: 10.18632/oncotarget.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y.S., Rhim C.C., Lee H.N., Lee K.H., Park J.S., Namkoong S.E. HPV status in sentinel nodes might be a prognostic factor in cervical cancer. Gynecol. Oncol. 2007;105(2):351–357. doi: 10.1016/j.ygyno.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Fule T., Csapo Z., Mathe M., Tatrai P., Laszlo V., Papp Z. Prognostic significance of high-risk HPV status in advanced cervical cancers and pelvic lymph nodes. Gynecol. Oncol. 2006;100(3):570–578. doi: 10.1016/j.ygyno.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Slama J., Fischerova D., Pinkavova I., Zikan M., Cibula D. Human papillomavirus DNA presence in pelvic lymph nodes in cervical cancer. Int J. Gynecol. Cancer. 2010;20(1):126–132. doi: 10.1111/IGC.0b013e3181c01cf0. [DOI] [PubMed] [Google Scholar]
- 28.Sutton B.C., Allen R.A., Moore W.E., Dunn S.T. Distribution of human papillomavirus genotypes in invasive squamous carcinoma of the vulva. Mod. Pathol. 2008;21(3):345–354. doi: 10.1038/modpathol.3801010. [DOI] [PubMed] [Google Scholar]
- 29.Munger K., Baldwin A., Edwards K.M., Hayakawa H., Nguyen C.L., Owens M. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004;78(21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campo L., Zhang C., Breuer E.K. EMT-inducing molecular factors in gynecological cancers. BioMed. Res. Int. 2015;2015:420891. doi: 10.1155/2015/420891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thain A., Jenkins O., Clarke A.R., Gaston K. CpG methylation directly inhibits binding of the human papillomavirus type 16 E2 protein to specific DNA sequences. J. Virol. 1996;70(10):7233–7235. doi: 10.1128/jvi.70.10.7233-7235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lillsunde Larsson G., Carlsson J., Karlsson M.G., Helenius G. Evaluation of HPV genotyping assays for archival clinical samples. J. Mol. Diagn. 2015;17(3):293–301. doi: 10.1016/j.jmoldx.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Swan D.C., Limor J.R., Duncan K.L., Rajeevan M.S., Unger E.R. Human papillomavirus type 16 variant assignment by pyrosequencing. J. Virol. Methods. 2006;136(1–2):166–170. doi: 10.1016/j.jviromet.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Lillsunde Larsson G., Helenius G. Digital droplet PCR (ddPCR) for the detection and quantification of HPV 16, 18, 33 and 45 - a short report. Cell. Oncol. (Dordr.). 2017;40(5):521–527. doi: 10.1007/s13402-017-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peitsaro P., Johansson B., Syrjanen S. Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique. J. Clin. Microbiol. 2002;40(3):886–891. doi: 10.1128/JCM.40.3.886-891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lillsunde Larsson G., Helenius G., Sorbe B., Karlsson M.G. Viral load, integration and methylation of E2BS3 and 4 in human papilloma virus (HPV) 16-positive vaginal and vulvar carcinomas. PloS One. 2014;9(11):e112839. doi: 10.1371/journal.pone.0112839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maggino T., Landoni F., Sartori E., Zola P., Gadducci A., Alessi C. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF study. Cancer. 2000;89(1):116–122. doi: 10.1002/1097-0142(20000701)89:1<116::aid-cncr16>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Jafri S.I.M., Ali N., Farhat S., Malik F., Shahin M. The tell-tale heart: a case of recurrent vulvar carcinoma with cardiac metastasis and review of literature. Gynecol. Oncol. Rep. 2017;21:20–23. doi: 10.1016/j.gore.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agrawal A., Wood K., Giede C., Chibbar R. A case of kidney metastasis in vulvar squamous cell carcinoma: a case report and review of literature. Case Rep. Clin. Med. 2013;2:306–309. [Google Scholar]
- 40.Prieske K., Haeringer N., Grimm D., Trillsch F., Eulenburg C., Burandt E. Patterns of distant metastases in vulvar cancer. Gynecol. Oncol. 2016;142(3):427–434. doi: 10.1016/j.ygyno.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Bechtold V., Beard P., Raj K. Human papillomavirus type 16 E2 protein has no effect on transcription from episomal viral DNA. J. Virol. 2003;77(3):2021–2028. doi: 10.1128/JVI.77.3.2021-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romanczuk H., Howley P.M. Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc. Natl. Acad. Sci. USA. 1992;89(7):3159–3163. doi: 10.1073/pnas.89.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung J.L., Cheung T.H., Yu M.Y., Chan P.K. Virological characteristics of cervical cancers carrying pure episomal form of HPV16 genome. Gynecol. Oncol. 2013;131(2):374–379. doi: 10.1016/j.ygyno.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Reinson T., Henno L., Toots M., Ustav M., Jr, Ustav M. The cell cycle timing of human papillomavirus DNA replication. PloS One. 2015;10(7):e0131675. doi: 10.1371/journal.pone.0131675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middleton K., Peh W., Southern S., Griffin H., Sotlar K., Nakahara T. Organization of human papillomavirus productive cycle during neoplastic progression provides a basis for selection of diagnostic markers. J. Virol. 2003;77(19):10186–10201. doi: 10.1128/JVI.77.19.10186-10201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsakogiannis D., Darmis F., Gortsilas P., Ruether I.G., Kyriakopoulou Z., Dimitriou T.G. Nucleotide polymorphisms of the human papillomavirus 16 E1 gene. Arch. Virol. 2014;159(1):51–63. doi: 10.1007/s00705-013-1790-8. [DOI] [PubMed] [Google Scholar]
- 47.Tsakogiannis D., Gortsilas P., Kyriakopoulou Z., Ruether I.G., Dimitriou T.G., Orfanoudakis G. Sites of disruption within E1 and E2 genes of HPV16 and association with cervical dysplasia. J. Med. Virol. 2015;87(11):1973–1980. doi: 10.1002/jmv.24256. [DOI] [PubMed] [Google Scholar]
- 48.Mirghani H., Moreau F., Lefevre M., Tam C., Perie S., Soussan P. Human papillomavirus type 16 oropharyngeal cancers in lymph nodes as a marker of metastases. Arch. Otolaryngol. Head Neck Surg. 2011;137(9):910–914. doi: 10.1001/archoto.2011.141. [DOI] [PubMed] [Google Scholar]
- 49.Chan P.K., Yu M.M., Cheung T.H., To K.F., Lo K.W., Cheung J.L. Detection and quantitation of human papillomavirus DNA in primary tumour and lymph nodes of patients with early stage cervical carcinoma. J. Clin. Virol. 2005;33(3):201–205. doi: 10.1016/j.jcv.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Badal V., Chuang L.S., Tan E.H., Badal S., Villa L.L., Wheeler C.M. CpG methylation of human papillomavirus type 16 DNA in cervical cancer cell lines and in clinical specimens: genomic hypomethylation correlates with carcinogenic progression. J. Virol. 2003;77(11):6227–6234. doi: 10.1128/JVI.77.11.6227-6234.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding D.C., Chiang M.H., Lai H.C., Hsiung C.A., Hsieh C.Y., Chu T.Y. Methylation of the long control region of HPV16 is related to the severity of cervical neoplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009;147(2):215–220. doi: 10.1016/j.ejogrb.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Johannsen E., Lambert P.F. Epigenetics of human papillomaviruses. Virology. 2013;445(1–2):205–212. doi: 10.1016/j.virol.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodrigues I.S., Lavorato-Rocha A.M., de M.M.B., Stiepcich M.M., de Carvalho F.M., Baiocchi G. Epithelial-mesenchymal transition-like events in vulvar cancer and its relation with HPV. Br. J. Cancer. 2013;109(1):184–194. doi: 10.1038/bjc.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holthoff E.R., Spencer H., Kelly T., Post S.R., Quick C.M. Pathologic features of aggressive vulvar carcinoma are associated with epithelial-mesenchymal transition. Hum. Pathol. 2016;56:22–30. doi: 10.1016/j.humpath.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]