Abstract
The tentative identification of bioactive compounds in the extract of Vernonia amygdalina leaf was carried out using positive ionization of Liquid chromatography-mass spectrometry quadrupole time of flight (LC-Q-TOF/MS). The positive ionization is associated with the presence of saponins, flavonoids, alkaloids, terpenoids, and glycosides. Tentative assignments of the secondary metabolites were performed by comparing the MS fragmentation patterns with Waters® UNIFY library which allows positive identification of the compounds based on the spectral match. All the metabolites compounds were estimated and presented in a BPI (Base peak intensity) plot. These data are the unpublished supplementary materials related to “Ethanolic extraction of bioactive compounds from V. amygdalina leaf using response surface methodology as an optimization tool” (Alara et al., 2018).
Specification table
| Subject area | Natural product research, Medicinal chemistry |
| More specific subject area | Analytical chemistry |
| Type of data | Figure, Table |
| How data were acquired | Liquid chromatography-mass spectrometry quadrupole time of flight |
| Data format | Analysed |
| Experimental features | The tentative estimation of secondary metabolites in V. amygdalina leaf was examined |
| Data source location | Centre of Excellence for Advanced Research in Fluid Flow (CARIFF), Universiti Malaysia Pahang, Gambang, Malaysia |
| Data accessibility | Data have been outlined within this article and Supplementary material |
| Related research article | Alara OR, Abdurahman NH, Olalere OA. Ethanolic extraction of bioactive compounds from Vernonia amygdalina leaf using response surface methodology as an optimization tool. J. Food Meas. Charact. 2018 12:1107–1122. |
Value of the data
-
•
The data can be used as baseline showing the present saponins, flavonoids, terpenoids, alkaloids, and glycosides in the extract of Vernonia amygdalina leaf.
-
•
The data can be used to provide information on LC-Q-TOF/MS conditions of the positive ionization secondary metabolites in V. amygdalina leaf.
-
•
The data presented in this manuscript can further be isolated, purified, characterized and quantified.
1. Data
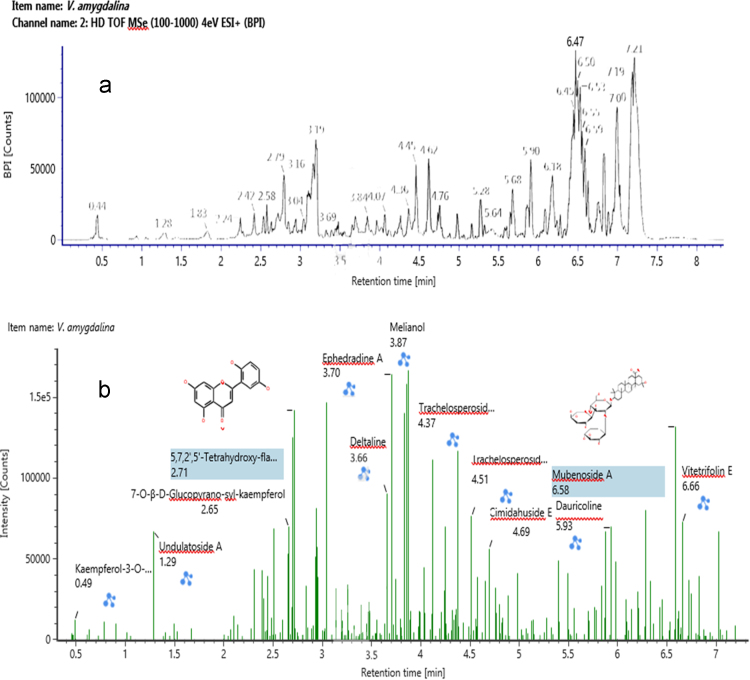
The data illustrate the LC-Q-TOF/MS base peak intensity profiles of saponins, flavonoids, terpenoids, alkaloids, and glycosides obtained from the extract of V. amygdalina leaf as compared to Waters® UNIFY library in the positive ionization (Fig. 1). The tentatively assigned secondary metabolites are presented in the Supplementary table 1.
Fig. 1.
LC-Q-TOF/MS base peak intensity profiles of positive ions secondary metabolites (a) and identified compounds (b).
2. Material, method and sample preparation
The powdered sample of V. amygdalina leaf collected from Gambang, Malaysia was extracted at the optimum conditions based on the previous report using an ETHOS X microwave-assisted extractor (Milestone, Italy) [1]. A 10 g sample of V. amygdalina leaf was irradiated for 4 min at the temperature of 70 °C, microwave power of 558 W, the solvent concentration of 76% v/v ethanol, and solid/solvent ratio of 1:10 g/mL. After the extraction process, the extract mixture was filtered using Whatman filter No. 1 and concentrated through a rotatory evaporator. Then, the extract was prepared for LC-Q-TOF/MS analysis.
The concentrated extract of V. amygdalina leaf was re-dissolved in HPLC grade methanol to make a concentration of 0.02 mg/mL prior to LC-Q-TOF/MS analysis. The LC-Q-TOF/MS equipped with Photodiode Array detector (Waters Vion IMS, USA) and a symmetry C18 column of 100 mm × 2.1 mm, 1.8 µm particle size (Waters Acquity UPLC HSS T3, USA) was employed. The mobile phases used for the analysis were A (water with 0.1% formic acid) and B (100% acetonitrile). The gradient elution as previously reported was employed [2], [3]. Briefly, the gradients elution were 90% A and 10% B (0.00 min) 90% A and 10% B (0.00–1.25 min), 45% A and 55% B (1.25–4.17 min), 10% A and 90% B (4.17–6.25 min), 90% A and 10% B (6.25–8.34 min), with the flow rate of 0.5 mL/min and injection volume of 20 µL. The metabolites in the plant extract were tentatively assigned using SYNAPT mass spectrometer (Waters) coupled with an electrospray ionization operated in positive ion mode, comprised of the following operating conditions: mass range of 100–1000 m/z, low collision energy of 4 keV, high collision energy ramp start at 10 keV, column temperature of 40 °C, sample temperature of 15 °C, gas flow of 0.5 mL/min, the source type was ESI with desolvation temperature of 550 °C, source temperature of 120 °C, cone gas of 50 L/h, capillary voltage of 1.50 kV, and desolvation gas flow rate of 800 L/h. The compounds were tentatively assigned using Waters® UNIFY Software 1.0.0.
Acknowledgement
The authors of this manuscript acknowledge the support of Universiti Malaysia Pahang, Malaysia under the soft grant of RDU180329.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.10.159.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.10.159.
Contributor Information
Oluwaseun Ruth Alara, Email: ruthoalao@gmail.com.
Nour Hamid Abdurahman, Email: abrahman@ump.edu.my.
Transparency document. Supplementary material
Supplementary material
.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Alara O.R., Abdurahman N.H., Olalere O.A. Ethanolic extraction of bioactive compounds from Vernonia amygdalina leaf using response surface methodology as an optimization tool. J. Food Meas. Charact. 2018;12:1107–1122. [Google Scholar]
- 2.Alara O.R., Abdurahman N.H., Ukaegbu C.I., Azhari N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018;122:533–544. [Google Scholar]
- 3.Alara O.R., Abdurahman N.H., Ukaegbu C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants. 2018;11:12–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material