Abstract
Objective:
The aim of this long-term follow-up study was to investigate the association of local and systemic cardiovascular complications with endothelium-dependent and-independent microvascular relaxations and blood biomarkers and biochemicals in patients with peripheral arterial disease (PAD) caused by atherosclerosis.
Methods:
This prospective study included 67 patients with PAD who had not undergone any endovascular intervention, peripheral arterial surgery, or major amputation. Changes in the microvascular blood flow were measured using laser Doppler imaging after iontophoresis of acetylcholine (ACh) and sodium nitroprusside (SNP). The biochemical markers of high sensitivity C reactive protein (hs-CRP), nitric oxide (NO), total antioxidant capacity (TAC), asymmetric dimethyl arginine (ADMA), and hydrogen sulfide (H2S) levels were measured from blood samples. All the patients were followed up for 5 years to determine the development of cardiovascular adverse events (CVAEs) and major amputation. At the end of the follow-up period, the patients were classified into two groups: those who had a CVAE [CVAE (+)] and those who did not experience CVAE [CVAE (−)]. Parameters such as demographic features, atherosclerotic risk factors, chronic ischemia category, microvascular endothelial functions, and plasma biomarkers were compared between the groups.
Results:
A total of 67 patients comprising 61 (91%) males and 6 (9%) females with a mean age of 62.3±9.7 years were included. During the follow-up period, 29 patients had CVAE (43.3%) and 38 patients did not have CVAE (56.7%). There was no difference between the groups in terms of ACh and SNP-induced vasodilation responses. Plasma high density lipoprotein (HDL) cholesterol values were lower in the CVAE (+) group [(CVAE+HDL: 38.4±9.1), (CVAE−HDL: 44.7±11.1), p=0.02]. Plasma hs-CRP values were significantly higher in the CVAE (+) group [(CVAE+ hs-CRP: 14.3±20.6), (CVAE−hs-CRP: 5.9±10.9), p=0.004]. No significant difference was observed between the groups in terms of plasma biomarkers and other biochemical levels.
Conclusion:
Based on the study findings, it was concluded that only low plasma HDL and high hs-CRP levels were risk factors for the development of CVAEs during follow-up of patients with PAD.
Keywords: atherosclerosis, cardiovascular adverse event, high density lipoprotein, high sensitive C-reactive protein, endothelial function
Introduction
Peripheral arterial diseases (PAD) are significant in terms of more serious distant organ involvements, which can occasionally result in death depending upon local ischemic symptoms in the extremities and the systemic effects of the disease. Atherosclerosis is the most prevalent form of PAD and has a high prevalence for cardiovascular events and mortality (1, 2). Endothelial dysfunction is known to be the first stage in atherosclerosis development in the mechanism of cardiovascular pathology in patients with PAD (3). The disruption of the vasodilator and antithrombotic and anti-inflammatory properties of the endothelium increases the risk of cardiovascular events.
Clinical studies have reported that both plasma biomarkers related to endothelial functions and vascular endothelial reactivity tests are abnormal in patients with atherosclerosis (4-6). This abnormality is believed to occur as a result of nitric oxide (NO) reduction, oxidative stress, and inflammation and the associated risk factors for atherosclerosis. The evaluation of endothelial function is accepted as a reliable criterion of vascular health, and this method can particularly be used for the prediction of cardiovascular events (7). Endothelial dysfunction plays a crucial role leading to structural changes and clinical symptoms in cardiovascular diseases. Therefore, researchers have recently focused on determining endothelial dysfunction at an early stage (4, 5, 8).
It has been hypothesized that the evaluation of endothelial function in the peripheral arteries would provide information from other vascular beds and could be a valuable method in terms of systemic and local risk estimation for patients. However, the relationship of long-term vascular bed events with endothelial function and blood biomarkers has not been evaluated. The aim of this study was to investigate the association of local and systemic cardiovascular complications with endothelium-dependent and -independent microvascular relaxations, blood biomarkers, and biochemicals in patients with PAD caused by atherosclerosis.
Methods
The approval for this prospective study of 67 patients with PAD was granted by the Local Ethics Committee (approval no: 20030809171). Informed consent for voluntary participation in the study was obtained from all participants.
Study design
The study included patients who had not undergone any endovascular intervention, peripheral arterial surgery, or major amputation (amputation of the lower limb above the ankle). The exclusion criteria were upper extremity arterial involvement, patients with Buerger`s disease, major infection, cancer and autoimmune diseases, a history of major vascular surgical operation, pregnancy, or age <18 years.
For each patient, demographic and atherosclerotic risk factors such as age, gender, obesity (body mass index ≥30.00), diabetes, hypertension, and smoking were recorded. All other risk factors of the patients in terms of pulmonary, renal, carotid, and cardiovascular systems were also determined. Peripheral arterial involvement was examined by measuring the ankle/brachial pressure index (ABPI). The clinical degree of peripheral ischemia was classified according to Rutherford’s Clinical Chronic Ischemia Category (9).
The microvascular vasodilation response as a measure of endothelial function was measured using laser Doppler imaging of cutaneous erythrocyte flux after iontophoresis of acetylcholine (ACh) and sodium nitroprusside (SNP). Plasma hemoglobin, glycated hemoglobin A1c (HbA1c), glucose, creatinine, total cholesterol, triglyceride, total bilirubin, uric acid, homocysteine, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, very low density lipoprotein (VLDL) cholesterol, high-sensitivity C-reactive protein (hs-CRP), white blood cell (WBC), nitric oxide (NO), total antioxidant capacity (TAC), asymmetric dimethyl arginine (ADMA), and hydrogen sulfide (H2S) levels were measured from blood samples. The patients were treated using either medical, open vascular, or endovascular intervention methods based on their clinical presentation and the physician’s preference. All patients were followed up for approximately 5 years to determine cardiovascular adverse events (CVAE) development. [CVAE: cardiovascular death, atherothrombotic stroke, acute coronary syndrome (unstable angina pectoris, without Q-wave acute myocardial infarction, and with Q-wave acute myocardial infarction) and major amputation (amputation of the lower limb above the ankle)].
CVAE development was determined by examining the medical history, physical examination, and related specialist physician reports on annual controls from the study initiation. The cardiovascular death status was determined by examining the medical history and death reports from relatives of patients who could not receive timely control. The patients were classified into two groups: those who had experienced a cardiovascular adverse event [CVAE (+)] and those who did not experience CVAE [CVAE (-)] during the follow-up period. There was no difference between the groups in terms of medical treatment of the patients. The two groups were compared in terms of demographic features, atherosclerotic risk factors, initial ischemia categories, microvascular endothelial functions, and plasma biomarkers.
Measurement of microcirculatory function
This method, described in detail by Henricson et al. (10), was used to measure the microvascular function. In the iontophoresis technique, charged molecules migrate across the skin under the influence of the applied electric field, and the effects in cutaneous vasodilation are observed using a laser Doppler imager. The vasodilator responses formed by iontophoresis of ACh (endothelium-dependent vasodilator) and SNP (endothelium-independent vasodilator) are quantified. Patients were fasted overnight and kept in a dark room at a temperature of 23±1°C for 20 min for acclimatization. Meanwhile, the volar aspect of the right forearms was lightly wiped with alcohol and deionized water. Subsequently, two iontophoresis chambers (LI 611 drug delivery electrodes; Perimed, Jarfalla, Sweden) were attached 5 cm below the medial condyle of the right forearm using double-sided adhesive rings. Precaution was taken to place the chambers at more than 10 cm apart, thus avoiding broken skin, superficial veins, and hair.
The anodal and cathodal chambers were filled with 0.25 mL 1% (w/v) ACh (Sigma-Aldrich Chemicals, UK) and 0.25 mL 1% (w/v) SNP (Sigma-Aldrich Chemicals, UK), respectively. Drug release from each chamber was provided with a battery-controlled constant current iontophoresis device (Perilont 382 power supply, Perimed, Jarfalla, Sweden). The microvascular erythrocyte flux of the forearm skin was assessed using a cumulative dose response protocol, which has been described in detail previously (10). Drug distribution was ensured by the incremental duration and fixed current. Thus, 0.1 mA was applied for 5, 10, 20, 40, and 80 s, which corresponded to 0.5, 1, 2, 4, and 8 milliCoulombs (mC). Skin perfusion was measured using a laser Doppler imager (PeriScan PIM II, Perimed, Jarfalla, Sweden; wavelength 670 nm, power 1 mW, and beam diameter 1 mm). The technique is based on the Doppler shift imparted by moving blood cells in the underlying tissue to the backscattered light. The laser is scanned over both chambers and through the cover slips in a raster manner. The backscattered light is collected using photo detectors and converted into a signal proportional to perfusion in arbitrary perfusion (flux) units (PU), which is displayed as a color-coded image on a monitor. Perfusion measurements were obtained using the imager manufacturer’s image analysis software (LDPIwin software, Perimed, Jarfalla, Sweden).
The baseline image was recorded before the drug and current application. After each iontophoretic drug application, eight laser images were scanned, with each image scan taking 30 s. The median current in the region where the images were scanned was determined by considering approximately 700 measurement points. In total, 44 images (including four before drug administration) were obtained after incremental duration and fixed current, according to the time protocol described above. The total perfusion response was shown by the area under the curve for 44 image scans. The numerical responses of all images were measured at baseline and after each iontophoretic charge (0.5, 1, 2, 4, and 8 mC). The flow increases were calculated using a percentage enhancement formula as follows: Flow increase=[(mean value of 8 calculations in each charge−basal value)/basal value]×100.
Assessment of plasma endothelial biomarkers
The blood samples were collected, centrifuged, and the separated plasmas were frozen. The plasma NO level was measured using the spectrophotometric method based on the Griess reaction (11). We used a modified version of the method previously described by Navarro-Gonzálvez et al. (11) other endothelial biomarkers of TAC and H2S plasma levels were measured using the spectrophotometric method as described previously (12, 13). Plasma TAC values were measured using neocuproine as the chromogenic agent and H2S levels were measured based on methylene blue absorption rates. Plasma ADMA levels were measured using ELISA kits (Immunodiagnostic A.G., Germany).
Statistical analysis
The statistical analysis was performed using IBM Statistical Package for Social Sciences (SPSS) ver. 18.0 (IBM Co., Armonk, NY, USA). The continuous data were reported as mean±standard deviation. The nominal data were reported as the number of subjects. For group comparisons, the Shapiro–Wilk test was used to examine whether the continuous variables were normally distributed. For normally distributed variables, the Student’s t-test was used to compare the mean differences between the two groups. For not normally distributed variables, (follow-up, glucose, creatinine, VLDL, triglyceride, total bilirubin, homocysteine, hs-CRP, WBC, ADMA, and H2S), the nonparametric Mann-Whitney U test was used, and median, minimum and maximum values were included in descriptive statistics. Categorical data were compared using the Chi-squared and Fisher’s exact tests. A value of p<0.05 was considered statistically significant.
Results
A total of 67 patients with PAD were included in the study, comprising 61 males (91%) and 6 females (9%) with a mean age of 62.3±9.7 years. When the treatment methods applied to the patients were evaluated, conventional surgery on 22 patients (32.9%; seven, aortofemoral bypass; one, iliofemoral bypass; seven, femoropopliteal bypass; six, femorodistal bypass; and one, popliteodistal bypass), percutaneous transluminal angioplasty on 12 patients (17.9%), and medical treatment for 33 patients (49.2%) were performed. During the follow-up period, 16 patients had acute coronary syndrome (unstable angina pectoris, non-Q-wave acute myocardial infarction, Q wave acute myocardial infarction; 23.9%), 12 patients died of cardiovascular reasons (17.9%), 10 patients had major lower limb amputation as the result of ischemic arterial disease (14.9%), and 3 patients had atherothrombotic stroke (4.5%). In total, 29 (43.3%) patients had CVAE (43.3%), and 38 (56.7%) patients did not experience any CVAE (Table 1).
Table 1.
Distribution of cardiovascular adverse events among patients
| Patient | Number of patients (n) | Percentage (%) |
|---|---|---|
| CVAE (+) | 29 | 43.3 |
| Cardiovascular death | 12 | 17.9 |
| Atherothrombotic stroke | 3 | 4.5 |
| Acute coronary syndrome | 16 | 23.9 |
| Major amputation | 10 | 14.9 |
| Below-the-knee | 6 | 8.9 |
| Over-the-knee | 4 | 6 |
CVAE- cardiovascular adverse events
The mean age of the patients in the CVAE (+) group was 62.7±10.2 years, and the follow-up period was 54.1±10.3 months. In the CVAE (−) group, the mean age was 62.0±9.4 years, and the mean follow-up was 51.3±12.7 months. Patients in both groups had similar age, gender, and follow-up period (Table 2). Both groups were similar based on the classification made according to the ABPI values and chronic ischemia category. There was no difference between the groups with respect to the initial atherosclerotic risk factors, including diabetes mellitus, smoking, hypertension, and obesity, or in the assessment conducted based on cardiac, pulmonary, renal, and carotid functions (Table 2). On evaluation of the microvascular endothelial functions of the patients, ACh-induced endothelium-dependent and SNP-induced endothelium-independent relaxation responses were not different between the groups (Fig. 1, 2).
Table 2.
Comparison between the groups in terms of demographic data, initially detected cardiovascular risk factors, and comorbidities
| Patient | CVAE (-) (n=38) Mean±SD | CVAE (+) (n=29) Mean±SD | P* | ||
|---|---|---|---|---|---|
| Age (years) | 62.7±10.2 | 62.0±9.4 | 0.772 | ||
| ABPI | 0.54±0.2 | 0.46±0.1 | 0.085 | ||
| Follow up (months) Mean±SD | 54.1±10.3 | 51.3±12.7 | 0.421 | ||
| Median (min.-max.) | 60 (25-60) | 60 (25-60) | |||
| n | % | n | % | P* | |
| Gender | |||||
| Male | 34 | 89.5 | 27 | 93.1 | 0.691 |
| Female | 4 | 10.5 | 2 | 6.9 | |
| DM | |||||
| No | 21 | 55.3 | 17 | 58.6 | |
| Type 2 (regulated by medicine) | 13 | 34.2 | 5 | 17.2 | 0.161 |
| Type 2 (regulated by insulin) | 4 | 10.5 | 7 | 24.1 | |
| Smoking | |||||
| Non-smoker | 12 | 31.6 | 7 | 24.1 | |
| Smoking for the previous 10 years | 13 | 34.2 | 12 | 41.4 | 0.836 |
| Smoking for the previous 1 year | 8 | 21.1 | 5 | 17.2 | |
| Smoking | 5 | 13.2 | 5 | 17.2 | |
| Obesity | |||||
| No | 36 | 94.7 | 27 | 93.1 | 1.000 |
| Yes | 2 | 5.3 | 2 | 6.9 | |
| Hypertension | |||||
| Normotensive | 17 | 44.7 | 20 | 69 | |
| Under control, with single medicine | 15 | 39.5 | 7 | 24.1 | 0.085 |
| Under control, with two medicines | 6 | 15.8 | 1 | 3.4 | |
| Out of control, with more than two medicines | 0 | 0 | 1 | 3.4 | |
| Cardiac | |||||
| Asymptomatic | 23 | 60.5 | 20 | 69 | |
| Asymptomatic and past MI or occult MI in ECG | 12 | 31.6 | 7 | 24.1 | |
| Stable angina | 2 | 5.3 | 1 | 3.4 | 0.882 |
| Unstable angina | 1 | 2.6 | 1 | 3.4 | |
| Renal | |||||
| Normal | 33 | 86.8 | 28 | 96.6 | |
| Creatinine <2.4 (mg/dL) | 5 | 13.2 | 0 | 0 | 0.071 |
| Creatinine 2.5–5.9 (mg/dL) | 0 | 0 | 1 | 3.4 | |
| Pulmonary | |||||
| Normal | 36 | 94.7 | 28 | 96.6 | 1.000 |
| Mild dyspnea | 2 | 5.3 | 1 | 3.4 | |
| Carotid | |||||
| Asymptomatic | 33 | 86.8 | 28 | 96.6 | |
| Asymptomatic but lesion (+) | 1 | 2.6 | 0 | 0 | 0.524 |
| TIA | 1 | 2.6 | 0 | 0 | |
| Stroke | 3 | 7.9 | 1 | 3.4 | |
| Chronic ischemia category | |||||
| Category 1 | 7 | 18.4 | 3 | 10.3 | |
| Category 2 | 13 | 34.2 | 8 | 27.6 | |
| Category 3 | 8 | 21.1 | 5 | 17.2 | 0.289 |
| Category 4 | 4 | 10.5 | 3 | 10.3 | |
| Category 5 | 6 | 15.8 | 6 | 20.7 | |
| Category 6 | 0 | 0 | 4 | 13.8 | |
CVAE - cardiovascular adverse events; ABPI - ankle/brachial pressure index; DM - diabetes mellitus; TIA - transient ischemic attack; SD - standard deviation; MI - myocardial infarction; ECG - electrocardiogram
Figure 1.
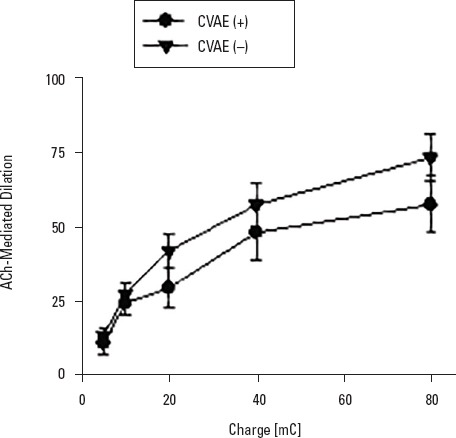
Comparison of ACh-induced endothelium-dependent microvascular flow increment between cardiovascular adverse events (+) and (–) groups. Ach-induced endothelium-dependent increase in microvascular perfusion of skin was not significantly different between the groups. Values are expressed as mean±SD (n=27-35)
Figure 2.
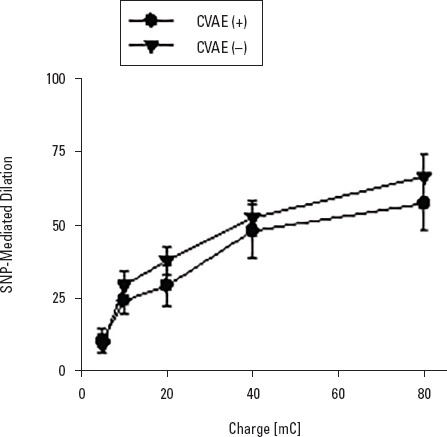
Comparison of SNP-induced endothelium-independent microvascular flow increment between adverse events (+) and (–) groups. SNP-induced endothelium-independent increase in microvascular perfusion of skin was not significantly different between the groups. Values are expressed as mean±SD (n=28-36)
The results obtained from the blood samples of the plasma biomarkers and biochemicals related to endothelial functions and the comparison of these results are presented in Table 3. No statistically significant differences for blood glucose, HbA1c, creatinine, uric acid, total cholesterol, LDL, VLDL, triglycerides, total bilirubin, homocysteine, WBC, and hemoglobin values were observed between the groups. Statistically significant differences in plasma HDL and hs-CRP levels were determines between the groups. Plasma HDL levels were lower in the CVAE (+) group than in the CVAE (−) group [(CVAE+HDL: 38.4±9.1), (CVAE−HDL: 44.7±11.1), p=0.02, p<0.05]. Plasma hs-CRP levels were significantly higher in the CVAE (+) group than in the CVAE (−) group [(CVAE+hs-CRP: 14.3±20.6), (CVAE−hs-CRP: 5.9±10.9), p=0.004, p<0.01]. For the biomarkers NO, ADMA, TAC, and H2S for evaluating endothelial functions, no statistically significant difference was determined between the groups.
Table 3.
Comparison between the groups in terms of laboratory values and endothelial biomarkers
| CVAE (–) | CVAE (+) Mean±SD | Median (min.-max.) | Mean±SD (min.-max.) | Median | P |
|---|---|---|---|---|---|
| *Glucose (mg/dL) | 118.7±45.6 | 104.5 (68-280) | 145.2±79.1 | 114 (61-380) | 0.238 |
| *Creatinine (mg/dL) | 1.1±0.3 | 1 (0.7-2.35) | 1.1±1 | 0.9 (0.1-5.1) | 0.077 |
| Total cholesterol (mg/dL) | 199.7±51.8 | 194.9±46.8 | 0.704 | ||
| HDL (mg/dL) | 44.7±11.1 | 38.4±9.1 | 0.020 | ||
| LDL (mg/dL) | 117.4±41.6 | 116±32.4 | 0.885 | ||
| *VLDL (mg/dL) | 39.1±24.5 | 31.5 (11-109) | 40.5±20.3 | 37 (8-64) | 0.384 |
| *Triglyceride (mg/dL) | 155.1±98.3 | 122 (55-479) | 157±55.8 | 161.5 (71-276) | 0.163 |
| *Total bilirubin (mg/dL) | 0.4±0.1 | 0.4 (0.1-0.9) | 0.6±0.3 | 0.5 (0.1-1.6) | 0.240 |
| *Homocysteine (µmol/L) | 12.4±6.8 | 12.3 (1.2-32) | 11.1±6.9 | 9.8 (1.2-32) | 0.332 |
| *hs-CRP (mg/L) | 5.9±10.9 | 2.9 (0.2-59) | 14.3±20.6 | 6.8 (0.8-90) | 0.004 |
| *WBC (µL) | 9±2.9 | 8.1 (4.9-16.8) | 9.5±3.1 | 8.7 (4.9-16.4) | 0.458 |
| Hemoglobin (g/dL) | 14.1±1.9 | 13.7±2.2 | 0.506 | ||
| Hemoglobin A1c (%) | 6.9±1.4 | 6.3±1.7 | 0.391 | ||
| Uric acid (mg/dL) | 5.8±1.4 | 4.9±1.5 | 0.082 | ||
| NO (µM) | 67.2±30 | 79±41.5 | 0.329 | ||
| TAC (µM) | 733.9±184.9 | 709.9±94.2 | 0.604 | ||
| *ADMA (µM) | 1.2±1.4 | 0.8 (0.1-6.4) | 2.1±3.1 | 0.7 (0.3-10.7) | 0.792 |
| *H2S (µM) | 52.9±35.8 | 41.7 (14.5-164.7) | 48±30.1 | 45.9 (12.1-110.8) | 0.695 |
These variables were compared with nonparametric Mann–Whitney U test because they were not normally distributed.
CVAE - cardiovascular adverse events; HDL - high density lipoprotein cholesterol; LDL - low density lipoprotein cholesterol; VLDL - very low density lipoprotein cholesterol; hs-CRP - high-sensitivity C-reactive protein; WBC - white blood cell; NO - nitric oxide; TAC - total antioxidant capacity; ADMA - asymmetric dimethyl arginine; H2S - hydrogen sulfide;
Discussion
In the present study, the association between the prognosis of PAD caused by atherosclerosis and endothelium-dependent and -independent microvascular relaxations and plasma biomarkers was evaluated. Although the microvascular endothelial functions of the CVAE (+) patients with PAD displayed a general decreasing trend from the values at baseline in contrast to the CVAE (−) patients, there was no statistically significant difference. Similarly, there was no difference in the levels of plasma biomarkers NO, ADMA, TAC, and H2S, which reflect endothelial functions. However, the results of this study revealed that the initial plasma HDL levels of CVAE (+) patients were lower and the hs-CRP values were higher than those of the CVAE (−) group.
Endothelial dysfunction is a deterioration of the balance between vasodilatation and vasoconstriction in the vascular system, with a predominance of vasoconstriction (14). When the lower or upper extremity arteries are occluded in PAD, many inflammatory and vasoactive mediators are released, leading to endothelial dysfunction in the remote organs because of the large surface area of the peripheral vascular bed. Generally, endothelial dysfunction occurs in the early phases of cardiovascular diseases, and the evaluation of endothelial function is a significant method to identify individuals at risk of these diseases (4).
In a study by Brevetti et al. (5, 8) in 131 PAD patients followed up for 23 months, it was determined that from the acute cardiovascular events perspective, the original FMD values of patients with an acute cardiovascular event were lower. In the same study, it was reported that the reliability of the prognosis estimation was increased if FMD and ABPI were used concomitantly in patients with PAD. In another similar study, 199 patients with PAD (a heterogeneous sample comprising patients with carotid and aorta aneurysm together with patients with lower extremity PAD) were followed up for 1.2 years after FMD measurement. The initial FMD values of 35 patients who experienced a cardiovascular event (myocardial infarction, unstable angina, and stroke) were determined to be significantly low (15).
The findings of these studies suggest that impaired endothelial function in patients with PAD is a measure that can be used as a prognostic parameter for the prediction of cardiovascular events. However, there are a limited number of studies that have investigated the prognostic importance of endothelial function in PAD in terms of general cardiovascular health (16-22). Moreover, many of those studies included a heterogeneous patient population and a limited follow-up period. Furthermore, cardiovascular events were not standardized in those studies. Although all the studies reported that endothelial dysfunction increased the risk of cardiovascular morbidity, there is no information regarding extremity protection or amputation levels.
The present study can be considered valuable because of the homogeneous distribution of the patient groups, the longer follow-up period compared to previous studies, and the inclusion of peripheral vascular morbidity, such as major lower extremity amputation. Moreover, to our knowledge, this is the first study to have investigated the relationship between cardiovascular mortality/morbidity of patients with PAD and endothelial functions using the iontophoretic laser Doppler flowmetry method. Although the relationship between microvascular dysfunction and prognosis was not clearly determined in this study, this could be attributed to the surplus of standard error mean (SEM) values and the low number of patients. Nevertheless, the study findings revealed that high plasma hs-CRP and low HDL level are risk factors for patients in terms of CVAE development.
CRP is an acute phase reactant determining cardiovascular events in healthy individuals or individuals known to have had a previous cardiovascular disorder (23, 24). Although CRP values do not have sufficient sensitivity to vascular disorders in the long term, hs-CRP has been shown to be more responsive and sensitive to atherosclerosis (25, 26). However, no association with other independent risk factors has been demonstrated. In the present study, hs-CRP levels were evaluated instead of plasma CRP levels because of its relationship with atherosclerosis and because it is more sensitive.
Vascular hs-CRP production is stimulated by cytokines such as IL-1 and IL-6. Vascular hs-CRP inhibits endothelial nitric oxide synthase, increases the expression of adhesion molecules, contributes to vasoconstriction with the expression of oxygen products reactive from monocytes and neutrophils and cytokines, and provides platelet activation and vascular smooth muscle cells migration and proliferation, thereby playing a critical role in atherosclerosis development (26, 27). Some studies have reported that the CRP level correlates with FMD (5, 28). However, no previous study has investigated the relationship of hs-CRP and microvascular function in patients with PAD. In the present study, the evaluation of cutaneous microvascular endothelial functions was applied instead of methods examining the endothelial functions of large conduit arteries, such as FMD of patients with atherosclerosis, because the method used in the present study can diagnose atherosclerosis in the early phases. Although the microvascular functions of CVAE (+) patients are not correlated with plasma hs CRP levels, determining high hs-CRP levels alone is also valuable. It has been previously reported that it is possible to average multiple measurements in plasma hs-CRP levels measurements and that evaluations with a single measurement are sufficient (28-30). The single measurement was preferred in the present study, both for patient comfort and cost efficiency.
In a study by Rost et al. (31), the plasma CRP levels of 591 males and 871 females were examined. In a 14-year follow-up period, 196 patients had ischemic stroke and temporary ischemic attack. The study reported that regarding ischemic stroke and transient ischemic attack, 25% higher CRP levels placed females at two-fold more risk and males at three-fold more risk. In a study by Ridker and Cook (32), it was determined that individuals with plasma hs-CRP levels >10 mg/L are at high risk of cardiovascular events. The current study supports these studies because significantly high plasma hs-CRP levels (14.3±20.6 mg/L) were detected in the CVAE (+) patients with PAD.
Previous studies have demonstrated that low plasma HDL levels increase the risk of cardiovascular event development (33-35). Similarly, in the present study, initial plasma HDL levels of the CVAE (+) patients were observed to be lower. The antiatherogenic property of HDL is based on cholesterol being returned from peripheral tissues to the liver and discarded in the bile, thus mediating contrary carriage (36). Another endothelial protective mechanism of HDL is the inhibition of caspase activation and the prevention of endothelial cell apoptosis (37). In a study of patients with hypercholesterolemia by Spieker et al. (38), intravenous reconstituted HDL infusion was reported to increase the bioavailability of NO and ensure a rapid return of endothelial-dependent vasodilatation to normal. In contrast to the study that reported beneficial effects of HDL on endothelial function in vivo, the results of the present study determined no correlation between the HDL level and microvascular endothelial functions. The reason for this difference could be because of the method by which endothelial functions were evaluated and the different patient groups.
Study limitations
Limitations of this study include; the high sensitivity of the evaluation method to external factors such as fixing the patient’s arm, the room temperature and the darkness, the high SEM values of the findings, and the low number of patients. In addition, only major amputations were considered as local complications of atherosclerosis.
Conclusion
In conclusion, the results of this study demonstrated that low plasma HDL levels and high hs CRP levels measured independently of endothelial functions of patients with PAD are risk factors for CVAE development in subsequent years. There is a need for further studies with greater patient numbers and longer follow-up periods to clarify the relationship between CVAE and microvascular endothelial function.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.A., C.K.; Design – M.A.; Supervision – M.A.; Fundings – None; Materials – C.K.; Data collection &/or processing – M.A.; Analysis &/or interpretation – S.E.U.; Literature search – S.T.; Writing – M.A.; Critical review – C.K., E.D.Y.
References
- 1.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 2.Steg PG, Bhatt DL, Wilson PW, D'Agostino R, Sr, Ohman EM, Röther J, et al. REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Brevetti G, Schiano V, Chiariello M. Endothelial dysfunction:a key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis. 2008;197:1–11. doi: 10.1016/j.atherosclerosis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Brevetti G, Silvestro A, Giacomo SD, Bucur R, Di Donato A, Schiano V, et al. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–9. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 6.Akkoca M, Usanmaz SE, Koksoy C, Bengisun U, Demirel-Yilmaz E. Plasma nitric oxide level is correlated with microvascular functions in the peripheral arterial disease. Clin Hemorheol Microcirc. 2017;65:151–62. doi: 10.3233/CH-16143. [DOI] [PubMed] [Google Scholar]
- 7.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 8.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease:additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–8. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 9.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia:revised version. J Vasc Surg. 1997;26:517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 10.Henricson J, Tesselaar E, Persson K, Nilsson G, Sjöberg F. Assessment of microvascular function by study of the dose-response effects of iontophoretically applied drugs (acetylcholine and sodium nitroprusside)--methods and comparison with in vitro studies. Microvasc Res. 2007;73:143–9. doi: 10.1016/j.mvr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Navarro-Gonzálvez JA, García-Benayas C, Arenas J. Semiautomated measurement of nitrate in biological fluids. Clin Chem. 1998;44:679–81. [PubMed] [Google Scholar]
- 12.Han S, Uludag MO, Usanmaz SE, Ayaloglu-Butun F, Akcali KC, Demirel-Yilmaz E. Resveratrol affects histone 3 lysine 27 methylation of vessels and blood biomarkers in DOCA salt-induced hypertension. Mol Biol Rep. 2015;42:35–42. doi: 10.1007/s11033-014-3737-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Moochhala SM, Bhatia M. Endogenous hydrogen sulfide regulates inflammatory response by activating the ERK pathway in polymicrobial sepsis. J Immunol. 2008;181:4320–31. doi: 10.4049/jimmunol.181.6.4320. [DOI] [PubMed] [Google Scholar]
- 14.Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med. 2008;18:109–16. doi: 10.1016/j.tcm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado FJ, Miralles Jde H, Aguilar EM, Gonzalez AF, García JR, García FA. Relationship between noninvasively measured endothelial function and peripheral arterial disease. Angiology. 2009-2010;60:725–31. doi: 10.1177/0003319708327787. [DOI] [PubMed] [Google Scholar]
- 17.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function:a prospective study. Circulation. 2002;105:1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 18.Blann AD, McCollum CN. Circulating endothelial cell/leukocyte adhesion molecules in atherosclerosis. Thromb Haemost. 1994;72:151–4. [PubMed] [Google Scholar]
- 19.Blann AD, McCollum CN. von Willebrand factor and soluble thrombomodulin as predictors of adverse events among subjects with peripheral or coronary atherosclerosis. Blood Coagul Fibrinolysis. 1999;10:375–80. doi: 10.1097/00001721-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Silvestro A, Brevetti G, Schiano V, Scopacasa F, Chiariello M. Adhesion molecules and cardiovascular risk in peripheral arterial disease. Soluble vascular cell adhesion molecule-1 improves risk stratification. Thromb Haemost. 2005;93:559–63. doi: 10.1160/TH04-07-0440. [DOI] [PubMed] [Google Scholar]
- 21.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–9. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makita S, Nakamura M, Murakami H, Komoda K, Kawazoe K, Hiramori K. Impaired endothelium-dependent vasorelaxation in peripheral vasculature of patients with thromboangiitis obliterans (Buerger's disease) Circulation. 1996;94(9 Suppl):II211–5. [PubMed] [Google Scholar]
- 23.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, et al. The prognostic value of C-reactive protein and serum-amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–24. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 25.Kampus P, Kals J, Ristimäe T, Fischer K, Zilmer M, Teesalu R. High-sensitivity C-reactive protein affects central haemodynamics and augmentation index in apparently healthy persons. J Hypertens. 2004;22:1133–9. doi: 10.1097/00004872-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Nurizal A, Antono D, Wijaia IP, Shatri H. Correlation between high-sensitivity C reactive protein and local arterial stiffness measured by radio frequency echotracking system in type 2 diabetic patients. Acta Med Indones. 2014;46:308–13. [PubMed] [Google Scholar]
- 27.Nakhai-Pour HR, Grobbee DE, Bots ML, Muller M, van der Schouw YT. C-reactive protein and aortic stiffness and wave reflection in middle-aged and elderly men from the community. J Hum Hypertens. 2007;21:949–55. doi: 10.1038/sj.jhh.1002255. [DOI] [PubMed] [Google Scholar]
- 28.Tan KC, Chow WS, Tam SC, Ai VH, Lam CH, Lam KS. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002;87:563–8. doi: 10.1210/jcem.87.2.8249. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 30.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Centers for Disease Control and Prevention;American Heart Association. Markers of inflammation and cardiovascular disease:Application to clinical and public health practice:A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 31.Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack:The Framingham study. Stroke. 2001;32:2575–9. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109:1955–9. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- 33.Goldbourt U, Yaari S, Medalie JH. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler Thromb Vasc Biol. 1997;17:107–13. doi: 10.1161/01.atv.17.1.107. [DOI] [PubMed] [Google Scholar]
- 34.Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem. 2001;276:34480–5. doi: 10.1074/jbc.M103782200. [DOI] [PubMed] [Google Scholar]
- 35.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 36.Barter P. CETP and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2029–31. doi: 10.1161/01.atv.20.9.2029. [DOI] [PubMed] [Google Scholar]
- 37.Ferrières J, Gousse ET, Fabry C, Hermans MP, French C EPHEUS Investigators. Assessment of lipid-lowering treatment in France the CEPHEUS study. Arch Cardiovasc Dis. 2008;101:557–63. doi: 10.1016/j.acvd.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Spieker LE, Sudano I, Hürlimann D, Lerch PG, Lang MG, Binggeli C, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]


