Abstract
Purpose:
Glioblastoma multiform (GBM) is the most aggressive glial neoplasm. Researchers have exploited the fact that GBMs are highly vascularized tumors. Anti-angiogenic strategies including those targeting VEGF pathway have been emerged for treatment of GBM. Previously, we reported the anti-inflammatory effect of atorvastatin on GBM cells. In this study, we investigated the anti-angiogenesis and apoptotic activity of atorvastatin on GBM cells.
Methods:
Different concentrations of atorvastatin (1, 5, 10µM) were used on engineered three-dimensional (3D) human tumor models using glioma spheroids and Human Umbilical Vein Endothelial cells (HUVECs) in fibrin gel as tumor models. To reach for these aims, angiogenesis as tube-like structures sprouting of HUVECs were observed after 24 hour treatment with different concentrations of atorvastatin into the 3-D fibrin matrix and we focused on it by angiogenesis antibody array. After 48 hours exposing with different concentrations of atorvastatin, cell migration of HUVECs were investigated. After 24 and 48 hours exposing with different concentrations of atorvastatin VEGF, CD31, caspase-3 and Bcl-2 genes expression by real time PCR were assayed.
Results:
The results showed that atorvastatin has potent anti-angiogenic effect and apoptosis inducing effect against glioma spheroids. Atorvastatin down-regulated the expression of VEGF, CD31 and Bcl-2, and induced the expression of caspase-3 especially at 10µM concentration. These effects are dose dependent.
Conclusion:
These results suggest that this biomimetic model with fibrin may provide a vastly applicable 3D culture system to study the effect of anti-cancer drugs such as atorvastatin on tumor malignancy in vitro and in vivo and atorvastatin could be used as agent for glioblastoma treatment.
Keywords: Angiogenesis, apoptosis, glioblastoma, VEGF, caspase-3, atorvastatin
Introduction
Brain cancers are composed of many interacting biotic (microglia, stem cells, astrocytes, endothelial cells, cancer cells) and abiotic (extracellular matrix [ECM], cytokines, growth factors) elements (Pong and Gutmann, 2011). The tumor microenvironment (TME) plays a critical role in tumor initiation, angiogenesis, cell proliferation, apoptosis, progression and responses to therapy (Quail and Joyce, 2013). Glioblastoma (GBM) is grade IV glioma, the most common adult brain tumor and the most aggressive glial neoplasm, which despite advances in medical management, the outcomes remain quite poor and median survival time of patients is only 6 months to 2 years (Arvold and Reardon, 2014). Researchers have exploited the fact that GBMs are highly vascularized tumors, this phenomenon makes development of anti-angiogenic therapies feasible (Lu and Bergers, 2013). Tumor growth is strongly dependent on the formation of new blood vessels that infiltrate the growing mass of tumor cells by providing the oxygen, nutrients and taking away metabolites (Dulak and Józkowicz, 2005). Without the supply of new blood vessels, the size of a tumor can only reach a volume of about 2 mm3 in animal tumor models. As diffusion of oxygen can occur at the distance of only 100–200 μm (Dulak and Józkowicz, 2005; Heymans, 1999). The cells at its core start to accumulate hypoxia inducible factors (HIFs) such as HIF-1α by experiencing hypoxia and nutrient deprivation, which triggers a phenotypic transition known as the angiogenic switch (Song et al., 2014; Bergers and Benjamin, 2003; Chung et al., 2010). Activation of the pathway leads to overexpression of cytokines, growth factors, and other soluble factors, such as vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8), to the microenvironment that breaks the balance between pro- and anti-angiogenic factors (Fischbach et al., 2007; Weis and Cheresh, 2011). This dysregulated cascade ultimately recruits new blood vessels to the tumor site (Chung et al., 2010). In 1971, Folkman proposed that the growth of tumors depends on angiogenesis (Folkman, 1971). This hypothesis catalyzed the development of anti-angiogenic therapy (Scribner et al., 2014). VEGF-A, a critical mediator of angiogenesis, is highly expressed in glioblastoma and regulates tumor angiogenesis, which its expression is significantly enhanced or induced by numerous mediators, including hypoxia, inflammatory cytokines, other growth factors, such as basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) (Dulak and Józkowicz, 2005; Xie et al., 2004). Preclinical studies have shown that VEGF inhibitors inhibit the growth of glioma cells (Gerstner et al., 2009). Anti-angiogenic agents, and particularly drugs that target the VEGF pathway, are increasingly being incorporated into treatment regimens (Elizabeth et al., 2012).
Statins are widely used as lipid-lowering agents to reduce cardiovascular risk with a favorable safety profile. The recent demonstration that several statins, inhibitors of 3-hydroxy-3-methylglutrayl coenzyme A (HMG-CoA) reductase, has possible application in anti-cancer therapy by influencing angiogenesis and inhibiting experimental tumor growth (Dulak and Józkowicz, 2005). The effects of statins were found to depend on their blood concentration (Weis et al., 2002). It has been shown that lovastatin, simvastatin, atorvastatin, fluvastatin and cerivastatin markedly reduced viability of cultured rat pulmonary vein endothelial cells leading to apoptosis (Kaneta et al., 2003).
Several experimental cancer models have shown that statins prevent cell proliferation and exert pro-apoptotic properties in various cells, including endothelial cells, an effect associated with decreased tumor vascularization (Dulak and Józkowicz, 2005; Chwalek et al., 2014; CIOFU, 2012). Some studies have shown that the anti-angiogenic effect of statins is related to the induction of endothelial cell apoptosis (Ghavami et al., 2010). Proposed mechanisms for statin-mediated apoptosis include an up-regulation of pro-apoptotic protein expression (e.g., Bax, Bim), combined with decreased anti-apoptotic protein expression (e.g., Bcl-2) (Wood et al., 2013). Statins have also been shown to activate caspase proteases involved in programmed cell death. To address these issues, investigators in the field of cancers are now utilizing three- dimensional (3D) culture models that are prevalent in the field of tissue engineering, which has allowed researchers to design systems that mimic the physiological cell–cell and cell–ECM interactions of a variety of tissue types (Song et al. 2014; Bian et al., 2013). For creating 3D in vitro models of tissues, hydrogels are used as hydrophilic polymeric networks. Variety of natural and synthetic hydrogel materials have been employed as 3D artificial tumor microenvironments to create engineered microenvironments for supporting cancer cell growth and angiogenesis, that can provide mechanical support and chemical structures of the cellular microenvironment while regulating tumor behaviors within the matrix (Drury and Mooney, 2003; Ko et al., 2013). Fibrinogen is a large glycoprotein found in plasma that plays a critical role in blood clotting, fibrinolysis, cellular and matrix interactions, inflammatory response, wound healing, and neoplasia (Mosesson, 2005). Fibrin hydrogels have been widely used as an artificial microenvironment because they have a nano/macro fibrous architecture that mimics the native ECMs (Liu et al., 2012). The aim of the present study was to determine the potential inhibitory effect of atorvastatin on angiogenesis and apoptosis in a three-dimensional fibrin matrix co- culture of human umbilical vein endometrial cells with glioblastoma spheroids in vitro as a possible glioblastoma 3D model. We focused on the mediators of angiogenesis by angiogenesis antibody array and real-time PCR analysis.
Materials and Methods
Cell cultures
A human U87 human primary glioblastoma cell line was purchased from the Cell Bank of the Pasteur Institute, Tehran, Iran and were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (complete medium). The U87 glioma cells were used in the first five passages after recovery. Human Umbilical Vein Endothelial cells (HUVECs) were acquire from the National Cell Bank, Pasteur Institute of Iran and were cultured in full RPMI-1640 media containing 1% antibiotics (Penicillin/Streptomycin, Gibco) and 10% FBS (Gibco) at second or third passages. Cells were cultured in an incubator with 5% CO2 and saturated humidity at 37°C.
Three-Dimensional in vitro tumor angiogenesis assays
Generally, three-dimensional in vitro tumor angiogenesis model involve the HUVECs seeded on top of the fibrin gel with encapsulated glioma spheroids inside the fibrin gel as describe below:
Glioma spheroids formation in 3D culture medium
The U87 cell line was cultured in non-adherent plate. Briefly, the cells were washed with PBS, added cell dissociation enzyme and incubated at 37°C for 2-5 min after that cell suspension was centrifuged and removed supernatant and cell pellet re-suspended in 1ml of complete growth medium. The cell suspension was transferred to a sterile non-adherent 8-well plate and incubated at 37°C, 5% CO2, 95% humidity. Tumor spheroid formation was confirmed visually 4 days later. Cultures were performed in 24-well culture plate; 0.5 mL of a solution of fibrinogen (3 mg/mL in M199 solution (Sigma, USA)) was added to each well and mixed with 15 μM of thrombin (50 National Institutes of Health U/mL in 0.15 M NaCl (Sigma, USA)) and 50μl of FBS. Glioblastoma spheroids were placed in the center of the wells after 1h to form a 3D network structure and were covered by an additional 0.5 mL per well of the fibrinogen–thrombin solution, to hold them at the same level between the two clots. After gel formation, 1 mL per well of Medium 199, supplemented with 5% FBS, and antibiotics (streptomycin, 50 mg/mL; penicillin, 50 IU/mL) added. Explants were cultured at 37°C in 5.5% CO2 and 95% humidity for 24 h. Spheroids explants were observed and photographed with a phase-contrast microscope (Olympus, Tokyo, Japan). Tissue cultures were divided into four groups on the basis of the concentrations of atorvastatin in the culture media: (Arvold and Reardon, 2014) 1 μM, (Lu and Bergers, 2013) 5 μM, (Dulak and Józkowicz, 2005) 10 μM and (Heymans, 1999) the control group.
Endothelial tube formation assay (in vitro angiogenesis)
Angiogenic activities of HUVECs were stimulated with a starving medium (a medium without growth factors or serum) according to the protocol (Francescone et al., 2011): 1) starve the HUVECs for 3-6 h just prior to performing the tube formation assay in Medium 200PRF without antibiotics 2) trypsinize to dislodge the cells from the surface of the flask, determine the concentration of HUVECs by counting the cells, and resuspend HUVECs at 4 x 105 per ml serum-free DMEM by pipetting several times to ensure a homogeneous single-cell suspension 3) thaw a 0.5 ml aliquot of the CM of target cell line collected before and supplement it with FBS to a final concentration of 1% for resuspending the HUVECs pellet(s) in previous step 4) Co-culture of HUVECs with glioma spheroids, HUVECs were layered on top of the prepared fibrin gel with encapsulated glioma spheroids in 24-well culture plate, incubate the plate at 37°C, 5% CO2 for 24 h) visualize the cells by phase-contrast microscopy (Olympus BX61; Olympus Corporation, Tokyo, Japan). Atorvastatin was provided from Sigma Aldrich and was dissolved in sterile DMSO (stock 10mM) and used in vitro, the final concentration of DMSO in any media did not exceed 0.1%. After tube formation, to investigate the effect of atorvastatin on angiogenesis and apoptosis, co-cultured HUVECs with encapsulated glioma spheroids in fibrin gel in 24-well plate were exposed to atorvastatin at 1 µM, 5 µM and 10 µM concentrations for 48h and the cell samples without atorvastatin were considered as control. capillary network and tube lengths were seen under a phase-contrast microscope (Olympus BX61; Olympus Corporation, Tokyo, Japan) after 24 h and 48 h.
MTT assay for cell proliferation
The effect of atorvastatin on the proliferation of HUVECs was determined by the MTT assay (thiazolyl blue tetrazolium bromide; Sigma). Briefly, HUVECs in fibrin gel were planted in 96-well plates. Atorvastatin was added into the medium in different concentrations for 48 h. Cells were added with 100 μl of 5 mg/ml MTT solution (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Sigma)) and cultured for another 4 h then the insoluble purple formazan product was dissolved by dimethyl sulfoxide (DMSO). The absorbance under 490 nm and 570 nm wave length was detected by a microplate reader (EL340 Bio-Tek Instruments, Hopkinton, MA). All determinations were performed in quadruplicate and each experiment was repeated at least three times.
Immunocytochemistry analysis
The co-cultured HUVECs for CD31 antibody as an endothelial cells marker were fixed with 4% paraformaldehyde (PFA (Sigma-Aldrich)) for 30 min at room temperature. The cells were permeabilized with 0.2% Triton-X 100 (Sigma-Aldrich) for 10 min, the cells were blocked with goat serum and incubated overnight at 4 °C with primary antibody CD31, mouse monoclonal anti-human (Abcam, 1:200), then to determine the specificity of immunofluorescence, labeled secondary antibody (Alexa Fluor@488 donkey anti mouse IgG, at a 1:500 dilution; Abcam, USA) was added for 2 h at room temperature in the dark. For negative controls, only the secondary antibody was used. After PBS washes, the nuclei of cells were stained by 4’,6-diamidino-2- phenylindole (DAPI, Sigma) in darkness for 5 min. The images were captured by fluorescence microscopy (Olympus BX51, Japan).
Scratch-wound healing assay
The wound healing assay was performed as a straightforward and economical method to study cell migration in vitro. HUVECs with a density of 2×105 were seeded in 6-well plates and grown in DMEM media containing all supplements with 10% FBS. After 24 h, confluent monolayers were gently scratched with a sterile 200μL tip to create a uniform wound area. After scratching, cells were incubated in fresh media with 1 μM, 5 μM and 10 μM of atorvastatin. Cell movement and migration into the wound area were examined at 0 and 48 h by phase-contrast microscopy using a 10x objective. The photographs were correlated and the number of cells migrated into the scratch were counted for quantitative analysis.
Determination of angiogenesis and apoptosis by quantitative real-time polymerase chain reaction
Total RNA was extracted from co-cultured HUVECs with encapsulated glioma spheroids in fibrin gel using an RNA Purification Kit (GeneJETTM Fermentas, Hungary), followed by treatment with a first strand cDNA Synthesis Kit for RT-qPCR (RevertAid, Thermo, Hungary). The purified RNA was reverse transcribed using SYBR Green qPCR Master Mix (Fermentas). Quantitative real-time PCR was performed using the kit according to the manufacturer’s instructions and the real time QRT-PCR of each cDNA sample was repeated in two individual runs, and the data from two separate experiments was taken for statistical analysis. The primers were designed using Primer 3 software and synthesized by Takara Biotechnology, and are listed in Table 1.
Table 1.
Primers Used for Real Time RT-PCR.
| Oligo | Oligo Sequence 5’---3’ | |
|---|---|---|
| VEGF | F | TAATCATTCCGAAGCAAGGTGTG |
| R | AGGTGCTAGCCATCTTATTCTTTCC | |
| CD31 | F | GAAATGTCCAGGCCAGCAGTAC |
| R | GTTTCTGACATCGTCATTGTGACC | |
| Bcl-2 | F | AAAATACAACATCACAGAGGAAGTAGACTG |
| R | TCAATCACGCGGAACACTTG | |
| Caspase-3 | F | AAAAGCACTGGAATGACATCTCG |
| R | GAAACATCACGCATCAATTCCAC | |
| GAPDH | F | CCTGCACCACCAACTGCTTAG |
| R | CAGTCTTCTGGGTGGCAGTGA |
Statistical analysis
All experiments were performed in three independent experiments and data reported as mean ± standard deviation (SD). Statistical significance was analyzed by using one-way analysis of variance (ANOVA) tests or unpaired Student’s t-test, P<0.05 was considered as a statistically significant difference. Statistical significance is expressed as ***P<0.001; **P<0.01; *P<0.05 in bar graphs.
Results
Vascular sprout formation and HUVECs co-cultures with glioblastoma spheroids
After adding HUVECs that were layered on top of the fibrin gel with encapsulated glioma spheroids (Figure 1), the HUVECs began to sprout and form short, narrow cord like structures in 1-2 days (Figure 1). Angiogenesis as tube-like structures sprouting into the 3-D fibrin matrix, the main cellular event, was observed during the 24 h of culture of HUVECs (Figure 1).
Figure 1.
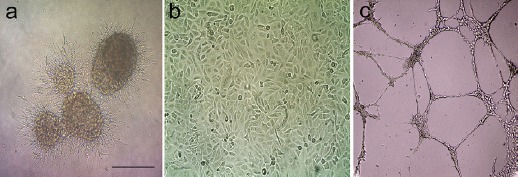
Morphology of Glioblastoma Spheroids and HUVECs. a) Encapsulated glioma spheroids inside the fibrin gel after 2 days. b) Morphology of HUVECs at passage 3 in 2D cultures. c) Representative phase contrast pictures (magnification 10×) of tube-like structures sprouting of HUVECs into the fibrin gel after 24 h as described in Materials and Methods. Scale bar=100 μm.
Atorvastatin inhibits morphogenesis of HUVECs
To investigate the effect of atorvastatin on angiogenesis in vitro, the tube formation test of HUVECs was performed with different concentration of atorvastatin. Tube formation and its inhibition is shown in Figure 2. Treatment by atorvastatin causes immense disruption of the capillary tubes network in contrast to untreated controls (Figure 2). The tubular structure in atorvastatin-treated wells was rather incomplete with fewer branch points and shorter tube length. Atorvastatin significantly reduced number of connected tubes specially at 5 μM and 10μM significantly reduced number of connected tubes, and total tube length compared to untreated controls (Figure 2). To address anti-angiogenic effect of atorvastatin, PECAM-1-specific antibody was used for immunostaining assay in co-cultured HUVECs with encapsulated glioma spheroids in fibrin gel (Figure 2a). As expected, expression of PECAM-1 protein at single cell level in treated group decreased compared untreated group (Figure 2a). Immunocistochemical staining demonstrated reduced tub formation in treated group compared with untreated group. There is also a significant difference between 1 µM and untreated group (Figure 2a). These data demonstrated that atorvastatin effectively inhibited HUVECs’ tube formation in a dose-dependent manner. This observation suggests that atorvastatin has an inhibitory effect on angiogenesis.
Figure 2.
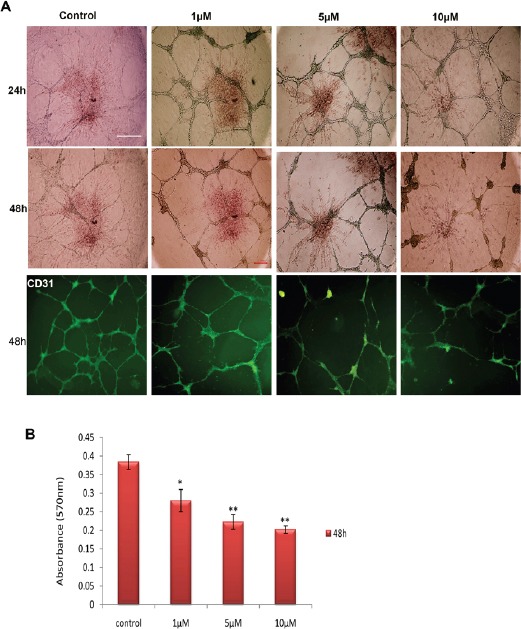
Effect of Different Concentrations of Atorvastatin on Tube Formation and Cell Proliferation. a) Dose dependent effect of atorvastatin on proliferation of the tubular structure. Atorvastatin at 5 μM and 10 μM significantly reduced number of connected tubes. Expression of PECAM-1 protein demonstrated reduced tub formation in treated group specially at 5 and 10 µM. Scale bar is 100 μm. b) The MTT assay of HUVECs seeded on top of fibrin. The proliferation rate was inhibited by all concentration, especially at 5 and 10μM. Data are expressed as mean ± SD of three independent experiments. *P<0.05, **P<0.01.
Atorvastatin inhibits the proliferation of HUVECs
Cell viability was examined by MTT assay, which measures the cell metabolic activity. We managed the treatments with different concentrations of atorvastatin (1 µM, 5 µM and 10 µM) for 48 h. As shown in Figure 2b, atorvastatin inhibited the proliferation of HUVECs in a dose-dependent manner.
Atorvastatin inhibits the migratory ability of HUVECs
HUVECs were treated with different concentrations of atorvastatin for 0 and 48h after which we observed that the migration of HUVECs was inhibited by atorvastatin treatment (Figure 4) in a dose-dependent manner. Quantitative determination of the invaded area showed a significant inhibitory effect of atorvastatin specially at 10 μM (Figure 3).
Figure 4.
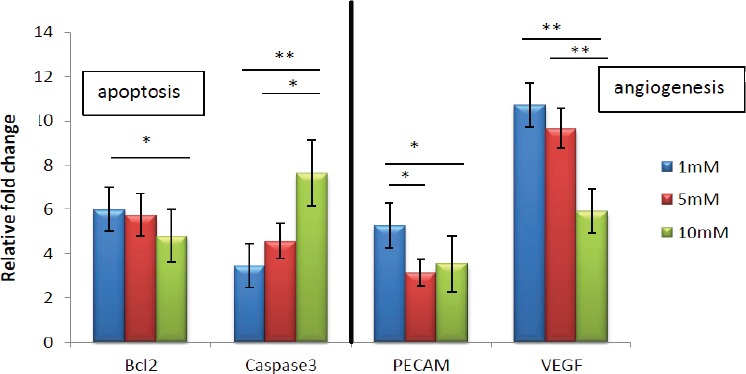
Real Time-PCR Analysis of the mRNA Levels of Angiogenesis Markers (VEGF and CD31), Pro- Apoptotic Gene (caspase-3), and Anti-Apoptotic Gene Bcl-2 in Co-Cultured HUVECs with Encapsulated Glioma Spheroids in Fibrin Gel that Treated with Atorvastatin in the Different Concentrations. Results are expressed as mean ± SD of three identical experiments made in three replicate followed by normalization with GAPDH protein amount. (*p < 0.05, ** p < 0.01, *** p < 0.001).
Figure 3.
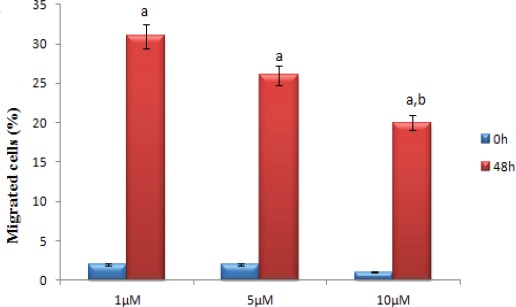
Effect of Atorvastatin on HUVECs Migration. Results are expressed as mean ± SEM (n=3). The groups normalized with control group and compared with each others, all groups in 48h were statistically significant compared with 0h(a ***p < 0.001) but the groups on 0h did not significance with each other. The groups on 48h statistically significance tow groups with each other (1µm compared with 10µm b *p < 0.05).
Inhibition of angiogenesis markers expression and induction of apoptosis in in-vitro glioblastoma model
After atorvastatin (1 μM ,5 μM and 10 μM) exposure, co-cultured HUVECs with encapsulated glioma spheroids in fibrin gel were lysed and markers of angiogenesis were screened using a real-time PCR. Atorvastatin treatment significantly down-regulated the expression of VEGF and CD31 (platelet endothelial cell adhesion molecule 1 [PECAM-1]), which are considered the main mediator of tumor angiogenesis (Figure 5). These markers are involved in mediating angiogenesis steps, including cell proliferation, tube formation and migration. Meanwhile the atorvastatin treatment induced upregulation in the levels of apoptotic marker expression such as caspase-3 specially at 10 μM (Figure 4). These markers play important roles in inhibition of endothelial tube formation, activation of anti-tumor agents, apoptosis and prevention of vascular growth.
Discussion
In this study, we showed that atorvastatin has potent anti-angiogenic effect and apoptosis inducing effect against glioma spheroids in 3D glioma model. Atorvastatin down-regulated the expression of VEGF, CD31 and Bcl-2, and induced the expression of caspase-3 especially at 10µM concentration. Angiogenesis has received considerable attention during the past few decades, in the hope that manipulating regulators of angiogenesis may provide biological therapies against disease processes that involve insufficient or exuberant vascularization in their pathogenesis (Yoo and Kwon, 2013). GBM is one of the most highly vascularized of all human tumors and, by virtue of increased abnormal endothelial cell proliferation and vessel structure, it provides us with an ideal model for studying tumor angiogenesis (Charalambous et al., 2006). However, most of the current in vitro glioma angiogenesis models are in two-dimensional (2D) cultures. It has sometimes been difficult to achieve results in animal studies that are similar to the results of 2D in vitro experiments. There is a growing consensus that three-dimensional (3D) in vitro angiogenesis assays offer a model which is much closer to the actual environment in vivo than that which can be achieved using 2D cultures (Chen et al., 2009; Nakatsu et al., 2003).
In the last two decades, the successful and widespread uses of statin drugs for hypercholesterolemia have revealed their potential anti-cancer effects (Boudreau et al., 2010). Although for all statins have been observed clear anti-cancer effects, the existing data suggest certain differences of individual statins in the anti-tumor effects (Jiang et al., 2014; Gbelcova et al., 2008). Recently, a study in Danish population with a diagnosis of cancer between 1995 and 2007 found that the use of statins is associated with reduced cancer-related mortality, with a reduction of up to 15% for the 13 cancer types examined (Nielsen et al., 2012). These data support reduced cancer incidence, recurrence, and mortality with the use of statins (Boudreau et al., 2010). The strong and consistent association of statins and lowered cancer risk has prompted 18 ongoing clinical trials in cancer (Clendening and Penn, 2012). In addition, statins are not primarily designed for cancer therapy, so the anti-cancer effects need to be carefully evaluated and compared before one can optimize the efficacy of statins as anti-cancer agents. The potency of atorvastatin in inhibition of angiogenesis and induction of apoptosis in glioblastoma 3D model has been investigated in our study. Understanding the link between statin and cell death in the form of apoptosis may help develop new approaches to cancer therapy. Recent studies have demonstrated that statins reduce cell migration in several cancers including glioma (Murai et al., 2011; Fromigue et al., 2008). This study found that atorvastatin slightly inhibited glioma cell migration. It should be mentioned inhibitory effect of atorvastatin on glioma migration processes at 1 μM ,5 μM and 10 μM concentrations (Esfandiari et al., 2007). Recent findings also have shown that atorvastatin has anti-tumor in several cancers including osteosarcoma and non-small-cell-lung carcinomas (NSCLCs) (Fromigue et al., 2008; Chen et al., 2012; Chen et al., 2012). The anti-tumor effects may include cytotoxicity, induction of and inhibitory effects on MMPs (Fromigue et al., 2008; Tapia-Pérez et al., 2011; He et al., 2012). We found that atorvastatin could induce cell apoptosis in a dose-dependent manner, atorvastatin could significantly reduce the expression of Bcl-2, an anti-apoptosis protein. The expression level of caspase-3 was up-regulated by atorvastatin. It is known that caspase-3 is death-promoting factors, whereas Bcl-2 protein is a death antagonist (Shu et al., 2014). Atorvastatin is potential chemo preventive agents for glioblastoma that can inhibit proliferation in glioblastoma, but the molecular mechanisms through which atorvastatin affects glioblastoma remain unclear (Kamat and Nelkin, 2005). In order to clarify the exact mechanisms of anti-tumor effect in glioblastoma, we investigated the influence of atorvastatin on the crucial proteins associated with angiogenesis and apoptosis. The results of this study indicated the usefulness of atorvastatin in efficient control of glioma.
In this study, atorvastatin reduced glioma angiogenesis and migration in 3D glioma model. Nehls et al., (1994) found that vascular smooth muscle cells encapsulated in 3D fibrin produce to elongated, capillary-like structures, whereas the same cells cultured in a 2D system displayed a stellate-shaped, flattened phenotype. 3D cultures will allow many more basic questions to be answered before having to turn to whole-animal research (Chen et al., 2009). Of course, to provide a superior test bed, such 3D systems should be reproducible and be able to mimic several of the major steps of angiogenesis (Chen et al., 2009). In this study we established a stable, reproducible, easily manipulated 3D human glioma-HUVECs co-culture system. Fibrin gels, which are comprised of hydrophilic cross-linked fibrils, are believed suitable for 3D cell culture (Eyrich et al., 2007). In the present study, HUVECs, a widely used experimental model for endothelial cells, were used to evaluate the anti-angiogenic mechanism of atorvastatin. Firstly, the HUVECs were identified by CD31, a marker of endothelial cells, CD31 specifically expresses in HUVECs, and the cell viability was evaluated by MTT assay to investigate the effect of atorvastatin. Co-culture of HUVECs with U87, T98, and NT2 cells indicated that the HUVECs co-cultures with U87 and T98 cells resulted in the vascular sprout formation, However, no vascular sprout formation and no produce VEGF were observed in HUVECs co-cultured with NT2 neuronal cells (Chen et al., 2009; Cao et al., 2001). Co-culture of HUVECs with U87 cells indicated the result in vascular sprout formation. These findings reinforce the idea that cellular secretion of growth factors such as VEGF appears to be needed to induce vascular sprouts. The addition of anti-VEGF antibody resulted in significant reduction of vascular sprout formation in both normoxic and hypoxic conditions. It is distinguished that tumors can regulate the responsiveness of associated endothelial cells by secreting multiple growth factors, cytokines, and mitogens (Chen et al., 2009; Khodarev et al., 2003). This study was performed to investigate the anti-tumor effect mechanism of atorvastatin on co-cultured HUVECs with encapsulated glioma spheroids in fibrin gel and also was investigated the possible mechanism of atorvastatin-induced inhibitory effect on VEGF-induced cell proliferation and tube formation. Results indicate that the atorvastatin has inhibitory effect on VEGF-induced cell proliferation and tube formation. Statins preserved the structure of the vascular wall, the effect ascribed to decreased expression of VEGF and hypoxia-inducible factor-1 (HIF-1) (Dulak and Józkowicz, 2005; Wilson et al., 2002). Interestingly, in vitro (Frick et al., 2003) and in vivo (Weis et al., 2002) experiments demonstrated the proangiogenic activity of statins, which occurs particularly at low, picomolar or nanomolar concentrations (Weis et al., 2002; Urbich et al., 2002) and anti-angiogenic effect of statin at micromolar concentration. In general, angiogenesis inhibitors can be classified into two main group of inhibitors: (i) direct inhibitors that target endothelial cells in the growing vasculature, and (ii) indirect inhibitors that target either tumor cells or the other tumor-associated stromal cells (El-Kenawi and El-Remessy, 2013) Direct endogenous inhibitors of angiogenesis, such as angiostatin, endostatin, arrestin, canstatin, tumstatin and others, are fragments released on proteolysis of distinct ECM molecules. Endogenous inhibitors prevent vascular endothelial cells from proliferating, migrating in response to a spectrum of angiogenesis inducers, including VEGF, bFGF, IL-8 and PDGF (rajabi and shaker, 2017) In this study has shown that atorvastatin has direct effects on endothelial cells by growth and migration inhibition.
Indirect inhibitors of angiogenesis classically prevent the expression or block the activity of pro-angiogenic proteins (Folkman, 2007). VEGF levels have also been described to be suppressed by atorvastatin in this 3D model.
In the present study, angiogenesis is crucial in tumor growth to acquire adequate blood supply, thus, inhibition of angiogenesis could be beneficial for tumor therapy. Our results suggest that atorvastatin could be used as an anti-angiogenic agent in cancer deserves future investigations, but to the best of our knowledge, numerous mechanisms are involved in anti-angiogenesis effect of atorvastatin that further research in vitro and in vivo is required to elucidate these.
In conclusion, the results of this study showed that atorvastatin could inhibit the cell viability of HUVECs and inhibits vascular endothelial growth factor (VEGF)-induced proliferation and tube formation of HUVECs. Meanwhile, atorvastatin also induces apoptosis via upregulation of caspase-3. These findings suggest that atorvastatin could regulate endothelial cell function and might be used in anti-angiogenesis treatment. It is interesting that the anti-angiogenic activities of statins are exerted at those doses that induce apoptosis of tumor cells, which might constitute the background for novel approaches in anti-cancer therapy. Further studies are, however, required to clarify that point of the actions of statins.
References
- Arvold ND, Reardon DA. Treatment options and outcomes for glioblastoma in the elderly patient. Clin Interv Aging. 2014;9:357–67. doi: 10.2147/CIA.S44259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A. 2013;110:10117–22. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau DM, Yu O, Johnson J. Statin use and cancer risk:a comprehensive review. Expert Opin Drug Saf. 2010;9:603–21. doi: 10.1517/14740331003662620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YJ, Shibata T, Rainov NG, et al. Hypoxia-inducible transgene expression in differentiated human NT2N neurons-a cell culture model for gene therapy of postischemic neuronal loss. Gene Ther. 2001;8:1357–62. doi: 10.1038/sj.gt.3301536. [DOI] [PubMed] [Google Scholar]
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature:insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–14. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- Chwalek K, Bray LJ, Werner C. Tissue-engineered 3D tumor angiogenesis models:potential technologies for anti-cancer drug discovery. Adv Drug Deliv Rev. 2014;15:30–9. doi: 10.1016/j.addr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- CIOFU C. The Statins as Anticancer Agents. Maedica (Buchar) 2012;7:377. [PMC free article] [PubMed] [Google Scholar]
- Charalambous C, Chen TC, Hofman FM. Characteristics of tumor-associated endothelial cells derived from glioblastoma multiforme. Neurosurg Focus. 2006;15:E22. doi: 10.3171/foc.2006.20.4.e22. [DOI] [PubMed] [Google Scholar]
- Chen J, Hou J, Zhang J, et al. Atorvastatin synergizes with IFN-γin treating human non-small cell lung carcinomas via potent inhibition of RhoA activity. Eur J Pharmacol. 2012;5:161–70. doi: 10.1016/j.ejphar.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Chen Z, Htay A, Dos Santos W, et al. In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells. J Neurooncol. 2009;2:121–8. doi: 10.1007/s11060-008-9742-y. [DOI] [PubMed] [Google Scholar]
- Chen J, Lan T, Hou J, et al. Atorvastatin sensitizes human non-small cell lung carcinomas to carboplatin via suppression of AKT activation and upregulation of TIMP-1. Int J Biochem Cell Biol. 2012;44:759–69. doi: 10.1016/j.biocel.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Chen Z, Htay A, Dos Santos W, et al. In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells. J Neurooncol. 2009;92:121–8. doi: 10.1007/s11060-008-9742-y. [DOI] [PubMed] [Google Scholar]
- Clendening JW, Penn LZ. Targeting tumor cell metabolism with statins. Oncogene. 2012;31:4967–78. doi: 10.1038/onc.2012.6. [DOI] [PubMed] [Google Scholar]
- Drury JL, Mooney DJ. Hydrogels for tissue engineering:scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Dulak J, Józkowicz A. Anti-angiogenic and anti-inflammatory effects of statins:relevance to anti-cancer therapy. Curr Cancer Drug Targets. 2005;5:579–94. doi: 10.2174/156800905774932824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizabeth R, Gerstner MD, Tracy T, Batchelor MD. Antiangiogenic Therapy for Glioblastoma. Cancer J. 2012;18:45–50. doi: 10.1097/PPO.0b013e3182431c6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kenawi AE, El-Remessy AB. Angiogenesis inhibitors in cancer therapy:Mechanistic perspective on classification and treatment rationales. Br J Pharmacol. 2013;170:712–9. doi: 10.1111/bph.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfandiari N, Khazaei M, Ai J, et al. Effect of a statin on an in vitro model of endometriosis. Fertil Steril. 2007;87:257–62. doi: 10.1016/j.fertnstert.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Eyrich D, Brandl F, Appel B, et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28:55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–60. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis:therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Francescone RA, Faibish M, Shao R. A Matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J Vis Exp. 2011;7:3040. doi: 10.3791/3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick M, Dulak J, Cisowski J, et al. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis. 2003;170:229–36. doi: 10.1016/s0021-9150(03)00299-5. [DOI] [PubMed] [Google Scholar]
- Fromigue O, Hamidouche Z, Marie PJ. Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J Biol Chem. 2008;283:30549–56. doi: 10.1074/jbc.M801436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbelcova H, Lenicek M, Zelenka J, et al. Differences in antitumor effects of various statins on human pancreatic cancer. Int J Cancer. 2008;122:1214–21. doi: 10.1002/ijc.23242. [DOI] [PubMed] [Google Scholar]
- Gerstner ER, Sorensen AG, Jain RK, Batchelor TT. Anti-vascular endothelial growth factor therapy for malignant glioma. Curr Neurol Neurosci Rep. 2009;9:254–62. doi: 10.1007/s11910-009-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S, Mutawe MM, Hauff K, et al. Statin-triggered cell death in primary human lung mesenchymal cells involves p53-PUMA and release of Smac and Omi but not cytochrome c. Biochim Biophys Acta. 2010;4:452–67. doi: 10.1016/j.bbamcr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- He Z, Mangala LS, Theriot CA, et al. Cell killing and radiosensitizing effects of atorvastatin in PC3 prostate cancer cells. J Radiat Res. 2012;53:225–33. doi: 10.1269/jrr.11114. [DOI] [PubMed] [Google Scholar]
- Heymans S, Luttun A, Nuyens D, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–42. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- Jiang P, Mukthavaram R, Chao Y, et al. In vitro and in vivo anticancer effects of mevalonate pathway modulation on human cancer cells. Br J Cancer. 2014;111:1562–71. doi: 10.1038/bjc.2014.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat AM, Nelkin GM. Atorvastatin:a potential chemopreventive agent in bladder cancer. Urology. 2005;66:1209–12. doi: 10.1016/j.urology.2005.06.075. [DOI] [PubMed] [Google Scholar]
- Kaneta S, Satoh K, Kano S, Kanda M, Ichihara K. All hydrophobic HMG-CoA reductase inhibitors induce apoptotic death in rat pulmonary vein endothelial cells. Atherosclerosis. 2003;170:237–43. doi: 10.1016/s0021-9150(03)00301-0. [DOI] [PubMed] [Google Scholar]
- Khodarev NN, Yu J, Labay E, et al. Tumour-endothelium interactions in co-culture:coordinated changes of gene expression profiles and phenotypic properties of endothelial cells. J Cell Sci. 2003;116:1013–22. doi: 10.1242/jcs.00281. [DOI] [PubMed] [Google Scholar]
- Ko DY, Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog Polym Sci. 2013;38:672–701. [Google Scholar]
- Liu J, Tan Y, Zhang H, et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater. 2012;8:734–41. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KV, Bergers G. Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS Oncol. 2013;2:49–65. doi: 10.2217/cns.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- Murai T, Maruyama Y, Mio K, et al. Low cholesterol triggers membrane microdomain-dependent CD44 shedding and suppresses tumor cell migration. J Biol Chem. 2011;286:1999–2007. doi: 10.1074/jbc.M110.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu MN, Sainson RC, Aoto JN, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels:the role of fibroblasts and Angiopoietin–1. Microvasc Res. 2003;66:102–12. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Nehls V, Schuchardt E, Drenckhahn D. The effect of fibroblasts, vascular smooth muscle cells, and pericytes on sprout formation of endothelial cells in a fibrin gel angiogenesis system. Microvasc Res. 1994;48:349–63. doi: 10.1006/mvre.1994.1061. [DOI] [PubMed] [Google Scholar]
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- Pong WW, Gutmann DH. The ecology of brain tumors:lessons learned from neurofibromatosis-1. Oncogene. 2011;30:1135–46. doi: 10.1038/onc.2010.519. [DOI] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi M, Mousa SA. The role of angiogenesis in cancer treatment. Biomedicines. 2017;5:34. doi: 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner E, Saut O, Province P, et al. Effects of anti-angiogenesis on glioblastoma growth and migration:model to clinical predictions. PLoS One. 2014;15:e115018. doi: 10.1371/journal.pone.0115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Q, Li W, Li H, Sun G. Vasostatin inhibits VEGF-induced endothelial cell proliferation, tube formation and induces cell apoptosis under oxygen deprivation. Int J Mol Sci. 2014;9:6019–30. doi: 10.3390/ijms15046019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HH, Park KM, Gerecht S. Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv Drug Deliv Rev. 2014;79-80:19–29. doi: 10.1016/j.addr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Pérez JH, Kirches E, Mawrin C, Firsching R, Schneider T. Cytotoxic effect of different statins and thiazolidinediones on malignant glioma cells. Cancer Chemother Pharmacol. 2011;67:1193–201. doi: 10.1007/s00280-010-1535-2. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dernbach E, Zeiher AM, Dimmeler S. Double-edged role of statins in angiogenesis signaling. Circ Res. 2002;90:737–4. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis:molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–45. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- Wilson SH, Herrmann J, Lerman LO, et al. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 2002;105:415–18. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]
- Wood WG, Igbavboa U, Muller WE, Eckert GP. Statins, Bcl-2 and apoptosis:Cell death or cell protection? Mol Neurobiol. 2013;48:308–14. doi: 10.1007/s12035-013-8496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Wei D, Shi Q, Huang S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev. 2004;15:297–324. doi: 10.1016/j.cytogfr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators Inflamm. 2013;2013:127170. doi: 10.1155/2013/127170. [DOI] [PMC free article] [PubMed] [Google Scholar]


