Abstract
Background:
Several studies indicate that chemokines play important roles in colorectal mucosal immunity.The chemokine CXCL5 which is expressed by epithelial cells within colorectal mucosa is a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. The purpose of this study was to investigate whether serum and tissue CXCL5 levels is altered in colorectal carcinomas (CRC) compared to colonic adenoma and normal mucosa. It also aimed to compare colon adenoma and colorectal cancer for blood CXCL5 and CEA levels, their sensitivity, and specificity.
Methods:
CXCL5 expression was assessed with immunohistochemistry staining in biopsy samples taken during colonoscopy in 22 colonic adenomas, 23 colorectal carcinomas and 23 normal colonic tissue samples. Also all patients’ serum CXCL5 and CEA levels were measured. This stduy was prospective observational study.
Results:
The number of cases who were stained positive with immunohistochemistry was found to be higher in the group with CRC. When compared with the other groups, both levels of serum CXCL5 and CEA were significantly high in the group CRC. Sensitivity and specificity of serum CXCL5 were found to be low as a result of the ROC analysis.
Conclusion:
Although the level of CXCL5 is high in CRC, its level in serum is not significant enough to support the early diagnosis of the disease.
Keywords: Colon, colorectal cancer, cytokine, immunohistochemistry, tumor
Introduction
Colorectal cancer (CRC) is the most common cancer in the gastrointestinal tract and is one of the three leading causes of cancer-related death among men and women in the Western world (Smink, 2015; Torre et al., 2015). Since early detection is critical for improving outcomes and reducing mortality of CRC patients, there is an urgent need to identify biomarkers for early diagnosis and prognosis of the disease. Currently, carcinoembryonic antigen (CEA) is the most commonly used circulating biomarker for CRC diagnosis (Nakamura et al., 1996; Duffy et al., 2003; Duffy et al., 2007). However, the elevation of CEA concentration rarely occurs in patients with early stages of CRC (Carpelan-Holmstrom et al., 2002). The sensitivity and validity of CEA detection are also not sufficient for early cancer detection (Locker et al., 2006). Except CEA, there are few other valuable biomarkers for monitoring CRC. Therefore, it is imperative to develop novel sensitive and specific circulating biomarkers for detection of CRC, especially in the early stages.
Genetic and environmental factors are effective in the development of colon cancer. Cellular oncogenes, growth factors, and receptors are accused of taking part in the development of colorectal cancer. Inflammation plays an important role in cancer development. The resolution of inflammation is an active coordinated process that requires the production of anti-inflammatory mediators, the termination of proinflammatory signalling pathways and the appropriate clearance or migration of inflammatory cells. Disruption of any of these processes can lead to chronic persistent inflammation and tumour growth (Lawrence, 2007). Chemokines (CKs) that were secreted from peritumoral neutrophils, cytokines, reactive oxygen species, proteinases, and proliferation of tumor cells have important roles in angiogenesis and metastasis (Gregory and Houghton, 2011). Besides being chemotactic for cells of the immune functions as well as a tool in acute and chronic inflammation, chemokines also promote angiogenesis and metastatic spread (Baier et al., 2005; Lazennec and Richmond, 2010). Epithelial neutrophil-activating peptide78 (ENA-78), also known as the CXCL5 chemoattractant for neutrophils, also contributes to the angiogenic properties and cancer progression. Epithelial cells of the colon and colorectal carcinomas were higher than in normal tissues (Keates et al., 1997; Rubie et al., 2008). Several reports have demonstrated relevance of CXCL5 expression and CRC(Baier et al., 2005; Dimberg et al., 2007; Speetjens et al., 2008). To date, no study has been performed on sensitivity and specificity of CXCL5 for CRC.
In this study, to facilitate the diagnosis of colorectal cancer and early diagnosis on behalf of a new role in the neutrophil-activating protein CXCL5, the authors will try to understand the disease course. It also aimed to compare colon adenoma and colorectal cancer for blood CXCL5 and CEA levels, their sensitivity, and specificity.
Materials and Methods
Patients and Samples. This study comprised blood and tissue samples that were obtained from 68 patients who underwent colonoscopy at the Department of Surgery, Samsun Training and Research Hospital, Samsun, Turkey, between November 2014 and May 2015. All blood and tissue samples were obtained with the informed written consent of the patients, and the protocol was approved by Ethics Committee of Ondokuz Mayis University Hospital, with 09.04.2014/747 as the decision number.
Patients were divided into three groups. The first group included 22 patients with colonic adenoma. During colonoscopy, blood samples for the determination of the CXCL5 level were taken from patients with 1–2 cm colorectal adenoma. Adenoma biopsies were obtained from the patients for CXCL5 determination. The second group included 23 patients with a malignant mass of colon and rectum. During colonoscopy, blood samples for determination of CXCL5 level were obtained in these patients. Malignant mass biopsies were obtained from these patients for CXCL5 determination. The third group included 23 patients with normal colon mucosa during colonoscopy. Blood samples and colonic biopsies for CXCL5 were obtained from these patients. In addition, serum CEA levels taken from patients in all three groups before the colonoscopy were measured.
Exclusion criteria. Patients with less than 1 cm and above 2 cm adenoma, patients using medication that could affect cytokine levels, patients with inflammatory disease, and pregnant women were excluded from the study.
ELISA for CXCL5. Serum CXCL5 levels were assayed by enzyme-linked immunosorbent assay (ELISA). Blood samples centrifuged quickly within 1 h. The separated serum was placed in an eppendorf tube. Plasma was stored at -80°C. First, samples and standards were prepared using the ENA-78 ELISA kit (Bioassay Technology, China) in the collected samples. The second, antibody labeled with biotin ELISA solutions, were added to samples and standards. The reaction was allowed to remain at 37°C for 60 minutes. Then, the plate was washed five times with an ELISA washer. Chromog A and B were added on top of the pit. It was incubated at 37°C for 10 minutes for coloring. A stock solution was added, the 450 nm wavelength was read on an ELISA reader, and serum CXCL5 was calculated. This process was completed in Tecan Brand Infinite m200PRO/NanoQuant reader and washer.
Immunohistochemistry staining for CXCL5. Immunohistochemistry in specimens were examined in the Ondokuzmayıs University Pathology Department. Specimens were routinely fixed in formalin and subsequently embedded in paraffin. Sections were taken at 4 µm thick from the selected block. An immunohistochemical study was performed by the streptavidin-biotin peroxidase method using the ENA-78 primary antibody (YZ-14, sc-73931, Santa Cruz Biotechnology, Santa Cruz, Calif., USA). Pancreas ductal adenocarcinoma was used as positive control. The best dilution ratio was determined by experimentation. The ENA-78 was used by diluting 1/50 percent. Endogenous peroxidase activity was quenched by treatment with 3% hydrogen peroxide for 10 minutes. Slides were allowed to cool at room temperature for 20 minutes after boiling a citrate buffer for 30 minutes. Block antibody to sections was performed for 10 minutes, followed by a 2 h administration of the ENA78 primer antibody. It was incubated with biotinylated anti-immunoglobulin and streptavidin-peroxidase conjugate for 20 minutes. 3.3’-diamino benzidine (DAB) containing kit (DAKO, Carpinteria, USA) as a coloring agent was used. Finally, the sections were stained for 4 minutes with Mayer’s hematoxylin (Histolab Products AB, Sweden). Sections were washed with pH 7.6 phosphate buffer until the DAB applications, then washed with distilled water after the DAB application. Operations were carried out at room temperature. Conclusions were evaluated with a light microscope Olympus BX51 (Olympus, USA, 1999). Cytoplasmic staining was considered positive.
Serum CEA levels taken before the operation were measured with Siemens Advice on Centaur XP immunoassay system. The normal reference range of serum CEA was considered 0-5 ng/ml for smokers and 0-2.5 ng / ml for nonsmokers. Cost of CXCL5 and CEA kits per patient were calculated.
Statistical Analysis
All analyses were performed with SPSS statistical software (version 16.0 for Windows; SPSS, Inc.). The Chi-square, Anova, and Kruskal-Wallis test was used for statistical evaluation. ROC curves were applied to analyze the diagnostic values of the CXCL5 and CEA. The Youden index (sensitivity + specificity-1) was chosen to identify the optimal cut-off threshold values. The average results were expressed as standard deviation and a P value < 0.05 was considered statistically significant.
Results
The study included 68 patients. Forty of patients were male (58.8%) and 28 of patients were women (41.2%); the mean age was 63,2±12, 63±12,8, 49,4±16,8 years old in group 1,2, and 3, respectively. The mean BMI was 25,8±3,4, 26,9±4,9, 27±3,1, kg/(m2) in group1,2, and 3, respectively.
CXCL5 expression was evaluated by immunohistochemistry in biopsy specimens. Positive staining of tumor epithelial cells in CRC tissues was observed. Weak staining was observed in apical epithelial cells within tumor neighbor tissues of CRC. Of 23 cases, 19 (82.6%) were positive staining (Figure 1 A, B). Focal weak staining was observed in the Group 1 adenomatous epithelium. In 22 cases, 8 (36.4%) were positive staining (Figure 1 C). For In 23 cases of adenomas and tumors not detected during colonoscopy and biopsies of the colon mucosa, 22 (95.6%) did not stain (Figure 1 D).
Figure 1.
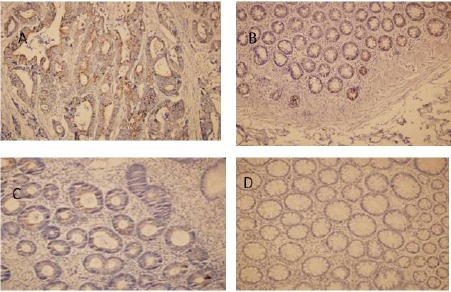
A-Representative Expression of CXCL5 in Colorectal Cancer. B-Focal weak immunostaining in apical epithelial cells within tumor neighbor tissues of CRC. C- Weak immunostainig in CRA specimens. D-No staining in normal colonic mucosa. Original magnification 400x.
When we compared the groups for immunohistochemical staining for CXCL5 expression; the p-value was 0,002, 0,010, and <0,001 in group 1 -2, group 1-3, and group 2- 3 respectively.
In Group 2, with colon cancer, greater staining was revealed than in the other two groups (p<0.001). The characteristic staining between groups; the rate was 8/22, 19/23, 1/23 in group 1, group 2, and group 3 respectively.
Serum CXCL5 and CEA values before the colonoscopy are shown in Table 1. Serum CXCL5 and CEA values were found significantly higher than in the other two groups in Group 2 (p<0.05; Kruskal-Wallis test).
Table 1.
Blood CXCL5 and CEA Levels
| Group 1 | Group 2 | Group 3 | P value | |
|---|---|---|---|---|
| CXCL5 mean (ng/L) | 66 | 89,6 | 66,6 | 0,016 |
| CEA Mean (ng/ml) | 1,3 | 9,8 | 1,16 | 0,026 |
Measured values of ENA-78(CXCL5) and CEA were compared with an area under the ROC curve. The areas under curve (AUC) for ENA-78 and CEA were 0.671 and 0.692, respectively (p=0.010, p=0.022). The cut-off value calculated for these two markers, PPDs, NPD, sensitivity and specificity values are shown (Table 2). The CEA ELISA kit cost was 1 euro, while the CXCL5 ELISA kit cost was 3 euros per patient.
Table 2.
Cut-off, PPV, NPV, Sensitivity and Specificity Values of CEA and CXCL5
| CEA | CXCL5 | |
|---|---|---|
| Cut-off value | 1,33 | 49,63 |
| PPV | 43,75% | 41,94% |
| NPV | 75,00% | 75,00% |
| Sensitivity | 61,00% | 57,00% |
| Specificity | 60,00% | 67,00% |
Discussion
Blood-based biomarkers for early detection of colorectal cancer could complement current approaches to CRC screening. Current methods widely deployed for colorectal cancer screening lack the necessary sensitivity and specificity required for population-based early disease detection. Cancer-specific protein biomarkers are thought to be produced by either the tumor itself or other tissues in response to the presence of cancers or associated conditions. Previously, CEA was identified as a potential CRC biomarker. Many studies have shown elevated CEA expression in Dukes’ stage C and D CRC (Hansen et al., 1974; Bray et al., 1975; Ahlemann et al., 1980; Goldenberg et al., 1981; Sadun, 1990; Japink et al., 2009). Also in our study, the serum CEA level of patients with colorectal cancer were found significantly higher than the other two groups, similar to previous studies (p=0.026). CEA expression significantly correlates with the presence of metastatic CRC, though it was not found to be an effective plasma biomarker of very early stage disease (Mahboob et al., 2015).
Today, it is known that chemokines have different roles in cancer biology. CKs regulate antitumor immunity, primarily by causing dendritic cells first to migrate towards the peripheral site and, after activation, to proceed towards draining lymph nodes (Caux et al., 2000). Previous studies demonstrated evidence that significantly increased CXCL5 expression associated with CRC (Baier et al., 2005; Dimberg et al., 2007; Rubie et al., 2008; Speetjens et al., 2008). First, Baier et al. reported that CXCL5 levels in colon carcinomas were higher than normal mucosa. Also, Rubie et al. showed a significant correlation between the expression of CXCL5 and CXCL1 in CRC and adenoma patients. Another study by Speetjens et al. was used to associate the level of CXCL5 RNA expression with prognosis. Higher expression in tumor tissue than in normal tissue was found in their experiments. However, they reported that absence of CXCL5 expression in tumor tissue was correlated with poor prognosis (Baier et al., 2005; Rubie et al., 2008; Speetjens et al., 2008). Dimberg et al. found that higher protein levels compared to normal colon mucosa CXCL5 in cancer tissue. Our study also provides evidence that significantly increased CXCL5 expression is associated with CRC, as also shown in other studies. We found higher immunohistochemical staining for CXCL5 in patients with CRC than the other groups (Dimberg et al., 2007).
Levels of plasma CXCL5 has been investigated with colorectal cancer patients and healthy controls. Kawamura et al. reported that serum CXCL5 was significantly elevated in patient with CRC compared with healthy volunteers (p=0.013). We also found serum CXCL5 values significantly higher in patients with CRC (p=0.016) than the other two groups (Kawamura et al., 2012). We assessed the sensitivity and specificity of CXCL5 for CRC, unlike other studies. In our study, we found that serum CXCL5 has low sensitivity (57%) and specificity (67%) for CRC. Sensitivity of CEA was 61% and specificity was 60% respectively. There was no significant difference between CXCL5 and CEA in the sensitivity and specificity. The cost of the CXCL5 assay was found to be higher than that of CEA (approximately three times higher).
However, CXCL5 was shown to have significantly higher staining in the CRC tissues in comparison to the CRA tissues. Since CRA constitutes a premalignant condition often preceding the development of colorectal malignancies, we hypothesized that a significant increase of chemokine expression in the CRC tissues with respect to the CRA tissues may indicate a transition from the premalignant condition to the development of the malignancy. Positive staining for CXCL5 may anticipate for carcinoma in suspicious adenomas.
Other methods (ELISA, PCR) were not used in CXCL5 expression, which has limited the study. However, this study is the first that evaluated sensitivity and specificity of CXCL5 for CRC.
In conclusion, we found that serum CXCL5 levels were increased in patients with colorectal cancer. Nevertheless, in spite of the high levels of CXCL5 in colorectal cancer tissue, levels in serum are not significant to support the early diagnosis of the disease. Due to the low sensitivity and specificity of serum CXCL5 levels were not found to be a suitable marker for the screening of CRC patients. However, the adenoma tissue compared to normal colon mucosa staining may be more important in the diagnosis of precancerous adenomas. In this regard, including an increased number of patients and detailed studies are needed to be done on different types of adenoma.
Acknowledgments
This study was not supported by any institution or company.
References
- Ahlemann LM, Staab HJ, Koch HL, et al. Carcinoembryonic antigen (CEA) measurements as an aid to management of patients with lung cancer treated by radiotherapy. Oncodev Biol Med. 1980;1:143–50. [PubMed] [Google Scholar]
- Baier PK, Eggstein S, Wolff-Vorbeck G, et al. Chemokines in human colorectal carcinoma. Anticancer Res. 2005;25:3581–4. [PubMed] [Google Scholar]
- Bray PF, Wu JT, Madsen AC. Carcinoembryonic antigen (CEA) assay. In diagnosis and management of cancer. Rocky Mt Med J. 1975;72:21–3. [PubMed] [Google Scholar]
- Carpelan-Holmstrom M, Louhimo J, Stenman UH, et al. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002;22:2311–6. [PubMed] [Google Scholar]
- Caux C, Ait-Yahia S, Chemin K, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- Dimberg J, Dienus O, Lofgren S, et al. Expression and gene polymorphisms of the chemokine CXCL5 in colorectal cancer patients. Int J Oncol. 2007;31:97–102. [PubMed] [Google Scholar]
- Duffy MJ, van Dalen A, Haglund C, et al. Tumour markers in colorectal cancer:European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348–60. doi: 10.1016/j.ejca.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Duffy MJ, van Dalen A, Haglund C, et al. Clinical utility of biochemical markers in colorectal cancer:European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 2003;39:718–27. doi: 10.1016/s0959-8049(02)00811-0. [DOI] [PubMed] [Google Scholar]
- Goldenberg DM, Neville AM, Carter AC, et al. CEA (carcinoembryonic antigen):its role as a marker in the management of cancer. J Cancer Res Clin Oncol. 1981;101:239–42. doi: 10.1007/BF00410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AD, Houghton AM. Tumor-associated neutrophils:new targets for cancer therapy. Cancer Res. 2011;71:2411–6. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- Hansen HJ, Snyder JJ, Miller E, et al. Carcinoembryonic antigen (CEA) assay. A laboratory adjunct in the diagnosis and management of cancer. Hum Pathol. 1974;5:139–47. doi: 10.1016/s0046-8177(74)80061-4. [DOI] [PubMed] [Google Scholar]
- Japink D, Leers MP, Sosef MN, et al. CEA in activated macrophages. New diagnostic possibilities for tumor markers in early colorectal cancer. Anticancer Res. 2009;29:3245–51. [PubMed] [Google Scholar]
- Kawamura M, Toiyama Y, Tanaka K, et al. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48:2244–51. doi: 10.1016/j.ejca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Keates S, Keates AC, Mizoguchi E, et al. Enterocytes are the primary source of the chemokine ENA-78 in normal colon and ulcerative colitis. Am J Physiol. 1997;273:G75–82. doi: 10.1152/ajpgi.1997.273.1.G75. [DOI] [PubMed] [Google Scholar]
- Lawrence T. Inflammation and cancer:a failure of resolution? Trends Pharmacol Sci. 2007;28:162–5. doi: 10.1016/j.tips.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Richmond A. Chemokines and chemokine receptors:new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–44. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–27. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- Mahboob S, Ahn SB, Cheruku HR, et al. A novel multiplexed immunoassay identifies CEA, IL-8 and prolactin as prospective markers for Dukes'stages A-D colorectal cancers. Clin Proteomics. 2015;12:10. doi: 10.1186/s12014-015-9081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tabuchi Y, Nakae S, et al. Serum carcinoembryonic antigen levels and proliferating cell nuclear antigen labeling index for patients with colorectal carcinoma. Correlation with tumor progression and survival. Cancer. 1996;77:1741–6. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1741::AID-CNCR49>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Rubie C, Frick VO, Wagner M, et al. ELR+CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8:178. doi: 10.1186/1471-2407-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadun AA. Distinguishing between clinical impairments due to optic nerve or macular disease. Metab Pediatr Syst Ophthalmol. 1990;13:79–84. [PubMed] [Google Scholar]
- Smink DS. Schwartz's principles of surgery, 10th Edition. Ann Surg. 2015;261:1026. [Google Scholar]
- Speetjens FM, Kuppen PJ, Sandel MH, et al. Disrupted expression of CXCL5 in colorectal cancer is associated with rapid tumor formation in rats and poor prognosis in patients. Clin Cancer Res. 2008;14:2276–84. doi: 10.1158/1078-0432.CCR-07-4045. [DOI] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]


