Abstract
The aim of this study was to use single-molecule, nanopore sequencing to explore the genomic environment of the resistance determinants in a multidrug-resistant (MDR) strain of enteroaggregative Escherichia coli serotype O51 : H30, sequence type (ST) 38. Sequencing was performed on the MinION Flow cell MIN-106 R9.4. Nanopore raw FAST5 reads were base-called using Albacore v1.2.1, converted to FASTA and FASTQ formats using Poretools v0.6.0, and assembled using Unicycler v0.4.2, combining the long-read sequencing data with short-read data produced by Illumina sequencing. The genome was interrogated against an antimicrobial resistance (AMR) gene reference database using blast. The majority of the 12 AMR determinants identified were clustered together on the chromosome at three separate locations flanked by integrases and/or insertion elements [region 1 –catA, bla OXA-1, aac(6′)-Ib-cr, tetA and bla CTX-M-15; region 2 – dfrA1 and aadA1; region 3 – catA, bla TEM-1, tetA and sul2]. AMR determinants located outside these three regions were a chromosomally encoded bla CMY-16, mutations in gyrA and parC, and two plasmid-encoded AMR determinants, bla OXA-181 and qnrS1 located on the same IncX3 plasmid. Long-read analysis of whole genome sequencing data identified mobile genetic elements on which AMR determinants were located and revealed the combination of different AMR determinants co-located on the same mobile element. These data contribute to a better understanding of the transmission of co-located AMR determinants in MDR E. coli causing gastrointestinal and extra-intestinal infections.
Keywords: nanopore sequencing, mobile genetic elements
Data Summary
Long- and short-read FASTQ sequences have been deposited in the NCBI Sequence Read Archive under the BioProject PRJNA315192 (SRP071789), Sample: SRS2937479, Experiment: SRX3676443, Accession numbers Nanopore FASTQ: SRR6702264 and Illumina FASTQ: SRR5470155. Assembly accession numbers: CP026723.1 (chromosome) and CP026724.1 to CP026728.1 (five individual plasmids).
Impact Statement.
The implementation of whole genome sequencing (WGS) for routine public health surveillance has enabled us to monitor trends in antimicrobial resistance (AMR) gene content in Escherichia coli and provides real-time data on emerging resistance patterns nationally and internationally. Understanding the genomic environment of these AMR determinants, with respect to whether they are chromosomally encoded or plasmid-encoded, and whether they are co-located is essential for monitoring transmission of AMR and assessing the risk to public health. Short-read WGS data can identify the presence or absence of AMR determinants but not their genomic architecture. In this study, long-read analysis of WGS data enabled us to determine the mobile genetic elements on which the key AMR determinants are located, and to characterize the combination of different AMR determinants co-located on the same mobile element. A combination of short- and long-read WGS data contributes to a better understanding of the transmission of co-located AMR determinants in multidrug-resistant E. coli.
Introduction
Enteroaggregative Escherichia coli (EAEC) cause a range of gastrointestinal symptoms including acute or persistent, watery or mucoid diarrhoea, often accompanied by severe abdominal pain [1]. Studies have shown that EAEC make a significant contribution to the burden of diarrhoeal disease globally, and are a common cause of travellers’ diarrhoea in the UK [2]. Certain sequence types (STs), such as ST38, also cause extra-intestinal infections, including sepsis and urinary tract infections [3]. There are no known animal reservoirs and transmission is most likely person-to-person via the faecal oral route.
The implementation of whole genome sequencing (WGS) at Public Health England (PHE) has improved surveillance of antimicrobial resistance (AMR) of gastrointestinal pathogens [4]. Analysis of these surveillance data indicates that the incidence of AMR in strains of E. coli belonging to the EAEC pathotype is high in comparison with other diarrhoeagenic E. coli pathotypes [2]. In 2017, we identified a strain of EAEC serotype O51 : H30 ST38, isolated from a patient who developed persistent diarrhoea shortly before returning to the UK from Pakistan, that had 12 AMR genetic determinants including bla OXA-181, bla CMY-16, bla CTX-M-15, bla TEM-1, bla OXA-1, aadA1b, aac(6′)-Ib-cr, gyrA[83 : S-L];parC[80 : S-I;84 : E-V], qnrS1, dfrA1, tetA, sul2 and catA [2]. As described previously, the isolate was confirmed phenotypically by antimicrobial susceptibility testing to be resistant to the third-generation cephalosporins, ciprofloxacin, streptomycin, trimethoprim, tetracycline, sulphonamide and chloramphenicol [2].
From the analysis of the short-read sequencing data, it was not possible to determine the whether the resistance determinants in this multidrug-resistant (MDR) strain of EAEC were plasmid-encoded or if they had been incorporated into the chromosome. The aim of this study was to use single-molecule, nanopore sequencing to explore the genomic environment of the resistance determinants in this MDR strain.
Methods
DNA extraction and nanopore sequencing
DNA was extracted using the Wizard Genomic DNA Purification kit (Promega). Library preparation was performed using the 1D Genomic DNA sequencing kit SQK-LSK108 (Oxford Nanopore Technologies) with the omission of DNA shearing and DNA repair steps to prevent further DNA fragmentation. Library preparation was initiated at the DNA end-prep step using NEB repair modules (New England Biolabs). All bead washing steps were performed using AMPure XP beads (Beckman Coulter). The final 80 µl prepared library was sequenced. Sequencing was performed on the MinION using a FLO-MIN-106 R9.4 Flow cell (Oxford Nanopore Technologies) using the MinKNOW software for the full 48 h run time with no alterations to any voltage scripts.
Genome assembly and annotation
Nanopore raw FAST5 reads were basecalled using Albacore (Oxford Nanopore Technologies) v1.2.1., converted to FASTQ formats using Poretools v0.6.0 and assembled using Unicycler v0.4.2 combining the long-read sequencing data with short-read data produced by Illumina sequencing [5, 6]. The genome was interrogated against the previously described PHE AMR gene reference database [2] using blast n (alignment length 100 %, query coverage and identity at 80 %), and annotated using Prokka v1.12 [7]. Further annotation was performed manually using the information from Prokka and National Center for Biotechnology Information (NCBI) blastn for any unknown coding DNA sequences. When complete, regions of interest were viewed by DNA features viewer v0.1.3 (https://github.com/Edinburgh-Genome-Foundry/DnaFeaturesViewer). Illumina sequencing and detection of AMR determinants from the short-read data were performed as described previously [2].
Long- and short-read FASTQ sequences have been deposited in the NCBI Sequence Read Archive under the BioProject PRJNA315192 (SRP071789), Sample: SRS2937479, Experiment: SRX3676443, Accession numbers Nanopore FASTQ: SRR6702264 and Illumina FASTQ: SRR5470155. Assembly accession numbers: CP026723.1 (chromosome) and CP026724.1 to CP026728.1 (five individual plasmids)
Results and Discussion
The genome of EAEC O51 : H30 ST38 assembled into a single chromosomal contig of 5 492 922 bp and five plasmids (Table 1). Located on the chromosome at positions 2 087 027–2 109 149 were genes encoding pap pili/fimbriae, characteristic of extra-intestinal E. coli. The largest plasmid was 129 627 bp (p266917_2_01) and belonged to replicon type IncFIB based on the repA sequence. This plasmid carried known EAEC virulence genes, including the universal regulator aggR, the dispersin-encoding aap, the aggregative transported aat and the aggA genes encoding fimbriae type I. The remaining four plasmids (p266917_2_02, p266917_2_03, p266917_2_04 and p266917_2_05) were smaller in size ranging from 33 288 to 97 124 bp (Table 1). These plasmids have been previously described in E. coli and Klebsiella species, and isolated in globally dispersed regions from clinical samples, food, animals and the environment (Table 1).
Table 1. Plasmids identified in EAEC O51 : H30 ST38.
| Plasmid name | Inc type | Replicon type | Size (bp) | Notes |
|---|---|---|---|---|
| p266917_2_01 | IncFIB | repFIB | 129 627 | pAA encoding aggR |
| p266917_2_02 | IncY | repA1 | 97 124 | CP009168 – Clinical isolate (USA) |
| CP015997 – Isolated from a chicken | ||||
| CP012494 – Isolated from food | ||||
| KU980950 – Korean clinical MDR isolate | ||||
| p266917_2_03 | IncI1 | repA2 | 83 010 | CP021208 – Clinical isolate |
| CP023362/CP023370 – MDR isolates from veterinary sources | ||||
| KY964068 – Isolated from a pig | ||||
| p266917_2_04 | IncX3 | repB | 51 479 | Harbours bla OXA-181 and qnrS1 |
| p266917_2_05 | IncX4 | repE | 33 288 | CP016037 – Clinical isolate (Germany) |
| JX981514 – Isolated from cattle | ||||
| KM580533 – Isolated from a pig |
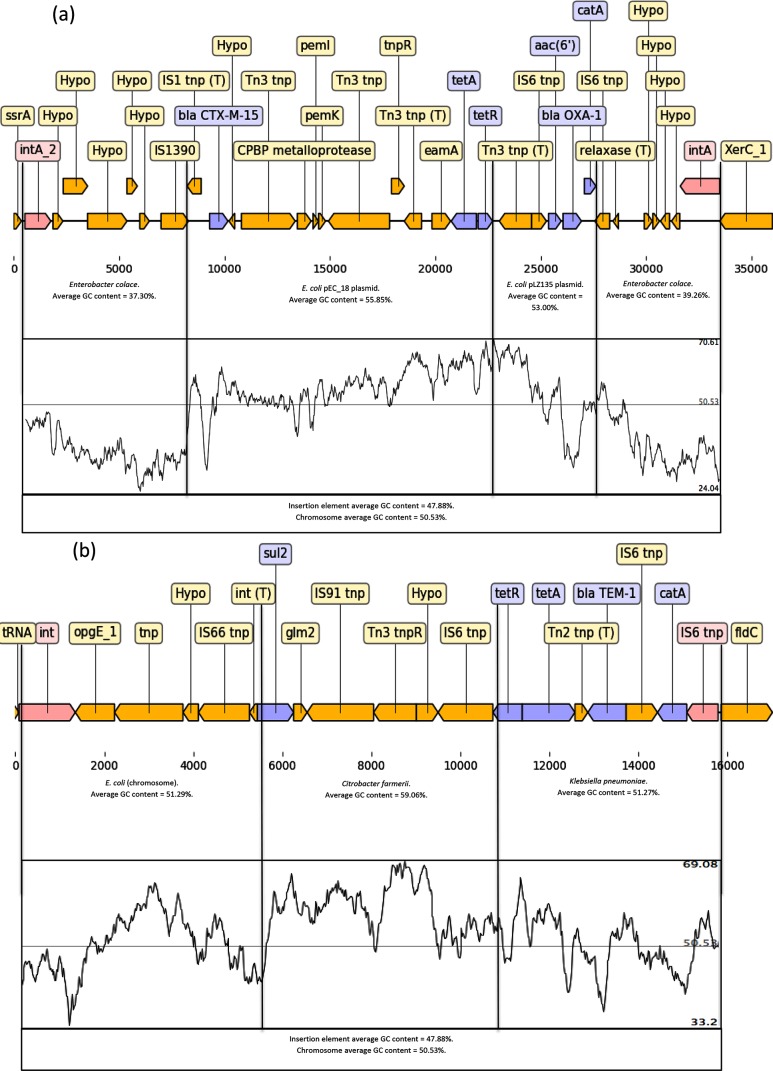
Despite the number of plasmids harboured by this isolate, analysis of the assembled genome indicated that the majority of the AMR determinants were clustered together on the chromosome in three separate regions flanked by integrases and/or insertion elements. AMR region 1 was located on the chromosome at positions 1 686 326–1 719 287 (32 961 bp) between a transfer messenger RNA gene (ssrA) and a tyrosine recombinase (xerC) (Fig. 1a). The AMR determinants located on this integron were catA, bla OXA-1, aac(6′)-Ib-cr, tetA and bla CTX-M-15, conferring reduced susceptibility to chloramphenicol, ampicillin, streptomycin and ciprofloxacin, tetracycline and the third-generation cephalosporins. bla CTX-M-15 is the most abundant CTX-M gene in ESBL (extended-spectrum beta-lactamase)-producing E. coli causing human infections [8]. Analysis using blastn indicated that the AMR determinants on this transposon originated from two different fragments of DNA originating from different plasmids (Accessions: NC_014384.1 and MF353155.1) inserted into a fragment of DNA that originated from a strain of Enterobacter cloace (Fig. 1a).
Fig. 1.
(a) Chromosomal AMR region 1 located at positions 1 686 326–1 719 287 between a transfer messenger RNA gene (ssrA) and a tyrosine recombinase (xerC) encoded catA, bla OXA-1, aac(6′)-Ib-cr, tetA and bla CTX-M-15. (b) Chromosomal AMR region 3 located at positions 3 674 464–3 693 662 between the tRNA-Leu (5′) gene and the (R)-phenyllactyl-CoA dehydratase beta subunit (fldC) (3′) gene encoded catA, bla TEM-1, tetA and sul2 AMR determinants highlighted in blue and intergrases and insertion elements highlighted pink.
AMR region 2 was identified as a Class 2 Tn7 and encoded dfrA1 and aadA1, conferring reduced susceptibility to trimethoprim and streptomycin. It was inserted at positions 3 044 997–3 057 993 (12 996 bp) between a phosphate-binding protein (pstC) and a glutamine-fructose-6-phosphate aminotransferase (glmS). Tn7 is a well-known representative of Class 2 integrons, and is found in many different bacterial species [9].
AMR region 3 was inserted between the tRNA-Leu gene and the phenyllactyl-CoA dehydratase beta subunit (fldC) gene at positions 3 674 464–3 693 662 (19 198 bp), and had four different AMR determinants, including catA, bla TEM-1, tetA and sul2 conferring reduced susceptibility to chloramphenicol, ampicillin, tetracycline and sulphonamide (Fig. 1b). Analysis using blastn indicated that this region comprised DNA fragments exhibiting homology with DNA from E. coli and a fragment of plasmid DNA (Accession: CP015026.1) previously identified in Klebsiella pneumoniae and Citrobacter farmerii (Fig. 1b). It was observed that both tetA and catA were present at two separate locations on the chromosome (AMR regions 1 and 3) (Fig. 1a, b). Furthermore, there were multiple genes encoding reduced susceptibility to streptomycin [aadA1b and aac(6′)-Ib-cr], ciprofloxacin [aac(6′)-Ib-cr, qnrS1 and the chromosomal mutations in gyrA and parC] and the β-lactams (bla CMY-16, bla CTX-M-15, bla OXA-1 and bla TEM-1) at different locations in the genome in this strain of EAEC (Fig. 1a, b).
The AMR determinants that were identified outside these three regions were bla CMY-16, located on the chromosome between positions 892 919 and 894 050, mutations in gyrA and parC, and two plasmid-encoded AMR determinants, the carbapenemase bla OXA-181 and the plasmid-mediated quinolone-resistant determinant (PMQR) qnrS1 (Table 1). bla CMY-16 has been previously described in Proteus mirabilis, Providencia stuartii, Salmonella enterica and Klebsiella pneumoniae [10–12]. In E. coli, bla CMY genes are usually found on plasmids but chromosomally encoded bla CMY have been described [13].
In addition to the PMQRs qnrS1 and aac(6′)-Ib-cr, which exhibit the potential to induce reduced susceptibility to the fluroquinolones, EAEC O51 : H30 also had one mutation in gyrA[83 : S-L] and two in parC[80 : S-I;84 : E-V]. The combination of all these fluroquinolone resistance determinants resulted in the isolate exhibiting an MIC>0.5 mg l−1 to ciprofloxacin.
Of the two plasmid-encoded AMR determinants, bla OXA-181 is a bla OXA-48-like carbapenemase conferring resistance to penicillins and carbapenems, and is commonly found on a 51 kb IncX3 plasmid [14], as observed in this study. The bla OXA-181 gene was initially identified in Enterobacter cloacae and Klebsiella pneumoniae isolates in India in 2007 [15]. Enterobacteriaceae isolates producing bla OXA-181 are globally distributed, although in most cases the patients report recent travel to the Indian subcontinent [16, 17]. The presence of bla OXA-181 on a self-transmissible IncX3 plasmid is of significance, as IncX3 plasmids have been found as a common vehicle mediating the dissemination of other carbapenemases, and have the potential to disseminate widely [15]. Due to the co-location of qnrS1 on the same plasmid, treatment with ciprofloxacin may indirectly select for resistance to the carbapenems, as well as the fluroquinolones.
A previous study in the UK showed that E. coli ST38 was the ST most commonly associated with bla OXA-48-like genes, and 18 % (62/351) had bla OXA-181 [17]. The authors suggested that the accumulation of bla OXA-48-like carbapenemases within the UK is due to repeated importations, coupled with both the spread of successful clones (e.g. E. coli ST38) and, in particular, the dissemination of successful plasmids [17].
Finally, an assembly utilizing only the ONT reads was generated with Unicycler (v0.4.2) with default parameters. To estimate the accuracy of the derived assembly, Illumina reads were mapped using BWA v0.7.13 and Samtools v1.1 and a VCF file was generated using GATK v2.6.5 with true positive SNPs defined as those with a mapping depth greater than 10, a consensus variant ratio of greater than 0.9 and mapping quality greater than 30. Illumina reads could map accurately to 81.15 % of the assembly and 152 030 variants were identified. This corresponded to an error rate in consensus assembly sequence of 3.18 % [152 030/(5 880 795−1 108 320)×100=3.18].
Conclusion
E. coli ST38 is a known cause of gastrointestinal disease and extra-intestinal infection, including sepsis and urinary tract infections, and has been associated with MDR in the UK and elsewhere [3, 18]. Short-read WGS data can identify the presence or absence of AMR determinants but not their genomic architecture. Long-read analysis of WGS data enables the characterization of mobile genetic elements on which the key AMR determinants are located, and identifies the combination of different AMR determinants co-located on the same mobile element, but may lack the accuracy required to identify AMR associated with mutations in chromosomal genes [19, 20]. A combination of short- and long-read WGS data facilitates the detailed analysis of AMR in E. coli, contributing to a better understanding of the transmission of co-located AMR determinants in MDR E. coli causing gastrointestinal and extra-intestinal infections.
Data bibliography
Greig DR, Dallman TJ, Jenkins C. NCBI Sequence Read Archive PRJNA315192 (2018).
Funding information
This work was supported by the National Institute for Health Research Health Protection Research Unit in Gastrointestinal Infections. Claire Jenkins and Tim Dallman are affiliated to the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Gastrointestinal Infections at University of Liverpool in partnership with PHE, in collaboration with University of East Anglia, University of Oxford and the Quadram Institute of Food Research. Claire Jenkins and Tim Dallman are based at PHE. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, the Department of Health or PHE.
Acknowledgements
We would like to thank Michel Doumith for his advice during the preparation of the manuscript. DG would also like to thank Nick Loman, Josh Quick, John Tyson, Matt Loose and all the organizers, instructors and participants at Pore Camp Birmingham July 2017.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AMR, antimicrobial resistance; EAEC, enteroaggregative Escherichia coli; MDR, multidrug resistance; PHE, Public Health England; PMQR, plasmid-mediated quinolone-resistant determinant; ST, sequence type; WGS, whole genome sequencing.
References
- 1.Okeke IN, Nataro JP. Enteroaggregative Escherichia coli . Lancet Infect Dis. 2001;1:304–313. doi: 10.1016/S1473-3099(01)00144-X. [DOI] [PubMed] [Google Scholar]
- 2.Do Nascimento V, Day MR, Doumith M, Hopkins KL, Woodford N, et al. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of enteroaggregative Escherichia coli isolated from cases of diarrhoeal disease in England, 2015–16. J Antimicrob Chemother. 2017;72:3288–3297. doi: 10.1093/jac/dkx301. [DOI] [PubMed] [Google Scholar]
- 3.Chattaway MA, Jenkins C, Ciesielczuk H, Day M, Donascimento V, et al. Evidence of evolving extraintestinal enteroaggregative Escherichia coli ST38 clone. Emerg Infect Dis. 2014;20:1935–1937. doi: 10.3201/eid2011.131845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day MR, Doumith M, do Nascimento V, Nair S, Ashton PM, et al. Comparison of phenotypic and WGS-derived antimicrobial resistance profiles of Salmonella enterica serovars Typhi and Paratyphi. J Antimicrob Chemother. 2018;73:365–372. doi: 10.1093/jac/dkx379. [DOI] [PubMed] [Google Scholar]
- 5.Loman NJ, Quinlan AR. Poretools: a toolkit for analyzing nanopore sequence data. Bioinformatics. 2014;30:3399–3401. doi: 10.1093/bioinformatics/btu555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wick RR, Judd LM, Gorrie CL, Holt KE. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genom. 2017;3:e000132. doi: 10.1099/mgen.0.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 8.Irrgang A, Falgenhauer L, Fischer J, Ghosh H, Guiral E, et al. CTX-M-15-producing E. coli isolates from food products in Germany are mainly associated with an IncF-type plasmid and belong to two predominant clonal E. coli lineages. Front Microbiol. 2017;8:2318. doi: 10.3389/fmicb.2017.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall RM, Collis CM. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist Updat. 1998;1:109–119. doi: 10.1016/S1368-7646(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 10.Arpin C, Thabet L, Yassine H, Messadi AA, Boukadida J, et al. Evolution of an incompatibility group IncA/C plasmid harboring bla CMY-16 and qnrA6 genes and its transfer through three clones of Providencia stuartii during a two-year outbreak in a Tunisian burn unit. Antimicrob Agents Chemother. 2012;56:1342–1349. doi: 10.1128/AAC.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour W, Haenni M, Saras E, Grami R, Mani Y, et al. Outbreak of colistin-resistant carbapenemase-producing Klebsiella pneumoniae in Tunisia. J Glob Antimicrob Resist. 2017;10:88–94. doi: 10.1016/j.jgar.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Villa L, Guerra B, Schmoger S, Fischer J, Helmuth R, et al. IncA/C plasmid carrying bla NDM-1, bla CMY-16, and fosA3 in a Salmonella enterica serovar corvallis strain isolated from a migratory wild bird in Germany. Antimicrob Agents Chemother. 2015;59:6597–6600. doi: 10.1128/AAC.00944-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phan MD, Peters KM, Sarkar S, Forde BM, Lo AW, et al. Third-generation cephalosporin resistance conferred by a chromosomally encoded bla CMY-23 gene in the Escherichia coli ST131 reference strain EC958. J Antimicrob Chemother. 2015;70:1969–1972. doi: 10.1093/jac/dkv066. [DOI] [PubMed] [Google Scholar]
- 14.Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, et al. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006–2007. Antimicrob Agents Chemother. 2011;55:1274–1278. doi: 10.1128/AAC.01497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruppé E, Armand-Lefèvre L, Estellat C, El-Mniai A, Boussadia Y, et al. Acquisition of carbapenemase-producing Enterobacteriaceae by healthy travellers to India, France, February 2012 to March 2013. Euro Surveill. 2014;19:20768. doi: 10.2807/1560-7917.ES2014.19.14.20768. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Feng Y, Wu W, Xie Y, Wang X, et al. First report of OXA-181-producing Escherichia coli in China and characterization of the isolate using whole-genome sequencing. Antimicrob Agents Chemother. 2015;59:5022–5025. doi: 10.1128/AAC.00442-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Findlay J, Hopkins KL, Loy R, Doumith M, Meunier D, et al. OXA-48-like carbapenemases in the UK: an analysis of isolates and cases from 2007 to 2014. J Antimicrob Chemother. 2017;72:1340–1349. doi: 10.1093/jac/dkx012. [DOI] [PubMed] [Google Scholar]
- 18.Turton JF, Doumith M, Hopkins KL, Perry C, Meunier D, et al. Clonal expansion of Escherichia coli ST38 carrying a chromosomally integrated OXA-48 carbapenemase gene. J Med Microbiol. 2016;65:538–546. doi: 10.1099/jmm.0.000248. [DOI] [PubMed] [Google Scholar]
- 19.Judge K, Harris SR, Reuter S, Parkhill J, Peacock SJ. Early insights into the potential of the Oxford Nanopore MinION for the detection of antimicrobial resistance genes. J Antimicrob Chemother. 2015;70:2775–2778. doi: 10.1093/jac/dkv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemon JK, Khil PP, Frank KM, Dekker JP. Rapid nanopore sequencing of plasmids and resistance gene detection in clinical isolates. J Clin Microbiol. 2017;55:3530–3543. doi: 10.1128/JCM.01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]