Abstract
Aims
This study assessed the performance of a new fully automated immunoassay, ARCHITECT B.R.A.H.M.S procalcitonin (PCT), comparing the results with other commercial assays on routine clinical specimens.
Methods
At nine sites from eight countries, precision analysis was carried out on controls by ANOVA. Threshold and linearity were verified according to standard procedures. Comparison of ARCHITECT B.R.A.H.M.S PCT with the Cobas®, LIAISON®, VIDAS® and Kryptor® PCT assays was evaluated using Passing-Bablok and Deming regression analyses.
Results
The within-laboratory standard deviation and %CV across all sites ranged from 0.005 to 0.008 and 2.7 to 4.1; 0.040 to 0.212 and 2.1 to 11.7; 1.628 to 4.191 and 2.5–6.3 for the three control levels, respectively. The mean slope (linearity analysis) across all sites ranged from 0.85 to 1.03, with a mean y-intercept ranging from –6.15 to + 1.71 and a correlation coefficient ranging from 0.94 to 1.00. The LoB, LoD, and LoQ claims were verified. Deming regression analysis of 1116 plasma or serum samples with PCT results detected across a dynamic assay range of 0.02–100 μg/l using the ARCHITECT B.R.A.H.M.S PCT assay yielded results of r = 0.989 vs. Roche Cobas®, r = 0.986 vs Kryptor® B.R.A.H.M.S, r = 0.987 vs BioMèrieux VIDAS® and r = 0.972 vs. Diasorin LIAISON®, respectively. Concordance at cut-offs of 0.25 μg/l and 0.50 μg/l were 96.9% and 98.1% with Roche Cobas®, 95.4% and 96.1% with B.R.A.H.M.S Kryptor®, 93.8% and 98.4% with BioMèrieux VIDAS®, and 92.7% and 93.9% with Diasorin LIAISON®.
Conclusions
Compared with other assays, ARCHITECT B.R.A.H.M.S PCT offers excellent precision and low-end sensitivity.
1. Introduction
Procalcitonin (PCT), a 13-kDa peptide precursor of calcitonin, has gained widespread acceptance as an early and specific biomarker for the presence of systemic bacterial infection [1]. Based on high plasma levels of PCT during systemic bacterial and fungal infections that decrease on recovery, and low concentrations during infections of viral origin or nonspecific causes, the use of PCT assays can be used to assist the clinical management of different patient groups in a range of clinical settings [1], [2]. In particular, PCT monitoring has demonstrated value in guiding antibiotic use for bacterial infections. Controlled, clinical studies have found that PCT-guided therapy can reduce antibiotic exposure in multiple indications including lower respiratory tract infections, urinary tract infections, sepsis, meningitis, and pneumonia [3], [4], [5], [6], [7], [8], [9], [10], [11].
In recent years, the B.R.A.H.M.S Kryptor® PCT assay has been used to validate other automated immunoassay platforms, including Roche's Elecsys, Cobas® and Modular E170 systems [2], the BioMérieux VIDAS® assay [12], [13], [14], Siemens ADVIA Centaur® [1], and the LIAISON® B.R.A.H.M.S PCT II GEN [15]. The ARCHITECT B.R.A.H.M.S PCT assay (Abbott Laboratories, Wiesbaden, Germany) is a novel PCT immunoassay intended for use with automated ARCHITECT instrumentation. In this study, the ARCHITECT B.R.A.H.M.S PCT assay was evaluated with the objective of assessing assay precision, linearity and sensitivity, and the performance of the assay in comparison with the Roche Cobas®, LIAISON®, BioMérieux VIDAS® and B.R.A.H.M.S Kryptor® systems.
2. Methods
The ARCHITECT B.R.A.H.M.S PCT assay is a two-step immunoassay using chemiluminescent microparticle immunoassay (CMIA) technology, with a 29-min assay completion time. The ARCHITECT B.R.A.H.M.S PCT assay was evaluated on Abbott ARCHITECT i2000SR immunoanalyzers (Abbott Laboratories, Abbott Park, IL, USA) using de-identified plasma or serum samples from patients tested at nine clinical laboratories in Australia, France, Germany (n = 2), Italy, South Africa, Turkey, Vietnam and Wales. The manufacturer had previously performed the equivalence of plasma and serum samples for the ARCHITECT B.R.A.H.M.S assay [16]. Plasma or serum samples were tested either fresh or following storage of the serum fraction at 2–8 °C for a maximum of 24 h. Each individual involved in executing the study was trained on the operation and maintenance of the ARCHITECT instrument systems and comparator instrument systems, as applicable. The study was conducted in compliance with the study protocol, Good Clinical Practice and applicable regulatory requirements. An informed consent waiver was obtained from the Independent Ethics Committee of each study site.
2.1. Analytical performance
Precision, linearity and sensitivity studies were conducted according to Clinical and Laboratory Standards Institute (CLSI) guidelines, EP15-A2 and EP17-A2 [17], [18]. For the precision analysis, a single ARCHITECT run was performed on each of 5 days, with runs consisting of 5 replicates of each level of quality control material (0.20 ng/mL, 2.00 ng/mL, 70.00 ng/mL; manufactured by Abbott Laboratories, Wiesbaden, Germany). Samples with known high analyte concentrations were used for the linearity analysis and diluted to a minimum of 5 linearity levels. For the sensitivity analysis, testing included 1 run on each of 3 days, with each run consisting of 2 samples tested in 4 replicates. Limit of blank (LOB), limit of detection (LOD) and limit of quantitation (LOQ) were assessed using leftover de-identified samples and targeted at or below the established claim for each limit.
2.2. Method comparison
The ARCHITECT B.R.A.H.M.S PCT assay was compared with the Roche Cobas®, Diasorin LIAISON®, BioMérieux VIDAS®, and widely accepted B.R.A.H.M.S Kryptor® systems based on guidance from the CLSI EP09-A3 protocol [19]. All instruments were calibrated according to their respective package inserts. Samples were analysed once on ARCHITECT i2000SR immunoanalysers and reanalysed on the LIAISON®, Kryptor®, VIDAS® and Cobas® for determination of PCT levels. For analytical comparison, we used 1116 plasma or serum samples tested at clinical laboratories from nine sites in eight countries, with three samples excluded from analyses due to no available comparator results. Samples were pooled across sites for each comparator system and samples for the ARCHITECT PCT assay were pooled by sites using the same comparator, respective to each analysis.
2.3. Statistical analyses
For the precision analysis, mean values, within-run and between-day variance (standard deviation [SD]), and percent coefficient of variation (%CV) were estimated for each control level using analysis of variance (ANOVA). Within-laboratory (total) variance was calculated as the accumulation of the within-run and between-day variance, and was used to estimate the total SD and total %CV. For each linearity sample level, the linearity relationship between the mean and the target value was evaluated. For LOB verification, the number and percentage of results across samples and days that were ≤ LOB claim were calculated. For LOD verification, the number and percentage of results across samples and days that were ≥ LOB claim were calculated. For LOQ, the number and percentage of results that were within the allowable total error around the target value of the samples, across samples and days, was calculated. Comparison of ARCHITECT B.R.A.H.M.S PCT with the Cobas®, LIAISON®, VIDAS® and Kryptor® PCT assays was evaluated using both Passing-Bablok and Deming regression analyses. The correlation coefficient (r), linear regression slope and intercept were calculated. Bias was evaluated using the Bland-Altman method. Samples with missing results were excluded from the analysis. All data analyses were performed using SAS software (version 9.3 or higher).
3. Results
3.1. Performance characteristics
For the 5-day precision analysis, the within-laboratory SD and %CV across all sites ranged from 0.005 to 0.008 and 2.7 to 4.1, respectively, for the low concentration control; 0.040 to 0.212 and 2.1–11.7, respectively, for the medium concentration control; and 1.628 to 4.191 and 2.5 to 6.3, respectively, for the high concentration control. For the medium control, if the highest value is excluded to remove long-tail outliers, the SD ranged from 0.040 to 0.058 and %CV from 2.1 to 3.0. For the high control, if we exclude the highest value, the SD ranged from 1.628 to 2.865 and %CV from 2.5 to 4.0. For the linearity analysis, the mean slope across all sites ranged from 0.85 to 1.03, with a mean y-intercept ranging from –6.15 to + 1.71 and a correlation coefficient ranging from 0.94 to 1.00. The highest observed LOB and LOD value was 0.00 ng/mL, with an observed proportion boundary of 87%. The LoB (0.00 ng/mL), LoD (0.00 ng/mL), and LoQ (0.01 ng/mL) claims were verified.
3.2. Method comparison
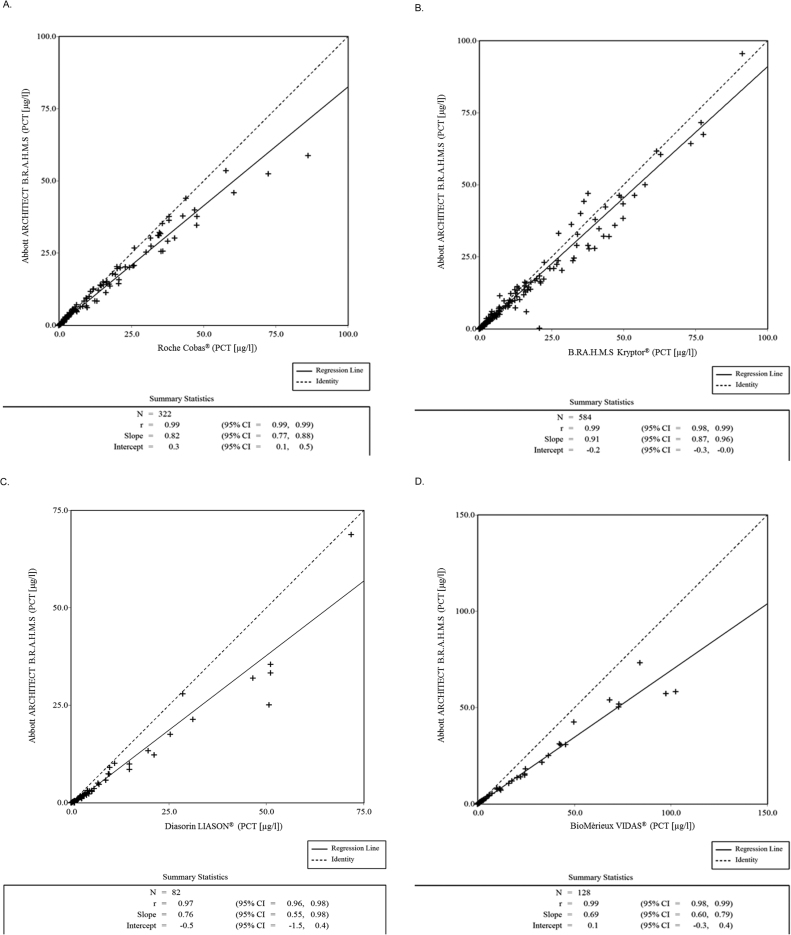
Deming regression analysis of 1116 plasma or serum samples with PCT results detected across a dynamic assay range of 0.02–100 µg/L using the ARCHITECT PCT assay showed a good correlation with comparators (Fig. 1). In 322 samples from sites in Germany, South Africa and Vietnam, the ARCHITECT PCT assay compared with the Roche Cobas® assay had a slope of 0.82 (95% confidence interval [CI], 0.77 to 0.88), y-intercept of 0.3 (95% CI, 0.1 to 0.5), and correlation coefficient of r = 0.989 (95% CI, 0.987 to 0.991). In 584 samples from sites in France, Germany, Turkey and Wales, the ARCHITECT PCT assay compared with the Kryptor® B.R.A.H.M.S assay had a slope of 0.91 (95% CI, 0.87 to 0.96), y-intercept of −0.2 (95% CI, −0.3 to 0.0), and r = 0.986 (95% CI, 0.984 to 0.988). In 128 samples from sites in Australia and Italy, the ARCHITECT PCT assay compared with the BioMèrieux VIDAS® assay had a slope of 0.69 (95% CI, 0.60 to 0.79), y-intercept of 0.1 (95% CI, −0.3 to 0.4), and r = 0.987 (95% CI, 0.981 to 0.991). In 82 samples from one site in Germany, the ARCHITECT PCT assay compared with the Diasorin LIAISON® assay had a slope of 0.76 (95% CI, 0.55 to 0.98), y-intercept of −0.5 (95% CI, −1.5 to 0.4), and correlation coefficient of r = 0.972 (95% CI, 0.956 to 0.982). The method comparison for Passing-Bablok and Deming regression analyses for samples from individual sites as well as pooled samples are summarized in the Table 1.
Fig. 1.
Deming regression scatter plots comparing ARCHITECT PCT assay with Roche Cobas® (A), B.R.A.H.M.S Kryptor® (B), Diasorin LIAISON® (C), and BioMèrieux VIDAS® (D) for measuring range of 0.02–100 µg/L.
Table 1.
Passing-Bablok and Deming regression analyses for plasma or serum samples collated across 9 sites including pooled samples (Germany, South Africa, Vietnam; France, Germany, Turkey, Wales; and Australia, Italy) with concentrations across the dynamic range of the assay (0.02 – 100 µg/L).
| N | SITE |
ARCHITECT |
Comparator |
Correlation coefficient (r) |
Passing-Bablok |
Deming regression |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Intercept |
Slope |
Intercept |
Slope |
||||||||||||||
| Min | Max | Name | Min | Max | r | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |||
| 276 | France | 0.0 | 95.6 | K | 0.0 | 91.5 | 0.990 | (0.988, 0.992) | − 0.0 | (−0.03, 0.0) | 0.96 | (0.95, 0.98) | − 0.0 | (−0.2, 0.1) | 0.97 | (0.90, 1.04) | |
| 152 | Altona, Germany | 0.0 | 58.9 | C | 0.0 | 86.5 | 0.990 | (0.987, 0.993) | − 0.0 | (0.0, 0.0) | 0.96 | (0.93, 0.98) | 0.6 | (0.2, 0.9) | 0.77 | (0.69, 0.85) | |
| 104 | Altona, Germany | 0.0 | 32.1 | K | 0.1 | 45.2 | 0.991 | (0.986, 0.994) | − 0.0 | (0.0, 0.0) | 0.81 | (0.79, 0.83) | 0.1 | (0.0, 0.1) | 0.71 | (0.68, 0.75) | |
| 52 | Italy | 0.0 | 25.3 | V | 0.1 | 36.9 | 0.999 | (0.998, 0.999) | 0.0 | (0.0, 0.0) | 0.69 | (0.67, 0.72) | 0.0 | (0.0, 0.1) | 0.66 | (0.63, 0.69) | |
| 82 | Gottingen, Germany | 0.0 | 68.9 | DL | 0.1 | 72.0 | 0.972 | (0.956, 0.982) | − 0.0 | (−0.1, 0.0) | 0.65 | (0.63, 0.68) | − 0.5 | (−1.5, 0.4) | 0.76 | (0.55, 0.98) | |
| 132 | Wales | 0.0 | 64.4 | K | 0.0 | 73.7 | 0.991 | (0.987, 0.993) | − 0.0 | (0.0, 0.0) | 0.85 | (0.81, 0.87) | − 0.1 | (−0.4, 0.1) | 0.89 | (0.82, 0.96) | |
| 76 | Australia | 0.0 | 73.4 | V | 0.1 | 102.9 | 0.985 | (0.977, 0.991) | 0.0 | (0.0, 0.0) | 0.74 | (0.72, 0.77) | 0.2 | (−0.3, 0.6) | 0.69 | (0.59, 0.80) | |
| 101 | Vietnam | 0.0 | 46.0 | C | 0.0 | 60.8 | 0.995 | (0.992, 0.997) | − 0.0 | (0.0, 0.0) | 0.87 | (0.84, 0.90) | 0.1 | (0.0, 0.2) | 0.78 | (0.73, 0.83) | |
| 69 | South Africa | 0.0 | 53.6 | C | 0.0 | 58.0 | 0.997 | (0.994, 0.998) | − 0.0 | (0.0, 0.0) | 0.93 | (0.91, 0.96) | − 0.1 | (−0.3, 0.1) | 0.93 | (0.90, 0.97) | |
| 73 | Turkey | 0.0 | 71.8 | K | 0.0 | 77.2 | 0.993 | (0.989, 0.995) | − 0.0 | (0.0, 0.0) | 0.84 | (0.80, 0.87) | − 0.2 | (−0.5, 0.1) | 0.88 | (0.79, 0.97) | |
| 322 | Germany, South Africa, Vietnam | 0.0 | 58.9 | C | 0.0 | 86.5 | 0.989 | (0.987, 0.991) | − 0.0 | (0.0, 0.0) | 0.94 | (0.93, 0.96) | 0.3 | (0.1, 0.5) | 0.82 | (0.77, 0.88) | |
| 584 | France, Germany, Turkey, Wales | 0.0 | 95.6 | K | 0.0 | 91.5 | 0.986 | (0.984, 0.988) | − 0.0 | (0.0, 0.0) | 0.87 | (0.86, 0.88) | − 0.2 | (−0.3, 0.0) | 0.91 | (0.87, 0.96) | |
| 128 | Australia, Italy | 0.0 | 73.4 | V | 0.1 | 102.9 | 0.987 | (0.981, 0.991) | 0.0 | (0.0, 0.0) | 0.73 | (0.71, 0.74) | 0.1 | (−0.3, 0.4) | 0.69 | (0.60, 0.79) | |
C, Roche Cobas®; CI, confidence interval; DL, Diasorin LIAISON® XL; Est, estimated; K, B.R.A.H.M.S Kryptor®; Max, maximum; Min, minimum; V, BioMèrieux VIDAS®.
The mean bias between the ARCHITECT B.R.A.H.M.S PCT assay and comparators at the measuring range of 0.02–100 µg/l was −0.8 µg/l (95% CI, −27.6–0.9 µg/l) for Roche Cobas®, −0.6 μg/l (95% CI, −20.7–9.3 µg/l) for B.R.A.H.M.S Kryptor®, −2.5 µg/l (95% CI, −44.5–0.1 µg/l) for BioMèrieux VIDAS®, and −2.3 µg/l (95% CI, −25.7–0.0 µg/l) for Diasorin LIASON®. Concordance for ARCHITECT B.R.A.H.M.S PCT assay with each comparator assay pooled across sample sites at the cut-offs of 0.25 µg/l and 0.50 µg/l, respectively, was 96.9% and 98.1% with Roche Cobas®, 95.4% and 96.1% with B.R.A.H.M.S Kryptor®, 93.8% and 98.4% with BioMèrieux VIDAS®, and 92.7% and 93.9% with Diasorin LIAISON®.
4. Discussion
In this study, a novel PCT assay was evaluated, including its analytical performance using the ARCHITECT i2000SR immunoanalyzer. Compared with existing assays, the ARCHITECT B.R.A.H.M.S PCT assay showed equivalent method comparison findings across a dynamic assay range of 0.02–100 µg/L. Previous studies have similarly found concordance between B.R.A.H.M.S Kryptor® and Roche systems [2], Kryptor® and ADVIA Centaur® [1], Kryptor® and VIDAS® B.R.A.H.M.S PCT® [12], [13], [14], and Kryptor® and LIAISON® B.R.A.H.M.S PCT II GEN [15].
The imprecision characteristics obtained from nine sites in eight countries indicated that the assay is robust and independent of the instrument. Results for LOB, LOD and LOQ were in agreement with the manufacturer's specifications and thus verified the low-end performance of the assay [16]. Current literature supports a PCT threshold of ≥ 0.5 and ≥ 2.0 µg/L for detecting possible and likely sepsis, respectively, with a threshold of ≥ 10 µg/L indicating a high likelihood of severe sepsis or septic shock [20], [21], [22].
The data shows an excellent correlation between the Architect B.R.A.H.M.S PCT assay in comparison with the other assays examined in this study. The slope in the Deming regression scatter plots of 0.91 with the B.R.A.H.M.S Kryptor® PCT reference assay indicates a good accordance. Nevertheless, the regression lines of the method comparisons performed were considerably different, with slopes ranging between 0.69 and 0.91. Most importantly, this difference is seen at higher concentrations across all of the methods and not at the low end where medical cut-offs are. This may suggest a lack of standardization of the respective calibration materials for the different PCT assays.
5. Conclusions
In summary, independent observations at nine different clinical laboratory sites confirmed the analytical characteristics of the ARCHITECT B.R.A.H.M.S PCT immunoassay. Compared with other assays, ARCHITECT B.R.A.H.M.S PCT offers excellent precision and low-end sensitivity.
Acknowledgements
We would like to acknowledge MIMS Pte Ltd for providing writing and editorial assistance.
Acknowledgments
Authorship and contributions
All authors contributed to the intellectual content of the paper and provided significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data. All authors reviewed and revised the paper and approved the final version.
Acknowledgments
Funding
Supported by a research grant from Abbott Laboratories.
Acknowledgments
Disclosures
MTS has served on advisory panels for Roche, and has received research support from Abbott and Becton Dickinson. AS, JLY, JH and AB are employed by Abbott Laboratories. MHH, OP, LB, GL, KM and KM-O have declared no conflicts of interest.
References
- 1.Sanders R.-J., Schoorl M., Dekker E., Snijders D., Boersma W.G., Ten Boekel E. Evaluation of a new procalcitonin assay for the Siemens ADVIA Centaur with the established method on the B.R.A.H.M.S Kryptor. Clin. Lab. 2011;57:415–420. [PubMed] [Google Scholar]
- 2.de Wolf H.K., Gunnewiek J.K., Berk Y., van den Ouweland J., de Metz M. Comparison of a new procalcitonin assay from Roche with the established method on the Brahms Kryptor. Clin. Chem. 2009;55:1043–1044. doi: 10.1373/clinchem.2008.117655. [DOI] [PubMed] [Google Scholar]
- 3.Schuetz P., Briel M., Christ-Crain M., Stolz D., Bouadma L., Wolff M., Luyt C.-E., Chastre J., Tubach F., Kristoffersen K.B., Wei L., Burkhardt O., Welte T., Schroeder S., Nobre V., Tamm M., Bhatnagar N., Bucher H.C., Mueller B. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin. Infect. Dis. 2012;55:651–662. doi: 10.1093/cid/cis464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuetz P., Müller B., Christ-Crain M., Stolz D., Tamm M., Bouadma L., Luyt C.E., Wolff M., Chastre J., Tubach F., Kristoffersen K.B., Burkhardt O., Welte T., Schroeder S., Nobre V., Wei L., Bhatnagar N., Bucher H.C., Briel M. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. In: Schuetz P., editor. Cochrane Database Syst. Rev. John Wiley&Sons, Ltd; Chichester, UK: 2012. (CD007498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dusemund F., Bucher B., Meyer S., Thomann R., Kühn F., Bassetti S., Sprenger M., Baechli E., Sigrist T., Schwietert M., Amin D., Hausfater P., Carre E., Schuetz P., Gaillat J., Regez K., Bossart R., Schild U., Müller B., Albrich W.C. ProREAL Study Team, Influence of procalcitonin on decision to start antibiotic treatment in patients with a lower respiratory tract infection: insight from the observational multicentric ProREAL surveillance. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:51–60. doi: 10.1007/s10096-012-1713-8. [DOI] [PubMed] [Google Scholar]
- 6.Vincent J.-L., Sakr Y., Sprung C.L., Ranieri V.M., Reinhart K., Gerlach H., Moreno R., Carlet J., Le Gall J.-R., Payen D. Sepsis occurrence in acutely Ill patients investigators, sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 7.Prkno A., Wacker C., Brunkhorst F.M., Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock--a systematic review and meta-analysis. Crit. Care. 2013;17:R291. doi: 10.1186/cc13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drozdov D., Schwarz S., Kutz A., Grolimund E., Rast A.C., Steiner D., Regez K., Schild U., Guglielmetti M., Conca A., Reutlinger B., Ottiger C., Buchkremer F., Haubitz S., Blum C., Huber A., Buergi U., Schuetz P., Bock A., Fux C.A., Mueller B., Albrich W.C. Procalcitonin and pyuria-based algorithm reduces antibiotic use in urinary tract infections: a randomized controlled trial. BMC Med. 2015;13:104. doi: 10.1186/s12916-015-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maseda E., Suarez-de-la-Rica A., Anillo V., Tamayo E., García-Bernedo C.A., Ramasco F., Villagran M.-J., Maggi G., Gimenez M.-J., Aguilar L., Granizo J.-J., Buño A., Gilsanz F. Procalcitonin-guided therapy may reduce length of antibiotic treatment in intensive care unit patients with secondary peritonitis: a multicenter retrospective study. J. Crit. Care. 2015;30:537–542. doi: 10.1016/j.jcrc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Vikse J., Henry B.M., Roy J., Ramakrishnan P.K., Tomaszewski K.A., Walocha J.A. The role of serum procalcitonin in the diagnosis of bacterial meningitis in adults: a systematic review and meta-analysis. Int. J. Infect. Dis. 2015;38:68–76. doi: 10.1016/j.ijid.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Wei T.-T., Hu Z.-D., Qin B.-D., Ma N., Tang Q.-Q., Wang L.-L., Zhou L., Zhong R.-Q. Diagnostic accuracy of procalcitonin in bacterial meningitis versus nonbacterial meningitis: a systematic review and meta-analysis. Medicine. 2016;95:e3079. doi: 10.1097/MD.0000000000003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagabrielle J.-F., Tachet A., Boin V. Dosage de la procalcitonine: comparaison des résultats obtenus sur Kryptor® (Brahms) et Vidas® (BioMérieux) Immuno-anal. Biol. Spécialisée. 2008;23:245–250. [Google Scholar]
- 13.Hausfater P., Brochet C., Freund Y., Charles V., Bernard M. Procalcitonin measurement in routine emergency medicine practice: comparison between two immunoassays. Clin. Chem. Lab. Med. 2010;48:501–504. doi: 10.1515/CCLM.2010.091. [DOI] [PubMed] [Google Scholar]
- 14.Schuetz P., Christ-Crain M., Huber A.R., Müller B. Long-term stability of procalcitonin in frozen samples and comparison of Kryptor and VIDAS automated immunoassays. Clin. Biochem. 2010;43:341–344. doi: 10.1016/j.clinbiochem.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Fortunato A. A new sensitive automated assay for procalcitonin detection: liaison® BRAHMS PCT® II GEN. Pract. Lab. Med. 2016;6:1–7. doi: 10.1016/j.plabm.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott ARCHITECT B.R.A.H.M.S PCT. Package insert. G1-0647/R03, 2016.
- 17.Clinical and Laboratory Standards Institute (CLSI) User Verification of Performance for Precision and Trueness. 2nd ed. CLSI; Wayne, PA: 2006. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute (CLSI) Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures. 2nd ed. CLSI; Wayne, PA: 2012. [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (CLSI) Measurement Procedure Comparison and Bias Estimation Using Patient Samples. 3rd ed. CLSI; Wayne, PA: 2013. [DOI] [PubMed] [Google Scholar]
- 20.Brunkhorst F.M., Wegscheider K., Forycki Z.F., Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000;26:S148–S152. doi: 10.1007/BF02900728. [DOI] [PubMed] [Google Scholar]
- 21.Müller B., Becker K.L., Schächinger H., Rickenbacher P.R., Huber P.R., Zimmerli W., Ritz R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit. Care Med. 2000;28:977–983. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Harbarth S., Holeckova K., Froidevaux C., Pittet D., Ricou B., Grau G.E., Vadas L., Pugin J. Geneva sepsis network, diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am. J. Respir. Crit. Care Med. 2001;164:396–402. doi: 10.1164/ajrccm.164.3.2009052. [DOI] [PubMed] [Google Scholar]