Abstract
Background:
Human papillomavirus (HPV) infection is associated with cervical cancer; however, it is controversial whether it is involved in non-cervical genital cancers.
Objective:
This study aimed to evaluate articles on the prevalence of HPV in penile cancer, vulvar cancer, colorectal cancer, prostate cancer and anal canal cancer in studies conducted in Brazil.
Methods:
The study was conducted in accordance with the Preferred Reporting of Systematic Reviews and Meta-Analysis Statement. Comprehensive searches for HPV and cancer for the years 2006 to 2016 were conducted in two databases (PubMed and Web of Knowledge) and Google Scholar system. We also tracked the references of all eligible articles to identify additional non-captured publications through online surveys.
Results:
Eighteen studies, with a combined sample size of 1,552 patients were analyzed. The overall prevalence of HPV was 43% (95% CI: 36–51%; p < 0.001). The pooled prevalence of HPV in penile cancer was 42% (95% CI: 32–55%; p < 0.001), in colorectal cancer it was 67% (95% CI: 64–70%; p < 0.001) and in vulvar cancer 43% (95% CI: 34–55%; p < 0.001). HPV 16 was the most prevalent in all sites evaluated, with prevalence estimated at 54% (95% CI: 44–66%; p < 0.001), followed by genotypes 33 (21%; 95% CI: 17–28; p < 0.001), 6 (15%; 95% CI: 8–26%; p < 0.001), 11 (13%; 95% CI: 5–32%; p < 0.001) and 18 (12%; 95% CI: 7–22%; p < 0.001), respectively. The pooled prevalence of single infection was 82% and infection by multiple genotypes of HPV was 22%.
Conclusion:
Our study demonstrated a high prevalence of HPV in non-cervical genital cancers in Brazil, with predominance of genotype 16, providing evidence for the need for preventive and control measures to avoid future harm to the population.
Keywords: Human papillomavirus, cancer, prevalence, Brazil
Introduction
Cancer is one of the leading causes of morbidity and mortality worldwide, with approximately 14 million new cases in 2012. Approximately 2.2 million (15.4%) of cases are attributed to carcinogenic infections, mainly human papillomavirus (HPV) (Plummer et al., 2016). HPV is associated with 99.7% of cervical cancer cases (Walboomers et al., 1999) and is implicated in the pathogenesis of other ano-genital malignancies (Crawford et al., 2011).
The carcinogenic effect of HPV depends on integration of the virus into the host-cell DNA and expression of the oncoproteins E6 and E7, which antagonize the functions of the tumor-suppressor proteins p53 and pRb, respectively (Damin et al., 2013). Twelve HPV types are classified as “high risk” (HPV: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59), and eight are probably or possibly oncogenic (HPV: 26, 53, 66, 67, 68, 70, 73, and 82) (Almeida et al., 2017).
HPV types 16 and 18, classified as high-risk viruses for cervical cancer, are currently considered as human carcinogens by the International Agency for Research on Cancer (Humans, 1995). However, the cancer-causing effects of HPV are not limited to the cervix; an estimated 50% of penile, 88% of anal, 43% of vulvar, 70% of vaginal, and 13–56% of oropharyngeal cancers are attributable to HPV, primarily HPV 16 and typically followed by HPV 18 (De Vuyst et al., 2009; WHO, 2013). Of these, anal cancer is the most strongly associated with HPV, yet much less is known of the natural history of anal compared to cervical infection (Crawford et al., 2011). Other studies have also shown the association of HPV and non-cervical cancers (Ang et al., 2011; De Vuyst et al., 2009; O’Sullivan et al., 2016; Zandberg et al., 2013).
HPV has a well-recognized etiological role in cervical and non-cervical cancers (Ang et al., 2011; Hu et al., 2015; Zandberg et al., 2013), however, the lack of consistent results from data on non-cervical cancer creates limitations to the planning of surveillance and control measures. The understanding of the prevalence of HPV in non-cervical cancers and the knowledge of the distribution of the viral subtypes are important epidemiological information that can help the development of local or regional public policies. We performed a systematic review and meta-analysis of studies carried out in Brazil on HPV infection and the development of prostate, anal, colorectal, penile and vulvar cancers, and in parallel, aimed to determine the prevalence and main HPV genotypes involved in cancers of the selected anatomical sites.
Materials and Methods
This review was conducted in accordance with the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement (Moher et al., 2015).
Search strategy
We searched the literature articles published from January 1, 2006 and December 31, 2016 that evaluated the prevalence and possible association between non-cervical genital cancer and HPV in Brazil. This stage was computed by five independent investigators (CMS, LDP, VLB, JP, and MT [“Group 1”]) that carried out the specific research to define the maximum possible Medical Subject Heading (MeSH) terms. Discrepancies or disagreements were resolved by consensus with support and validation of a specialist (JJVT). In the first phase, the researchers from Group 1 conducted the search in PubMed, Web of Knowledge databases, and Scholar Google system to find original articles, using as search criterion a combination of the following keywords organized in three blocks: Block 1: “penile neoplasms”, “Vulvar neoplasms”, “Vaginal neoplasms”, “Rectal Neoplasms”, “Prostatic Neoplasms”, “Adenocarcinoma”, “Anus Neoplasms”, “Carcinoma, Adenosquamous”, “Carcinoma, Squamous Cell”, “Genotype”, “Papillomaviridae”, “Papillomavirus infections”, Block 2: “Clinical Laboratory Techniques”, “Genotyping techniques”, “Human Papillomavirus DNA Tests”, “In Situ Hybridization”, “Molecular biology”, “ Multiplex Polymerase Chain Reaction”, “Polymerase Chain Reaction”, “Polymorphism, Restriction Fragment Length”, “Random Amplified Polymorphic DNA Technique”, “Real-Time Polymerase Chain Reaction”, “Reverse Transcriptase Polymerase Chain Reaction”, and Block 3: “Brazil”.
The PubMed search used a combination of Medical Subject Heading Terms; search on Web of Knowledge was made by topic; and the freely accessible Scholar Google system was used to perform a search using the same MeSH terms in different combinations. The identified studies were compiled into a database, and citations were initially screened by title and abstract to assess the potential eligibility.
The strategy used to design this study is described in the flow diagram (Figure 1). Abstracts were reviewed, and full-text articles of potentially relevant studies were examined independently by investigators in Group 1 and Group 2 (JJVT, CG, VS, FG and MELC) for the updated search against pre-specified selection criteria. Data were independently extracted by Group 1 and compared. If there was doubt about the suitability of the paper based on the abstract, the full text was reviewed. We also manually searched the references of selected articles for retrieval of publications not selected in the search. Any disagreements or discrepancies were resolved by consensus-based discussions. Cross-checking of bibliographies from other published reviews and from all retrieved articles was conducted to identify additional publications. The corresponding author was contacted whenever we had questions about the eligibility of the article or critical data were missing. Online searches were conducted between May 10, 2017 and August 31, 2017.
Figure 1.
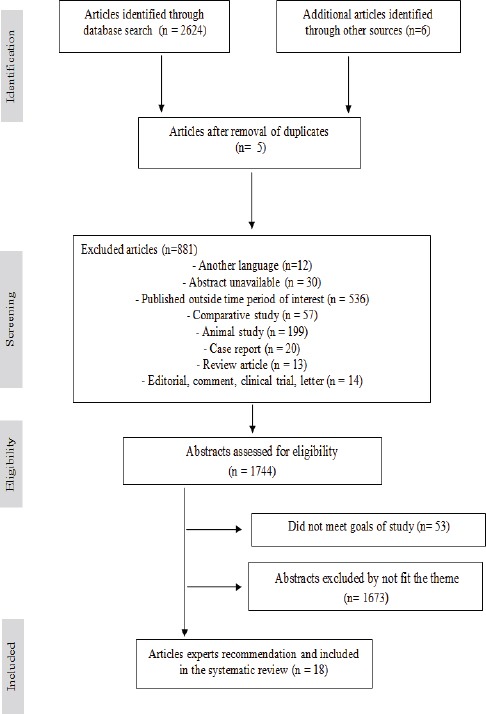
Flow Diagram of Selected Studies for Systematic Review. In Total, 18 Studies Met the Inclusion Criteria
Inclusion and exclusion of studies
We have included only the primary studies conducted in Brazil between 2006 and 2016 published in the medical literature that reported the prevalence of HPV in non-cervical genital cancers (prostate, penile, vulvar, colorectal, anal canal), as well as those reporting genotypes present in each case, detected by molecular biology techniques. Individuals were classified as having cancer by pathological analysis.
We excluded studies that evaluated the presence of HPV by cytological methods, systematic reviews and reviews, clinical trials, letters, comparative studies, those mainly with HIV positive or negative cases, case reports, studies that did not present analysis of the HPV genotypes, and duplicate publications. No restrictions on sex and age were applied.
Data extraction
For data extraction, researchers from Group 1 located articles in PDF format to check for the retention or otherwise of the publications in the study. The articles were randomized and distributed among Group 1 researchers for independent reading, followed by validation through consensus. The entries were checked by researchers from Group 2. This group also analyzed the content extracted from each article individually and included it in the tables, and any discrepancies in the results described in the tables were resolved by consensus. Whenever there was a doubt about the suitability of the paper based on the abstract, the full text was reviewed.
We extracted the following information from all eligible studies: study objectives, method used, period of sampling, age of patients, anatomical location of the cancers, number of patients who were part of the study, prevalence of HPV in the samples, prevalence of HPV genotypes (6, 11, 16, 18, 26, 28, 31, 33, 35, 39, 42, 45, 51, 52, 53, 54, 58, 62, 67, 68, 69, 70, 71, 73, 82 and 84), and prevalence of simple infections and coinfections among HPV genotypes.
For studies in which only the patient numbers were reported, prevalences were estimated in relation to the genotyped samples number. The total prevalence of each specific genotype in each study was estimated independent of the presence of single infection or coinfection. For the determination of the prevalence of simple infection/coinfection, where this was not described, it was estimated based on the approximate values reported in the articles. Ethical approval was not required for this study as it was based on data/information retrieved from published studies already available in the public domain.
Statistical analysis
All the statistical analyses for the meta-analysis were developed in Stata 9.0® software (Stata Corporation, College Station, TX, USA), by means of the Metan Command, with statistical significance at p < 0.05. The data extracted from the papers for the development of the forest plot are described in Table 1. The estimated effect measure grouped for the variability among the groups was determined by the odds ratio (OR), with a confidence interval of 95%. Fixed effects models and random effects models were initially tested. HPV prevalences at each anatomical site and prevalences of HPV genotypes were obtained from the studies to determine the consistency of the results. To estimate the pooled prevalence of total HPV and at each anatomical site, the pooled prevalence of each HPV genotype and the presence of simple infection and coinfection, the random effects method was used (DerSimonian and Nan Laird, 2015), with a 95% confidence interval.
Table 1.
Characteristics of Included Studies in Systematic Review
| Study | Local (city/state) and sampling period (years) | Brief HPV related objectives of the study | Patients (n) | Age group of CA pacients (years) | Diagnosis (Detection and genotyping) | HPV in CA patients n (%) | HPV16 in HPV/CA patiens (%) | Single HPV infection prevalence n (%) | Multiple HPV infection prevalence n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Scheiner et al, 2008 | Rio de Janeiro, RJ 1995-2000 | To assess the prevalence of HPV infection in penile carcer. | Total: 80 With CA: 80 | Mean age: 57.6 (36 - 86) | PCR and RFLP | 58 (72.5) | 12 (52) Evaluated: 23 | 23 (100) Evaluated: 23 | 0 (0) |
| Afonso et al, 2012 | Rio de Janeiro, RJ 2007-2010 | To determine HPV prevalence rates and main genotypes present in penile cancer. | Total: 135 With CA: 135 | Mean age: 58.5 (21 - 87) | PCR and RFLP | 82 (60.7) | 27 (29.7) | NR | 7 (9.2) |
| Fonseca et al, 2013 | Belém, PA 2001-2008 | To evaluate the prevalence, distribution and association of HPV infection with worse prognosis in penile cancer and to determine predictive value metastasis. | Total: 82 With CA: 82 | Median age: 58 (22 - 91) | PCR | 50 (60.9) | 15 (30) | NR | 25 (50) |
| Sousa et al, 2015 | São Luiz, MA 2001- 2011 | To determine the prevalence of HPV-DNA in penile cancer and to correlate the virus presence to histopathological factors. | Total: 76 With CA: 76 | Mean age: 60.7 (26 - 97) | PCR | 48 (63.1) | 10 (21.3) | NR | 25 (51.0) |
| Termini et al, 2015 | São Paulo, SP 1953 – 2000 | To evaluate the association of SOD2 immunoexpression with HPV DNA presence in penile cancer. | Total: 125 With CA: 125 | <50 (34.4%) 50–59(32.8%) ≥60 (32.8%) | PCR | 26 (20.8) | 17 (65.4) | NR | 4 (15,4) |
| Calmon et al, 2013 | São José do Rio Preto and São Paulo, SP Belém, PA NR | To identify novel genes expressed in penile cancer HPV positive and evaluate a correlation between HPV positivity, the expression of the genes and the subtypes of penile cancer. | Total: 47 With CA: 47 | Mean age: 63 (31 - 95) | INNO-LiPA | 23 (48.9) | 19 (82.6) | 21 (91.3) | 2 (8.7) |
| Busso Lopes et al,2014 | Barretos e São Paulo – SP 2000 – 2010 | To identify potential molecular markers in penile cancer and evaluate the viral role in penile tumors biology. | Total: 46 With CA: 46 | NR | Linear Array | 16 (34.8) | 14 (87.5) | 13 (81,2) | 3 (18.7) |
| Kuasne et al, 2015 | São Paulo e Barretos, SP NR | To identify molecular markers for penile cancer and to determine if HPV status influenced in gene expression levels and methylation patterns. | Total: 87 Without CA: 43 With CA: 44 | Mean age: 57.2 (24 - 92) | Linear Array | 17 (38.6) | 15 (88,2) | NR | NR |
| Araujo-Neto et al, 2016 | Teresina, PI 2011 – 2013 | To examine the prevalence of HPV infections in prostate câncer | Total: 104 With CA: 104 | Mean age: 68 (45 - 90) | Nested PCR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Silvestre et al, 2009 | Belém, PA NR | To detect HPV DNA in samples of prostate cancer and in prostatic hyperplasia. | Total: 71 Without CA: 6 With CA: 65 | Mean age: With CA: 62 | Linear Array | 2 (3) | 2 (100) | 0 (0) | 2 (100) |
| Soares et al, 2011 | Belém, PA 1998 - 2000 | To identify HPV types in the carriers of anal canal cancer, relating them to the degree of cellular differentiation and staging of the lesion | Total: 75 Without CA: 42 With CA: 33 | Mean age: With CA: 48.5 | PCR and Dot blot | 20 (60.6) | 14 (70) | 19 (95) | 1 (5) |
| Picanç-Junior et al, 2015 | Belém, PA 1999 – 2003 | To correlate the HPV presence with the staging and degree of cell differentiation among colorectal cancer the possibility of this relationship with prognostic factors. | Total: 144 Without CA: 65 With CA: 79 | Mean age: 57,8 (25 - 85) | PCR and Dot blot | 41/144 (45.6) | 36 (100) | 36 (100) | 0 (0) |
| Damin et al, 2007 | Porto Alegre, RS 2004-2005 | To investigate the presence of HPV in colorectal cancer and the correlation of the viral infection with prognostic factors for the disease outcome. | Total: 72 With CA: 72 | Mean age: 64.1 (39 - 75) | Nested PCR | 60 (83.3) | 41 (68.3) | 41 (68.3) | 19 (31.7) |
| Lavorato Rocha et al, 2013a | São Paulo, SP 1979 – 2006 | To evaluate the prognostic relevance of CDKI in vulvar cancer and their relation with HPV infection. | Total: 139 With CA: 139 | Mean age: 69 (15 - 98) | Linear Array | 43 (41) Evaluated: 105 | 18 (41.9) | 33/41 (76.6) Related: 41 | 8/41 (17) Related: 41 |
| Rodrigues et al,2013 | São Paulo, SP 1979 – 2006 | To evaluate the expression of E-cadherin, b-catenin, Snail, Slug, Twist and Vimentin in vulvar cancer and associated their expression with clinical data and the presence of HPV. | Total: 87 With CA: 87 Total: 139 | Mean age: 68.7 (46 - 90) | Linear Array | 34 (39.1) | 17 (49.9) | 26 (76.6) | 8 (23.5) |
| Lavorato Rocha et al, 2013b | São Paulo, SP 1979 -2006 | To determine the prognostic role of p14ARF in vulvar cancer its involvement in the p53 pathway, and in the context of HPV infection, and correlating these results with clinical and pathological data. | With CA:139 | Mean age: 69 (15- 98) | Linear Array | 38 (32.8) Evaluated: 85 | 19 (49.9) | 28 (70.6) | 10 (26.3) |
| Akagi et al, 2014 | São Paulo, SP 1990 – 2010 | To determine the prognostic value of ROCK1 gene and protein analysis in vulvar cancer, and correlated with clinicopathological characteristics. | Total: 96 With CA: 96 | Mean age: 75 (30 – 103) | Linear Array | 46 (48) | 22 (48) | NR | NR |
| Maia et al,2012 | São Paulo, SP 1979 - 2006 | To determine the prognostic importance of c-KIT by evaluation its protein and mRNA expression in vulvar cancer and correlating with clinicopathological features and HPV infection. | Total: 103 With CA: 103 | Mean age: 69 (15 – 98) | Linear Array | 60 (58,2) | 26 (44.2) | 46 (77) | 7 (11.6) |
CA – cancer, MA – Maranhão, PA – Pará, PI – Piauí, RJ – Rio de Janeiro, RS – Rio Grande do Sul, SP – São Paulo, NR – Not related, CDKI - cyclin-dependent kinase inhibitors
Subgroup analysis
The subgroups were analyzed according to the proportions between the presence and absence of HPV in the general prevalence of non-cervical genital cancers and at each anatomical site. The pooled total prevalence of HPV genotypes 6, 11, 16, 18, 31, 33, 35, 45 and 53, as well as the pooled prevalence of genotypes 6, 11, 16, 18 and 45 in penile cancer, 16, 18, 33, 35 and 53 in vulvar cancer, and 16 in colorectal cancer were determined.
Heterogeneity and publication bias
The heterogeneity between the studies was analyzed according to Cochran’s Q statistical test (p < 0.10) as indicative of significance. The publication bias was verified using Begg’s (Begg and Mazumdar, 1994) and Egger’s (Egger et al., 1997) methods, as well as the funnel plots, with statistical significance at p < 0.05. The inconsistency of the findings was performed by I2 statistic according to the classification: low (25%), moderate (50%), and high (75%) (Higgins et al., 2003).
Results
The electronic search yielded 2,630 citations. Based on titles and abstracts, 886 publications were eliminated. After abstract review, 1,726 publications were further excluded. The remaining 18 studies were included in the analysis (Figure 1 and Table 1) (Afonso et al., 2012; Akagi et al., 2014; Araujo-Neto et al., 2016; Busso-Lopes et al., 2015; Calmon et al., 2013; Damin et al., 2007; Fonseca et al., 2013; Kuasne et al., 2015; Lavorato-Rocha et al., 2013a; Lavorato-Rocha et al., 2013b; Melo Maia et al., 2012; Picanço-Junior et al., 2014; Rodrigues et al., 2013; Scheiner et al., 2008; Silvestre et al., 2009; Soares et al., 2011; Sousa et al., 2015; Termini et al., 2015). The total number of individuals included in the study was 1,708, of which 1,552 had cancer. According to the geographical distribution of the studies, 72 individuals were from the Southern region (Damin et al., 2007), 994 from the Southeast region (Afonso et al., 2012; Akagi et al., 2014; Busso-Lopes et al., 2015; Kuasne et al., 2015; Lavorato-Rocha et al., 2013a; Lavorato-Rocha et al., 2013b; Rodrigues et al., 2013; Scheiner et al., 2008; Termini et al., 2015), 180 from the Northeast region (Araujo-Neto et al., 2016; Sousa et al., 2015) and 259 from the Northern region of Brazil (Fonseca et al., 2013; Picanço-Junior et al., 2014; Silvestre et al., 2009; Soares et al., 2011;). Only one study, with 47 individuals, comprised samples from the Southeast and Northeast regions (Calmon et al., 2013). According to publication years, 3/18 (16.67%) (Damin et al., 2007; Scheiner et al., 2008; Silvestre et al., 2009), 2/18 (11.11%) (Afonso et al., 2012; Soares et al., 2011), and 13/18 (72.22%) (Akagi et al., 2014; Araujo-Neto et al., 2016; Busso-Lopes et al., 2015; Calmon et al., 2013; Fonseca et al., 2013; Kuasne et al., 2015; Lavorato-Rocha et al., 2013a; Lavorato-Rocha et al., 2013b; Sousa et al., 2015; Termini et al., 2015; Rodrigues et al., 2013), of the studies were published in the periods 2006–2009, 2010–2012, and 2013–2016, respectively. The mean/median age of individuals was specified in 17/18 (94.44%) of the studies, ranging from 15 to 103 years. Sample sizes across the studies ranged from 33 to 139 and the sampling period ranged from 1 to 47 years. This information was not presented in 3/18 (16.66%) of the studies.
Of the total number of studies selected in this review, 8/16 (50%) corresponded to the research performed on penile cancer (Afonso et al., 2012; Busso-Lopes et al., 2015; Calmon et al., 2013; Fonseca et al., 2013; Kuasne et al., 2015; Scheiner et al., 2008; Sousa et al., 2015; Termini et al., 2015), 2/18 (11.11%) in prostate cancer (Araujo-Neto et al., 2016; Silvestre et al., 2009), 1/18 (5.55%) in anal canal cancer (Soares et al., 2011), 2/18 (11.11%) colorectal cancer (Damin et al., 2007; Picanço-Junior et al., 2014) and 5/18 (27.28%) in vulvar cancer (Akagi et al., 2014; Lavorato-Rocha et al., 2013a; Lavorato-Rocha et al., 2013b; Rodrigues et al., 2013; Melo Maia et al., 2012). For HPV diagnosis (detection and genotyping), 3/18 (16.67%) of the studies used only polymerase chain reaction (PCR) (Fonseca et al., 2013; Sousa et al., 2015; Termini et al., 2015), 2/18 (11.11%) used PCR and Restriction Fragment Length Polymorphism (RFLP) (Afonso et al., 2012; Scheiner et al., 2008), 2/18 (11.11%) PCR and Dot Blot Hybridization (Dot blot) (Picanço-Junior et al., 2014; Soares et al., 2011), 2/18 (11.11%) Nested PCR (Araujo-Neto et al., 2016; Damin et al., 2007), 8/18 (44.44%) Linear Array HPV Genotyping (Linear Array) (Akagi et al., 2014; Busso-Lopes et al., 2015; Kuasne et al., 2015; Lavorato-Rocha et al., 2013a; Lavorato-Rocha et al., 2013b; Melo Maia et al., 2012; Rodrigues et al., 2013; Silvestre et al., 2009) and 1/18 (5.55%) INNO-LiPA® HPV Genotyping Extra text (INNO-LiPA) (Calmon et al., 2013). Table 1 summarizes the study retrieval steps. In 14/18 (77.8%) of the studies, only cancer samples were used for HPV screening; however, in 4/18 (22.2%) of the studies, the prevalence of HPV in normal tissue was also sought.
The prevalence of HPV ranged from 0 to 83.3%, with an overall prevalence of 43% (95% CI: 36–51%; p < 0.001) (Figure 2), with great variation according to the study sites. For penile cancer, the prevalence of HPV ranged from 20.8 to 72.5%, yielding a pooled prevalence of 42% (95% CI: 32-55%, p < 0.001) (Figure 3A). For colorectal cancer, two studies showed a prevalence of HPV of 45.6 and 83.3% and a pooled prevalence of 67% (95% CI: 64-70%, p < 0.001) (Figure 3B). For vulvar cancer, HPV ranged from 39.1 to 58.2%, yielding a pooled prevalence of 43% (95% CI: 34-55%, p < 0.001) (Figure 3C).
Figure 2.
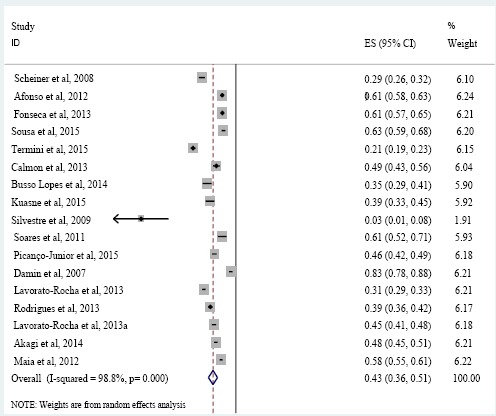
HPV Pooled Prevalence in Non-cervical Genital Cancer Patients
Figure 3.
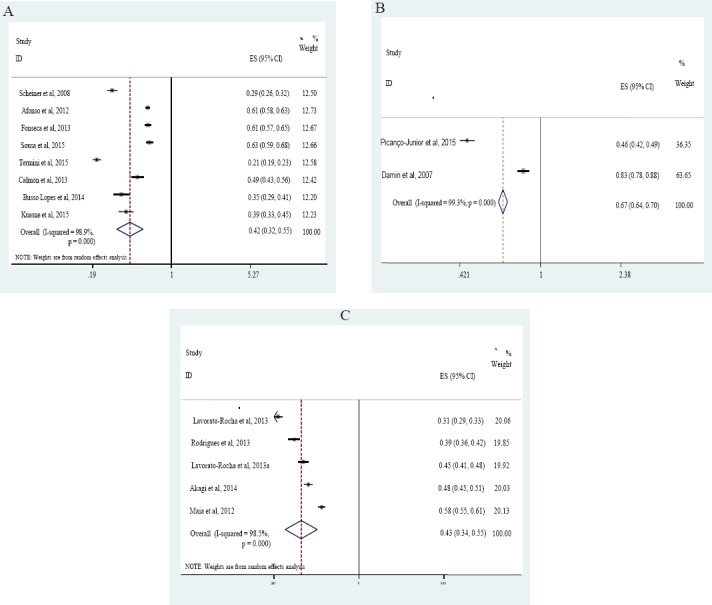
HPV Pooled Prevalence in Penile Cancer Patients (A), Colorectal Cancer Patients (B) and Vulvar Cancer Patients (C)
Genotype 16 was the only one found and reported in all cancers presented in this review. This genotype was also the most prevalent (54%, 95% CI: 44-66%, p < 0.001), followed by HPV 33 (21%, 95% CI 17-28%, p < 0.001), HPV 6 (15%, 95% CI 8-26%, p < 0.001) and HPV 11 (13%, 95% CI 5-32%, p < 0.001). In the samples of penile cancer, the most prevalent genotypes were 16 (51%, 95% CI: 35-73%, p < 0.001) and 6 (17%, 95% CI: 8-33%, p < 0.001). In vulvar cancer, a higher prevalence of genotypes 16 (46%, 95% CI: 43-49%, p = 0.293) and 33 (26%, 95% CI: 23-29%, p = 0.256) were found. For colorectal cancer, it was only possible to determine the prevalence of genotype 16 (78%, 95% CI 73-83%, p < 0.001). The pooled prevalence of prostate and anal canal cancers were not determined because the prevalence of HPV in prostate cancer reported in one of the articles was equal to 0 and only one article was identified for cancer of the anal canal (Table 2, Table 3 and Supplementary file 1).
Table 2.
Pooled Prevalence of Human Papillomavirus Genotypes in Cancer Patients
| Total | Number of studies | Number of cases | Pooled HPV prevalence | 95% CI | p | I2 (%) |
|---|---|---|---|---|---|---|
| Genotypes HPV | ||||||
| 16 | 17 | 324 | 54 | 44 - 66 | <0.001 | 96.5 |
| 33 | 9 | 62 | 21 | 17 - 28 | <0.001 | 75.5 |
| 6 | 6 | 40 | 15 | 8-26 | <0.001 | 93.0 |
| 11 | 7 | 56 | 13 | 5-32 | <0.001 | 97.3 |
| 18 | 12 | 73 | 12 | 7-22 | <0.001 | 95.5 |
| 53 | 6 | 22 | 10 | 6-16 | 0.009 | 67.2 |
| 35 | 7 | 13 | 7 | 4-11 | 0.281 | 19.6 |
| 31 | 7 | 11 | 4 | 3-7 | 0.942 | 00.0 |
| 45 | 7 | 27 | 4 | 1-15 | <0.001 | 79.6 |
| Penile HPV genotypes | ||||||
| 16 | 8 | 129 | 51 | 35 - 73 | <0.001 | 96.7 |
| 6 | 4 | 32 | 17 | 8-33 | <0.001 | 94.6 |
| 11 | 5 | 48 | 15 | 5-41 | <0.001 | 97.1 |
| 18 | 6 | 19 | 8 | 4-18 | <0.001 | 86.2 |
| 45 | 4 | 24 | 6 | 1-29 | 0.002 | 80.0 |
| Vulvar HPV genotypes | ||||||
| 16 | 5 | 102 | 46 | 43-49 | 0.293 | 19.1 |
| 33 | 5 | 57 | 26 | 23-29 | 0.256 | 24.8 |
| 18 | 4 | 16 | 13 | 10-17 | 0.252 | 26.7 |
| 53 | 3 | 11 | 10 | 7-14 | 0.662 | 00.0 |
| 35 | 3 | 9 | 9 | 5-14 | 0.267 | 24.3 |
| Colorectal HPV genotypes | ||||||
| 16 | 2 | 77 | 78 | 73 - 83 | <0.001 | 96.7 |
HPV, Human papillomavirus; OR, odds ratio; CI, confidence interval
Table 3.
HPV Genotypes Prevalence Related in Cancer Patients of Included Studies in Systematic Review
| Genotype prevalence (%) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anatomic site | Study | 6 | 11 | 16 | 18 | 26 | 28 | 31 | 33 | 35 | 39 | 40 | 42 | 45 | 51 | 52 | 53 | 54 | 58 | 62 | 67 | 68 | 69 | 70 | 71 | 73 | 82 | 84 |
| * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | ||||||||||||||
| 1 | 17.4 | - | 52.1 | 4.3 | - | 8.7 | 4.3 | 4.3 | - | - | - | - | 4.3 | - | - | - | - | - | - | - | - | - | - | 4.3 | - | - | - | |
| 2 | 9.9 | 2.4 | 29.7 | 5.5 | 1.2 | 2.4 | 4.9 | 1.2 | 1.2 | - | - | - | 23.1 | - | - | 1.2 | - | - | 2.4 | - | - | - | 1.2 | 2.4 | 1.2 | - | - | |
| 3 | 32 | 64 | 30 | 2 | - | - | - | 4 | - | - | - | - | 2 | 2 | 2 | 18 | - | 2 | - | - | 2 | - | - | - | - | - | - | |
| 4 | - | 12.8 | 21.3 | 8.5 | - | - | - | - | - | - | - | - | 2.1 | - | - | - | - | - | - | - | - | 4.2 | - | - | - | - | - | |
| 5 | 11.5 | 11.5 | 65.4 | 26.9 | - | - | - | - | 3.8 | 3.8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 6 | - | 21.7 | 82.6 | - | - | - | - | - | 4.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 7 | - | - | 87.5 | - | - | - | - | - | - | - | - | - | - | - | - | 6.2 | - | - | 6.2 | - | - | - | - | - | - | - | - | |
| Penis | 8 | - | - | 88,2 | □ | - | - | □ | □ | - | - | 12.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Prostate | 9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 10 | - | - | 100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 100 | |
| Anal canal | 11 | 5 | 5 | 70 | 25 | - | - | 5 | 5 | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Colorectal | 12 | - | - | 100 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 13 | 11.7 | 11.7 | 68.3 | 50 | - | - | 1.7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 14 | - | - | 42 | 7 | - | - | - | 25 | 6 | - | - | 4 | 2 | - | - | 9 | 4 | - | - | 5 | - | 2 | - | 5 | - | 2 | 2 | |
| 15 | - | - | 50 | 11.7 | - | - | - | 29.4 | 11.7 | - | - | 2.9 | 2,9 | - | - | 11.8 | 2.9 | - | - | - | - | - | - | 5.9 | - | 2.9 | 2.9 | |
| 16 | - | - | 49.9 | 13.1 | - | - | 2.6 | 26.2 | 7.8 | - | - | 5.2 | 2.6 | - | - | 7.9 | 5.2 | - | - | 5.3 | - | 2.6 | - | 2.6 | - | 2.6 | 2.6 | |
| 17 | - | - | 48 | 15 | - | - | - | 24 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Vulva | 18 | - | - | 44.2 | - | - | - | - | 27.9 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
□Presence reported, but with prevalence not described. Asterisks indicate high-risk HPV genotypes. Studies, 1 - Scheiner et al., 2008; 2 - Afonso et al., 2012; 3 - Fonseca et al., 2013; 4 - Sousa et al., 2015; 5 - Termini et al., 2015; 6 - Calmon et al., 2013; 7 - Busso Lopes et al., 2014; 8 - Kuasne et al., 2015; 9 - Araujo-Neto et al., 2016; ; 10 - Silvestre et al., 2009; 11 - Soares et al., 2011; 12 - Picanço-Junior et al., 2015; 13 - Damin et al., 2007; 14 - Lavorato-Rocha et al., 2013a; 15 - Rodrigues et al., 2013; 16 - Lavorato Rocha et al., 2013b; 17 - Akagi et al., 2014; 18 - Maia et al., 2012
Infections by one and multiple HPV genotypes were reported in 12/18 (66.67%) (Araujo-Neto et al., 2016; Busso-Lopes et al., 2015; Calmon et al., 2013; Damin et al., 2007; Lavorato-Rocha et al., 2013a; Lavorato-Rocha et al., 2013b; Melo Maia et al., 2012; Picanço-Junior et al., 2014; Rodrigues et al., 2013; Scheiner et al., 2008; Silvestre et al., 2009; Soares et al., 2011) and in 16/18 (88.89%) (Afonso et al., Araujo-Neto et al., 2016; 2012; Busso-Lopes et al., 2015; Calmon et al., 2013; Damin et al., 2007; Fonseca et al., 2013; Lavorato-Rocha et al., 2013a; Lavorato-Rocha et al., 2013b; Melo Maia et al., 2012; Picanço-Junior et al., 2014; Rodrigues et al., 2013; Scheiner et al., 2008; Silvestre et al., 2009; Sousa et al., 2015; Soares et al., 2011; Termini et al., 2015), of the studies, respectively. The prevalence of infection by a single HPV genotype was 82% (95% CI: 75-90%, p < 0.001), the most common being genotype 16 (60%). Infection by multiple HPV genotypes was described in 72.2% of the studies in this review, with a mean prevalence of 22% (95% CI: 15-30%, p < 0.001), with coinfection by genotypes 16 and 18 being 34% (Table 4 and Supplementary file 2).
Table 4.
Prevalence of HPV Genotypes in Multiple Infections in Genital Non Cervical Cancer Patients
| HPV multiple infections | Patients n (%) | Anatomic site | Studies |
|---|---|---|---|
| 16*/11 | 1 (1.7) | Penis | Calmon et al., 2013 |
| 16*/18* | 12 (20.3) | Colorectal | Damin et al., 2007 |
| Vulva | Lavorato-Rocha et al., 2013b | ||
| 16*/31* | 2 (3.4) | Colorectal | Damin et al., 2007 |
| Vulva | Lavorato-Rocha et al., 2013b | ||
| 16*/ 33* | 6 (10.2) | Vulva | Lavorato-Rocha et al., 2013a; |
| Lavorato-Rocha et al., 2013b; | |||
| Rodrigues et al., 2013 | |||
| 16*/40 | 1 (1.7) | Penis | Busso Lopes et al., 2014 |
| 16*/ 45* | 1 (1.7) | Penis | Afonso et al., 2012 |
| 16*/62 | 1 (1.7) | Penis | Busso Lopes et al., 2014 |
| 16*/84 | 2 (3.4) | Prostate | Silvestre et al., 2009 |
| 16*/6/11 | 5 (8.5) | Penis | Termini et al., 2015; |
| Colorectal | Damin et al., 2007 | ||
| 16*/33*/35* | 2 (3.4) | Vulva | Lavorato-Rocha et al., 2013a; |
| Lavorato-Rocha et al., 2013b; | |||
| Rodrigues et al., 2013 | |||
| 16*/18*/33* | 3 (5.1) | Vulva | Lavorato-Rocha et al., 2013b; |
| Lavorato-Rocha et al., 2013a; | |||
| Rodrigues et al., 2013 | |||
| 16*/18*/39* | 1 (1.7) | Penis | Termini et al., 2015 |
| 16*/33*/ 84 | 3 (5.1) | Vulva | Lavorato-Rocha et al., 2013a; |
| Lavorato-Rocha et al., 2013b; | |||
| Rodrigues et al.,2013 | |||
| 18*/40 | 1 (1.7) | Penis | Busso Lopes et al., 2014 |
| 18*/33*/35* | 2 (3.4) | Vulva | Lavorato-Rocha et al., 2013a |
| 6/11 | 2 (3.4) | Penis | Afonso et al., 2012; |
| Termini et al., 2015 | |||
| 6/11/18* | 3 (5.1) | Colorectal | Damin et al., 2007 |
| 6/11/45* | 1 (1.7) | Penis | Afonso et al., 2012 |
| 6/31* | 1 (1.7) | Penis | Afonso et al., 2012 |
| 6/45* | 1 (1.7) | Penis | Afonso et al., 2012 |
| 11/35* | 1 (1.7) | Penis | Calmon et al., 2013 |
| 31*/33*/82* | 3 (5.1) | Vulva | Lavorato-Rocha et al., 2013a; |
| Lavorato-Rocha et al., 2013b; | |||
| Rodrigues et al., 2013 | |||
| 42/54 | 3 (5.1) | Vulva | Lavorato-Rocha et al., 2013a; |
| Lavorato-Rocha et al., 2013b; | |||
| Rodrigues et al., 2013 | |||
| 6/11/31*/33*/35* | 1 (1.7) | Anal canal | Soares et al., 2011 |
| Total | 59 (100.0) |
Asterisks indicate high-risk HPV genotypes.
Discussion
The causative role of HPV has been extensively studied in uterine cervical lesions; however, only a few such studies have been performed in prostate, anal canal, colorectal, penile, and vulvar tumors in Brazil and the world, clearly showing that more evidence is needed for the understanding of HPV in these cancers. It is known that cervical cancer remains an important health problem; however, relatively uncommon cancers of the anus, penis, and vulva have been shown to have increased incidence in recent years. It is believed that the changing occurrence of anogenital cancer may be mainly due to increased HPV transmission, due to changes in sexual behavior (Wakeham and Kavanagh, 2014).
The actual prevalence of HPV in non-cervical cancers remains controversial. While some studies have shown high prevalence (Urgoiti et al., 2014; Wakeham et al., 2017), others report that HPV is present in these cancers, but the prevalence is low; however, additional studies are needed for its confirmation (Bae, 2015; Yang et al., 2015). On the other hand, other studies claim that HPV has no influence on the development of cancer (Taherian et al., 2014). Our results reinforce the idea that HPV plays an important role in the development of cancer. The overall pooled prevalence in the studied cancers was high, being above 40%.
Infection by oncogenic HPV genotypes is a necessary cause for the development of cervical cancer (Walboomers et al., 1999), in contrast, HPV DNA detection was relatively less common in invasive cases of penile, prostate, vulvar, anal, and colorectal cancers (Backes et al., 2009; Damin et al., 2013; Urgoiti et al., 2014; Yow et al., 2014; Wakeham et al., 2017). The incidence of penile, vulvar and colorectal cancer is lower compared to cervical cancer (INCA, 2016), probably due to the lower susceptibility of tissues present in these organs to malignant transformation compared to the cervix (Palefsky, 2007). Already, studies available in the literature show that anal carcinoma rarely occurs in the absence of HPV, reinforcing the role of the virus as the main risk factor for this type of tumor (Abramowitz et al., 2011). Several concordant HPV genotypes, both oncogenic and non-oncogenic, can be found in anal specimens of women with coexisting HPV cervical infection, which may suggest similar susceptibility to the development of cancer in the anal canal, similar to that seen in the cervix (Guler et al., 2013).
Studies report that unlike cervical cancer, there may be several etiologies for non-cervical genital cancers, one being related to HPV and the other due to factors unrelated to HPV infection. Old age, country of birth, longstanding ulcerative colitis, red meat diet, obesity (Doubeni et al., 2012; Haggar et al., 2009), lifestyle, and genetic predisposition are related to colorectal cancer. Men who have sex with men, tobacco and immunosuppression are associated with anal cancer (Nelson and Benson, 2017). Immunosuppression, smoking, vulvar intraepithelial neoplasia, history of cervical cancer, and vulvar dystrophy are associated with vulvar cancer (Jones et al., 1997; Palefsky, 2009). Phimosis with chronic inflammation, lack of circumcision, inadequate hygiene practices, presence of other sexually transmitted diseases, number of sexual partners, and smoking (Calmon et al., 2011) are related to penile cancer. Regarding prostate cancer, little is known about the exact mechanisms involved in its development; however, environmental and hereditary components such as age, race, and family history play a crucial role in carcinogenesis (Shavers et al., 2009), it is believed that genital infections such as sexually transmitted infections, including HPV (Bae, 2015) are related to the etiology of prostatic inflammation which may possibly progress to prostate cancer (Puhr et al., 2016).
It is estimated that 40 to 50% of vulvar cancers have been associated with HPV (Bosch et al., 2013). Wakeham et al., (2017) found high-risk HPV genotype prevalence in 52% of vulvar cancer. In men, HPV DNA is found varying from 30 to 100% in penile cancers (“Human Papillomavirus and Related Diseases Report,” 2017). Studies report a prevalence of approximately 48% (Backes et al., 2009; Miralles-Guri et al., 2009). A recent study published in Brazil (Afonso et al., 2017), described a 63.6% prevalence of HPV in penile cancer. HPV 16 was the most prevalent genotype (40.5%), followed by HPV 6 (16.5%) and HPV 45 (11.4%).
For prostate cancer, the prevalence varies from 0 to 41% (Singh et al., 2015; Yang et al., 2015; Yow et al., 2014). In both sexes, HPV DNA is detected in anal cancer (60.1 to 100%) (Aguiar et al., 2014) and colorectal cancer (14.1% to 60.8%) (Damin et al., 2013). For the meta-analysis, we investigated penile, vulvar and colorectal cancers, in which the prevalences were 42%, 43% and 67%, respectively. For prostate cancer, it was not possible to perform meta-analysis because the prevalence reported in the studies that we found corresponded to 0 and 3%. For anal canal cancer, meta-analysis was not performed because we found only one report during the period studied. In 2017, Guimarães et al. reported a prevalence of 81.5% of HPV in anal canal cancer in a study conducted in Brazil, with HPV 16 (100% of cases), followed by HPV 52 (31.8%).
Genotype 16 was the most frequent in all the studies that were part of this systematic review, corresponding to a prevalence of 54%, followed by genotypes 33, 6, 11, 18, 53, 35, 31 and 45. HPV 16 is the most common genotype of HPV reported in publications (Aguiar et al., 2014; Backes et al., 2009; Bae, 2015; Damin et al., 2013; De Vuyst et al., 2009; Hoots et al., 2009; “Human Papillomavirus and Related Diseases Report,” 2017; Tewari et al., 2007; Yang et al., 2015;), followed by genotypes 18, 31, 33, and 45 (Bosch et al., 2013). Genotypes 6 and 11, of low risk, have also been presented as being of great importance for the development of neoplasias (Cornall et al., 2013).
It is believed that HPV, when associated with tissues, leads to the development of precursor lesions caused by viral infection. Researchers argue that the pathway is similar to HPV-mediated cervical carcinogenesis. Virus-induced carcinogenesis involves several steps: resistant infection caused by HPV is the initial causal event, with genetic alterations (Alves et al., 2001) and epigenetics (Afonso et al., 2017). The infected cell would present the malignant phenotype, due to the integration of the virus into the host-cell DNA and expression of the E6 and E7 oncoproteins, which antagonize the functions of the tumor-suppressor proteins p53 and pRb, respectively (Damin et al., 2013).
The mean age of the patients studied was approximately 63.2 years, and the great majority of the cases were verified to be above 60 years. The accumulation of exposure to different risk factors throughout life contributes to the higher prevalence of tumors at more advanced ages, especially coinfections with HPV (Bosch et al., 2013). Coinfection by two or more high- and low-risk HPV genotypes was found in all cancers studied. Coinfection was also identified in other studies and was related to the development of cancer (Afonso et al., 2017; Muñoz et al., 2003). Some genotypes of low risk, such as HPV 6, are well known for causing the majority of condylomas, and have been found to be important in several cancer cases, with a major presence of multiple coinfections with high-risk genotypes (Ferlay et al., 2013).
The distribution, type of HPV, and rate of infection may vary by geographical region (Giuliano et al., 2016), HPV detection method, study population and histological subtype of cancer (“Human Papillomavirus and Related Diseases Report,” 2017). Different methods of detecting HPV show different sensitivities of detection of HPV DNA. Nevertheless, the results should be interpreted with caution, since DNA detection methods can easily suffer sample contamination which could probably bias the result (Abreu et al., 2012).
The high percentage of HPV infection in the studied cancers emphasizes the importance of control measures, with emphasis on HPV vaccination in an immunization program for both women and men. Two vaccines are available for the Brazilian population, the quadrivalent HPV vaccine, Gardasil®/Silgard® (Sanofi Pasteur MSD/Merck Sharp and Dohme), which protects against infection from HPV 6, 11, 16, and 18, and the bivalent HPV vaccine, Cervarix® (GlaxoSmithKline Biologicals), which protects against infection with HPV 16 and 18 (Lehtinen and Dillner, 2013). In 2015, the 9-valent HPV vaccine, Gardasil9 (Sanofi Pasteur MSD/Merck Sharp and Dohme), was licensed in Europe for the prevention of cancers and precancerous lesions of the cervix, vulva, vagina, and anus, as well as genital warts caused by HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58 (European Medicines Agency). This vaccine protects against five high-risk HPV types not included in first-generation HPV vaccines (HPV 31, 33, 45, 52, and 58) (Joura et al., 2015). Although available vaccines have shown good results in reducing HPV infections and as a consequence of some types of cancer, there is still a need for public awareness.
Strengths and limitations of the study
This systematic review presents high robustness and considerable precision in the search for articles that present HPV prevalence in non-cervical genital cancers published in Brazil between 2006 and 2016. The MeSH terms and articles that were part of the search were decided by consensus among several professionals in this area, which increased the sensitivity of the work. In addition, meta-analyses were performed to determine the prevalence of HPV in cancer of three anatomical sites, as well as to determine the prevalence of HPV genotypes in these cancers. The main findings reported in the studies that have been done in this review were organized and detailed in numerous tables and figures, ensuring a good and realistic presentation of the data.
There are some inherent limitations to the research method: only two databases were surveyed, and although the total number of patients included in our meta-analysis is large, for many specific associations the number of studies that could actually be combined to provide absolute quantitative information or descriptive statistics was relatively small. This is a limitation that, unfortunately, cannot be overcome without access to primary data from all included publications. It is also assumed that the heterogeneity observed in the present review as well as in comparison with other studies conducted elsewhere in the world can be explained by regional differences in HPV prevalence, HPV type, distribution and/or non-risk factors related to HPV, as well as the various methods used for research, such as inclusion criteria, in the individual studies. Despite inherent limitations of the meta-analysis to some degree, our results are in agreement with the published literature, bringing a positive contribution regarding the development of cancer due to the presence of HPV.
Final considerations
The systematic review and meta-analysis demonstrated high pooled HPV prevalence. High prevalence of HPV infection has also been found in penile cancer, in colorectal cancer, and in vulvar cancer. Although the highest prevalence of HPV 16 was confirmed in all cancers studied, significant prevalence of high- and low-risk genotypes 33, 6, 11, 18, 53, 35, 31 and 45 of HPV were also found. For the future, there is a need for prospective, multicenter, randomized studies to determine optimal treatment for patients based on HPV status, as well as the development of potential prevention programs, thus minimizing the possible development of tumors.
Acknowledgements
We thank to all contacted authors who kindly provided additional data and greatly contributed to improve the quality of this review.
References
- Abramowitz L, Jacquard AC, Jaroud F, et al. Human papillomavirus genotype distribution in anal cancer in France:the EDiTH V study. Int J Cancer. 2011;129:433–39. doi: 10.1002/ijc.25671. [DOI] [PubMed] [Google Scholar]
- Abreu ALP, Souza RP, Gimenes F, Consolaro MEL. A review of methods for detect human papillomavirus infection. Virol J. 2012;9:1–9. doi: 10.1186/1743-422X-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso LA, Carestiato FN, Ornellas AA, et al. Human papillomavirus, Epstein-Barr virus, and methylation status of p16ink4a in penile cancer. J Med Virol. 2017;89:1837–43. doi: 10.1002/jmv.24833. [DOI] [PubMed] [Google Scholar]
- Afonso LA, Moyses N, Alves G, et al. Prevalence of human papillomavirus and Epstein-Barr virus DNA in penile cancer cases from Brazil. Mem Inst Oswaldo Cruz. 2012;107:18–23. doi: 10.1590/s0074-02762012000100003. [DOI] [PubMed] [Google Scholar]
- Aguiar MTM, Bosso NCC, Leal CBQS, et al. Clinicopathological aspects and prevalence of human papillomavirus in anal cancer. J Coloproctol. 2014;34:76–82. [Google Scholar]
- Akagi EM, Lavorato-Rocha AM, de Maia B, Rodrigues IS, Carvalho KC, et al. ROCK1 as a novel prognostic marker in vulvar cancer. BMC Cancer. 2014;14:822. doi: 10.1186/1471-2407-14-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida LM, Martins LFL, Pontes VB, et al. Human papillomavirus genotype distribution among cervical cancer patients prior to Brazilian national HPV immunization program. J Environ Public Health. 2017;???:1645074. doi: 10.1155/2017/1645074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves G, Heller A, Fiedler W, et al. Genetic imbalances in 26 cases of penile squamous cell carcinoma. Genes, Chromosomes Cancer. 2001;31:48–53. doi: 10.1002/gcc.1117. [DOI] [PubMed] [Google Scholar]
- Ang KKP, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2011;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo-Neto AP, Ferreira-Fernandes H, Amaral CMM, et al. Lack of detection of human papillomavirus DNA in prostate carcinomas in patients from northeastern Brazil. Genet Mol Biol. 2016;39:24–9. doi: 10.1590/1678-4685-GMB-2015-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449–57. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- Bae JM. Human papillomavirus 16 infection as a potential risk factor for prostate cancer:an adaptive meta-analysis. Epidemiol Health. 2015;37:e2015005. doi: 10.4178/epih/e2015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- Bosch FX, Broker TR, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31:11–31. doi: 10.1016/j.vaccine.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Busso-Lopes AF, Marchi FA, Kuasne H, et al. Genomic profiling of human penile carcinoma predicts worse prognosis and survival. Cancer Prev Res. 2015;8:149–56. doi: 10.1158/1940-6207.CAPR-14-0284. [DOI] [PubMed] [Google Scholar]
- Calmon MF, Mota MTO, Babeto É, et al. Overexpression of ANXA1 in penile carcinomas positive for high-risk HPVs. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0053260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmon MF, Tasso Mota M, Vassallo J, Rahal P, Rahal P. Penile carcinoma:Risk factors and molecular alterations. Sci World J. 2011;11:269–82. doi: 10.1100/tsw.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornall AM, Roberts JM, Garland SM, et al. Anal and perianal squamous carcinomas and high-grade intraepithelial lesions exclusively associated with “low-risk” HPV genotypes 6 and 11. Int J Cancer. 2013;133:2253–58. doi: 10.1002/ijc.28228. [DOI] [PubMed] [Google Scholar]
- Crawford R, Grignon AL, Kitson S, et al. High prevalence of HPV in non-cervical sites of women with abnormal cervical cytology. BMC Cancer. 2011;11:473. doi: 10.1186/1471-2407-11-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damin DC, Caetano MB, Rosito MA, et al. Evidence for an association of human papillomavirus infection and colorectal cancer. Eur J Surg Oncol. 2007;33:569–74. doi: 10.1016/j.ejso.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Damin DC, Ziegelmann PK, Damin AP. Human papillomavirus infection and colorectal cancer risk:a meta-analysis. Color Dis. 2013;15:420–8. doi: 10.1111/codi.12257. [DOI] [PubMed] [Google Scholar]
- De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus:A meta-analysis. Int J Cancer. 2009;124:1626–36. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Nan L. Meta-analysis in clinical trials revisited:HHS public access. Contemp Clin Trials. 2015;45:139–45. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubeni CA, Major JM, Laiyemo AO, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104:1353–62. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide:IARC CancerBase. No. 11 [Internet]. [WWW Document] Lyon, Francer: International Agency for Research on Cancer; 2013. [accessed on:October 02, 2017]. Avaliable from: http://globocan.iarc.fr . [Google Scholar]
- Fonseca AG, Soares FA, Burbano RR, Silvestre RV, Pinto LOAD. Human papilloma virus:Prevalence, distribution and predictive value to lymphatic metastasis in penile carcinoma. Int Braz J Urol. 2013;39:542–50. doi: 10.1590/S1677-5538.IBJU.2013.04.12. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Nyitray AG, Kreimer AR, et al. EUROGIN 2014 roadmap:differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int J Cancer. 2015;136:2752–60. doi: 10.1002/ijc.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler T, Uygur D, Uncu M, Yayci E, Atacag T, et al. Coexisting anal human papillomavirus infection in heterosexual women with cervical HPV infection. Arch Gynecol Obstet. 2013;288:667–72. doi: 10.1007/s00404-013-2821-0. [DOI] [PubMed] [Google Scholar]
- Haggar FA, Boushey RP. Colorectal cancer epidemiology:incidence, mortality, survival , and risk factors. Clin Colon Rectal Surg. 2009;6:191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–83. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- Hu Z, Zhu D, Wang W, et al. Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet. 2015;47:158–63. doi: 10.1038/ng.3178. [DOI] [PubMed] [Google Scholar]
- Information Centre on HPV and Cancer. Human Papillomavirus and Related Diseases Report. 2017. Avaliable at: http://www.hpvcentre.net/statistics/reports/XWX.pdf .
- Humans IWG. IARC working group on the evaluation of carcinogenic risks to humans. IARC Monogr Eval Carcinog Risks Hum. 1995;64:1–378. [PMC free article] [PubMed] [Google Scholar]
- INCA. Instituto Nacional de Câncer (National cancer institute) Incidence of cancer in Brazil – 2016 estimative. 2016. Avaliable at: http://www.inca.gov.br/
- Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva:The influence of vulvar intraepithelial neoplasia. Obstet Gynecol. 1997;90:448–52. doi: 10.1016/s0029-7844(97)00298-6. [DOI] [PubMed] [Google Scholar]
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- Kuasne H, Cólus IMDS, Busso AF, et al. Genome-wide methylation and transcriptome analysis in penile carcinoma:uncovering new molecular markers. Clin Epigenetics. 2015;7:46. doi: 10.1186/s13148-015-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorato-Rocha AM, Rodrigues ISA, Melo Maia B, et al. Cell cycle suppressor proteins are not related to HPV status or clinical outcome in patients with vulvar carcinoma. Tumor Biol. 2013;34:3713–20. doi: 10.1007/s13277-013-0955-0. [DOI] [PubMed] [Google Scholar]
- Lavorato-Rocha AM, Melo Maia B, Rodrigues IS, et al. Prognostication of vulvar cancer based on p14ARF status:Molecular assessment of transcript and protein. Ann Surg Oncol. 2013;20:31–9. doi: 10.1245/s10434-012-2560-7. [DOI] [PubMed] [Google Scholar]
- Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat Rev Clin Oncol. 2013;10:400–10. doi: 10.1038/nrclinonc.2013.84. [DOI] [PubMed] [Google Scholar]
- Melo Maia B, Lavorato-Rocha A, Rodrigues I, et al. Prognostic significance of c-KIT in vulvar cancer:bringing this molecular marker from bench to bedside. J Transl Med. 2012;10:150. doi: 10.1186/1479-5876-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles-Guri C, Bruni L, Cubilla AL, et al. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62:870–8. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Nelson VM, Benson AB. Epidemiology of anal canal cancer. Surg Oncol Clin N Am. 2017;26:9–15. doi: 10.1016/j.soc.2016.07.001. [DOI] [PubMed] [Google Scholar]
- O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S):a multicentre cohort study. Lancet Oncol. 2016;17:440–51. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4:52–6. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palefsky JM. HPV infection in men. Dis Markers. 2007;23:261–72. doi: 10.1155/2007/159137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picanço-Junior OM, Oliveira ALT, et al. Association between human papillomavirus and colorectal adenocarcinoma and its influence on tumor staging and degree of cell differentiation TT. ABCD Arq Bras Cir Dig. 2014;27:172–6. doi: 10.1590/S0102-67202014000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012:a synthetic analysis. Lancet Glob Health. 2016;4:609–16. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed] [Google Scholar]
- Puhr M, De Marzo A, Isaacs W, et al. Inflammation, microbiota, and prostate cancer. Eur Urol Focus. 2016;2:374–82. doi: 10.1016/j.euf.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Rodrigues IS, Lavorato-Rocha AM, de M Maia B, et al. Epithelial-mesenchymal transition-like events in vulvar cancer and its relation with HPV. Br J Cancer. 2013;109:184–94. doi: 10.1038/bjc.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner MA, Campos MM, Ornellas AA, et al. Human papillomavirus and penile cancers in Rio de Janeiro, Brazil:HPV typing and clinical features. Int Braz J Urol. 2008;34:467–74. doi: 10.1590/s1677-55382008000400009. [DOI] [PubMed] [Google Scholar]
- Shavers VL, Underwood W, Moser RP. Race/ethnicity and the perception of the risk of developing prostate cancer. Am J Prev Med. 2009;37:64–7. doi: 10.1016/j.amepre.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre RV, Leal MF, Demachki S, et al. Low frequency of human papillomavirus detection in prostate tissue from individuals from Northern Brazil. Mem Inst Oswaldo Cruz. 2009;104:665–7. doi: 10.1590/s0074-02762009000400024. [DOI] [PubMed] [Google Scholar]
- Singh N, Hussain S, Kakkar N, et al. Implication of high risk human papillomavirus HR-HPV infection in prostate cancer in Indian population-a pioneering case-control analysis. Sci Rep. 2015;5:7822. doi: 10.1038/srep07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares PC, Ferreira S, Villa LL, Matos D. Identification of human papillomavirus in patients with anal squamous cell carcinoma. J Coloproctol. 2011;31:8–16. [Google Scholar]
- Sousa IDB, Vidal FCB, Vidal JPCB, et al. Prevalence of human papillomavirus in penile malignant tumors:viral genotyping and clinical aspects. BMC Urol. 2015;15:13. doi: 10.1186/s12894-015-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taherian H, Tafvizi F, Fard ZT, Abdirad A. Lack of association between human papillomavirus infection and colorectal cancer. Prz Gastroenterol. 2014;9:280–4. doi: 10.5114/pg.2014.46163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termini L, Fregnani JH, Boccardo E, et al. SOD2 immunoexpression predicts lymph node metastasis in penile cancer. BMC Clin Pathol. 2015;15:3. doi: 10.1186/s12907-015-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M, Shukla HS, Kumar M. Nd:YAG laser treatment of early stage carcinoma of the penis preserves form and function of penis. Asian J Surg. 2007;30:126–30. doi: 10.1016/S1015-9584(09)60145-7. [DOI] [PubMed] [Google Scholar]
- Urgoiti GBR, Gustafson K, Klimowicz AC, et al. The prognostic value of HPV status and p16 expression in patients with carcinoma of the anal canal. PLoS One. 2014;9:1–10. doi: 10.1371/journal.pone.0108790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeham K, Kavanagh K. The burden of HPV-associated anogenital cancers. Curr Oncol Rep. 2014;16:1–11. doi: 10.1007/s11912-014-0402-4. [DOI] [PubMed] [Google Scholar]
- Wakeham K, Kavanagh K, Cuschieri K, et al. HPV status and favourable outcome in vulvar squamous cancer. Int J Cancer. 2017;140:1134–46. doi: 10.1002/ijc.30523. [DOI] [PubMed] [Google Scholar]
- Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- WHO. HPV Information Centre. 2013. Avaliable at: http://www.hpvcentre.net/
- Yang L, Xie S, Feng X, et al. Worldwide prevalence of human papillomavirus and relative risk of prostate cancer:A Meta-analysis. Nat Publ Gr. 2015;2017:1–10. doi: 10.1038/srep14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yow MA, Tabrizi SN, Severi G, et al. Detection of infectious organisms in archival prostate cancer tissues. BMC Cancer. 2014;14:579. doi: 10.1186/1471-2407-14-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandberg DPDP, Bhargava R, Badin S, Cullen KJKJ. The role of human papillomavirus in nongenital cancers. CA Cancer J Clin. 2013;63:58–81. doi: 10.3322/caac.21167. [DOI] [PubMed] [Google Scholar]


