Abstract
The secreted growth factor granulin (GRN) is upregulated during diverse epithelial cancers. GRN stimulates cell growth and development while inhibiting apoptosis. Orthologues of vertebrate granulins evolved in other animals including the liver fluke Opisthorchis viverrini. Curiously, liver fluke granulin, termed Ov-GRN-1 promotes cholangiocarcinogenesis during chronic opisthorchiasis but, by contrast, limited information is available concerning mammalian GRN during liver fluke infection-induced cholangiocarcinoma (CCA). Here we investigated the expression of mammalian granulin in the O. viverrini-associated a hamster model of opisthorchiasis and liver fluke infection-induced CCA. Male Syrian golden hamsters were assigned to one of four treatment groups, each group included 30 hamsters: 1) normal (control), 2) infected with O. viverrini (OV); 3) exposed to N-dimethylnitrosamine in drinking water (DMN); and 4) infected with O. viverrini and exposed to DMN (OVDMN). Immunohistochemistry using an anti-granulin specific probe for mammalian granulin was undertaken to monitor expression and location in hepatobiliary tissues of the hamsters. In parallel, cognate studies of transcription of mRNA and protein. Histopathological examination revealed development of proliferative lesions from the onset and eruption of CCA onwards, an outcome that was most prominent in the OVDMN hamsters. Proliferating cell nuclear antigen (PCNA) index rose continuously from initiation of infection and increased with lesion progression in OV, DMN and markedly in OVDMN hamsters. Expression of GRN in biliary was elevated in biliary epithelial cells in CCA lesions in hamsters in the DMN and OVDMN groups. Expression of GRN as assayed by western blot and RT-PCR reflected the same trend as seen with PCNA. Together the histopathogical and molecular assay based findings revealed marked expression of granulin during cholangiocarcinoma in these hamsters, and highlighted the prospect that granulin represents a potential prognostic marker for cholangiocarcinoma.
Keywords: Cholangiocarcinoma, granulin, hamster model, Opisthorchis viverrini, carcinogenesis
Introduction
Bateman and Bennett, (2009) described granulin a generation ago, and subsequently charcaterized many of its attributes and functions. Various termed progranulin (PGRN), granulin-epithelin precursor (GEP), acrogranin, and other synonyms, granulin is an extracellualr growth factor with functional roles in embryogenesis, inflammation, and neuronal cell survival, malignancy, metastasis and cancer stem cells (Wong et al., 2014). Granulins are widely conserved in evolution, and fish and many invertebrates evolved several granulin genes and forms. Mammals and other mammals have a single granulin gene, which encodes a secreted glycoprotein that is cleaved from a precursor with 7.5 repeats of a distinctive, conserved 12-cysteine granulin/epithelin motif. In mammals, granulin is known to inhibit cellular apoptosis, stimulate cell growth and development, promote anchorage-independent growth, participate in wound healing, and also is over-expressed in various malignant tumors (Bateman and Bennett, 2009; Ong and Bateman, 2003). Specifically, expression of GRN in epithelial cancers has been investigated in in breast cancer (Serrero, 2003), renal carcinoma (Donald et al., 2001), invasive ovarian cancer (Jones et al., 2003), glioblastoma (Liau et al., 2000), and hepatocellular carcinoma (Cheung et al., 2004; Ho et al., 2008; Wong et al., 2014). However, information is limited concerning expression of granulin in cholangiocarcinoma tissue and cells.
By contrast, a secreted granulin family member termed Opisthorchis viverrini granulin 1 (Ov-GRN-1) has been characterized from the liver fluke O. viverrini, and an orthologue is known from Clonorchis sinensis. In similar fashion to human PGRN/ GEP, Ov-GRN-1 positively promotes cellular proliferation of mammalian epithelial cells including cholangiocytes, promoted wound healing, inhibits apoptosis, is pro-angiogenic and, notably, pro-tumorigenic (Smout et al., 2009; Mulvenna et al., 2010; Smout et al., 2011; Papatpremsiri et al., 2014; Smout et al., 2015; Bansal; Smout et al., 2017; Wang et al., 2017). Opisthorchiasis is a human liver fluke infection caused by eating raw cyprinid fish that carry metacercariae of Opisthorchis viverrini (O. viverrini). The disease is considered as one of the most important public health problems in Southeast Asia, including Thailand, PDR Laos, Vietnam, and Cambodia, where high infection incidences have been reported especially in the superendemic areas locating in the vicinity of river basins and lakes (Sithithaworn et al., 2012). Pathological changes of biliary passages, including biliary cell proliferation and periductal fibrosis, are inductive consequences during chronic opisthorchiasis. Intriguingly, eruption of these alterations reflects the genetic susceptibility to cholangiocarcinoma (CCA) which is a fetal sequel of this chronic disease (Sripa et al., 2012). Damage to biliary passages has been proved to be the combined insults of direct physical abrasion, toxic free radicals and cell proliferative inducers. Studies in mice and in vitro demonstrated that Ov-GRN-1 accelerates wound healing and induces angiogenesis in vivo, and these investigators propose that Ov-GRN-1 facilitates the establishment of a microenvironment in the infected biliary duct conducive to tumorigenesis and the establishment and progression of CCA (Smout et al., 2015). Moreover, this linkage of between the wound repair attributes of O. viverrini granulin during opisthorchiasis to cancer progression has been highlighted by Botelho et al., (2016).
Since several lesions manifest during development of the liver fluke infection-induced CCA, including injury and healing in the duct ducts, cell proliferation, and periductal fibrosis, it seems likely that granulin plays roles in these developmental stages. The present investigation was primed by the paucity and indeed absence of information of granulin expression in the biliary tract and in cholangiocarcinoma. The study was designed to monitor and compare the temporal expression of granulin using immunohistochemical, immunoblot and other molecular approaches in an established rodent model of liver fluke infection-induced cholangiocarcinogenesis.
Materials and Methods
Hamsters, paraffin blocks and frozen tissues
The study was approved by the Animal Ethics Committee of Khon Kaen University according to the Ethics of Animal Experimentation of the National Research Council of Thailand (ACUC KKU 9/2558, 55/2554 and 5/2550). Since the hamster model of cholangiocarcinogenesis has been established (Thamavit et al., 1987) and currently used in the other research at the Tropical Disease Research Center, Faculty of Medicine, Khon Kaen University and given the necessity to limit use and numbers of vertebrate animals in biomedical research, research materials investigated here were partially shared with the other previous and ongoing studies in the sphere of liver fluke infection-induced CCA in our laboratory. In each set of CCA induction, a total of 120 male Syrian golden hamsters aged three 21 to 28 days at the start of the study were used. The rodents were assigned to one of four treatment groups, with 30 hamsters in each group: 1) No treatment (control group); 2) Infection with O. viverrini, with a single inoculum of 50 metacercariae per hamster (OV group); 3) N-nitroso dimethylamine, administered at 12.5 ppm in drinking water for eight weeks (DMN group); and 4) O. viverrini infection and NDMA administration (OVDMN group), inoculated with O. viverrini as well as administered with DMN, as above. All hamsters were housed in standard conventional conditions with access to commercial chow and clean water ad libitum. Five hamsters of each group were euthanized at weeks 1, 2, 4, 8, 12 and 24 following the commencement of the treatment. At necropsy, the liver and biliary tract, were removed, and portions preserved frozen or proceeded for paraffin tissue blocks. Five-micrometer sections from each paraffin block were prepared either for the histopathological evaluation using H and E staining or immunohistochemical investigation, as described (Lvova et al., 2012). Non-CCA and CCA frozen tissues in hamster at 4 and 24 weeks from similar study were investigated for molecular studies.
Histopathological study
Histopathological classification, following Lvova et al., (2012), was evaluated based on the increasing numbers of biliary cells (biliary proliferation), increasing number of bile ducts (biliary hyperplasia), biliary periductal fibrosis (cholangiofibrosis), cell type transformation of biliary cells (biliary metaplasia), and cancer of bile duct (CCA).
Immunohistochemical studies
PCNA immunohistochemistry
The slides were deparaffinized in an incubator at 65°C for 30 minutes, and rehydrated in xylene and serial dilutions of ethanol. Antigen was retrieved in 0.1M sodium citrate in a pressure cooker for five minutes. Non-specific antigens were blocked with 5% normal horse serum for 30 minutes, after which the sections were probed with polyclonal rabbit anti-PCNA (Proliferating cell nuclear antigen) antibody (Abcam plc, Cambridge, UK) diluted 1 in 200 in PBS, 0.05% NaN3 at room temperature overnight. Subsequently, sections were probed with goat anti-rabbit antibody (Sigma-Aldrich, St. Louis, MO, USA) adiluted 1 in 300 in PBS for one hour. After several washes in PBS, positive signals were detected using 0.03% diaminobenzidine (DAB) in 0.003% hydrogen peroxide in Tris-HCl pH 7.2, as the chromogenic substrate. Counterstaining with Mayer’s hematoxylin was performed before mounting the slides.
Granulin immunohistochemistry
A technique generally similar to that for PCNA was employed. Polyclonal rabbit anti-human GRN (HPA008763, Sigma-Aldrich, St. Louis, MO), an antibody that is specific for human and rodent granulin, was raised to an epitope tagged (PrEST) recombinant antigen (product data sheet). Human hepatoma (Cheung et al., 2004) and normal hamster liver tissue served as positive and negative control tissues for expression of granulin, respectively.
Molecular studies for granulin
Western blots
Protein extraction was performed as described (Ericsson and Nister, 2011). Samples were resolved using 10% SDS-PAGE gels and transferred to nitrocellulose membrane using a wet tank system. Following transfer of proteins, the membranes were blocked by incubation in 5% non-fat skimmed milk in PBS. Membranes were probed with polyclonal rabbit anti-GRN antibody, diluted 1 in 1,000 in PBS, followed by anti-rabbit immunoglobulin conjugated with horseradish peroxidase (HPA008763, Sigma-Aldrich, St. Louis, MO, USA). Positive signals were detected using chromogenic substrate DAB, as above. Human hepatoma and normal liver tissue served as positive and negative controls.
Real time quantitative RT-PCR
Levels of GRN transcripts were evaluated by RT-PCR GRN-forward primer (5´-CAGTCGGGAGACAGAATGGT-3´) and GRN-reverse primer (5´CACACAGCATGGGGTAACTG-3´), as described by Bustin (2000), with SBY green I presenter (SsoFasttmEvagreen®Supermix, Bio-Rad, USA). Beta-actin was used as internal control (Papatpremsiri et al., 2014).
Data analysis
The pathological outcome for each treatment was described qualitatively. Proliferation activity of biliary cells from PCNA immunohistochemistry was formulated in a PCNA index where PCNA index (%) = positive biliary cells*100/total 1000 biliary cells. Percentage of granulin-positive cells was semi-quantitatively evaluated: granulin positive cells (%) = positive biliary cells *100/1,000 biliary cells, and used as categories for grading as follows, Grade 1 <1%, Grade 2 = 1-25%, Grade 3 = 26-50%, Grade 4 > 50%. Staining intensity was recorded as mild, moderate or strong. The presence of granulin was confirmed by a positive western blot. Transcriptional level of granulin in samples of non-CCA and CCA liver was determined by RT-PCR.
Kolmogorov-Smirnov test (K-S test) was used to test the distribution of data. Relationship between the PCNA index and granulin grades was tested by Spearman’s rho correlation. Statistical differences among each group and treatment period were compared by Kruskal-Wallis test. RT-PCR data was analyzed by a non-parametric one-way ANOVA. P values of <0.05 were considered to be statistically significant.
Results
Histopathology study
Figure 1 documents examples of the histopathological changes in the biliary passages including proliferation, periductal fibrosis, the precancerous lesions dysplasia and cholangiofibrosis, cyst and CCA. In tandem, Figure 2 provides the timeline of the onset of these lesions. The lesions were often found at where the parasite resided. During the early pathological changes in the OV and OVDMN groups, eosinophils were found to be the most predominant inflammatory cells infiltrating in the fibrous tissue along the portal tract and periportal area where migration and maturation of the liver flukes had taken place. In all treatment groups, mononuclear cells, particularly lymphocytes, macrophages and plasma cells also migrated to the inflammatory sites to form lymphoid follicles. Follicle formation was evident by four weeks and the OVDMN hamsters displayed the most marked responses. Initial infiltration of mononuclear cells along the portal tract in the OV group was found earlier starting since week 1. Increasing infiltration of mononuclear cells likely was time-related. Focal lymphoid aggregations around the large bile ducts were seen at weeks 4 and 8, but these had decreased at week 24. In the DMN group, infiltration of lymphocytes along the portal tract started at week 1, and gradual increase was seen at weeks 8 and 12 and 24. These distinctive pathological features of inflammed bile ducts were not observed in normal livers.
Figure 1.
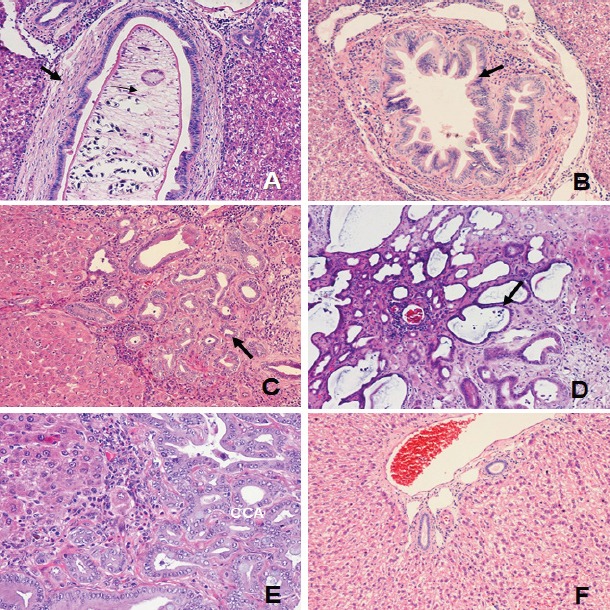
Histopathological Changes of Biliary Passages. Proliferative, precancerous and other lesions arose in all treatment groups; A, Periductal fibrosis of large bile duct (arrow, x100); B, Dysplasia of large bile duct (arrow, x100); C, Bile duct proliferation in early cholangiofibrosis (arrow, x100); D, Cholangiofibrosis and cyst (arrow) (x100); E, CCA (x200); and F, Normal bile duct x100)
Figure 2.
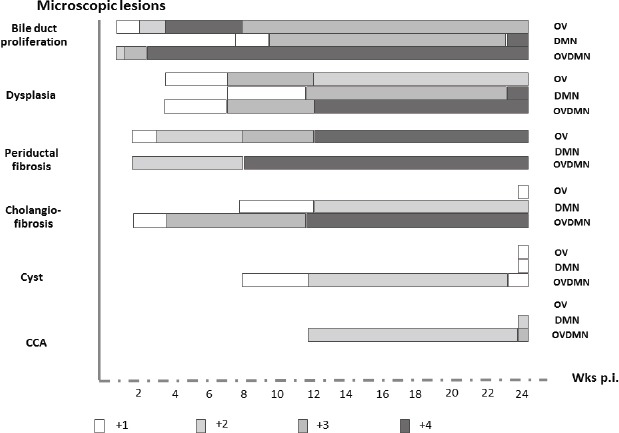
Schematic that Outlines Timing of Histopathological Changes of Biliary Passages in the OV, DMN and OVDMN Groups. The changes appeared in different grades and time frames. Proliferation, dysplasia and periductal fibrosis initially appeared at low grade (+1) in weeks 2 or 4, then became medium (+2 and +3) or high (+4) grades in a later period. Apparently, the high graded bile duct proliferation, dysplasia, periductal fibrosis and cholangiofibrosis was seen mainly in the OVDMN group at different time frames. Low graded cholangiofibrosis in the OV group and cyst in the OV and DMN groups could not be found until week 24. CCA was seen only in the DMN at week 24 and OVDMN since week 12.
Marked disparity in types and grades of lesions among treatment groups was observed (Figure 2). The OV group appeared to exhibit all grades of proliferation, dysplasia, and periductal fibrosis. This group developed only low-grade cholangiofibrosis and cyst; however, CCA was not found in this group. The DMN group contained all grades of proliferation, dysplasia, low and medium graded cholangiofibrosis, low-grade cyst and medium-grade CCA. Biliary periductal fibrosis was not seen in this group. All types of lesions were found in the OVDMN group at different grades: all grades of proliferation and dysplasia, medium and high grade periductal fibrosis and cholangiofibrosis, low grade cyst and medium-grade CCA.
Different lesions appeared at staggered intervals following the treatment (Figure 2). However, whereas the timing of initial eruption of the lesions was not uniform, lesions generally manifested with low grade during weeks one to four. Precancerous lesions including dysplasia and cholangiofibrosis usually erupted and were seen from week 12 while periductal fibrosis was predominant from week eight and increased through to week 24. CCA was evident only in the OVDMN and DMN hamsters. The earliest development of CCA that was detectable occurred at week 12 in the OVDMN group. CCA was seen also at 24 weeks. Bile duct proliferation, dysplasia and periductal fibrosis appeared during weeks 1 to 4 in OV and OVDMN hamsters whereas these lesions did not erupt until week eight in the DMN group was not seen until the week 8. Periductal fibrosis was evident by week 2 in the OV and DMN groups. Cholangiofibrosis of the OV, DMN and OVDMN was found in the weeks 24, 8, and 2, respectively. Cystic formation and dilatation in the OV and DMN groups erupted at week 24; these lesions were evident by week 8 in the OVDMN group. CCA was evident in OVDMN hamsters by week 14, and by week 24 in the DMN group.
Immunohistochemical staining for PCNA: marked cellular proliferation in the biliary epithelia during chronic opisthorchiasis and liver fluke infection- and nitrosamine-induced carcinoma
PCNA positive staining was demonstrated in nuclei of the proliferating cells (Figure 3). The proliferation activity is concomitant to the histopathological results. Pattern of the proliferation index in all groups was generally similar: minimal activity from weeks 1 to 4, an abrupt increase by week 8, which was maintained as sustained PCNA expression until week 24 when the experiment concluded (Figures 3, 4). The most marked PCNA expression was seen in the OV and OVDMN groups. The PCNA index of the OV group was steady from week 8 to 24, proliferation in DMN group increased abruptly from week 8, and the highest activity was seen the OVDMN group. Significant differences for the PCNA index among the four treatment groups were observed on weeks 1 (p=0.002), 2 (p=0.002), 4 (p=0.002), 8 (p=0.001), 12 (p=0.003) and 24 (p=0.002). In addition, differences in PCNA expression were apparent in the in time series for the DMN (p<0.001) and OVDMN (p=0.002) groups but not in the control group (p=0.897).
Figure 3.

Immunohistochemistry of PCNA Demonstrated Proliferation Activity of Bile Duct. Non-dividing biliary cells in the normal group showed negative staining (A). Higher activity was seen in proliferative lesions such as hyperplasia (B) and dysplasia (C). The highest activity was detected in CCA (D). (Original magnification: A, B, D = 200x; C = 100x)
Figure 4.

PCNA Index Indicated Proliferation Activity of Biliary Cells in the Normal, OV, DMN and OVDMN Groups. The activity was quite constant throughout the whole experiment, except that of the DMN group of which increased rapidly at week 8. The lowest activity was found in the DMN group but the highest activity was in the OVDMN group
Granulin immunolocalized in cholangiocarcinoma
The micrographs of thin sections of hamster liver in Figure 5 revealed expression of granulin as detected using the granulin specific rabbit antibody. The findings revealed clearly predominant expression of granulin only at during late stage of the CCA malignant lesion. Expression of granulin was found concomitantly with CCA formation in the OVDMN group at weeks 12 and 24. By contrast, expression was evident only by week 24 in the DMN group. (Statistical analyses were not performed with these findings because specimen blocks in some groups were not available.)
Figure 5.

Immunohistochemical Localization Targeting Granulin Unambiguously Revealed Positive Staining was in the Cancerous Tissue (CCA) that had Erupted the Hepatobiliary Tract of Hamsters in the DMN Group at Week 24 (F) and the OVDMN Group at Week 12 (H) and 24 (I). Negative or very weak staining was found in the non-cancerous tissues in the OV (A, B, C), DMN (D, E) and OVDMN (G) groups. (Original magnification: A, C, G = 100x; B, D, E, F, H, I = 200x)
Molecular studies of granulin
Western blots
The presence of granulin in the frozen liver tissues from cancerous and non-cancerous groups of hamsters was investigated by immunoblot analysis. Target band of ~88 kDa indicating granulin was seen in human hepatoma (positive control for granulin) and in all hepatobiliary tissues of the OVDMN group at week 24 (Figure 6).
Figure 6.

Western Blotting to Monitor Hepatic Tissue Expression of Granulin. A, Control (human hepatoma); B, Normal group at week 4; C, OV group at week 4; D, DMN group at week 4; E, OVDMN group at week 4; F, DMN group at week 24; G, OVDMN group at week 24; M, Size standards for molecular mass in kilodaltons (kDa).
Up-regulation of granulin expression during cholangiocarcinoma
Table 1 presents the findings for granulin expression as assesses by real-time RT-PCR, indicated the transcription level of granulin is predominantly higher statistically in CCA in the DMN and OVDMN groups (p <0.05). At week 4, the relative mRNA level in the DMN and OVDMN groups was not different from that in the tissue from the normal and OV groups. The differences of the mRNA level between weeks 4 and 24 in the same treatment increased dramatically in the group with developing CCA (Figure 7). Statistical significance also presented in the cancerous tissue of the DMN and OVDMN groups at week 24. The OVDMN tissue had higher transcription level (p = 0.003).
Table 1.
Statistical Analysis Revealing Differences among Treatment Groups. One-way ANOVA to compare levels of transcription level demonstrated increased expression of GRN in cancerous tissue at 24 weeks in OVDMN and DMN compared to livers of the normal, control group of hamsters and those in the OV group; 95% confidence.
| Group | Group | P value |
|---|---|---|
| Normal 4 week | OV 4 week | 1.000 |
| DMN 4 week | 1.000 | |
| OVDMN 4 week | 1.000 | |
| DMN 24 week | 0.014* | |
| OVDMN 24 week | <0.001* | |
| OV 4 week | DMN 4 week | 1.000 |
| OVDMN 4 week | 1.000 | |
| DMN 24 week | 0.014* | |
| OVDMN 24 week | <0.001* | |
| DMN 4 week | OVDMN 4 week | 1.000 |
| DMN 24 week | 0.018* | |
| OVDMN 24 week | <0.001* | |
| OVDMN 4 week | DMN 24 week | 0.020* |
| OVDMN 24 week | <0.001* | |
| DMN 24 week | OVDMN 24 week | <0.001* |
Statistically significance at P ≤ 0.05
Figure 7.
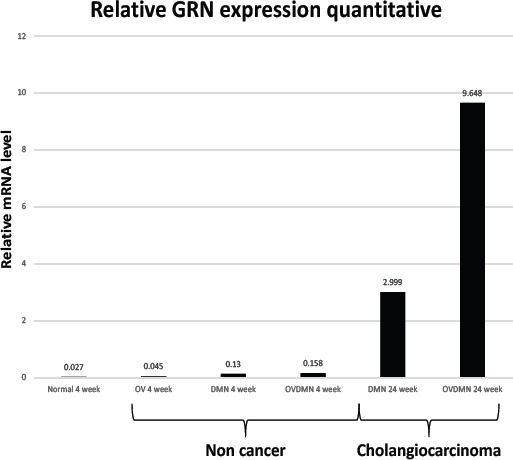
Relative mRNA Levels Encoding Granulin Elevated at Weeks 4 and 24 in the OVDMN and DMN Treatment Groups.
Discussion
The findings revealed the expression of granulin during cholangiocarcinoma in these hamsters. By histological examination, marked differences in lesional appearance, particularly in chronic stage, were evident among all four groups of hamsters. The liver of hamsters of the OVDMN group exhibited the most progressive pathological features.. The activities of the liver flukes certainly can be expected to have particpated in the pathological eruption, both through physical damage and by immunopathological processes (Sripa et al., 2012). Eosinophil infiltration was a prominent feature at the outset of liver fluke infection. Similar phenomena are known from other helminth infection-cancer related studies (Samaras et al., 2010; Tong et al., 2017). Eosinophils were prominent early in infection in the connective tissue surrounding the lesions. With the concept of hypersensitivity type I, eosinophils are chemotactically recruited in response to helminth infection to release mediates including cytokines and enzymes, stored in the intracellular granules, into the vicinity of the parasite. In the chronic stage (8 weeks), infiltration of mononuclear cells, such as lymphocytes and plasma cells, to aggregate and form lymphoid follicle was evident, as noted previously as the characteristic pathological immune process during opisthorchiasis (Sripa and Kaewkes, 2000). These pathogenic changes were evident in the OV and OVDMN groups.
NDMA is a known carcinogen (Lijinsky, 1994). However, the amount given to hamsters in the DMN and OVDMN groups was in a sub-carcinogenic dose. It is a fact that low NDMA dose could merely activate or produce lesions slowly and less vigorously in the early NDMA treatment. However, the long term feeding of this chemical would be able to exert a strong carcinogenic effect. This statement was seen in our experiment. The accumulative effect of this chemical could produce a medium graded CCA in the later stage of this experiment. Thus, hamsters in the DMN group did not show any CCA lesions until week 24. Accumulation of this chemical to the level can damage the genetic materials crucially for the apoptotic process. Once apoptosis is interrupted, proliferation of abnormal cells bearing the genetic defects is allowable (Labi and Eriacher, 2015). Progression from carcinoma in situ to CCA can be anticipated at this point (Sripa, 2003). Synergy between the tumorigenic risks from NDMA and O. viverrini infection expedite cholangiocarcinogenesis, and CCA erupted by week 12 in the hamsters.
Proliferation activity of biliary cells was observed in the OV, DMN and OVDMN groups by PCNA index. The highest activity was found in the OVDMN group that was in concordance with the histopathological severity. Perhaps the proliferation of biliary cells in the OVDMN group has been induced by O. viverrini in the early phase. Subsequently, the synergistic interaction between opisthorchiasis and the carcinogen NDMA potentially promotes cholangiocarcinogenesis at at weeks 12 and 24. Effects of O. viverrini in promoting cell proliferation had been shown in the OV and OVDMN groups. In the OV group, the cell proliferation is the best prove that O. viverrini alone can induce cell division, probably either through direct physical and chemical stimuli. Excretory secretory (ES) products of O. viverrini promote cell proliferation both in vitro and in vivo (Chaiyadet et al., 2015; Smout et al., 2009; Thuvajit et al., 2006). Liver fluek granulin is secreted (Mulvenna et al., 2010) and, as noted, is a potent promoter of cellular proliferation of chaolangiocyes and of wound healing (Smout et al., 2015). Thus synergism of the sub-carcinogenic dose of NDMA and the liver fluke infection undoubtedly could exert effects on the development of CCA through damaging genetic materials that control driving of abnormal condition and transformation into neoplasia as described above.
In addition to liver fluke granulin (Smout et al., 2009), mammals also expresses granulin to function in many aspects, including embryo development, inflammation, wound healing, tumorigenesis, (Ong and Bateman, 2003). The lack of granulin can cause neurological diseases and susceptible to metabolic diseases (Nguyen et al., 2013). By contrast, abnormal expression of granulin could lead to cancer development. Granulin may be play roles in at least one third of the known cancers via signaling pathway. It plays role in stimulation and development of cell inhibition of apoptosis and stimulating the formation of angiogenesis (He et al., 2003). Granulin has been often reported in cancers of epithelial origins (see Bateman and Bennett, 2009). Granulin appears to promote carcinogenesis in most epithelial cancers, by activating phosphorylation of SHC-transforming protein 1 and p44/42 MAPK pathway in extracellular signaling or ERK. In addition, it might stimulate PI3K, AKT/protein kinase B and p70S6kinase and lead cell to divisional stages (Bateman and Bennett, 2009). However, statistical significance between granulin and PCNA was not apparent here because the missing cases. It seems that proliferation activity gradually had increased concordance with progressive pathological lesions but granulin abruptly increases when malignant transformation occurs. So far, granulin is believed to involve in cancers at least in three alternatives: 1) increase in various cancers, 2) excessive granulin could stimulate cancer formation and growth, and 3) granulin inhibitors such as antibody or grn antisense could stop granulin formation and decrease cancer development (Cheung et al., 2004; Jone et al., 2003; Ong and Bateman, 2003). In breast cancer, granulin expression significantly correlated with fibrosis and poor survival (McAllister and Weinberg, 2014). Furthermore, granulin-secreted macrophage was revealed to play necessary roles in metastasis of pancreatic cancer to liver by inducing fibrosis (Nielsen et al., 2016). Although there are many studies regarding the existence of granulin in epithelial cancers, the study in CCA is very limited. The studies of Smout et al., (2009) suggested a role of liver fluke granulin (Ov-GRN-1) in inducing cell proliferation that is probably involved in CCA development. Ov-GRN-1 stimulates mammalian cells and promote wound healing (Smout et al., 2011; Smout et al., 2015). Granulin has been reported in other flukes such as Fasciola hepatica (Machicado et al., 2016) and Clonorchis sinensis (Wang et al., 2017) of which the latter is a risk factor for CCA. Nonetheless, those researchers also believed that granulin is secreted by O. viverrini. In human bile duct cancer, detection of overexpression of granulin by immunohistochemistry could show correlation between the poor progression and free survival in the advanced cases. This could be applied as a predictive factor after receiving chemotherapy (Kim et al., 2016). Here we detected granulin clearly in all cancerous CCA tissue Similar findings have been reported in breast cancer. (Jone et al., 2003), hepatocellular carcinoma (Cheung et al., 2004; Wong et al., 2014) and ovarian cancer (Davidson et al., 2004).
The histopathological findings using PCNA and granulin specific antibody probes on fixed thin section of hamster liver were collaborated by the western blot and RT-PCR outcomes. Protein detection using western blot showed only from the tissue of hamster liver from week 24 in the OVDMN group. In this group, histopathological findings indicated CCA occupied widely in liver, as well as the high PCNA index. Therefore, it is possible that the amount of cancerous tissue at other times or groups exhibiting CCA was below detection limits in the RT-PCRs and westerns. Further investigation using real-time RT-PCR may be able to reflect the transcription level of granulin that is predominant in the CCA tissue.
A limitaion of this study was the unavailbility of some tissue samples and paraffin blocks of fixed livers for the present treatment groups. Specifically, frozen tissue was available for investigation for only the weeks four and 24 (samples were shared from other studies that had employed the same treatment protocols). Nonetheless, informative comparison between the non-cancerous and cancerous tissues was made, and indeed the statistical analysis showed significant differences between the cancerous and non-cancerous tissues.
To conclude, marked expression of hamster granulin was evident during infection with O. viverrini and infection-associated CCA. Predominant expression of granulin was evident in CCA, irrespective of how and when it was induced. Further investigation in human CCA may enable evaluation of granulin as a prognostic marker for CCA. Last, we speculate that liver fluke granulin may complement or exacerbate the effects of endogenous hamster (or human) granulin during cholangiocarcinoma.
Conflict of interest
No conflicts of interest have been declared.
Acknowledgements
We thank the staff of the Tropical Disease Research Center (TDRC) for their kind support. We also thank the Graduate School, Khon Kaen University for the student scholarship. This investigation was supported by the National Institute of Allergy and Infectious Diseases (NIAID), award number P50AI098639, the National Cancer Institute (NCI), award number CA164719, and partially supported by the Thailand Research Fund (TRF) (RTA 5680006). BS is a TRF Senior Research Scholar. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID, NCI or the NIH or the funders.
References
- Bansal PS, Smout MJ, Wilson D, et al. Development of a potent wound healing agent based on the liver fluke granulin structural fold. J Med Chem. 2017;60:4258–66. doi: 10.1021/acs.jmedchem.7b00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun. 1990;173:1161–8. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- Bateman A, Bennett HP. The granulin gene family:from cancer to dementia. Bioessays. 2009;31:1245–54. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- Botelho MC, Alves H, Richter J. Wound healing and cancer progression in Opisthorchis viverrini associated cholangiocarcinoma. Parasitol Res. 2016;115:2913–4. doi: 10.1007/s00436-016-5090-6. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–93. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Chaiyadet S, Smout M, Johnson M, et al. Excretory/secretory products of the carcinogenic liver fluke are endocytosed by human cholangiocytes and drive cell proliferation and IL6 production. Int J Parasitol. 2015;45:773–81. doi: 10.1016/j.ijpara.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S.T, Wong SY, Leung KL, et al. Granulin-epithelin precursor overexpression promotes growth and invasion of hepatocellular carcinoma. Clin Cancer Res. 2004;10:7629–36. doi: 10.1158/1078-0432.CCR-04-0960. [DOI] [PubMed] [Google Scholar]
- Davidson B, Alejandro E, Flørenes VA, et al. Granulin epithelin precursor is a novel prognostic marker in epithelial ovarian carcinoma. Cancer. 2004;100:2139–47. doi: 10.1002/cncr.20219. [DOI] [PubMed] [Google Scholar]
- Donald CD, Laddu A, Chandham P, et al. Expression of progranulin and the epithelin/granulin precursor acrogranin correlates with neoplastic state in renal epithelium. Anticancer Res. 2001;21:3739–42. [PubMed] [Google Scholar]
- Ericsson C, Nistér M. Protein extraction from solid tissue. Methods Mol Biol. 2011;675:307–12. doi: 10.1007/978-1-59745-423-0_17. [DOI] [PubMed] [Google Scholar]
- He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–9. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- Ho JC, Ip YC, Cheung ST, et al. Granulin-epithelin precursor as a therapeutic target for hepatocellular carcinoma. Hepatology. 2008;47:1524–32. doi: 10.1002/hep.22191. [DOI] [PubMed] [Google Scholar]
- Jones MB, Michener CM, Blanchette JO, et al. The granulin-epithelin precursor/PC-cell-derived growth factor is a growth factor for epithelial ovarian cancer. Clin Cancer Res. 2003;9:44–51. [PubMed] [Google Scholar]
- Kim JH, Do IG, Kim K, et al. Progranulin as a predictive factor of response to chemotherapy in advanced biliary tract carcinoma. Cancer Chemother Pharmacol. 2016;78:1085–92. doi: 10.1007/s00280-016-3170-z. [DOI] [PubMed] [Google Scholar]
- Labi V, Erlacher M. How cell death shapes cancer. Cell Death Dis. 2015;6:e1675. doi: 10.1038/cddis.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijinsky W. Chemical structure of nitrosamines related to carcinogenesis. In: Loeppky RN, Michejda CJ, editors. ‘Nitrosamine and related N-nitrosocompounds’. Washington DC: American Chemical Society; 1994. pp. 250–66. [Google Scholar]
- Liau LM, Lallone RL, Seitz RS, et al. Identification of a human glioma-associated growth factor gene, granulin, using differential immuno-absorption. Cancer Res. 2000;60:1353–60. [PubMed] [Google Scholar]
- Lvova MN, Tangkawattana S, Balthaisong S, et al. Comparative histopathology of Opisthorchis felineus and Opisthorchis viverrini in a hamster model:an implication of high pathogenicity of the European liver fluke. Parasitol Int. 2012;61:167–72. doi: 10.1016/j.parint.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Machicado C, Marcos LA, Zimic M. Hypothetical granulin-like molecule from Fasciola hepatica identified by bioinformatics analysis. Springerplus. 2016;5:773. doi: 10.1186/s40064-016-2443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16:717–27. doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AD, Nguyen TA, Martens LH, Mitic LL, Farese RV., Jr Progranulin:At the interface of neurodegenerative and metabolic diseases. Trends Endocrinol Metab. 2013;24:597–606. doi: 10.1016/j.tem.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SR, Quaranta V, Linford A, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol. 2016;18:549–60. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CHP, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesis. Histol Histopathol. 2003;18:1275–88. doi: 10.14670/HH-18.1275. [DOI] [PubMed] [Google Scholar]
- Papatpremsiri A, Smout MJ, Loukas A, et al. Suppression of Ov-grn-1 encoding granulin of Opisthorchis viverrini inhibits proliferation of biliary epithelial cells. Exp Parasitol. 2015;148:17–23. doi: 10.1016/j.exppara.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrero G. Autocrine growth factor revisited:PC-cell-derived growth factor (progranulin), a critical player in breast cancer tumorigenesis. Biochem Biophys Res Commun. 2003;308:409–13. doi: 10.1016/s0006-291x(03)01452-9. [DOI] [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Nguyen VD, et al. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012;61:10–6. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras V, Rafailidis PI, Mourtzoukou EG, Peppas G, Falagas ME. Chronic bacterial and parasitic infections and cancer:a review. J Infect Dev Ctries. 2010;4:267–81. doi: 10.3855/jidc.819. [DOI] [PubMed] [Google Scholar]
- Smout MJ, Mulvenna J, Jones MK, Loukas A. Expression, refolding and purification of Ov-GRN-1, a granulin-like growth factor from the carcinogenic liver fluke, that causes proliferation of mammalian host cells. Protein Expr Purif. 2011;79:263–70. doi: 10.1016/j.pep.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Smout MJ, Sotillo J, Laha T, et al. Carcinogenic parasite secretes growth factor that accelerates wound healing and potentially promotes neoplasia. PLoS Pathog. 2015;11:e1005209. doi: 10.1371/journal.ppat.1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout MJ, Laha T, Mulvenna J, et al. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B. Pathobiology of opisthorchiasis:an update. Acta Trop. 2003;88:209–20. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, et al. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 2012;10:359–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthochiasis. Int J Parasital. 2000;30:735–40. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- Thamavit W, Kongkanuntn R, Tiwawech D, Moore MA. Level of opisthorchis infestation and carcinogen dose-dependence of cholangiocarcinoma induction in Syrian golden hamsters. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54:52–8. doi: 10.1007/BF02899196. [DOI] [PubMed] [Google Scholar]
- Thuwajit C, Thuwajit P, Uchida K, et al. Gene expression profiling defined pathways correlated with fibroblast cell proliferation induced by Opisthorchis viverrini excretory/secretory product. World J Gastroenterol. 2006;12:3585–92. doi: 10.3748/wjg.v12.i22.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Brindley PJ, Meyer CG, Velavan TP. Parasite infection, carcinogenesis and human malignancy. EBioMedicine. 2017;15:12–23. doi: 10.1016/j.ebiom.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lei H, Tian Y, et al. Clonorchis sinensis granulin:identification, immunolocalization, and function in promoting the metastasis of cholangiocarcinoma and hepatocellular carcinoma. Parasit Vectors. 2017;10:262. doi: 10.1186/s13071-017-2179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong NC, Cheung PF, Yip CW, et al. Antibody against granulin-epithelin precursor sensitizes hepatocellular carcinoma to chemotherapeutic agents. Mol Cancer Ther. 2014;13:3001–12. doi: 10.1158/1535-7163.MCT-14-0012. [DOI] [PubMed] [Google Scholar]


