Abstract
Antimicrobials are used to maintain good health and productivity of food animals. Misuse of antibiotics in livestock contributes to development of antimicrobial resistance, an emerging One Health issue. This study assessed pastoralists' knowledge and practices regarding antimicrobial usage, explore pathways for resistant pathogens emergence and associated social drivers for antimicrobial misuse in pastoral herds of North-central Nigeria. An interview questionnaire-based cross-sectional survey was conducted in randomly selected pastoral households. Descriptive and analytical statistical analyses were performed at 95% confidence level. All the 384 pastoralists participated in the study. Majority (58%) of respondents had no formal education. Only 8.1% of respondents knew antibiotic misuse to be when given under-dose and 70.1% of them did not know what misuse entailed. About 58.3% reported self-prescription of antimicrobials used on animals, while 67% of them reported arbitrary applications for dosage determination. Most frequently used antimicrobials were tetracycline (96.6%), tylosin (95.6%) and penicillin (94.0%). Identified pathways for antimicrobial resistant pathogens spread to humans were through contaminated animal products; contaminated animals and fomites; and environmental wastes. Improper antimicrobial usage (p < 0.001), non-enforcement of laws regulating antimicrobial usage (p < 0.001), weak financial status (p < 0.001), low education and expertise (p < 0.001), and nomadic culture (p < 0.001), influenced antimicrobials misuse in livestock. The study revealed low levels of knowledge and practices regarding antimicrobial usage in livestock. Socio-cultural activities significantly influenced antimicrobials misuse in livestock. Improve pastoralists' knowledge about effects of antimicrobials misuse and promotion of prudent usage in livestock will mitigate antimicrobial resistance menace in animals and humans.
Keywords: Antimicrobials, Antimicrobial resistance, Livestock, Pastoralist, Public health, Nigeria
Highlights
-
•
Only 8.1% pastoralists knew antimicrobials misuse to be when given under-dose.
-
•
About 58% pastoralists reported self prescription of antimicrobials used on animals.
-
•
Most frequently used antimicrobial was tetracycline (96.6%).
-
•
Nomadic culture influenced antimicrobials misuse in livestock.
-
•
‘OneHealth’ approach is needed for efficient antimicrobial usage surveillance.
1. Introduction
Livestock production has always been important in Nigeria, and the rapidly emerging livestock sector now ranks second among the 20 developing economies in terms of dependent on livestock [1]. Approximately 70% of pastoralist population in Nigeria lives in rural areas, with nomadic herdsmen herding about 90% of ruminants and practice seasonal transhumance or year-round nomadism [2]. Pastoralism has been evolving in Nigeria, with herders often combining cattle rearing with crop cultivation [3]. Cattle are usually extensively managed, either under nomadic or agro pastoral systems [4].
Antimicrobials are used in livestock production to maintain good health and productivity of the animals [5]. Inappropriate use of antimicrobials in the livestock sector contributes to development of antimicrobials resistance, an emerging One Health issue that can be transmitted between animals, humans and the environment and also spread to other countries [5]. Improper dosing of antimicrobials (too little, for too short a period, or use of wrong antimicrobials) can predispose to antimicrobial resistance [6]. The global antimicrobial usage in food animals was estimated at 63000 tons annually in 2015 and projected to increase by almost 70% in 2030 [7]. Apart from the top consumers (China, United States and Brazil), the largest relative increase is projected to take place in the developing countries; with Myanmar, Indonesia and Nigeria taking the lead among those that may experience an increase of >200% [7].
Antimicrobial consumption in livestock has received comparatively little attention, unlike in humans where their consumptions are now being tracked in most high-income and some middle-income countries through databases on antimicrobial sales [8,9], but with on coverage in less-income countries. Expert opinion suggests that global consumption of antimicrobials in animals is twice that of humans [10].
The misuse of antimicrobials creates selective evolutionary pressure that enables antimicrobial resistant pathogens to increase in numbers more rapidly than antimicrobial susceptible pathogens and thus increases the opportunity for individuals to become infected by resistant pathogens, posing a serious public health threat [7]. The trends of antimicrobials misuse are exponentially on the increase and considered as a major global public and animal health threats [11]. Massive use of antimicrobials is one of the most important factors promoting the emergence, selection, and dissemination of antimicrobial resistant pathogens in both veterinary and human medicine [12,13]. In addition to its public health impact, antimicrobial misuse causes many negative impacts on livestock production to farmers, such as increase mortality, decrease production and high financial losses, due to unsuccessful treatments [14]. The widespread use of antimicrobials for therapeutic, prophylactic and growth promotion purposes in livestock production has intensified the risk for the emergence and spread of resistant pathogens [15]. About half of the world's antimicrobials production is used in food animals, mostly without regulations [16].
Most of the antimicrobials used in food animals in Nigeria can be obtained without veterinarian's prescription, while laws regulating antimicrobial usage in animals are not always enforced. Control and prevention of antimicrobials misuse in food animals will depend heavily on herders' knowledge and compliance behaviour with antimicrobials guidelines. This will depends also on their level of risk perceptions on the effects of antimicrobials misuse, resistant pathogens emergence and spread through food chain. However, science-based information on antimicrobial usage by livestock farmers in food animals in Nigeria is not readily available. Exploration of pastoralists' local knowledge about antimicrobial usage is crucial for the development of effective antimicrobials surveillance, control and prevention of antimicrobials misuse in animals and humans.
The objectives of this study were to: (1) assess pastoralists' knowledge and practices towards antimicrobial usage in livestock; and (2) explore pathways for resistant pathogens emergence and spread from livestock to humans in pastoral settlements of North-central Nigeria. We hypothesized that pastoralists' socio-cultural activities cannot influence antimicrobial misuse in livestock. The findings are expected to be valuable in improving awareness on the need for proper antimicrobial usage in food animals as well as minimize associated public health challenges in pastoral communities of developing countries.
2. Materials and methods
2.1. Study area, structure of target population and livelihood
The study was conducted in Niger State, located at the Southern Guinea savannah zone of Nigeria, between latitude 8° 20′ N and 11° 30′ N, and longitude 3° 30′ E and 7° 20′ E. It provides transit routes for pastoral nomads on seasonal migrations from the northern parts to the southern areas of the country. The state has three agro-geographical zones (Southern zone, Eastern zone, and Northern zone), with variable climatic conditions, which provide favourable environments for livestock rearing. It has an estimated cattle population of about 2.5 million cattle and 1.9 million sheep and goats that are mostly in the custodies of nomads and agro pastoralists [17].
The target populations were pastoral herd owners, aged 20 years and above, with herds of local breeds of cattle, sheep and goats, domiciled in remote areas of the state during the study period. Average number of households that formed a pastoral settlement was 28, each managed by household head (a man, his wives and children, or an elderly widow and her children). A household constituted a herd and farmer's family that owned it. Average number of animals in a pastoral herd was 86 cattle, 11 sheep and 5 goats of variable ages. In Nigeria, pastoralists' settlements are either nomadic, who practice livestock rearing as main source of livelihood and transhumance as social order; or agro-pastoralists, who keep livestock in medium size, cultivates few crops, semi-settled, with limited livestock movements, and are on low-range graze land near environs, often given supplementary feeds of crop residues, particularly during the critical period of dry season.
2.2. Study design, sample size and sampling procedure
An interview questionnaire-based cross-sectional survey was conducted in randomly selected pastoral households between November 2015 and March 2016. The sample size was calculated with the use of Open Source Epidemiologic Statistics for Public Health (OpenEpi) version 2.3.1 software [18], with power set at 50% and 5% margin of error at 95% confidence level. A sample size of 384 households was obtained and enrolled into the study.
A multi-stage sampling method was used. In the first stage, the three Agro-geographical zones in the state were purposively considered. In the second stage, 30 pastoral settlements were selected across the study area, with 10 from each Agro-geographical zone. Systematic random sampling was used to select the 384 households in the final stage of the procedure, with 128 pastoral households selected in each zone. Sampling interval of two was used, obtained by dividing the total number of expected households in the three zones (n = 384) by the desired number of households to be sampled in each (n = 128).
2.3. Questionnaire design, pretesting and data collection
A structured questionnaire was designed and contained mostly close-ended questions, to ease data processing, minimize variation and improve precision of responses [19]. The questionnaire consisted of four sections that included: (i) pastoralist's socio-demographic characteristics: age, gender, marital status, occupation and formal education; (ii) knowledge/awareness about antimicrobial usage in food animals: antimicrobials misuse, antimicrobial resistance, and effects of resistance in animals and humans; (iii) practices of antimicrobial usage in food animals; (iv) identification of pathways for transmission of antimicrobial resistant pathogens emergence and dissemination from food animals to humans; and (v) identification of factors that influence antimicrobials misuse, which can predisposed to resistant pathogens emergence through food chain to humans in pastoral settlements.
The questionnaire was subjected to a pre-test on fifteen households in one nomadic pastoral settlement before administered in its final form on respondents. The pre-test was aimed at identifying problems and eliminate them for adequate delivery of required data. The questionnaire was designed in English and verbally translated into local Hausa language during process of questioning, as most of the pastoralists do not possessed formal education. Six enumerators fluent in English and Hausa languages were trained and carried out interviewer-administered questionnaires. They asked questions in Hausa and recorded the responses in English. The daily administrations of the questionnaires were monitored, and filled forms checked for purpose of quality control.
Objectives of the survey were explained to respondents at the beginning of each section of questionnaire administration. Their informed consent was verbally obtained before commencement and none declined to participate. They were assured of voluntary participation, confidentiality of responses and the opportunity to withdraw at any time without prejudice in line with the Helsinki Declaration (World Medical Association Declaration of Helsinki [20].
Advocacy visits were made to each selected pastoral settlement a week prior to the proposed interview and necessary permission obtained from Ardos (community leaders). The study protocols were approved by the Niger State Ministry of Livestock and Fisheries Development Internal Research Ethics Committee.
2.4. Data management and analysis
Participants' responses were summarized into Microsoft Excel 7 (Microsoft Corporation, Redmond, WA, USA) spreadsheets. Descriptive analysis was performed and results expressed in frequencies and proportions. Analytical analysis was carried out to determine associations.
The levels of knowledge about antimicrobial usage was determined according to the following learning outcome criteria; the words “very low” represented a proportion of respondents with “know” knowledge level that ranges between 1% and 24%; the word “low” represented a proportion of respondents with “know” knowledge level that ranges between 25% and 49%; the word “high” represented a proportion of respondents with “know” knowledge level that ranges between 50% and 74%; and the words “very high” represented a proportion of respondents with “know” knowledge level that ranges between 75% to 100%. A similar assessment was performed in evaluating levels of practices of antimicrobial usage.
To assess influence of pastoralists' socio-cultural activities on antimicrobial misuse, independent (explanatory) variables were created from the activities, while respondents' overall responses constituted the dependent (outcome) variables. However, to create outcome variables, a unique scoring system was used for the responses. Each respondent was assigned a response score within a range of 1–20 points and converted to 100%. These scores reflected stringency of their responses to questions. The score range was further categorized into ‘poor’ or ‘satisfactory’ to keep them as binary variables. Response scores that fell within 1–10 points were considered ‘poor’ (≤49%), and those that fell within 11–20 points were considered ‘satisfactory’ (≥50%).
Associations between explanatory and outcome variables were first subjected to univariable analysis using Chi-square tests [21]. Factors found to be statistically significant at this analysis were further subjected to likelihood stepwise backward multivariable logistic regression models to control for confounding and test for effect modification. The Hosmer and Lemeshow test was used to assess for goodness of fit of the final model and was found to be good. P < 0.05 was considered statistically significant in all analyses. All data were analyzed using the OpenEpi 2.3.1 software [18].
3. Results
3.1. Socio-demographic characteristics of participants
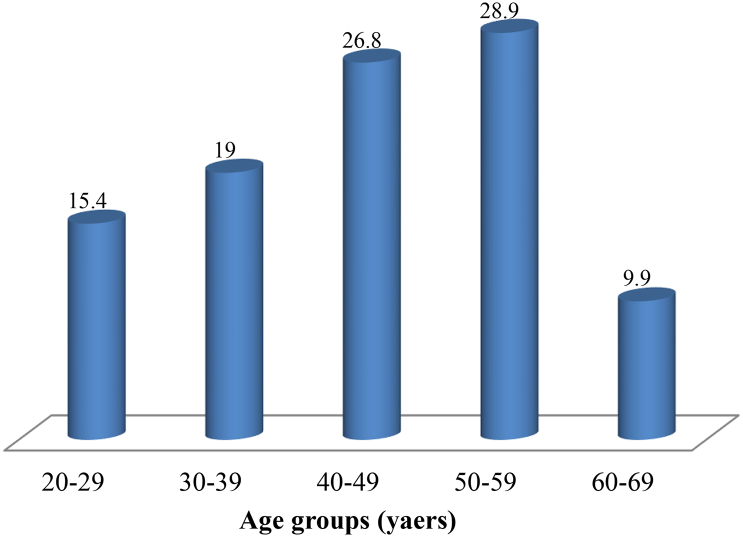
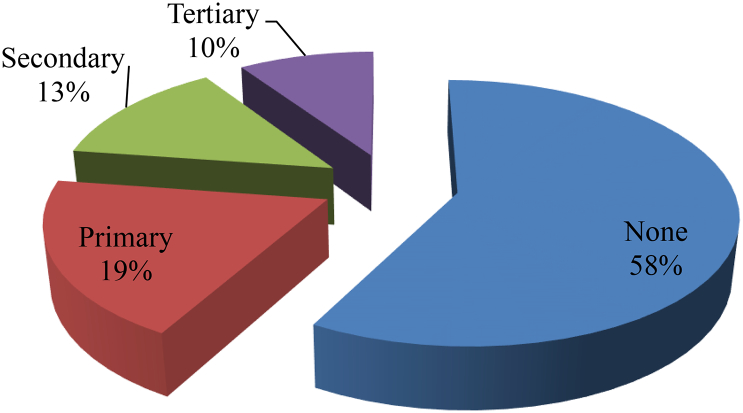
All recruited pastoralists participated in the study, with a mean age of 44.4 ± 12.1 SD years, and most (28.9%) were in age group 50–59 years (Fig. 1). Majority of the respondents were males (74.5%) and about 84.9% were married, while 9.6% (n = 37) and 5.5% (n = 21) were single and widows, respectively. On the pastoralists' occupations, majority (54.4%) were nomadic pastoralists, while 45.6% were agro pastoralists. Majority (58.0%, n = 223) of them had no formal education and only very few (9.4%, n = 36) had tertiary education (Fig. 2).
Fig. 1.
Proportions of age distribution of pastoralists in Niger State, North-central Nigeria.
Fig. 2.
Proportions of formal educational levels of Fulani nomadic pastoralists in Niger State, North-central Nigeria.
3.2. Knowledge and awareness about antimicrobial usage
All respondents mentioned to have heard about antimicrobials. They obtained information about antimicrobials from animal health personnel (71.6%, n = 275) and relatives (28.4%, n = 109). When asked about uses of antimicrobials in livestock, 54.4% and 23.2% of respondents mentioned treatment of infections and growth promotion, respectively. However, 3.4% of them did not know what antimicrobials are used for in livestock. Also, only 8.1% of them knew antimicrobials misuse to be when given under-dose, 7.3% indicated when given over-dose and 70.1% of them did not know what misuse entailed. Regarding whether antimicrobials misuse in livestock can predispose to resistant pathogens emergence, 29.7% of the participants agreed and 44.8% of them disagreed. Concerning knowledge about effects of antimicrobial resistance, 64.8% and 54.9% of them did not know the consequences in animals and humans, respectively (Table 1). Generally, only 28% of respondents mentioned antimicrobial resistance to be a phenomenon that happens when bacteria change and become resistant to antimicrobials used to treat infections, while 67% of them did not know and 5% were indifferent.
Table 1.
Pastoralists' knowledge and awareness about antimicrobial usage on livestock in North-central Nigeria.
| Variable | Frequency |
Proportion |
95% CI |
|---|---|---|---|
| (n) | (%) | ||
| Know antimicrobials to be used | |||
| To treat infections in animals | 209 | 54.4 | 49.4, 59.4 |
| To prevent infections in animals | 73 | 19.0 | 15.3, 23.2 |
| To promote growth in animals | 89 | 23.2 | 19.2, 27.6 |
| Don't know | 13 | 3.4 | 1.9, 5.6 |
| Antimicrobials misuse is when | |||
| Administered under-dose | 31 | 8.1 | 5.6, 11.1 |
| Administered over-dose | 28 | 7.3 | 5.0, 10.2 |
| Administered in normal dose | 56 | 14.6 | 11.3, 18.4 |
| Don't know | 269 | 70.1 | 65.3, 74.5 |
| Effects of antimicrobial resistance in animals | |||
| Non response to bacterial infection treatment | 99 | 25.8 | 21.6, 30.3 |
| Extra costs on treatment of bacterial infection | 36 | 8.6 | 6.1, 11.7 |
| Don't know | 249 | 64.8 | 60.0, 69.5 |
| Antimicrobials misuse in livestock can predispose to resistant pathogens emergence | |||
| Agree | 114 | 29.7 | 25.3, 34.4 |
| Disagree | 172 | 44.8 | 39.9, 49.8 |
| Don't know | 58 | 15.1 | 11.8, 19.0 |
| Antimicrobial resistant pathogen in animals can pass to humans through | |||
| Eating raw cheese | 16 | 4.2 | 2.5, 6.5 |
| Drinking raw milk | 11 | 2.9 | 1.5, 4.9 |
| Eating undercooked meat | 42 | 10.9 | 8.1, 14.4 |
| Touching aborted foetus | 33 | 8.6 | 6.1, 11.7 |
| Contacts of herders with animals | 12 | 3.1 | 1.7, 5.3 |
| Don't know | 270 | 70.3 | 65.5, 74.7 |
| Effects of antimicrobial resistance in humans | |||
| Non response to bacterial infection treatment | 75 | 19.5 | 15.8, 23.7 |
| Extra costs on treatment of bacterial infection | 26 | 6.8 | 4.6, 9.6 |
| Longer duration of illness and treatment | 32 | 8.3 | 5.9, 11.4 |
| Don't know | 211 | 54.9 | 49.9, 59.9 |
CI – Confidence interval.
3.3. Practices of antimicrobial usage in livestock
On personnel that prescribed antimicrobials for usage in livestock, 30.5% of the participants mentioned animal health officials and 58.3% of them practiced self prescription. Majority (59.6%) of pastoralists purchased antimicrobials from veterinary drug shops, while 34.9% of them patronized animal drug hawkers. Also, 65.4% of respondents practiced self-administration of antimicrobials on animals, and 34.6% contracted services of animal health officials. On frequency of antimicrobial usage on sick animals, about 27% of respondents followed prescribed instructions, 28% of them administered antimicrobials only once on sick animals until they recovered, while 45.6% administered the drugs once daily on sick animals until they recovered. Regarding dosage determination of antimicrobials used on livestock, 33% of respondents followed instructions on labels, while 67% of them practiced arbitrary applications. However, very few (15.9%) pastoralists observed withdrawal periods after antimicrobial usage, and most (84.1%) of them indicated non-compliance with withdrawal periods. On purpose of antimicrobial usage, 54.4% of the respondents used antimicrobials for therapeutic purpose, 40.6% of them used antimicrobials for preventive purpose, and 4.9% of them used the drugs as growth promoters (Table 2).
Table 2.
Practices of antimicrobial usage on livestock by pastoralist in North-central Nigeria.
| Practice | Frequency |
Proportion |
95% CI |
|---|---|---|---|
| (n) | (%) | ||
| Personnel that prescribe antimicrobials for usage in animals | |||
| Animal health officials | 117 | 30.5 | 26.0, 35.2 |
| Self prescription | 224 | 58.3 | 53.4, 63.2 |
| Friends and relations | 43 | 11.2 | 8.3, 14.7 |
| Purchasing places of antimicrobials | |||
| Veterinary drug shops | 229 | 59.6 | 54.7, 64.5 |
| Human drug shops | 21 | 5.5 | 3.5, 8.1 |
| Animal drug hawkers | 134 | 34.9 | 30.3, 39.8 |
| Who administer antimicrobials on animals | |||
| Self administered | 251 | 65.4 | 60.5, 70.0 |
| Animal health officials | 133 | 34.6 | 30.0, 39.5 |
| Frequency of antimicrobial usage on sick animals | |||
| As prescribed | 103 | 26.8 | 22.6, 31.4 |
| Once | 109 | 28.4 | 24.0, 33.1 |
| Once daily until recovered | 172 | 45.6 | 40.6, 50.6 |
| Dosage determination before antimicrobials use | |||
| From instructions on the label | 125 | 32.6 | 28.0, 37.4 |
| Arbitrary | 259 | 67.4 | 62.4, 72.0 |
| Frequently used route of administration | |||
| Injection | 215 | 56.0 | 51.0, 60.9 |
| Mouth (POS) | 96 | 25.0 | 20.9, 29.5 |
| On the skin (topical) | 42 | 10.9 | 8.1, 14.4 |
| In feed | 31 | ||
| Observe about withdrawal periods | |||
| Yes | 61 | 15.9 | 12.5, 19.8 |
| No | 323 | 84.1 | 80.2, 87.5 |
| Purpose for antimicrobials usage | |||
| Treatment of infections | 209 | 54.4 | 49.4, 59.4 |
| Prevention of infections | 156 | 40.6 | 35.8, 45.6 |
| Growth promotion | 19 | 4.9 | 3.1, 7.5 |
CI – Confidence interval.
3.4. Antimicrobials frequently used on livestock
Pastoralists used range of different classes of antimicrobials on livestock. However, most frequently mentioned antimicrobials to be used on livestock include: tetracycline (96.6%), tylosin (95.6%), penicillin (94.0%), streptomycin (93.0%), sulfonamides (92.4%), gentamicin (75.5%), neomycin (67.2%) and ciprofloxacin (39.6%) (Table 3).
Table 3.
Antimicrobials frequently used by pastoralists on livestock in North-central Nigeria.
| Antibiotic type | Always |
Occasionally |
|---|---|---|
| n (%) | n (%) | |
| Penicillin | 361 (94.0) | 23 (6.0) |
| Gentamicin | 294 (75.5) | 94 (24.5) |
| Streptomycin | 357 (93.0) | 27 (7.0) |
| Tetracycline | 371 (96.6) | 13 (3.4) |
| Ciprofloxacin | 152 (39.6) | 232 (60.4) |
| Tylosin | 367 (95.6) | 17 (4.4) |
| Neomycin | 258 (67.2) | 126 (32.8) |
| Sulfonamides | 355 (92.4) | 29 (7.6) |
n – Number of respondents; % – Proportion of respondents.
3.5. Pathways for transmission of antimicrobial resistant pathogens from livestock to humans
Regarding risk pathways for transmission of antimicrobial resistant pathogens from livestock to humans, consumption of contaminated food animal products, direct or indirect contacts with contaminated animals and fomites, and environmental contaminated releases (manures) were identified. However, 25.0% of the participants identified consumption of contaminated raw milk as risk pathway for antimicrobial resistant pathogen spread to humans and 37.0% of them reported consumption of under-cooked contaminated. Also, 76.6% of pastoralists identified contacts with contaminated animals, while 8.3% identified indirect contacts with contaminated fomites as risk pathways. Furthermore, 55.7% of the participants identified environmental releases and wastes, such as discharged contaminated faeces (manure) in the herd settlements, as risk pathways, and 24.2% of them identified flies attracted to the contaminated manure as environmental risk pathway (Table 4).
Table 4.
Identification of pathways for transmission and spread of antimicrobial resistant pathogens from livestock to humans in North-central Nigeria.
| Pathway | Frequency |
Proportion |
95% CI |
|---|---|---|---|
| (n) | (%) | ||
| Contaminated food animal products | |||
| Raw milk | 96 | 25.0 | 20.9, 29.5 |
| Raw cheese | 101 | 26.3 | 22.1, 30.9 |
| Under cooked meat | 142 | 37.0 | 32.3, 41.9 |
| Don't know | 45 | 11.7 | 8.8, 15.2 |
| Contacts: direct or indirect | |||
| Humans with contaminated animals | 294 | 76.6 | 72.1, 80.6 |
| Humans with contaminated fomites | 32 | 8.3 | 5.9, 11.4 |
| Don't know | 58 | 15.1 | 11.8, 18.9 |
| Environmental releases and wastes | |||
| Discharged contaminated faeces (manure) | 214 | 55.7 | 50.7, 60.7 |
| Aerosols from herd facilities | 56 | 14.6 | 11.3, 18.4 |
| Flies attracted to the contaminated faeces | 93 | 24.2 | 20.1, 28.7 |
| Don't know | 21 | 5.5 | 3.5, 8.1 |
CI – Confidence interval.
3.6. Factors that influence antimicrobials misuse on livestock
Many factors were identified to influence antimicrobials misuse on livestock, which ca predispose to emergence and dissemination of resistant pathogens through food animal chain to humans. At univariable analysis, improper antimicrobial usage, non-enforcement of laws regulating antimicrobial usage, weak financial status of pastoralists, low education and expertise of pastoralists, nomadic and transhumance culture of pastoralists, and extensive husbandry management system significantly (p < 0.05) influenced antimicrobials misuse on livestock. At multivariable analysis, improper antimicrobial usage was twenty eight times more likely (OR: 28.00; 95% CI: 13.06, 60.02) to satisfactorily influenced antimicrobials misuse on livestock, while non-enforcement of laws regulating antimicrobial usage was five times more likely (OR: 4.59; 95% CI: 2.67, 7.91) to influenced antimicrobials misuse. Also, weak financial status was five times more likely (OR: 4.69; 95% CI: 2.69, 8.17) to influenced antimicrobials misuse on livestock. And low education and expertise of pastoralists was nine times more likely (OR: 9.03; 95% CI: 4.89, 16.69) to influenced antimicrobials misuse on food animals. Further, nomadic and transhumance culture of the pastoralists and their extensive husbandry management system were more likely [(OR: 9.75; 95% CI: 5.47, 17.38) and (OR: 3.05; 95% CI: 1.73, 3.57), respectively] to influenced antimicrobials misuse on livestock (Table 5).
Table 5.
Socio-cultural factors that influence antimicrobials misuse on livestock by pastoralists in North-central Nigeria.
| Activity | Poor influence |
Satisfactory influence |
Odds ratio |
95% CI | P-value |
|---|---|---|---|---|---|
| (%) | (%) | (OR) | |||
| Improper antimicrobial usage | |||||
| No | 28 (63.6) | 16 (36.4) | 1.00 | ||
| Yes | 20 (5.9) | 320 (94.1) | 28.00 | 13.06, 60.02 | <0.001 |
| Non-enforcement of laws regulating antimicrobial usage | |||||
| No | 37 (52.1) | 34 (47.9) | 1.00 | ||
| Yes | 60 (19.2) | 253 (80.8) | 4.59 | 2.67, 7.91 | <0.001 |
| Weak financial status of pastoralists | |||||
| No | 37 (56.1) | 29 (43.9) | 1.00 | ||
| Yes | 68 (21.4) | 250(78.6) | 4.69 | 2.69, 8.17 | <0.001 |
| Low education and expertise of pastoralists | |||||
| No | 46 (73.0) | 17 (27.0) | 1.00 | ||
| Yes | 74 (23.1) | 247 (76.9) | 9.03 | 4.89, 16.69 | <0.001 |
| Nomadic and transhumance culture of pastoralists | |||||
| No | 55 (73.3) | 20 (26.7) | 1.00 | ||
| Yes | 68 (22.0) | 241 (78.0) | 9.75 | 5.47, 17.38 | <0.001 |
| Extensive husbandry system | |||||
| No | 32 (54.2) | 27 (45.8) | 1.00 | ||
| Yes | 91 (28.0) | 234 (72.0) | 3.05 | 1.73, 3.57 | 0.001 |
Statistically significant at p < 0.05.
4. Discussion
Antimicrobial usage in livestock is one of the least surveyed issues in pastoral communities of Nigeria, and to our knowledge, this study was the first to investigate antimicrobial usage in livestock by nomadic pastoralists in North-central of Nigeria. Antimicrobial resistance both in veterinary and human medicine has now been recognized as a significant emerging threat to global food security and public health [7,22,23].
Despite the frequency of antimicrobial usage by pastoralists to maintain good livestock health and production in the studied settlements, there was overall low level of knowledge and awareness about proper usage. This has had influence on antimicrobials misuse with potential risks of antimicrobial resistant pathogens emergence and dissemination to humans. The low knowledge level may be due to high proportion (58%) of pastoralists do not possessed formal education. Sensitization of pastoralists, especially among those domiciled in hard-to-reach settlements is therefore advocated. Bridging the knowledge gap will increase their perceptions of risks associated with antimicrobials misuse in food animals.
Regarding practices of antimicrobial usage in livestock, this study found high proportions of pastoralists practicing self-prescription and self-administration of antimicrobials on animals without professional advices and services. Where antimicrobials can be bought and used on livestock without prescriptions, the emergence and spread of antimicrobial resistant pathogens is made worse due to misuse. We also found some antimicrobials to be frequently used by pastoralists on livestock. About half of the world's antimicrobials productions have been reported to be frequently used in farm animals [16,24]. In Nigeria, the use of non-prescribed antimicrobials in food animals has been related to the unavailability of veterinary services and extra cost of veterinary services [25,26]. Also, this study found antimicrobials to be used for therapeutic and prophylactic purposes as well as for growth promotions. This is consistent with some findings that reported similar uses in food animals [[25], [26], [27], [28]]. The administration of antimicrobials to food animals at low doses for extended durations for growth promotion and disease prevention has been banned in many high income countries (HIC) because of the global health crisis of antimicrobial resistance in animals and humans, but still frequently used in low and medium income countries (LMIC) for these purposes [24]. Irrational use of antimicrobials in food animals have been also reported to have contributed significantly to the emergence, persistence, and spread of resistant bacteria from animals to humans [29,30]. Excessive use and misuse of antimicrobials have been recognized as some of the major drivers of antimicrobial resistance, due to selection pressure imposed on animal microbiota [[31], [32], [33], [34]].
It is worthy to mention that this study found regular practice of low dosages of antimicrobials administration on livestock by pastoralists. Antimicrobials at low dosages (i.e. residual levels, sub-lethal or sub-therapeutic dosages) have been reported to predispose to resistant pathogens emergence through promotion of genetic and phenotypic variability in exposed bacteria due to selection bias [[35], [36], [37]]. Also, a noteworthy observation was the high frequency of tetracycline, penicillin, streptomycin, sulfonamides and tylosin usage by pastoralists in livestock. This is consistent with previous studies that reported these antimicrobials to be frequently used in food animals in Nigeria [[38], [39], [40], [41], [42]]. Frequent use of antimicrobials in food animals has also been reported in other African countries (South Africa and Tanzania), where tetracycline, sulphadimidines, and penicillin-streptomycin were most commonly used in livestock [16,43]. Similar levels of antimicrobials usage have been reported in India, where penicillin and tetracycline were most commonly used in food animals [44].
This study has identified the key pathways for emergence and spread of antimicrobial resistant pathogens from food animals to humans. These were through: contaminated food animal products (raw milk, raw cheese, under cooked meat); direct or indirect contacts with contaminated animals and fomites; and antimicrobials contaminated environmental releases and wastes (faeces and their aerosols). These observations are in consonance with study findings that reported transmission of resistant pathogens from livestock to humans through food consumption, or through direct contact with animals or their waste in the environment [[45], [46], [47], [48], [49], [50], [51]].
Socio-cultural activities of improper antimicrobial usage, non-enforcement of laws regulating antimicrobial usage, weak financial status of pastoralists, low education and expertise of pastoralists, nomadic and transhumance culture of pastoralists, and extensive husbandry management system were found to significantly influence antimicrobials misuse on livestock. Improper antimicrobial usage and lack of enforcement of regulations guiding the use of antimicrobials in food animal are major factors contributing to increase in antimicrobial resistance [52]. To mitigate antimicrobials misuse and resistance rates in livestock, we advocate strengthening of awareness campaign in pastoralist communities through mass media, especially radio. For a successful intervention in reform of socio-cultural activities, inter-disciplinary collaborations involving veterinary and public health authorities, anthropologists, and sociologists are needed to educate pastoralists to change their perceptions and knowledge beforehand towards proper antimicrobial usage on livestock. Previous studies have indicated collaborative efforts to be the most efficient strategies for the modification of behaviours, achievement and enactment of effective cultural change among antimicrobials users for diseases control and prevention [[53], [54], [55], [56]].
Although most of the antimicrobials used by pastoralists were within the first categories of the OIE List [57], the major problem was with practice of appropriate dosages. The observed interconnected social factors that driven antimicrobials misuse could create complex challenges associated with antimicrobial resistance in food animals, humans and the environment. Single, isolated interventions may, therefore, have limited impact. Strategies to address current gaps in knowledge and practices regarding prudent antimicrobial usage in food animals should be through “One Health” approach because of human and environment involvement. This will also aligned with the OIE strategy on antimicrobial resistance and prudent use of antimicrobials and WHO Global Action Plan that recognizes importance of an approach involving human and animal health, agricultural and environmental needs [58].
This study used questionnaire tool designed in English and translated to Hausa language since majority of the participants do not possessed formal education to sufficiently answer questions satisfactorily, which is a major limitation. In this case, the questionnaire was pilot tested after translation to make sure that no information was lost in the process and the questions are interpreted in the same way as in the original questionnaire.
5. Conclusion
The current study has revealed overall low levels of knowledge and practices regarding antimicrobial usage in livestock among pastoralists surveyed. Improve pastoralists' knowledge about effects of antimicrobials misuse and promotion of prudent usage in livestock will mitigate antimicrobial resistance menace in food animals for direct benefits to humans. Antimicrobial resistant pathogens can be spread to humans through contaminated animal products; contaminated animals and fomites; and environmental wastes (faeces). Consideration of these pathways is crucial for the surveillance, control and prevention of antimicrobials misuse. Socio-cultural activities significantly influenced antimicrobials misuse among pastoralists. Gradual reform of these activities through collaborations, in line with ‘One Health’ approach, will assure food safety, food security, and animal, public and environmental health.
Authors' contributions
NBA contributed to the study design, data collection, study execution, data analysis and interpretation. TOI contributed to the study design, study execution, data analysis and interpretation. All authors read and approved the final version of the manuscript.
Acknowledgments
Acknowledgement
Our acknowledgement goes to all animal health officers in the Area Veterinary Offices who have helped us to identify pastoralists and their settlements and helped us in the data collection. Great thanks are extended to the pastoralists who voluntarily participated in this study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.ILRI . ILRI; Nairobi, Kenya: 2012. Mapping of Poverty and Likely Zoonoses Hotspots.http://cgspace.cgiar.org/handle/10568/21161 [Google Scholar]
- 2.Suleiman H. Proceedings of the National Conference on Pastoralism in Nigeria. Ahmadu Bello University Zaria; Nigeria: 1988. Policy issues in agropastoral development in Nigeria. [Google Scholar]
- 3.Iyayi E.A., Okoruwa V.O., Babayemi O.J., Busari A.A., Peters O.F. Livestock production pattern of agro-pastoralists in peri-urban centres of south-west Nigeria. Niger. J. Anim. Prod. 2003;30:87–92. [Google Scholar]
- 4.Lawal-Adebowale O.A. Dynamics of ruminant livestock management in the context of the Nigerian agricultural system. INTECH. 2012;4:61–80. [Google Scholar]
- 5.Robinson T.P., Bu D.P., Carrique-Mas J., Fevre E.M., Gilbert M., Grace D., Hay S.I. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016;110(7):377–380. doi: 10.1093/trstmh/trw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO, World Health Organization's First Global Report on Antibiotic Resistance. http://www.who.int/mediacentre/news/releases/2014/amr-report/en/, 2014a (accessed 14 June 2016).
- 7.Van Boeckel T.P., Brower C., Gilbert M., Grenfella B.T., Levina S.A., Robinson T.P. Global trends in antimicrobial use in food animals. PNAS. 2014;12(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goossens H., Ferech M., Vander Stichele R., Elseviers M., ESAC Project Group Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. 2005. [DOI] [PubMed] [Google Scholar]
- 9.Van Boeckel T.P., Gandra S., Ashok A., Caudron Q., Grenfell B.T., Levin S.A. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014;14(8):742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 10.Aarestrup F. Sustainable farming: get pigs off antibiotics. Nature. 2012;486(7404):465–466. doi: 10.1038/486465a. [DOI] [PubMed] [Google Scholar]
- 11.WHO, Antimicrobial resistance. World Health Organization Fact Sheet, No. 194. http://www.who.int/mediacentre/factsheets/fs194/en, 20156 (accessed 22 August, 2016).
- 12.Neu H.C. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 13.Witte W. Medical consequences of antibiotic use in agriculture. Science. 1998;279:996–997. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- 14.Komalate O. Antibiotic resistance in bacteria—an emerging public health problem. Malawi Med. J. 2003;15(2):63–67. doi: 10.4314/mmj.v15i2.10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . Report of the 2nd WHP Expert Meeting, 29–31 May, Copenhagen. 2007. Critically important antimicrobials for human medicine: categorization for the development of risk management strategies to contain antimicrobial resistance due to nonhuman antimicrobial use. [Google Scholar]
- 16.Katakweba A.A.S., Mtambo M.M.A., Olsen J.E., Muhairwa A.P. Awareness of human health risks associated with the use of antibiotics among livestock keepers and factors that contribute to selection of antibiotic resistance bacteria within livestock in Tanzania. Livest. Res. Rural. Dev. 2012;24:170. Art. #. [Google Scholar]
- 17.MLFD . 2015 Annual Livestock Report of the Ministry of Livestock and Fisheries Development (MLFD) Minna; Niger State, Nigeria: 2016. Estimated livestock population in Niger State; pp. 1–49. [Google Scholar]
- 18.Dean A.G., Sullivan K.M., Soe M.M. 2009. Open Source Epidemiologic Statistics for Public Health (OpenEpi), Version 2.3.1. [Google Scholar]
- 19.Thrusfield M. 3rd ed. Blackwell Science Ltd, a Blackwell Publishing company; 9600 Garsington Road, Oxford OX4 2DQ, UK: 2009. Veterinary Epidemiology; pp. 228–238. [Google Scholar]
- 20.WMADH World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001;79:373–374. [PMC free article] [PubMed] [Google Scholar]
- 21.Dohoo I., Martin W., Studahl H. Measures of association. In: McPike S.M., editor. Veterinary Epidemiologic Research. 2nd ed. University of Prince Edward Island; Charlottetown, PE, Canada: 2009. pp. 136–148. [Google Scholar]
- 22.Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc. Natl. Acad. Sci. U. S. A. 1973;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aminov R.I., Mackie R.I. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol. Lett. 2007;271:147–161. doi: 10.1111/j.1574-6968.2007.00757.x. [DOI] [PubMed] [Google Scholar]
- 24.Landers T.F., Cohen B., Wittum T.E., Larson E.L. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127(1):4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okoli I.C., Nwosu C.I., Okoli G.C., Okeudo N.J., Ibekwe V. Drug management of anti-microbial resistance in avian bacterial pathogen in Nigeria, Intern. J. Environ. Health Human Dev. 2002;3(1):39–48. [Google Scholar]
- 26.Okoli I.C., Anyaegbunam C.N., Etuk E.B., Opara M.N., Udedibie A.B.I. Entrepreneurial characteristics and constraints of poultry business in Imo state, Nigeria. J. Agric. Soc. Res. 2005;5(1):25–32. [Google Scholar]
- 27.Sirdar M.M., Picard J., Bisschop S., Gummow B.A. A questionnaire survey of poultry layer farmers in Khartoum State, Sudan, to study their antimicrobial awareness and usage patterns. Onderstepoort J. Vet. Res. 2012;79(1) doi: 10.4102/ojvr.v79i1.361. 8 pages. [DOI] [PubMed] [Google Scholar]
- 28.Oluwasile B.B., Agbaje M., Ojo O.E., Dipeolu M.A. Antibiotic usage pattern in selected poultry farms in Ogun state. J. Vet. Sci. 2014;12(1):45–50. [Google Scholar]
- 29.Morgan D.J., Okeke I.N., Laxminarayan R., Perencevich E.N., Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect. Dis. 2011;11:692–701. doi: 10.1016/S1473-3099(11)70054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larissa J.P., Verhegghe M., Crombé F., Dewulf J., Bleecke Y.D. Evidence of possible methicillin-resistant Staphylococcus aureus ST398 spread between pigs and other animals and people residing on the same farm. Prev. Vet. Med. 2013;109:293–303. doi: 10.1016/j.prevetmed.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Novo A., Andre S., Viana P., Nunes O.C., Manaia C.M. Antibiotic resistance, antimicrobial residues and bacterial community composition in urban wastewater. Water Res. 2013;47:1875–1887. doi: 10.1016/j.watres.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 32.PHE . 2014. New Report Reveals Increase in Use of Antibiotics Linked to Rising Levels of Antibiotic Resistance. News Release. London. [Google Scholar]
- 33.WHO . World Health Organization Geneva; Switzerland: 2014. Antimicrobial Resistance Global Report on Surveillance. [Google Scholar]
- 34.McEwen S.A. Antibiotic use in animal agriculture: what have we learned and where are we going? Anim. Biotechnol. 2006;17:239–250. doi: 10.1080/10495390600957233. [DOI] [PubMed] [Google Scholar]
- 35.Martinez J.L. Antibiotics and antibiotic resistance genes in natural environments. Science. 2008;321:365–367. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- 36.Andersson D.I., Hughes D. Microbiological effects of sub-lethal levels of antibiotics. Nat. Rev. Microbiol. 2014;12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- 37.You Y., Silbergeld E.K. Learning from agriculture: understanding low-dose antimicrobials as drivers of resistome expansion. Front. Microbiol. 2014;5:284. doi: 10.3389/fmicb.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabir J., Umoh V.J., Audu-Okoh E., Umoh J.U., Kwaga J.K.P. Veterinary drug use in poultry farms and determination of antimicrobial drug residue in commercial eggs and slaughtered chicken in Kaduna state, Nigeria. Food Control. 2004;15:99–105. [Google Scholar]
- 39.Oluyege A.O., Dada A.C., Ojo A.M., Oluwadare E. Antibiotic resistance profile of bacterial isolates from food sold on a university campus in south-western Nigeria. Afr. J. Biotechnol. 2009;8:5883–5887. [Google Scholar]
- 40.Olatoye I.O., Amosun E.A., Ogundipe G.A.T. Multidrug-resistant Escherichia coli O157 contamination of beef and chicken in municipal abattoirs of southwest Nigeria. Nat. Sci. 2012;10(8):125–132. [Google Scholar]
- 41.Adesokan H.K., Akanbi I.O., Akanbi I.M., Obaweda R.A. Pattern of antimicrobial usage in livestock animals in south-western Nigeria: the need for alternative plans. Onderstepoort J. Vet. Res. 2015;82(1) doi: 10.4102/ojvr.v82i1.816. Art #816. 6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojo O.E., Fabusoro E., Majasan A.A., Dipeolu M.A. Antimicrobials in animal production: usage and practices among livestock farmers in Oyo and Kaduna States of Nigeria. Trop. Anim. Health Prod. 2016;48:189–197. doi: 10.1007/s11250-015-0939-8. [DOI] [PubMed] [Google Scholar]
- 43.Eagar H., Swan G., Van Vuuren M. A survey of antimicrobial usage in animals in South Africa with specific reference to food animals. J. South Afr. Vet. Assoc. 2012;83(1) doi: 10.4102/jsava.v83i1.16. Art #16. 8 pages) [DOI] [PubMed] [Google Scholar]
- 44.Sahoo K.C., Tamhankar A.J., Johansson E., Lundborg C.S. Antibiotic use, resistance development and environmental factors: a qualitative study among healthcare professionals in Orissa, India. BMC Public Health. 2010;10(629):1–10. doi: 10.1186/1471-2458-10-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong P.Y., Yannarell A., Mackie R.I. CABI; Wallingford: 2011. The Contribution of Antibiotic Residues and Antibiotic Resistance Genes from Livestock Operations to Antibiotic Resistance in the Environment and Food Chain. [Google Scholar]
- 47.Marti R., Scott A., Tien Y.C., Murray R., Sabourin L., Zhang Y., Topp E. Impact of manure fertilization on the abundance of antibiotic-resistant bacteria and frequency of detection of antibiotic resistance genes in soil and on vegetables at harvest. Appl. Environ. Microbiol. 2013;79:5701–5709. doi: 10.1128/AEM.01682-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capita R., Alonso-Calleja C. Antibiotic-resistant bacteria: a challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013;53:11–48. doi: 10.1080/10408398.2010.519837. [DOI] [PubMed] [Google Scholar]
- 49.Aam A.A.O.M. American Academy of Microbiology; Washington, DC: 2009. Antibiotic Resistance: An Ecological Perspective on an Old Problem. [Google Scholar]
- 50.Woolridge M. Evidence for the circulation of antimicrobial-resistant strains and genes in nature and especially between humans and animals. Rev. Sci. Technol. Off. Int. Epiz. 2012;31:231–247. doi: 10.20506/rst.31.1.2109. [DOI] [PubMed] [Google Scholar]
- 51.Mceachran A.D., Blackwell B.R., Hanson J.D., Wooten K.J., Mayer G.D., Cox S.B. Antibiotics, bacteria, and antibiotic resistance genes: aerial transport from cattle feed yards via particulate matter. Environ. Health Perspect. 2015;123:337–343. doi: 10.1289/ehp.1408555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mtenga A., Emanuel M., Mabula J., Peter R. Alliance for the Prudent Use of Antibiotics (APUA); 136 Harrison Avenue, Boston: 2011. Consumer and Practitioner Education: Status of Antibiotic Resistance. [Google Scholar]
- 53.Prabhakar S.V.R.K., Sano D., Srivastava N. Sustainable Consumption and Production in the Asia-Pacific Region: Effective Responses in a Resource Constrained World White Paper III. Institute for Global Environmental Strategies; Hayama: 2010. Food safety in the Asia-Pacific Region: current status, policy perspectives and away forward; pp. 215–298. [Google Scholar]
- 54.Mensah P., Mwamakamba L., Kariuki S., Fonkoua M.C., Aidara-Kane A. Strengthening foodborne diseases surveillance in the WHO African region: a essential need for disease control and food safety surveillance. Afr. J. Food Agric. Nutr. Dev. 2012;12:6336–6353. [Google Scholar]
- 55.Mwamakamba L., Mensah P., Fontannaz-Aujoulat F., Hlabana M., Maiga F., Bangoura F. The WHO five keys to safer food: a tool for food safety health promotion. Afr. J. Food Agric. Nutr. Dev. 2012;16:6245–6259. [Google Scholar]
- 56.Harbarth S., Balkhy H.H., Goossens H., Jarlier V., Kluytmans J., Laxminarayan R. Antimicrobial resistance: one world, one fight! Antimicrob Resist Infect Control. 2015;4:49. [Google Scholar]
- 57.OIE . The World Organisation for Animal Health; Paris, France: 2015. List of Antimicrobial Agents of Veterinary Importance. [Google Scholar]
- 58.OIE . The World Organisation for Animal Health; Paris, France: 2016. The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. [Google Scholar]