Abstract
Background:
Breast cancer is the most common cancer among women worldwide and the obesity is one of the factors related to the risk of breast cancer mainly in postmenopausal women. This study investigated the association between obesity in pre- and postmenopausal women with the development of breast cancer and the expression of estrogen, progesterone, HeR-2 and triple-negative (TN) receptors.
Methods:
A case-control study was conducted on 100 patients with recently diagnosed breast cancer and 400 age-matched controls. The women were divided into pre- and post-menopausal groups.
Results:
The multivariate analysis showed that postmenopausal women with a BMI ≥ 30 kg/m2 at pre-diagnosis and at the most recent measurement were 1.50 (95% CI 1.06-2.13) and 1.56 (95% CI 1.11-2.21) times more likely to develop breast cancer, respectively. These women had a prevalence of obesity of 27.7% when considering pre-diagnosis BMI and 29.4% when analyzing the indicator of recent BMI. When only the cases regarding the presence of obesity with clinicopathological variables were analyzed, a total of 95.2% of the postmenopausal women with pre-diagnostic obesity according to BMI presented the positive estrogen receptor (ER) subtype.
Conclusions:
In Brazilian women, there is an association between obesity and the risk of breast cancer postmenopause; moreover, there is an association between the occurrence of the positive ER subtype in postmenopausal women and pre-diagnostic obesity according to BMI.
Keywords: Obesity, breast cancer, cancer risk
Introduction
Breast cancer is the most common cancer among women worldwide. An estimated 1.67 million new cases were diagnosed in 2012, corresponding to 25% of all cancers. It is anticipated that in 2020, breast cancer will be diagnosed in more than 1.97 million women worldwide, and 622,000 women will die of this disease. Breast cancer is currently ranked as the fifth leading cause of death from cancer in general (522,000 deaths), the most frequent cause of cancer death in women in less developed regions (324,000 deaths, 14.3% of the total) and the second most frequent cause of cancer deaths in more developed regions (198,000 deaths, 15.4% of the total) (International Agency of Research Cancer, 2012). In 2016, the expected number of new cases of breast cancer in Brazil is 57,960 (Brasil, 2015), with a mortality of 17,872 (International Agency of Research Cancer, 2012).
Obesity is one of the factors related to the risk of breast cancer (Bhaskaran et al., 2014; Benedetto et al., 2015). In 2014, more than 600 million adults were obese. Overall, approximately 15% of the world’s women were obese in 2014 (World Health Organization, 2015). In addition, obesity is associated with a poor prognosis for breast cancer, with an increased risk of disease recurrence and with mortality (Kamineni et al., 2013; World Health Organization, 2015). Researchers have concluded that morbid obesity increases the risk of death from breast cancer by 2.26 times (Kwan et al., 2014). A study conducted in Germany showed that obese patients had significantly shorter disease-free survival and overall survival than non-obese patients (Sholz et al., 2015).
The relationship between obesity and the risk of breast cancer has been the subject of many studies (Amadou et al., 2013; Bhaskaran et al., 2014; Harding et al., 2015; Rosner et al., 2015). However, there is little data investigating the relationship obesity and risk of breast cancer in Brazilian women. Most studies are carried out with American women (Rosner et al., 2015), African, Asian (Amadou et al., 2013), European (Bhaskaran et al., 2014) and Australian (Harding et al., 2015). Thus, the following question arose: does the association between obesity and breast cancer in women in Brazil differs from women of other nationalities?
For postmenopausal women with breast cancer, obesity is considered a risk factor (Bhaskaran et al., 2014; Harding et al., 2015), whereas it is inversely related to the incidence of breast cancer in premenopausal women (Bhaskaran et al., 2014). Sangrajrang et al., (2013), demonstrated a positive association with the increased risk of breast cancer in postmenopausal women (odds ratio [OR] = 1.67). Munsell et al., (2014), showed in a meta-analysis that a BMI of ≥ 30 kg/m2 was associated with increased risk compared to women of normal weight.
Adiposity measures at age 18 and 20 and weight gain have also been discussed in the literature as factors related to breast cancer (Amadou et al., 2013; Rosner et al., 2015). Women of low weight at age 20 were less likely to develop premenopausal breast cancer (Sangrajrang et al., 2013). A weight gain of ≥ 10 kg after the twenties was considered to present an increased risk of breast cancer in postmenopausal women (Suzuki et al., 2013). Some studies show that central adiposity is an independent predictor of breast cancer (Kabat et al.,2015; Harding et al., 2015), and many studies have indicated an increased risk associated with a higher waist circumference (WC) and a higher waist-hip ratio in women who are premenopausal (Amadou et al., 2013; Bandera et al., 2013; Wang et al., 2013).
Obesity is an independent risk factor for breast cancer in postmenopausal women, in particular for women with the positive estrogen receptor (ER) (Printz, 2014; Bandera et al., 2015) and positive progesterone receptor (PR) subtypes (Munsell et al., 2014). However, positive associations between obesity and the risk of breast cancer have also been demonstrated for triple-negative (TN) breast cancer (Pierobon and Frankenfel, 2013; Mowad et al., 2013).
Given the importance of the subject and because of the need for research on Brazilian women, the objective of this study was to investigate the association between obesity in pre- and postmenopausal women and the development of breast cancer and the expression of estrogen, progesterone, human epidermal growth factor receptor 2 (HeR-2)and TN receptors.
Materials and Methods
Study population
A case-control study was conducted with a group of 500 women in a philanthropic hospital run by the North Paranaense Association Against Cancer (Associação Norte Paranaense de CombateaoCâncer) with recognized local and regional representation for cancer care; this hospital is located in a Brazilian municipalityin the North Central ParanaenseMesoregion, state of Paraná, southern Brazil. The hospital serves approximately 150 state municipalities, with more than 80% of the treatment being provided through the National Health System (SistemaÚnico de Saúde - SUS). Data were collected between October 2014 and October 2015.
Women with a confirmed histological diagnosis of breast cancer that was discovered in the 6 months preceding the interview were selected as cases. Women who attended the hospital to perform routine breast examinations during the same period were randomly selected as controls. Women who had breast cancer recurrence, those who had any history of cancer and those who were diagnosed more than 6 months before the date of interview were excluded from the study.
The sample size was estimated based on the identification of patients diagnosed with breast cancer in the period selected for the survey (October 2013 to October 2014); this provided a total of 100 eligible women. For the controls, data were collected at a ratio of 1 case per 4 controls, resulting in a total of 400 controls, who were matched for age (standard deviation [SD] = 5 years).
From this total, the women were divided into premenopausal (n = 32 cases and n = 128 controls) and postmenopausal (n = 68 cases and n = 272 controls) groups (flowchart 1).
Flowchart 1.
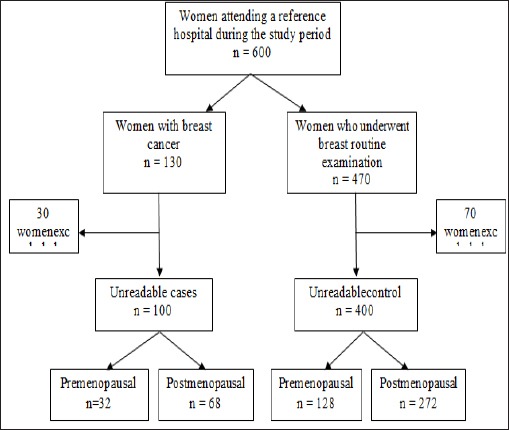
Delineation of women selected for the case-control study.
Women with natural menopause or those who had undergone total hysterectomy or bilateral oophorectomy were considered postmenopausal. Women who reported having undergone hysterectomy, but without the removal of one or both ovaries, were classified as premenopausal.
This study was approved by the Standing Committee on Ethics in Human Research of the State University of Maringa (Universidade Estadual de Maringá) under decision no.353,649.
Data collection
After confirming eligibility, the data were collected by conducting face-to-face interviews with the use of a structured questionnaire after obtaining the written consent of the participants.
The study variables collected for nutritional status (obesity and abdominal obesity) werecurrent weight, height, pre-diagnostic weight (self-reported weight - weight in the last 6 months prior to diagnosis), weight at age 20 (self-reported), BMI and WC. The BMI was calculated by the formula: weight (kg)/height (m)2, which was classified according to the World Health Organization criteria (World Health Organization, 2013); the results were stratified into BMI < 30.0 kg/m2 and BMI ≥ 30.0 kg/m2. WC was measured according to the criteria established by the World Health Organization (2005) and was classified into WC < 80 cm and WC > 80 cm.
The medical records of the women with breast cancer were analyzed to obtain the following clinicopathological variables: estrogen (negative/positive), progesterone (negative/positive), HeR-2 (negative/positive) and TN receptors.
The following sociodemographic data were collected: age (calculated in full years at the time of the interview and categorized as < 40 and ≥ 40 years; civil status (with or without a partner); educational level (according to the last completed grade, categorized as < 8 years or ≥ 8 years); and race/color (white or non-white).
Other information relating to potential breast cancer risk factors were collected, such as a family history of breast cancer in a 1st degree relative (yes/no); past contraceptive use (yes/no); age at menarche (< 13 and ≥ 13 years); nulliparity (yes/no); breastfeeding duration (< 12 and ≥ 12 months); age at menopause (< 55 or ≥ 55 years); and past hormone replacement therapy (HRT) use (yes/no).
Statistical analysis
For the statistical analysis, the information was tabulated using a descriptive analysis (mean and standard deviation) and was analyzed using the chi-square and Fisher´s exact tests, where applicable, to test the association between the selected characteristics of the study population. A crude OR analysis was performed using the chi-square test in Epi Info 3.5.1 to evaluate the effects of BMI at age 20, pre-diagnostic BMI, current BMI and WC with regard to the risk of breast cancer in the menopausal groups. A multivariate logistic regression model was used to determine the OR adjusted for potential confounding variables(age, family history of breast cancer, contraceptive use, age at menarche, nulliparity, breastfeeding, age at menopause and HRT use) using Statistica 7.1. The level of statistical significance was set at p < 0.05 and the confidence interval (CI) at 95%.
Results
The mean age of the 100 cases and 400 controls was 57.4 (SD 11.8) and 52.3 (SD 11.6) years, respectively. The characteristics of the study population by menopausal status are shown in Table 1. No significant differences were observed between cases and controls. However, in the premenopausal and postmenopausal groups, a higher frequency of women who had a family history of breast cancer of the first degree and nulliparity were observed in the group of breast cancer cases. Contraceptive use was observed to a greater extent among women with premenopausal breast cancer.
Table 1.
Selected Characteristics of Study Population by Menopausal Status. Brazil, 2017.
| Characteristics | Premenopausal | p value | Postmenopausal | p value | ||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||
| (n=32) | (n=128) | (n=68) | (n=272) | |||
| n (%) | n (%) | n (%) | n (%) | |||
| Age (years) | ||||||
| < 40 | 6 (11.5) | 46 (88.5) | 0 (0.0) | 1 (100.0) | ||
| ≥ 40 | 26 (24.1) | 82 (75.9) | 0.09 | 68 (20.1) | 271 (79.9) | 0.80* |
| Education (years of study) | ||||||
| < 8 | 10 (17.0) | 49 (83.0) | 0.54 | 45 (21.1) | 168 (78.9) | 0.50 |
| ≥ 8 | 22 (21.8) | 79 (78.2) | 23 (18.1) | 104 (81.9) | ||
| Marital Status | ||||||
| With partner | 23 (19.3) | 96 (80.7) | 40 (17.8) | 185 (82.2) | ||
| Without a partner | 9 (22.0) | 32 (78.0) | 0.71 | 28 (24.3) | 87 (75.7) | 0.15 |
| Race / Color | ||||||
| White | 25 (20.0) | 100 (80.0) | 56 (20.1) | 223 (79.9) | ||
| Nowhite | 7 (20.0) | 28 (80.0) | 1.00 | 12 (19.7) | 49 (80.3) | 0.94 |
| Family History of | ||||||
| Breast Cancer 1º degree | ||||||
| Yes | 4 (33.3) | 8 (66.7) | 0.22* | 5 (21.7) | 18 (78.3) | 0.50* |
| No | 28 (19.0) | 120 (81.0) | 63 (19.9) | 254 (80.1) | ||
| Hormone replacement therapy | ||||||
| Yes | - | - | 10 (13.7) | 63 (86.3) | 0.12 | |
| No | - | - | 58 (21.7) | 209 (78.3) | ||
| Contraceptive use | ||||||
| Yes | 25 (24.5) | 77 (75.5) | 0.06 | 38 (17.3) | 182 (82.7) | 0.08 |
| No | 7 (12.1) | 51 (87.9) | 30 (25.0) | 90 (75.0) | ||
| Age at menarche (years) | ||||||
| < 13 | 14 (18.2) | 63 (81.8) | 0.57 | 29 (20.0) | 116 (80.0) | 1.00 |
| ≥ 13 | 18 (29.7) | 65 (78.3) | 39 (20.0) | 156 (80.0) | ||
| Nulliparity | ||||||
| Yes | 5 (31.3) | 11 (68.7) | 0.23* | 9 (33.3) | 18 (66.7) | 0.07 |
| No | 27 (18.7) | 117 (81.3) | 59 (18.8) | 254 (81.2) | ||
| Time of breastfeeding | ||||||
| < 12 months | 11 (22.5) | 38 (77.5) | 0.41 | 14 (17.9) | 64 (82.1) | 0.87 |
| ≥ 12 months | 13 (16.7) | 65 (83.3) | 39 (18.7) | 169 (81.3) | ||
Fisher´s exact test
After stratification according to menopause, obesity in postmenopausal women appeared to be a risk factor for breast cancer. These women had a prevalence of obesity of 27.7% when considering pre-diagnosis BMI and 29.4% when analyzing the indicator of recent BMI. Based on the multivariate regression analysis with the potential risk factors for breast cancer, women with a BMI ≥ 30 kg/m2 at pre-diagnosis and at the most recent measurement were 1.50 and 1.56 times more likely, respectively, to develop breast cancer (Table 2 and 3).
Table 2.
Bivariate Analysis and Multivariate Logistic Regression of Association of BMI, WC and Breast Cancer Risk by Menopausal Status. Brazil, 2017
| Characteristics | Premenopausal | |||||
|---|---|---|---|---|---|---|
| Cases | Control | OR crude | 95% CI | OR adjusteda | 95% CI | |
| (n=32) | (n=128) | |||||
| n (%) | n (%) | |||||
| cPrediagnosis BMI (kg/m2) | ||||||
| <30 | 20 (16.5) | 101 (83.5) | 1.0 | |||
| ≥30 | 12 (30.8) | 27 (69.2) | 2.24 | 0.90-5.57 | 1.64 | 0.95-2-85 |
| p value | 0.05 | 0.07 | ||||
| dCurrent BMI (kg/m2) | ||||||
| <30 | 24 (19.0) | 102 (81.0) | 1.0 | |||
| ≥30 | 8 (23.5) | 26 (76.5) | 1.31 | 0.48-3.51 | 1.23 | 0.70-2.18 |
| p value | 0.56 | 0.56 | 0.47 | |||
| eBMI at age 20 years (kg/m2) | ||||||
| <30 | 31 (19.9) | 125 (80.1) | 1.0 | |||
| ≥30 | 1 (25.0) | 3 (75.0) | 1.34 | 0.22-7.07 | 1.06 | 0.27-4.24 |
| p value | 0.59* | 0.93 | ||||
| Waist circumference (cm) | ||||||
| <80 | 3 (13.0) | 20 (87.0) | 1.0 | |||
| ≥80 | 29 (21.2) | 108 (78.8) | 1.79 | 0.46-8.16 | 1.25 | 0.60-2.62 |
| p value | 0.27* | 0.54 | ||||
Adjusted for age, contraceptive use, age at menarche, nulliparity,
reastfeeding, family history of breast cancer;
Based on self-reported prediagnosis weight at least 6 month before breast cancer diagnosis and measured height at interview;
BMI measured at interview;
Based on self-reported weight with age 20 years and measured height at interview;
Fisher´s exact test; OR, odds ratio; CI, confidence interval.
Table 3.
Bivariate Analysis and Multivariate Logistic Regression of Association of BMI, WC and Breast Cancer Risk by Menopausal Status. Brazil, 2017
| Characteristics | Postmenopausal | |||||
|---|---|---|---|---|---|---|
| Cases | Control | OR crude | 95% CI | OR Adjusteda | 95% CI | |
| (n=68) | (n=272) | |||||
| n (%) | n (%) | |||||
| cPrediagnosis BMI (kg/m2) | ||||||
| <30 | 45 (17.5) | 212 (82.5) | 1.0 | |||
| ≥30 | 23 (27.7) | 60 (72.3) | 1.81 | 1.02-3.34 | 1.50 | 1.06-2.13 |
| p value | 0.04 | 0.02 | ||||
| dCurrent BMI (kg/m2) | ||||||
| <30 | 43 (16.9) | 212 (83.1) | 1.0 | |||
| ≥30 | 25 (29.4) | 60 (70.6) | 2.05 | 1.12-3.77 | 1.56 | 1.11-2.21 |
| p value | 0.01 | 0.01 | ||||
| eBMI at age 20 years (kg/m2) | ||||||
| <30 | 67 (20.0) | 268 (80.0) | 1.0 | |||
| ≥30 | 1 (20.0) | 4 (80.0) | 1.00 | 0.17-5.85 | 0.99 | 0.27-3.65 |
| p value | 0.67* | 0.99 | ||||
| Waist circumference (cm) | ||||||
| <80 | 5 (13.5) | 32 (86.5) | 1.0 | |||
| ≥80 | 63 (20.8) | 240 (79.2) | 1.68 | 0.59-5.13 | 1.38 | 0.80-2.40 |
| p value | 0.29 | 0.24 | ||||
Adjusted for age, contraceptive use, age at menarche, nulliparity,
reastfeeding, family history of breast cancer, age of menopause, hormone replacement therapy;
Based on self-reported prediagnosis weight at least 6 month before breast cancer diagnosis and measured height at interview
BMI measured at interview;
Based on self-reported weight with age 20 years and measured height at interview;
Fisher´s exact test; OR, odds ratio; CI, confidence interval.
When analyzing the cases of breast cancer regarding the presence of obesity with only clinicopathological variables no relationship was found between obesity and these variables in premenopausal women (Table 3). As for postmenopausal women, it was verified that the ER Status variable was significant. (Table 4). A total of 95.2% of the postmenopausal women with pre-diagnostic obesity according to BMI had a positive ER status (Table 5).
Table 4.
Association of BMI and WC with Clinicophatological Variable among Premenopausal Breast Cancer. Brazil, 2017
| Characteristics | ER Status | PR Status | HeR-2 Status | TN | ||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | Yes | No | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Prediagnosis BMI (kg/m2) | ||||||||
| <30 | 1 (6.3) | 15 (93.8) | 3 (20.0) | 12 (80.0) | 3 (27.3) | 8 (72.7) | 0 (0.0) | 11 (100.0) |
| ≥30 | 2 (25.0) | 6 (75.0) | 2 (25.0) | 6 (75.0) | 2 (28.6) | 5 (71.4) | 1 (14.3) | 6 (85.7) |
| p value | 0.24* | 0.58* | 0.67* | 0.38* | ||||
| Current BMI (kg/m2) | ||||||||
| <30 | 3 (16.7) | 15 (83.3) | 5 (29.4) | 12 (70.6) | 4 (25.0) | 12 (75.0) | 1 (6.3) | 15 (93.8) |
| ≥30 | 0 (0.0) | 6 (100.0) | 0 (0.0) | 6 (100.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 2 (100.0) |
| p value | 0.40* | 0.18* | 0.49* | 0.88* | ||||
| BMI at age 20 years (kg/m2) | ||||||||
| <30 | 3 (13.0) | 20 (87.0) | 5 (22.7) | 17 (77.3) | 5 (27.8) | 13 (72.2) | 1 (5.6) | 17 (94.4) |
| ≥30 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1(100.0) | - | - | - | - |
| p value | 0.87* | 0.78* | NA | NA | ||||
| Waist circumference (cm) | ||||||||
| <80 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) | - | - | - | - |
| ≥80 | 3 (13.0) | 20 (87.0) | 5 (22.7) | 17 (77.3) | 5 (27.8) | 13 (72.2) | 1 (5.6) | 17 (94.4) |
| p value | 0.87* | 0.78* | NA | NA | ||||
ER, estrogen receptor; PR, progesterone receptor; HER-2, Human Epidermal growth factor Receptor 2; TN, triple-negative;
Fisher´s exact test NA, no applicable
Table 5.
Association of BMI and WC with Clinicophatological Variable among Postmenopausal Breast Cancer. Brazil, 2017
| ER Status | PR Status | HeR-2 Status | TN | |||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | Yes | No | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Prediagnosis BMI (kg/m2) | ||||||||
| <30 | 13 (31.7) | 28 (68.3) | 15 (37.5) | 25 (62.5) | 9 (28.1) | 23 (71.9) | 6 (18.8) | 26 (81.3) |
| ≥30 | 1 (4.8) | 20 (95.2) | 3 (14.3) | 18 (85.7) | 6 (35.3) | 11 (64.7) | 0 (0.0) | 17 (100.0) |
| p value | 0.01* | 0.05* | 0.60 | 0.06* | ||||
| Current BMI (kg/m2) | ||||||||
| <30 | 6 (15.4) | 33 (84.6) | 10 (26.3) | 28 (73.7) | 9 (31.0) | 20 (69.0) | 27 (93.1) | 2 (6.9) |
| ≥30 | 8 (34,8) | 15 (65.2) | 8 (34.8) | 15 (65.2) | 6 (30.0) | 14 (70.0) | 16 (80.0) | 4 (20.0) |
| p value | 0.07 | 0.48 | 0.93 | 0.17* | ||||
| BMI at age 20 years (kg/m2) | ||||||||
| <30 | 13 (21.3) | 48 (78.7) | 17 (28.3) | 43 (71.7) | 15 (31.3) | 33 (68.8) | 6 (12.5) | 42 (87.5) |
| ≥30 | 1 (100.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| p value | 0.22* | 0.29* | 0.69* | 0.87* | ||||
| Waist circumference (cm) | ||||||||
| <80 | 1 (20.0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | 2 (40.0) | 3 (60.0) | 1 (20.0) | 4 (80.0) |
| ≥80 | 13 (22.8) | 44 (77.2) | 17 (30.4) | 39 (69.6) | 13 (29.5) | 31 (70.5) | 5 (11.4) | 39 (88.6) |
| p value | 0.68* | 0.53* | 0.48* | 0.49* | ||||
ER, estrogen receptor; PR, progesterone receptor; HER-2, Human Epidermal growth factor Receptor 2; TN, triple-negative;
Fisher´s exact test
Discussion
To our knowledge, data on the effects of obesity on breast cancer in Brazilian women are scarce. From this, the present study was conducted in order to answer whether the association between obesity and breast cancer in women in Brazil differs from women of other nationalities?
In this study, after investigating the association between obesity and the development of breast cancer and with the expression of ER, PR, HeR-2 and TN, it was identified that pre-diagnostic and recent obesity were risk factors for breast cancer in postmenopausal women and that postmenopausal cases with pre-diagnostic obesity according to BMI had a positive ER status.
Because the sample size is set the approach to power is based on minimum detectable differences in prevalence of obesity between cases and controls. Power calculations are based on a two-sided, two-group continuity corrected Chi-square test with alpha=0.05. We assume that the prevalence of obesity in cases (those with cancer) will be higher than those in the controls (without cancer). Because we have a set sample size our approach is to determine the minimum detectable difference based on the estimated prevalence of obesity in cases. Based on the aforementioned test we will have 80% power to detect the following diferences.
The present study identified a risk of developing breast cancer of 1.50 (95% CI 1.06-2.13) and 1.56 (95% CI 1.11-2.21) among postmenopausal women with BMI ≥ 30 kg/m2 at the time of pre-diagnosis and in the recent measurement, respectively.
This association between obesity and postmenopausal breast cancer agrees with research based on multicenter data from the Women’s Health Initiative (USA) involving 7,039 breast cancer patients (Kabat et al., 2015). Research conducted in Australia and New Zealand showed that postmenopausal women with obesity according to their BMI had a 1.06 times greater risk (CI 1.01 to 1.12) of developing breast cancer (Harding et al., 2015), whereas in Japanese women, the risk was 2.03 times greater (CI 1.59 to 2.61) (Tamaki et al., 2014).
In Brazil, a single study developed in the northeast region to investigate associations between the diagnosis of breast cancer and nutritional factors in women evaluated in two cancer hospitals showed that overweight or obesity and waist circumference> 88 cm were more prevalent in the group of cases, presenting respectively a risk chance of 2.70 (95% CI 1.28-5.70) and a risk of 3.10 (CI 95% 1.46-6.56) compared to the control group (Queiroz et al., 2018).
The finding in the present study between obesity and the risk of breast cancer presents some justifications. The increased risk of breast cancer in postmenopausal obese women can be explained by the increase in estradiol bioavailability as a result of the higher conversion rate of androgenic precursors to estradiol through the activity of aromatase, an enzyme present in adipose tissue (Goday et al., 2015). Furthermore, obesity is related to hyperinsulinemia, which in turn inhibits the hepatic secretion of sex hormone-binding globulin (SHBG), thus increasing the bioactivity of circulating estradiol (Boonyaratanakornkit and Pateetin, 2015).
Another factor that may justify the association between obesity and breast cancer identified in this study is that obesity is now also recognized as an inflammatory condition, and the role of inflammatory mediators such as prostaglandin E2 (PGE2) is to act as one of the main engines of aromatase expression in breast adipose stromal cells (Brown and Simpson, 2015). Subbaramaiah et al., (2012) showed that the expression of aromatase in the breasts of obese women was greater than that in non-obese women, and it was correlated with increased levels of PGE2. The increase in aromatase expression therefore results in the formation of estrogen in the breast, which would lead to the increased proliferation of breast cancer (Boonyaratanakornkit and Pateetin, 2015; Brown and Simpson, 2015).
Furthermore, obesity results in a decrease in adiponectin circulation and increased leptin, which in turn exerts a stimulatory effect in promoting the survival, proliferation, migration and invasion of ER-positive and ER-negative breast cancer cell lines (Dubois et al., 2014; Brown and Simpson, 2015). Adiponectin acts by exerting anticancer effects, increasing insulin sensitivity and decreasing insulin/IGF-1 (growth factor similar to type 1 insulin) (Benedetto et al., 2015).
Another result encountered in this study was that the higher prevalence of positive ER status in women with breast cancer was found only for those with postmenopausal obesity. Research conducted with African American women found that in postmenopause, a high recent BMI led to a 1.31 (1.02-1.67) times greater risk of having a positive ER subtype (Bandera et al., 2015). A meta-analysis investigating ER and PR identified a correlation between a BMI ≥ 30 kg/m2 and the incidence of postmenopausal breast cancer for ER-positive/PR-positive subtypes, but not for ER-negative/PR-negative cancer subtypes (Munsell et al., 2014).
The limitations of this study were as follows: the related use of HRT in postmenopausal women (amount, type and duration of medication) was not evaluated in this study; this can be a primary limitation because positive ER levels may also be linked to the use of HRT and not just to self-reported pre-diagnostic obesity. A second limitation is related to the fact that pre-diagnostic weight was self-reported by the women. Although self-reported weight is often used in epidemiological studies, whether in investigator-administered or self-administered questionnaires, or in telephone interviews, the memory factor in remembering weight becomes a limitation.
In conclusion, according to the analysis, our study shows that Brazilian women have outcomes regarding obesity and breast cancer risk that are similar to women of other nationalities. This study established that there is an association between obesity and breast cancer risk in postmenopausal women and also between the occurrence of the positive ER subtype cancer in postmenopausal women and pre-diagnostic obesity according to BMI. The results of this study may have important implications for women’s health. Health professionals working in primary care should begin to direct health education towards obesity as a risk factor; especially aimed at promoting the best prognosis with weight maintenance after the diagnosis of breast cancer (Bradshaw et al., 2012). These actions may lead to a reduction in breast cancer and mortality. Further studies with Brazilian women, preferably of a longitudinal and prospective nature, are required to determine whether lifestyle interventions such as weight loss could be an appropriate and preventive strategy against breast cancer.
Acknowledgements
The authors would like to thank to the Hospital, by the authorization for this research and the enterprise “American Journal Experts” by the translation of this article into English. The authors gratefully acknowledge especially to all the women who agreed to participate this research.
References
- Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity:a systematic review and dose-response meta-analysis. Obes Rev. 2013;14:665–78. doi: 10.1111/obr.12028. [DOI] [PubMed] [Google Scholar]
- Bandera EV, Chandran U, Zirpoli G, et al. Body fatness and breast cancer risk in women of African ancestry. BMC Cancer. 2013;13:475. doi: 10.1186/1471-2407-13-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandera EV, Chandran U, Hong CC, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150:655–66. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto C, Salvagno F, Canuto EM, Gennarelli G. Obesity and female malignancies. Best Pract Res Clin Obstet Gynaecol. 2015;29:528–40. doi: 10.1016/j.bpobgyn.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Bhaskaran K, Douglas I, Forbes H, et al. Body-mass index and risk of 22 specific cancers:a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Pateetin P. The Role of ovarian sex steroids in metabolic homeostasis, obesity, and postmenopausal breast cancer:Molecular mechanisms and therapeutic Implications. Biomed Res Int. 2015;2015:140196. doi: 10.1155/2015/140196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw PT, Ibrahim JG, Stevens J, et al. Post-diagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology. 2012;23:320–7. doi: 10.1097/EDE.0b013e31824596a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil. Ministério da Saúde. jInstituto nacional de câncer. Estimativa de câncer no Brasil 2016. Coordenação de Prevenção e Vigilância / Divisão de Vigilância Rio de Janeiro, 2015. 2015. [Accessed December 2015]. Available: http://www.inca.gov.br/wcm/dncc/2015/estimativa-2016.asp .
- Brown KA, Simpson ER. Estrogens, obesity, inflammation, and breast cancer-What is the link? Semin Reprod Med. 2015;33:208–12. doi: 10.1055/s-0035-1552581. [DOI] [PubMed] [Google Scholar]
- Dubois V, Jarde T, Delort L, et al. Leptin induces a proliferative response in breast cancer cells but not in normal breast cells. Nutr Cancer. 2014;66:645–55. doi: 10.1080/01635581.2014.894104. [DOI] [PubMed] [Google Scholar]
- Goday A, Barneto I, García-Almeida JM, et al. Obesity as a risk factor in cancer:A national consensus of the Spanish society for the study of obesity and the Spanish society of medical oncology. Clin Transl Oncol. 2015;17:763–71. doi: 10.1007/s12094-015-1306-y. [DOI] [PubMed] [Google Scholar]
- Harding JL, Shaw JE, Anstey KJ, et al. Comparison of anthropometric measures as predictors of cancer incidence:A pooled collaborative analysis of 11 Australian cohorts. Int J Cancer. 2015;137:1699–708. doi: 10.1002/ijc.29529. [DOI] [PubMed] [Google Scholar]
- International Agency of Research Cancer, World Health Organization 2012. GLOBOCAN 2012:Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012. [Accessed September 2015]. Available: http://globocan.iarc.fr .
- Kabat GC, Xue X, Kamensky V, et al. Risk of breast, endometrial, colorectal, and renal cancer in postmenopausal women in association with a body shape index and other anthropometric measures. Cancer Causes Control. 2015;26:219–29. doi: 10.1007/s10552-014-0501-4. [DOI] [PubMed] [Google Scholar]
- Kamineni A, Anderson ML, White E, et al. Body mass index, tumor characteristics, and prognosis following diagnosis of early stage breast cancer in a mammographically-screened population. Cancer Causes Control. 2013;24:305–12. doi: 10.1007/s10552-012-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan ML, John EM, Caan BJ, et al. Obesity and mortality after breast cancer by race/ethnicity:The California breast cancer survivorship consortium. Am J Epidemiol. 2014;179:95–111. doi: 10.1093/aje/kwt233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowad R, Chu QD, Li BDL, et al. Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res. 2013;184:253–9. doi: 10.1016/j.jss.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–36. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierobon M, Frankenfel CL. Obesity as a risk factor for triple-negative breast cancers:a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137:307–14. doi: 10.1007/s10549-012-2339-3. [DOI] [PubMed] [Google Scholar]
- Printz C. Obesity associated with higher mortality in women with ER-positive breast cancer. Cancer. 2014;120:3267. doi: 10.1002/cncr.29079. [DOI] [PubMed] [Google Scholar]
- Queiroz SA, Sousa IM, Silva FRM, Lyra CO, Fayh APT. Nutritional and environmental risk factors for breast cancer:a case-control study. Sci Med. 2018;28:1–8. [Google Scholar]
- Rosner B, Eliassen AH, Toriola AD, et al. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res Treat. 2015;150:643–53. doi: 10.1007/s10549-015-3344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangrajrang S, Chaiwerawattana A, Ploysawang P, et al. Obesity, diet and physical inactivity and risk of breast cancer in Thai women. Asian Pac J Cancer Prev. 2013;14:7023–27. doi: 10.7314/apjcp.2013.14.11.7023. [DOI] [PubMed] [Google Scholar]
- Scholz C, Andergassen U, Hepp P, et al. Obesity as an independent risk factor for decreased survival in node-positive high-risk breast cancer. Breast Cancer Res Treat. 2015;151:569–76. doi: 10.1007/s10549-015-3422-3. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Morris PG, Zhou XK, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–65. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Suzuki S, Kojima M, Tokudome S, et al. Obesity/weight gain and breast cancer risk:Findings from the Japan collaborative cohort study for the evaluation of cancer risk. J Epidemiol. 2013;23:139–45. doi: 10.2188/jea.JE20120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki K, Tamaki N, Terukina S, et al. The correlation between body mass index and breast cancer risk or estrogen receptor status in Okinawan women. Tohoku J Exp Med. 2014;234:169–74. doi: 10.1620/tjem.234.169. [DOI] [PubMed] [Google Scholar]
- Wang XL, Jia CX, Liu LY, et al. Obesity, diabetes mellitus, and the risk of female breast cancer in Eastern China. World J Surg Oncol. 2013;16:11–71. doi: 10.1186/1477-7819-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Obesity and overweight. Fact sheet n°311. 2015. [Accessed August 2015]. Available: http://www.who.int/mediacentre/factsheets/fs311/en/
- World Health Organization. Obesity and overweight. Fact sheet N°311. 2013. [Accessed July 2015]. Available: http://www.who.int/mediacentre/factsheets/fs311/en/
- World Health Organization. Preventing chronic diseases:a vital investment. Geneva: World Health Organization/Ottawa:Public Health Agency of Canada; 2005. [Accessed July 2015]. Available: http://whqlibdoc.who.int/publications/2005/9241563001_eng.pdf . [Google Scholar]


