Abstract
Objectives:
To determine and compare the serum lipid profiles and anthropometric parameters of newly diagnosed BC patients and healthy women.
Methods:
Serum total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), very low density lipoprotein cholesterol (VLDL-C), triglyceride (TG) and TC: HDL-C were measured in consent obtained newly diagnosed BC patients (n=155) and age matched apparently healthy females (n=75). Weight (W), height (H), waist circumference (WC), hip circumference (HC) and mid upper arm circumference (MUC) of each women were recorded. Cut off values for each parameter was found by receiver operative characteristic (ROC) curves and risk associated with was calculated using SPSS version 16.
Results:
Majority (67%) of BC women were postmenopausal. The mean TC, HDL-C, LDL-C, VLDL-C, TC: HDL-C, TG concentrations of BC patients who were not on cholesterol lowering drugs (n= 126) were 234 mg/dL (±51), 43 mg/dL (±10), 164 mg/dL (±44), 27 mg/dL (±14), 5.7(±1.7) and 135 mg/dL (±69) respectively. TC, LDL-C and TC: HDL-C of BC patients were significantly elevated when compared with healthy females. Significant difference in serum lipid profile parameters was not observed (p> 0.05) according to the menopausal status of BC and healthy women. One third (30.3%) of BC patients were overweight and 45% were obese. Majority had elevated WC (72%), W: H ratios (89%) and MUC (89%). BMI, W: H and MUC of BC women were significantly higher (p<0.05) when compared with healthy females.
Conclusions:
The lipid parameters TC, LDL-C and TC: HDL-C above 203 mg/dL, 139 mg/dL and 3.9 respectively were risk factors. Among anthropometric measures, BMI>25 kg/m2 showed the highest risk while elevated W:H and MUC were also significant risk factors among the study group.
Keywords: Breast cancer, lipid profile, anthropometry, menopausal status
Introduction
Breast cancer (BC) is the most common carcinoma among women worldwide (Bray et al., 2004). The incidence rates vary from 19.3 per 100,000 women in Eastern Africa to 89.7 per 100,000 women in Western Europe. The crude breast cancer incidence in Sri Lanka is 19 per 100,000 population in the year 2008 (National Cancer Control Programme, 2007). Breast cancer incidence is reported to rise in the younger age groups among Asian women. The age group that reports the peak prevalence of BC in Western countries is 50 to 59 years while 40 to 49 years among Asian women (Ahmadin and Samah, 2012).
According to WHO (Curado, 2011) the incidence of breast cancer is increasing in the developing world due to increased life expectancy, increased urbanization and adoption of western lifestyles. Studies on the contribution of various modifiable risk factors, to the overall breast cancer burden conclude that 21% of all breast cancer deaths worldwide are attributable to alcohol use, overweight and obesity, and physical inactivity (Danaei et al., 2005; Bertram et al., 2011). The prevalence of being obese and overweight among Sri Lankans has shown an increasing trend as in all Asian countries. Recent data on prevalence of overweight (17.1%), obese (28.8%) and centrally obese (30.8%) indicate that almost half of the population is either over-weight or obese. Men compared to women were less overweight, less obese and less abdominally obese (Katulanda et al., 2012) indicating that females are at more risk of developing chronic non communicable diseases including cancer.
Being overweight or obese and having elevated lipid profiles could be a result of unhealthy diet and lack of physical exercises. Positive associations between height (Okobia et al., 2005; Wirén et al., 2014) BMI, (Singh et al., 2011), waist to hip ratio (WHR) (Horn et al., 2014) and breast cancer risk are reported. Further, adiposity is negatively associated with premenopausal breast cancer risk and positively associated with postmenopausal breast cancer risk (Tehard and Clavel-Chapelon, 2006). Regular exercises lower the breast cancer incidence and 10%-70% BC risk reduction is reported in women involved in moderate to vigorous exercise (3-4 hours per week) (Friedenreich et al., 2001).
Studies on associations of serum lipid profiles and breast cancer risk are reported with contradictory results. Developing breast cancer is considered as one of the factors that contribute to the alterations in serum lipid profile levels (Abdelsalamet al., 2012). Studies also reveal that cholesterol accelerates and enhances tumour formation together with the tumor aggressiveness (Llaverias et al., 2011). Some studies indicate high total cholesterol (TC) concentrations in BC patients (Al-Swelmien, 2014) while contradictory results are reported on high density lipoprotein cholesterol (HDL-C) and breast cancer risk (Furberg et al., 2005; Touvier et al., 2015). However, serum triglyceride (TG) is found to be not associated with premenopausal BC women while a positive association is reported among postmenopausal BC women (Ulmer et al., 2009 ; Potischman et al., 1991). Elevated low density lipoprotein cholesterol (LDL-C) level is also found to promote BC progression (Santos et al., 2014). However, studies also reveal no significant differences in serum TC, HDL-C, LDL-C or TG among BC patients (Fiorenza and Branchi, 2000).
However, data on lipid parameters, anthropometric measures, attitudes and involvement in physical exercises among Sri Lankan BC patients is not reported. Hence the study was developed with the intention of studying the serum lipid profiles and anthropometric parameters of newly diagnosed Sri Lankan BC patients and apparently healthy females.
Materials and Methods
Methodology
A prospective cross sectional study was conducted during the years 2013 to 2015. The sample size was calculated with breast cancer prevalence 18.7 per 100,000 population with standard normal variant at 5% type 1 error (1.96) using the following equation:
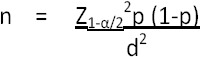
n = Sample size
Z1-α/2 = Standard normal variant at 5% type 1 error (1.96)
p = Round estimate of proportion of occurrence of event being measured.
d = Absolute error of precision
The calculated sample size was 28 as the proportion of breast cancer in Sri Lanka is 0.0187%. However, in order to improve the accuracy of the results, 150 newly diagnosed breast cancer patients who have not undergone any treatment (chemotherapy, radiotherapy, surgery etc) was enrolled for the study from the National Cancer Institute Maharagama, Sri Lanka. Age matched apparently healthy females (n=75) were enrolled for the comparative study.
Questionnaire study
Patients age, menopausal status, knowledge on serum lipid profile, if patients were on cholesterol lowering drugs, knowledge and attitudes towards physical exercises and types of physical exercises involved in were collected using a standardized, interviewer administered questionnaire. Women who have not had a naturally occurring menstrual cycle during the past 12 months or undergone a hysterectomy without complete oophorectomy before menopause were considered as postmenopausal.
Anthropometric parameters
Body weight was measured to the nearest 0.5 kg with participants dressed in lightweight clothing being bare feet. Height was measured using a wall mount ruler, while participant was standing straight, bare feet together with heels and buttocks, shoulders and back of the head touching the upright and measurement was made to the nearest 0.5 cm. BMI was calculated by dividing weight (kg) by height squared (m2). BMI ≥ 23 and ≥ 25 were considered as overweight and obese respectively.
Waist circumference (WC) was measured using a non stretchable tape around the trunk between the lower costal margin and iliac crest and hip circumference (HC) was measured at the wide area of the buttock and WHR was calculated. Over 80 cm of WC and WHR of ≥ 0.80 were considered as risk category. Mid upper arm circumference (MUC) was measured at the midpoint of the left upper arm between the shoulder and the tip of the elbow, while arm was hanging down side of the body and was kept relaxed. Measures were taken to protect the privacy of the patients and apparently healthy participants during the measurement of anthropometric parameters.
Lipid profile assays
Venous blood samples from 10-12 hours fasted patients were collected and serum lipid profile analyzed using appropriate biochemical kits (Biolabo, France) using KONE 20XT biochemical analyzer.
Serum LDL-C was calculated using the following Friedewald equation (Friedewald et al., 1972).
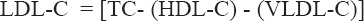
VLDL-C concentration of serum was calculated according to the following equation:
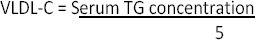
The BC patients on cholesterol lowering drugs were excluded from the statistical data analysis.
Statistical analysis
Statistical analysis was carried out using SPSS version 16.0 (2007, SPSS for Windows, SPSS Inc., Chicago, IL, USA) package. The quantitative data normally distributed was presented as mean±1 standard deviation (SD). The qualitative data was expressed by calculating the frequency and percentage. P value of less than 0.05 (p<0.05) was considered to be significant. Chi-square test was used to analyse significant association of categorical variables. Independent sample t-test and paired sample t-test were used for analysis of parametric variables. Receiver operative characteristic curves were plotted for the determination of cut off values of biochemical parameters that were significantly different among breast cancer and healthy women.
Results
Anthropometric measures of Sri Lankan breast cancer patients are illustrated in Table 1. Nearly one third (30.3%) of newly diagnosed breast cancer patients were overweight and 45% obese. BMI was significantly higher (p<0.05) among breast cancer patients in comparison to apparently healthy. Nearly 72% of breast cancer patients had their waist circumference above the proposed cut-off value (>80 cm) for Asia-Pacific region (Nestel et al., 2007) and was significantly higher than apparently healthy (p <0.05). In addition, 88.7% of breast cancer patients were at risk with respect to waist: hip ratio which was also significantly higher (p<0.05) when compared with healthy women. Eighty nine percent (89%) of diseased women had mid upper arm circumference (MUC) above the reference range (24 cm) which was significantly higher (p<0.05) than in healthy women. Sri Lankan females with BMI > 25 kg/m2, WC> 80 cm, W: H > 0.88 and MUC > 24 cm were found to have 4.5 (CI 2.04-10.03), 2.6 (CI 1.22-5.63), 1.8 (CI 1.34-4.78) and 1.5 (CI 1.73-3.09) times odds of having breast cancer among the study sample.
Table 1.
Anthropometric Parameters of Healthy Women and Breast Cancer Patients
| Parameters | Women with breast cancer n= 155 | Healthy females n= 75 |
|---|---|---|
| Weight (kg) | 57 ±11a | 54 ±7a |
| Height (m) | 1.51 ±0.1b | 1.51 ±0.1b |
| BMI (kg/m2) | 25.1 ±4.4c | 23.6 ±2.5d |
| WC (cm) | 83.5 ±13.5e | 80.0 ±6.6f |
| HC (cm) | 94 ±10.7g | 92 ±7.2g |
| W:H ratio | 0.89 ±0.1h | 0.87 ± 0.1i |
| MAC (cm) | 28.5± 4.2h | 26 ±2.7i |
Different superscripts along a row indicate significant differences at 95% confidence interval; BMI, body mass index; WC, Waist circumference; HC, Hip circumference; W:H, Waist to hip ratio; MAC, Mid upper Arm Circumference.
The lipid profiles of the breast cancer and apparently healthy females are illustrated in Table 2. Majority (67%) of Sri Lankan breast cancer patients were postmenopausal. Eighty one percent (n=126) of women with breast cancer were unaware of their serum lipid profile values. When tested 76% (n= 96) of them had serum total cholesterol (TC) levels above 200 mg/dL of which 41% (n=52) had TC above 240 mg/dL. Nearly one fifth (19%, n=29) of women with breast cancer was on cholesterol lowering drugs and were excluded from the analysis.
Table 2.
Serum Lipid Profile of Breast Cancer and Healthy Women
| Test (Reference range- mg/dL) | Breast cancer patients without CLD (n= 126) | Breast cancer patients with CLD (n= 29) | Healthy females (n= 75) | |||
|---|---|---|---|---|---|---|
| Pre (n=50) | Post (n=76) | Pre (n=8) | Post (n= 21) | Pre (n=30) | Post (n=45) | |
| 1TC (< 200) | 226±40a | 231±48a | 167±43b | 203±63c | 178±23b | 194±19b |
| 1LDL-C (< 100) | 158±34c | 161±44c | 100±37d | 133±52d | 111±23d | 114±23d |
| 1HDL-C (> 40) | 43±10e | 42±12e | 43±7.7e | 45±12e | 44±8.3e | 45±7.2e |
| 1TG (<150) | 122±58f | 139±64f | 117±71f | 122±74f | 108±45f | 123±13f |
| 1TC:HDL-C (< 5) | 5.5±1.4g | 5.8±1.8g | 3.9±1.2h | 4.8±1.8h | 4.2±0.9h | 4.2±0.7h |
CLD, Cholesterol lowering drugs; Pre, Premenopausal; Post, Postmenopausal; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High density lipoprotein cholesterol; TG, Triglycerides; TC:HDL-C, Total cholesterol: high density lipoprotein cholesterol; 1Third Report of the National Cholesterol Education Programme (Adult Treatment Panel III), 2002; Different superscripts along a row indicate significant differences at 95% confidence interval.
When considering the premenopausal breast cancer patients 40% had serum total cholesterol between 200-239 mg/dL and 36% had values above 240 mg/dL. Among postmenopausal patients 78% were unaware of their serum lipid profiles, of which 76% had serum cholesterol above 200 mg/dL of which 43% had total cholesterol above 240 mg/dL.
Serum TC, LDL-C and TC: HDL-C ratio of breast cancer patients those who were not on cholesterol lowering drugs were significantly elevated (p<0.05) than apparently healthy women (Table 2). These patients’ TC, LDL-C and TC: HDL-C were not significantly different according to the menopausal status (p>0.05). Even among the breast cancer patients on cholesterol lowering drugs, postmenopausal women had significantly higher levels (p<0.05) of TC, LDL-C, TG and TC: HDL-C when compared to premenopausal women on drugs. However, lesser number of premenopausal breast cancer patients was on cholesterol lowering drugs when compared with postmenopausal breast cancer patients.
ROC curves were used to determine the cutoff values for TC, LDL-C and TC: HDL-C to ascertain the risk in comparison to apparently healthy women in Sri Lanka. All three parameters interpreted results accurately with over 72% significance (p<0.005) and significant confidence intervals. According to the ROC curve, the cutoff value for TC for the study sample was 203 mg/dL with 66% sensitivity and 86% specificity. Considering the age at diagnosis of the breast carcinoma, majority of women were in the age category of 51-60 years with the highest average serum lipid parameters followed by women between 41-50 years (Table 3).
Table 3.
Average Lipid Profile Values of Breast Cancer Patients at Diagnosis of Breast Cancer (Excluding Patients on Cholesterol Lowering Drugs)
| Lipid parameter (mg/dL) | Age category (years) | ||||
|---|---|---|---|---|---|
| 31-40 (n=14) | 41-50 (n=33) | 51-60 (n=36) | 61-70 (n=29) | 71-80 (n=14) | |
| TC | 236 ± 60 | 223 ± 34 | 254 ± 41 | 236 ± 43 | 233 ± 49 |
| HDL-C | 40 ± 12 | 43 ± 8 | 41 ± 11 | 47 ± 13 | 42 ± 10 |
| LDL-C | 170 ± 50 | 156 ± 32 | 183 ± 39 | 163 ± 13 | 166 ± 45 |
| VLDL-C | 28 ± 19 | 25 ± 10 | 32 ± 17 | 27 ± 9 | 26 ± 8 |
| TG | 139 ± 92 | 127 ± 51 | 158 ± 84 | 133 ± 47 | 129 ± 42 |
| TC: HDL-C | 6.2 ± 1.9 | 5.3 ± 1.1 | 6.6 ± 1.9 | 5.2 ± 1.1 | 5.8 ± 1.6 |
TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High density lipoprotein cholesterol; VLDL-C, Very low density lipoprotein cholesterol; TG, Triglycerides; TC:HDL-C, Total cholesterol: high density lipoprotein cholesterol.
The mean age of menopause of breast cancer patients in the study sample was 50±3 years and women in the age category of 51-60 years had noticeably higher mean values of TC, LDL-C, TG and TC: HDL-C.
Majority of breast cancer patients (84%) did not engage in any physical exercise prior to the diagnosis of the disease. They were of the belief that the routine household activities provide adequate exercise per day. Only 16% of breast cancer patients exercised. Jogging or walking was the commonest exercises among patients as well as healthy women. Majority (95%) was unaware of the fact that exercises could reduce the risk of breast cancer.
Discussion
The common age categories of diagnosis of breast carcinoma among premenopausal and postmenopausal women were 41-50 and 51-60 years respectively and the mean age category at diagnosis was 51-60 years. This observation is compatible with the age group reported in Western countries for breast carcinoma (Anders et al., 2009; Brandt et al., 2015). Accordingly the highest breast cancer incidence diagnosis in pre- and postmenopausal women was a decade before and after the mean age of menopause respectively. Breast cancer incidence is found to increase with the age (Barginear et al., 2014). Caucasian women have the highest breast cancer incidence when 40 years or older and 50-59 age group is more vulnerable to breast cancer (DeSantis et al., 2014). Present study findings are also in agreement with above findings.
Women with BMI above 23 kg/m2 and 25 kg/m2 were 1.5 and 4.5 times more likely to have breast cancer respectively. This could be due to adiposity related secretion of oestrogen and other hormones that could stimulate the breast cell growth. Waist circumference and waist: hip of breast cancer patients were significantly higher (p<0.05) than apparently healthy females. Majority of breast cancer patients were in the risk category with respect to waist circumference (72%) and waist: hip ratio (88.7%) according to the proposed cut-off values for Asia-Pacific region (Misra et al., 2006). Waist: hip ratio is a significant predictor of breast cancer risk in Nigerian women (Okobia et al., 2005) with a two and half (2.5) fold increased risk in premenopausal and a 2 fold increased risk in postmenopausal women. According to current study results 2.6 (CI 1.22-5.63) fold increased risk is found in breast cancer patients when having high waist circumferences irrespective of menopausal status and 1.8 (CI 1.34-4.78) fold risk when having elevated waist: hip ratios. Hence risk related to central obesity cannot be considered negligible with respect to breast cancer among both pre and postmenopausal women.
Women with breast cancer had significantly high mid upper arm circumferences than apparently healthy (p<0.05). Women in the study sample having a mid upper arm circumference above 24 cm were at 1.5 times risk. This is the first study in Sri Lanka that reports a significantly high mid upper arm circumference among women with breast cancer. In parallel, an Indian study revealed the presence of statistically higher mid upper arm circumferences among breast cancer patients when compared with the controls (Singh et al., 2011).
Breast density is a risk factor for breast cancer (Boyd et al., 2010; Bertrand et al., 2013). In addition, excess weight gain and higher breast mass might make it difficult to detect the easily curable breast carcinomas at early stages. Among the study sample, mid upper arm circumference of women is significantly associated with weight (r=0.695, p=0.000) and BMI (R=0.674, P=0.000). Thus considerable awareness is required in maintaining healthy anthropometric measures to lower the risk associated with breast cancer.
Total cholesterol (TC) concentration, LDL-C and TC: HDL-C ratio of lipid profiles were significantly higher (p<0.05) among breast cancer patients than apparently healthy females irrespective of menopausal status. However, majority (n= 126, 81%) of patients was unaware of high serum lipid parameters (TC >200 mg/dL) which had not been reported earlier in Sri Lanka in breast cancer patients. Some studies reveal inverse association between total cholesterol and risk of breast cancer (Touvier et al., 2015). However, some report, similar results to what was observed in the present study (Abu-Bedair et al., 2003; Llaverias et al., 2011) and in addition regard plasma cholesterol to be an important determinant involved in the control of breast cancer (Llaverias et al., 2011). A protective effect of cholesterol in relation to breast cancer (Llanos et al., 2012) was not observed in the present study sample. According to receiver operative characteristic (ROC) curve women with total cholesterol above 203 mg/dL had 12 (CI 5.43- 25.91) times odds of developing breast cancer. Proliferating cells, such as cancer cells have an increased requirement for cholesterol (Baenke et al., 2013) which may lead to increased synthesis in the body. Thus it would be beneficial for women above 40 years to have regular health screening to minimize the risk.
LDL transport cholesterol from the liver to peripheral tissues (Hegele, 2009) and is found to promote breast cancer cell proliferation and migration (Santos et al., 2014). LDL-C of breast cancer patients in the present study sample were significantly elevated (p<0.05) and clearly supports the above theory. Women having LDL-C concentrations above 139 mg/dL were 27 times more likely to have breast cancer with 64% sensitivity and 94% specificity. These results are parallel to a study that report high LDL-C levels promote breast cancer progression (Santos et al., 2014) and oppose the findings that low plasma levels of LDL-C increase the risk of breast carcinoma (Benn et al., 2011). Moreover, a woman having TC: HDL-C ratio above 3.9 (cut off point with 82% sensitivity and 44% specificity) has 3.3 times odds of having breast carcinoma (CI 1.75-6.34). According to the current guidelines it is recommended that pre and postmenopausal women ideally maintain TC: HDL-C less than 3.1 and 3.7 respectively. As such 100% and 95% of pre and postmenopausal BC women in the study were in the risk category indicating that these parameters even though are nonspecific to breast cancer, are good parameters for the risk assessment.
Irrespective of not being involved in regular physical exercise breast cancer patients (84%) in the study sample had HDL-C concentrations within the normal reference range with a mean value of 43±10 mg/dL. Also HDL-C of women with breast cancer was not significantly different when compared with healthy females. Though a significant increase in breast cancer risk with low HDL-C levels are reported (Llanos et al., 2012), present study findings do not support the fact. Even though strong association of triglyceride levels and breast cancer risk is reported among Indian population (Kapil et al., 2013) neither the serum triglyceride concentrations nor VLDL-C of studied breast cancer patients were significantly different (p>0.05) compared with disease condition or menopausal status irrespective of 75% of breast cancer patients having a BMI ≥23. Assessing serum lipid profiles regularly, engaging in physical exercise to increase HDL-C and also knowledge on genetic susceptibility to hypercholesterolemia (so that pharmacological interventions could be taken as necessary) are some steps that could be beneficial in reducing the risk of developing BC.
Among the study population majority was either overweight or obese. Among anthropometric parameters WHR was a better parameter than BMI, WC, HC or MUC. Minority was involved in regular exercises yet had elevated anthropometric parameters. Majority of the breast cancer patients (95%) were aware that regular exercises could reduce the cancer risk including breast cancer even though not engaging in exercise. Thus it can be recommended that irrespective of the menopausal status all women should maintain healthy normal BMI, WC and W: H ratios and engage in regular physical exercises to reduce the burden of breast cancer risk.
In conclusion, among the studied Sri Lankan women, the highest breast cancer incidence was found among the women in the age category of 51-60 years with highest average lipid parameters. A Sri Lankan woman having TC, LDL-C and TC: HDL-C above 203 mg/dL, 139 mg/dL and 3.9 is at a higher risk of developing breast cancer irrespective of menopausal status. Among the lipid parameters LDL-C had the highest risk. However, no impact of HDL-C, triglyceride or VLDL-C in breast cancer was observed. Elevated BMI, W: H ratio and MUC were significant risk factors among anthropometric measures in Sri Lankan breast cancer patients while having a BMI above 25 kg/m2 showed the highest risk.
List of Abbreviations
BC = Breast Cancer, TC= Total Cholesterol, HDL-C= High density lipoprotein cholesterol, LDL-C = Low density lipoprotein cholesterol, VLDL-C = Very low density lipoprotein cholesterol, TG= Triglyceride (TG), W= Weight, H= Height, WC= Waist circumference, HC = Hip circumference, MUC= Mid upper arm circumference, ROC curve= Receiver operative characteristic curve
Declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from Ethics review committee of Faculty of Medical Sciences, University of Sri Jayewardenepura, Sri Lanka (Approval No 651/12, 28/14). The participation of both patients and healthy women were voluntary and informed written consent was obtained prior to the commencement of the study.
Statement conflict of Interest
Authors declare that they have no conflicts of interest.
Acknowledgements
This work was financially supported by grants ASP/06/RE/MED/2012/20 and ASP/ 06/RE/MED/ 2013/ 30 of University of Sri Jayewardenepura, Sri Lanka.
The newly diagnosed breast cancer patients, apparently healthy age matched females, medical officers and nursing staff of National Cancer Institute, Maharagama, Sri Lanka who contributed to the study are gratefully acknowledged.
References
- Abdelsalam Kamal EA, Ikhlas KH, Isam AS. The role of developing breast cancer in alteration of serum lipid profile. J Res Med Sci. 2012;17:562–5. [PMC free article] [PubMed] [Google Scholar]
- Abu-Bedair Ahmed F, El-Gamal BA, Ibrahim NA, El-Aaser AA. Serum lipids and tissue DNA content in Egyptian female breast cancer patients. Jpn J Clin Oncol. 2003;33:278–82. doi: 10.1093/jjco/hyg059. [DOI] [PubMed] [Google Scholar]
- Ahmadin M, Samah AA. A literature review of factors influencing breast cancer screening in Asian countries. Life Sci. 2012;9:585–94. [Google Scholar]
- Al-Swelmien Ali. Serum lipid profile in breast cancer patients. Rawal Med J. 2014;39:254–6. [Google Scholar]
- Anders Carey K, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–49. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenke F, Peck B, Miess H, Schulze A. Hooked on fat:The role of lipid synthesis in cancer metabolism and tumour development. DMM. 2013;6:1353–63. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barginear MF, Muss H, Kimmick G, et al. Breast cancer and aging:Results of the U13 conference breast cancer panel. Breast Cancer Res Treat. 2014;146:1–6. doi: 10.1007/s10549-014-2994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn M, Hansen AT, Stender S, et al. Low-density lipoprotein cholesterol and the risk of cancer:A mendelian randomization study. JNCI. 2011;103:508–19. doi: 10.1093/jnci/djr008. [DOI] [PubMed] [Google Scholar]
- Bertram Cadmus LA, Marcia L Stefanick, Saquib N, et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors:Findings from the WHEL study. Cancer Causes Control. 2011;22:427–35. doi: 10.1007/s10552-010-9714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand Kimberly A, Tamimi RM, Scott CG, et al. mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013;15:R104. doi: 10.1186/bcr3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd Norman F, Lisa J Martin, Bronskill M, et al. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102:1224–37. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Garne J, Tengrup I, Manjer JE. Age at diagnosis in relation to survival following breast cancer:A cohort study. World J Surg Oncol. 2015;13:33. doi: 10.1186/s12957-014-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, McCarron P, D Maxwell P. The changing global patterns of female breast cancer incidence and mortality. BCR. 2004;6:229–39. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Hoorn SV, Lopez AD, et al. Causes of cancer in the world:Comparative risk Assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–93. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- DeSantis C, Jiemin M, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- Fiorenza AM, Branchi A, Sommariva D. Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int J Clin Lab Res. 2000;30:141–5. doi: 10.1007/s005990070013. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM. Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev. 2001;10:15–32. doi: 10.1097/00008469-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Furberg Sofie A, Jasienska G, Bjurstam N, et al., editors. Metabolic and hormonal profiles:HDL cholesterol as a plausible biomarker of breast cancer risk. The norwegian EBBA study metabolic and hormonal profiles:HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. 2006;2005:33–40. [PubMed] [Google Scholar]
- Hegele Robert A. Plasma lipoproteins:Genetic influences and clinical implications. Nat Rev Genet. 2009;10:109–21. doi: 10.1038/nrg2481. [DOI] [PubMed] [Google Scholar]
- Horn J, Alsaker MDK, Signe O, et al. Anthropometric factors and risk of molecular breast cancer subtypes among postmenopausal Norwegian women. Int J Cancer. 2014;135:2678–86. doi: 10.1002/ijc.28912. [DOI] [PubMed] [Google Scholar]
- Kapil U, Bhadoria AS, Sareen N, et al. Total cholesterol and triglyceride levels in patients with breast cancer. J Breast Cancer. 2013;16:129–30. doi: 10.4048/jbc.2013.16.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katulanda P, Priyanga R, Jayawardana R, et al. Metabolic syndrome among Sri Lankan adults:prevalence, patterns and correlates. Diabetol Metab Syndr. 2012;4:24. doi: 10.1186/1758-5996-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos Adana A, Makambi KH, Cynthia A Tucker. Cholesterol, lipoproteins, and breast cancer risk in African American Women. Ethn Dis. 2012;22:281–7. [PMC free article] [PubMed] [Google Scholar]
- Llaverias G, Danilo C, Mercier I, et al. Role of cholesterol in the development and progression of breast cancer. Am J Pathol. 2011;178:402–12. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra A, Vikram NK, Gupta R, et al. Waist circumference cutoff points and action levels for Asian Indians for identification of abdominal obesity. Int J Obes. 2006;30:106–11. doi: 10.1038/sj.ijo.0803111. [DOI] [PubMed] [Google Scholar]
- National Cancer Control Programme. Sri Lanka 2008: Cancer Incidence Data; 2007. [Google Scholar]
- Nestel P, Lyu R, Low LP, Sheu WHH, Nitiyanant W. Metabolic syndrome:Recent prevalence in East and Southeast Asian populations. Asia Pac J Clin Nutr. 2007;16:362–7. [PubMed] [Google Scholar]
- Okobia Michael N, Bunker CH, Zmuda JM, et al. Anthropometry and breast cancer risk in Nigerian women. Breast J. 2005;12:462–6. doi: 10.1111/j.1075-122X.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- Potischman N, McCulloch CE, Byers T, et al. Associations between breast cancer, plasma triglycerides, and cholesterol. Nutr Cancer. 1991;15:205–15. doi: 10.1080/01635589109514128. [DOI] [PubMed] [Google Scholar]
- Santos D, Rodrigues C, Domingues G, et al. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Kapil U, Shukla NU, Deo S, Dwivedi S. Association of overweight and obesity with breast cancer in India. Indian J Community Med. 2011;36:259–62. doi: 10.4103/0970-0218.91326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehard B, Chapelon CF. Several anthropometric measurements and breast cancer risk:Results of the E3N cohort study. Int J Obes. 2005;30:156–63. doi: 10.1038/sj.ijo.0803133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touvier M, Fassier P, His M, et al. Cholesterol and breast cancer risk:A systematic review and meta-analysis of prospective studies. Br J Nutr. 2015;2015:1–11. doi: 10.1017/S000711451500183X. [DOI] [PubMed] [Google Scholar]
- Ulmer Borena H, Rapp W, Klenk J, Strasak A, et al. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer. 2009;101:1202–6. doi: 10.1038/sj.bjc.6605264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirén S, Häggström C, Ulmer H, Manjer J, et al. Pooled cohort study on height and risk of cancer and cancer death. Cancer Causes Control. 2014;25:151–9. doi: 10.1007/s10552-013-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


