ABSTRACT
Oncological treatment of colorectal cancer (CRC) has been available in Greenland since 2004. Treatment is provided by Queen Ingrid´s Hospital (QIH), under supervision from the Department of Oncology, Rigshospitalet, Denmark. The study describes patient characteristics, oncological treatment and survival for the first 8 years of treatment. The study was a registry-based observational study of all patients in Greenland diagnosed with histologically verified CRC from August 2004 to August 2012. Analyses were stratified according to stage and discussed in relation to reported data from patients with CRC in Denmark. 180 patients were included. . Stage I, II, III, and IV comprised 15, 34, 23, and 23%, respectively. 5% presented with unknown stage. A total of 51% received oncological treatment. 79% of patients with Stage III disease received adjuvant chemotherapy, 61% of patients with metastatic CRC received palliative chemotherapy. Five-year survival was 48 and 53% for colon and rectum cancer, respectively. An insignificant trend towards higher survival in men than in women was seen; adjusted hazard ratio for death (women vs men) = 1.46 (95% CI = 0.97–2.19). In conclusion; Stage distribution, provision of oncological treatment and 5-year survival were comparable to patients diagnosed and treated in Denmark.
KEYWORDS: Colorectal cancer, Greenland, oncology, treatment, survival, gender disparities
Introduction
CRC is the third-most commonly diagnosed malignancy and the fourth leading cause of cancer death in the world [1]. In the Inuit population, the incidence of CRC has increased rapidly over the last decades, possibly reflecting a change towards a more sedentary lifestyle and energy-dense diet [2]. However, it could also be a result of improved detection of CRC. In Greenland, part of the Danish Kingdom, the age-standardised incidence of CRC is slightly lower than in Denmark [3]. Approximately, 20–25 patients are diagnosed with CRC annually in Greenland [4].
Oncological treatment for the three most common cancers, i.e. lung-, breast – and colorectal cancer, has been available at the national Queen Ingrid´s Hospital, Nuuk (QIH) since 2004. Treatment is provided by local specialists in Internal Medicine. The oncology unit at QIH are in regular contact with the Department of Oncology, Rigshospitalet, and receives annual fly-in visits by an oncologist from Denmark. The treatment algorithm at QIH is in accordance with Danish guidelines. The primary treatment for CRC is surgical. Patients with Stages I–III colon cancer are offered curative-intended resection. After resection, patients with Stage III or high-risk Stage II disease are offered adjuvant chemotherapy to reduce the risk of recurrence. Patients with Stage III or high-risk Stage II rectal cancer are treated with radio – or chemoradiotherapy prior to surgery. The treatment strategy for CRC patients with non-resectable and/or Stage IV disease is palliative chemotherapy [5].
Numerous aspects could potentially have a negative impact on outcome when bringing specialised treatment to Greenland. The health care system is challenged by factors such as infrastructure, limited resources, and geographical disparities in health care access [6]. The population is small, divided over a huge geographical area and displays a heterogeneous health status according to region and urbanisation level [7]. Geographical remoteness in Greenland is a challenge, with two thirds of the inhabitants living in smaller towns and settlements along the coastline [8]. Data from a recent study in Greenland evaluating 113 CRC patients indicated that patients living on the coastline were exposed to a significant diagnostic delay compared to their counterparts in Nuuk [9]. Geographical remoteness has also been shown to cause disparities in treatment patterns and outcome for cancer patients living in Australia, USA and Canada [10–13]. Globally, survival is generally better in countries with good access to specialised care [14].
Bringing oncological treatment to Greenland is attractive from both a patient perspective and an economic perspective, and the Greenland national plan for cancer care and treatment recommends expansion of the field of medical oncology [15]. Therefore, an evaluation of the current treatment efforts is important. The objective of this study was to evaluate patient and cancer characteristics, oncological treatment patterns and survival rates from the first 8 years of CRC treatment and to discuss outcome with reported data from CRC patients in Denmark.
Methods
Design
Registry-based observational study.
Materials and methods
The source population consisted of 56.000 inhabitants living in Greenland, approximately 90% of the population is of Inuit origin. The median life expectancy in 2012 was 67 and 72 for men and women, respectively [8]. Study subjects were identified using the The Danish Pathology Data Bank at Rigshospitalet, Copenhagen. The Danish Pathology Data Bank is a national registry responsible for handling all tissue – and cell samples obtained in both Denmark and Greenland. Study subjects were identified by a pathologist, inclusion criteria were A) Permanent address in Greenland by the time of diagnoses (identified by a Greenlandic commune code) and B) Histologically verified CRC during the period 1 August 2004 to 1 August 2012. According to the International Classification of Diseases for Oncology (ICD-O-3) [16] the following histological subtypes were included in the search: adenocarcinomas, undifferentiated adenocarcinomas, mucinous adenocarcinoma, adenosquamous carcinoma, medullary carcinoma and signet ring cell carcinoma. Patients were excluded, if the pathological diagnosis was severe dysplasia without malignant transformation, or if the adenocarcinoma proved to originate from outside the colon or rectum.
In order to protect patient privacy, data regarding less than 5 cases will be censored to ‘< 5’.
Medical files were obtained from QIH and following information was captured: patient demographics (age and gender), surgical pathology report (number of removed lymph nodes, biopsy from metastases or recurrence suspected tumours), imaging modalities (x-ray, ultrasound (US) or computed tomography (CT)), presence/absence of surgery for primary tumour, chemotherapy (including type and number of treatment lines), radiotherapy, and surgery for metastatic disease. Documented recurrence during a minimum follow up of 5 years was registered. Data on mortality and residency status at the time of diagnosis were provided by the Greenlandic National Registry, a registry where all Greenlandic citizens can be identified by a unique, personal identification number (CPR-number). The registry contains information on name, addresses, dates of emigration and death. The registry does not include cause of death.
Data are disused in relation to annual reports (from 2001 to 2012) published by the Danish Colorectal Cancer Group (DCCG) [17].
Treatment
Oncological treatment with chemotherapy and targeted drugs is administered at QIH, including adjuvant and palliative chemotherapy. Surgical treatment for colon cancer is performed at the Surgical Department at QIH, whereas neoadjuvant radiotherapy/chemoradiotherapy and surgical treatment of most rectal cancers are performed in Denmark. Surgery for metastatic disease (liver and lung) is performed at Rigshospitalet, Copenhagen.
Patients with Stage III and high-risk Stage II disease are offered adjuvant chemotherapy with 5-Floururacil and Leucoverin or Capecitabine and Oxaliplatin. Patients with metastatic CRC are offered palliative chemotherapy with Oxaliplatin – and Irinotecan-based doublets with or without targeted therapy against VEGF (vascular endothelial growth factor) or EGFR (endothelial growth factor receptor). Complications during chemotherapy are treated either at QIH or at local hospitals with professional support from QIH as needed. All patients have optional phone access to the oncology unit at QIH during their cancer treatment.
Statistics
Baseline characteristics were described as proportions for dichotomous variables, and medians with range for continuous variables. Overall survival was computed from time-to-event data counting days from diagnosis to death (event) or to 31 August 2017 (censoring). Survival curves were constructed using the method of Kaplan and Meier [18]. Five-year survival was described as proportions with 95% confidence intervals (CI). The following independent variables were included: age, sex (male/female), residency: Nuuk (capital) vs. Coast (towns and settlements along the coastline outside the capital) and UICC Stages (I, II, III, IV). Multivariable Cox regression analysis was used to control for potential confounders. For all tests, p < 0.05 was considered the level of significance. The SPSS statistical software system for Windows (SPSS version 19.0, Chicago, IL) was used for the statistical analysis
Ethics
The study was approved by the Ethical Committee of the Greenlandic National Board of Health (Project No. 2017–15504).
Results
Demographic and clinical characteristics
From August 2004 to August 2012, 193 patients were identified in the histopathological databases with carcinomas with suspected origin from the colon or rectum. In total, 13 patients (7%) were excluded. Twelve due to incorrect histology diagnosis (eight patients with severe dysplasia without malignant transformation and five with adenocarcinomas from outside the colon or rectum) and one who had no hospital admission record. This left 180 for analyses. One patient was excluded from survival analyses due to missing mortality data. Patient demographics and cancer characteristics are listed in Tables 1 and 2, respectively.
Table 1.
Demographics for patients with CRC in Greenland 2004–2012.
| No (%) | |
|---|---|
| Gender | |
| Female | 80 (45%) |
| Male | 100 (55%) |
| Geographical location | |
| Nuuk | 51 (28%) |
| Coastal towns | 129 (72%) |
| Median age at diagnosis | |
| All | 65 (range 28–92) |
| Female | 66 (range 29–92) |
| Male | 64 (range 28–88) |
Table 2.
Cancer characteristics for patients with CRC in Greenland 2004–2012.
| No (%) | |
|---|---|
| Total number of CRC | 180 (100%) |
| Colon cancer | 125 (69%) |
| Rectum cancer | 55 (31%) |
| Histopathological subtypes | (n = 180) |
| Adenocarcinoma | 159 (88%) |
| Undifferentiated carcinoma | 12 (7%) |
| Mucinous adenocarcinoma | 6 (3%) |
| Other | < 5 |
| Total stage distribution | (n = 180) |
| UICC I | 27 (15%) |
| UICC II | 61 (34%) |
| UICC III | 42 (23%) |
| UICC IV | 41 (23%) |
| Unknown stage | 9 (5%) |
| Substaging UICC III (AJCC 6. edition) | (n = 39)* |
| UICC IIIA (T1-2N1M0) | <5 |
| UICC IIIB (T3-4N1M0) | 23 (59%) |
| UICC IIIC (anyTN2M0) | 14 (36%) |
* < 5 patients excluded due to unkonwn T or N status
The diagnostic imaging modality applied was chest x-ray and abdominal US for 99 patients (55%). 52 patients (29%) received a full CT of chest and abdomen. The remaining 29 patients (16%) received either a chest x-ray and CT of abdomen or the imaging modality was not mentioned in the medical record.
Surgical treatment
Of the 92 patients with colon cancer, 98% received surgical treatment in Nuuk. The majority of the 38 patients with rectal cancer was resected in Denmark (79%). Twelve patients received surgery for metastatic disease. The surgical pathology report indicated that 58% of the patients who received curatively intended resection had more than 12 lymph nodes examined.
Oncological treatment
Of the 180 patients with histologically verified CRC, 92 (51%) received oncological treatment. Some patients were offered both adjuvant treatment after primary resection, and in the case of recurrence, either adjuvant treatment after surgery for metastatic disease or palliative chemotherapy. Out of 42 patients with Stage III disease, 9 patients (21 %) did not receive adjuvant treatment, either because of patient choice, comorbidity, non-resectability or lack of referral. 16 (39%) Stage IV patients received best supportive care alone. Reasons were poor performance status, early deterioration, patient choice or lack of referral. Oncological treatment characteristics and chemo – and/or radiotherapeutics regimens are listed in Tables 3 and 4, respectively.
Table 3.
Oncological treatment characteristics for patients with CRC in Greenland 2004–2012.
| No | Percentage of subgroup | |
|---|---|---|
| Adjuvant treatment (n = 50) | ||
| UICC II (high risk) | 11 | 18% of all st II |
| UICC III | 33 | 79% of all st III |
| After surgery for metastatic disease | 8 | |
| Palliative treatment (n = 44) | ||
| UICC IV | 25 | 61% of all st IV |
| Recurrence | 19 | 46% of all with recurrence |
Table 4.
Chemo – and/or radiotherapeutic regimens for patients with CRC in Greenland 2004–2012.
| No | |
|---|---|
| Neoadjuvant thearpy (rectum cancer) | (n = 20) |
| Radio – or chemoradiotherapy | 20 |
| Adjuvant treatment | (n = 50) |
| Oxaliplatin and Capceitabine | 31 |
| 5-floururacil and leucoverin | 19 |
| Palliative treatment | (n = 44) |
| First-line chemotherapy | 44 |
| Capecitabine and Oxaliplatin +/ – Bevacizumab | 36 |
| Other combinations | 8 |
| Second-line chemotherapy | 27 |
| Monothearpy with Irinotecan was treatment choice for 85% of patients | |
| Third-line chemotherapy | 11 |
Survival
One-year survival was 82% (95% CI 76–89). Five-year survival was 49% (95% CI 42–57), 48% (95% CI 39–57) for colon cancer and 53% (95% CI 39–66) for rectum cancer.
5-year survival according to stage was 77% (95% CI 56–91) for Stage I, 77% (95% CI 65–87) for Stage II, 38% (95% CI 24–54) for Stage III and 10% (95% CI 3–23) for Stage IV disease.
Five-year survival for patients classified with unknown stage was 11% (95% CI 0–48).
Kaplan Meier curves for survival stratified by stage is presented in Figure 1.
Figure 1.
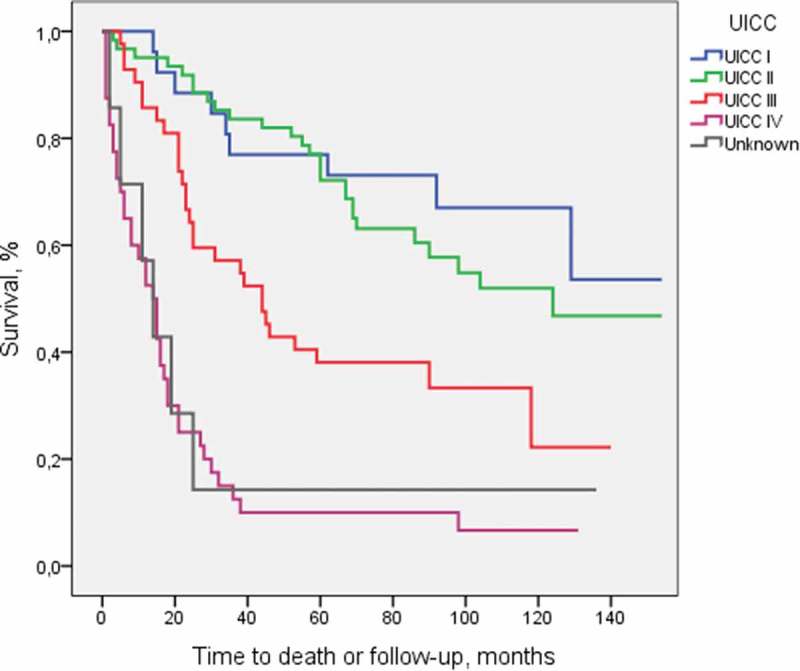
Overall survival stratified by stage.
Patients with Stage IV disease had a median survival of 14 months (95% CI 9–19). 17 months (95% CI 14–20) in those receiving chemotherapy and 2 months (95% CI 0–4) in those receiving best supportive care alone.
Multivariable analysis showed that stage and age had significant independent prognostic value. There was no association between place of residence and mortality. Cox regression analyses showed a higher mortality among women than men; hazard ratio for death = 1.52 (95% CI = 1.04–2.22). This association persisted but was no longer statistically significant after adjusting for stage, age, and residence; adjusted hazard ratio for death = 1.46 (95% CI = 0.97–2.19). Chi-Square test revealed no difference in stage distribution between men and women (p = 0.407).
Cox regression analyses are presented in Table 5.
Table 5.
Multivariate Cox regression analyses of patients with CRC in Greenland 2004–2012.
| Unadjusted |
Adjusted |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Sex, male = 1 | 1.52 | 1.04–2.22 | 0.031 | 1.46 | 0.97–2.19 | 0.063 |
| Residence, coast = 1 | 1.07 | 0.70–1.63 | 0.761 | 0.85 | 0.54–1.31 | 0.486 |
| Age at diagnosis | 1.02 | 0.98–1.04 | 0.076 | 1.02 | 1.00–1.04 | 0.033 |
| UICC, St I = 1 | ||||||
| UICC II | 1.30 | 0.61–2.77 | 0.494 | 1.31 | 0.6–2.73 | 0.488 |
| UICC III | 2.78 | 1.31–5.91 | 0.008 | 2.78 | 1.30–5.93 | 0.008 |
| UICC IV | 8.37 | 3.99–17.54 | 0.000 | 8.80 | 4.10–18.12 | 0.000 |
| Unknown | 6.33 | 2.23–17.89 | 0.001 | 5.32 | 1.81–14.94 | 0.002 |
Kaplan–Meier curves for survival stratified by gender are presented in Figure 2.
Figure 2.
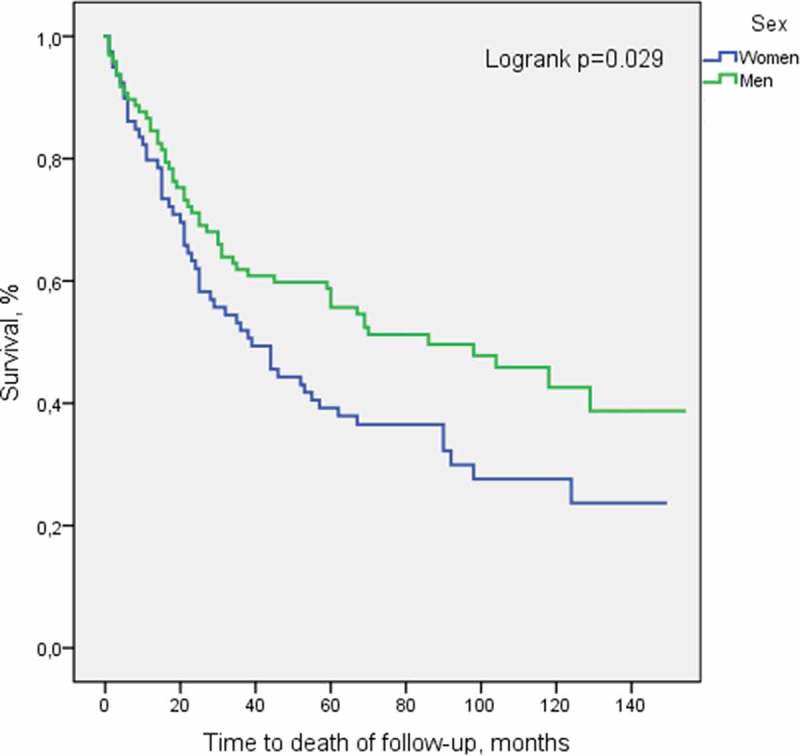
Overall survival stratified by gender.
Discussion
This observational study is the first to examine broad patterns of CRC management in Greenland. We found that stage distribution of 15, 34, 23 and 23% for Stages I, II, III, and IV, respectively, were similar to the distribution of 14, 29, 23 and 26% seen in Denmark. Adenocarcinomas of glandular type was the most common histological subtype in both Greenland and Denmark (88% vs. 82%) [19]. Our data suggest that provision of oncological treatment in Greenland was in accordance with Danish standards. A high percentage of CRC patients with Stage III disease received adjuvant therapy, 79% compared to 69% in Denmark [19]. Palliative chemotherapy for metastatic CRC was initiated as often in Greenland as in Scandinavia, 61% vs. 61% received palliative treatment [20]. The present study indicates that 5-year survival was similar in Greenland and Denmark for both colon cancer (48% vs. 49%) and rectum cancer (53% vs. 50%) [16]. Among patients with metastatic CRC, median survival in Greenland was similar to patients in Scandinavia: 17 vs. 15.8 months for patients treated with chemotherapy and 2 vs. 2.8 months for patients receiving best supportive care alone [20]. Despite a previous study in Greenland suggesting a significant diagnostic delay for patients living in remote areas outside Nuuk [6], and international research reporting on significant disparities in cancer-related outcomes in rural and remote areas [10–13], we found no significant difference in stage distribution or survival rates between patients from Nuuk and patients living along the coastline outside Nuuk.
Surprisingly, 5-year survival for Stage III differed markedly between Greenland (38%) and Denmark (54%) [17]. Part of this difference might be explained by under-staging due to inadequate diagnostic imaging or insufficient lymph node harvest. Only 29% in Greenland vs. 92% in Denmark (2012) received a full CT scan of chest and abdomen [19]. This might be partly due to changing national guidelines during 2004–2012, featuring a transition from chest X-ray and abdominal US to chest and abdominal CT. Today it is well known that CT is more sensitive than abdominal US and chest X-ray in detection of hepatic and pulmonary CRC metastasis [21,22]. Another important difference during primary staging was lymph node harvest. A minimum of 12 examined lymph nodes are recommended to obtain correct staging [23] and the number of identified lymph nodes has been reported to increase overall survival [24]. Of all curatively resected patients in Greenland (2004–2012) only 57% were able to meet the criteria of a minimum of 12 examined lymph nodes, in comparison this aim has been obtained by more than 75% in Denmark since 2007 [25]. Both nodal status and depth of invasion for stage III CRC patients have also been reported to have a significant effect on survival [26], but the distribution of sub-staging in our study did not indicate a majority of high risk stage III patients in Greenland; the distribution of Stages IIIa, IIIb and IIIc was 5, 59 and 36% compared to 6, 43, 51% in Greenland and Denmark, respectively [27]. Our data indicated that the oncological treatment was less likely to account for the survival gap, since more Stage III patients in Greenland than in Denmark seemed to receive adjuvant chemotherapy (79% vs. 69%) or neoadjuvant therapy (36% vs. 28%) [19]. Furthermore, adjuvant therapy for Stage III colon cancer leads to a maximal improved survival of 5–15% points [28], and neoadjuvant radiotherapy for Stage III rectal cancer solely improves the risk of recurrence, not survival [29]. The present study did not evaluate surgical treatment characteristics, but a previous study of 113 CRC patients in Greenland revealed no excess in surgical complications compared to international reports, and a low 30-day mortality in Greenland compared to Denmark (1.4% vs. 4.6%) [30]. We found similar 1-year survival rates in Greenland and Denmark [19], supporting the assumption that fatal complications during surgical or oncological treatment can be excluded as a cause of the observed difference in 5-year survival.
The tendency to a higher mortality among women in our study was not explained by factors such as geography, age difference or stage distribution. Across the world, CRC incidence patterns and mortality are comparable between men and women [31]. A health disadvantage for women in Greenland has been suggested in previous research. A study evaluating diagnostic interval for 113 patients with CRC in Greenland found a longer primary care interval for women than men (70 days vs. 15 days) [6], and a nationwide population survey from 2014, including 5559 Inuit Greenlanders, found higher proportions of poor self-rated health and chronic illnesses in women than in men [32]. Comorbidity is known to have a major negative impact on survival after CRC [17]. Hence, gender differences in chronic disease patterns and comorbidity might explain our observed difference in survival. Age and ethnicity might also be of relevance. Statistics from Korea and Japan have indicated that women over 65 years show a higher mortality and lower 5-year survival compared to their age-matched male counterparts [33,34]. Further, a review from 2015 covering gender impact on CRC suggests that both genetic factors, dietary habits and socio-cultural environmental factors may attribute to a gender-specific disparity [35]. A review from 2012 reports that women are more prone to right-sided colon cancer than men, associated with a poorer prognosis [36]. Women have also been found to have a higher percentage of high-grade and microsatellite-unstable tumours [37], and there is growing but complex evidence for an oestrogenic role in CRC, suggesting oestrogen as initially protective against CRC, but once developed might increase proliferation [38].
In our study, CRC patients in Greenland were 7 years younger than in Denmark at the time of diagnosis. Median age for women and men in Greenland was 64 and 66 years, respectively, compared to 71 and 73 years in Denmark. Parts of this discrepancy might be explained by the difference in overall life expectancy in Greenland, which is almost a decade shorter than in the Nordic countries [39]. Hence, a smaller proportion of the population is alive in the age group 70–80 years, which is where the highest number of cases are diagnosed in Denmark [19]
No hereditary diseases patterns (i.e. familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC)) have yet been observed in Greenland [3], however, lifestyle and nutritional risk factors may also be implicated in the observed age difference. Smoking and regular alcohol consumption are known to be associated with younger ages at the onset of CRC [40,41]. Alcohol consumption in Greenland is lower than in Denmark [42], but smoking is markedly higher with 66% of the adult population smoking in 2004 [43]. Finally, epidemiological studies have suggested an inverse relationship between CRC and fresh vegetable and fruit consumption [44]. These food products are extremely expensive and often inaccessible for people living in Greenland.
In Denmark, a national screening program for patients age 50–74 was introduced in 2014. Since then, more patients are being diagnosed at age > 50 years. The same effect could be expected in Greenland. The possibility of a national screening program is discussed in a previously published study from 2017 [9].
Since age is significantly associated with mortality, and the Greenlandic CRC patients were on average 7 years younger than Danish patients, a direct age-adjusted comparison could potentially expose a less favourable outcome for patients with CRC in Greenland.
Strengths and limitations
The strengths of this study were the ability to identify a complete population of persons diagnosed with CRC in Greenland. With both treatment and histopathological diagnosis being limited to each a single institution, selection bias is unlikely. We consider that data on diagnostic methods and oncological treatment are reliable. Further, the national registries of death allowed us to obtain valid and complete survival data. Our study had limitations as well. The relatively small study population decreased the statistical precision of our results, and the wide confidence intervals on survival estimated should be kept in mind before conclusions are drawn, particular regarding subgroup analyses. Further limitations were the lack of data on comorbidity, socioeconomic status and ethnicity; and limited data regarding adherence to prescribed treatment or participation in recommended follow-up. The distribution of these potential confounders might explain part of the gender – and stage-specific survival gaps.
Conclusion
Several aspects could potentially have a negative impact on outcome when bringing specialised cancer care to Greenland. Among others are the challenging geography, a health care system with limited resources and implications of handling specialised treatment without a resident oncologist. Nevertheless, evaluation of the first 8 years of CRC treatment in Greenland showed that stage distribution and overall 1- and 5-year survival for CRC was comparable to patients diagnosed in Denmark. Provision of oncological treatment in Greenland was in accordance with Danish standards. It is noteworthy that our data showed no geographical disadvantage for CRC patient living along the coastline outside Nuuk.
Surprisingly, a tendency towards worse outcome in women than in men warrants further investigations in women’s health in Greenland. In conclusion, oncological treatment for CRC in Greenland is attractive for several reasons, and the present study documents that the current treatment effort is satisfying with outcomes comparable to CRC patients in Denmark.
Funding Statement
The study was supported by ‘Sundhedspuljen’ Ministry of Health, Government of Greenland and by ‘Jubilæumsfonden af 1985’, The Department of Oncology, Rigshospitalet, Denmark. None of the funding agencies had any role in study design, the collection or interpretation of data.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. BMJ. 2017;66:683–8. [DOI] [PubMed] [Google Scholar]
- [2].Friborg JT, Melbye M.. Cancer patterns in inuit populations. Lancet Oncol. 2008;9:892–900. [DOI] [PubMed] [Google Scholar]
- [3].Naalakkersuisut Dept. of Health. Government of Greenland. Kræftredegørelsen (Report on Cancer status); 2011. Avalible from:https://naalakkersuisut.gl
- [4].Association of the Nordic Cancer Registries (NORDCAN) Cancer statistics; 2014. Avalible from:http://www-iarc.fr/NORDCAN.htm
- [5].Danish Colorectal Cancer Group (DCCG) DCCG’s nationale retningslinier for behandling af kolorektal cancer (DCCG’s National Guidelines for Treatment of Colorectale Cancer); 2012. Avalible from:https://dccg.dk/.
- [6].Niclasen B, Mulvad G. Health care and health care delivery in Greenland. Int J Circumpolar Health. 2010;69:437–447. [DOI] [PubMed] [Google Scholar]
- [7].Iburg KM, Brønnum-Hansen H, Bjerregaard P. Health expectancy in Greenland. Scand J Public Health. 2001;29:5–12. [DOI] [PubMed] [Google Scholar]
- [8].Statistics Greenland Greenland in Figures; 2012. Avalible from:http://www.stat.gl/.
- [9].Tolstrup J, Madsen RC, Sneftrup MV, et al. Greenlandic patients with colorectal cancer: symptomatology, primary investigations and differences in diagnostic intervals between Nuuk and the rest of the country. Int J Circumpolar Health. 2017;76:1344086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ireland MJ, March S, Crawford-Williams F, et al. A systematic review of geographical differences in management and outcomes for colorectal cancer in Australia. BMC Cancer. 2017;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beckmann KR, Bennett A, Young GP, et al. Treatment patterns among colorectal cancer patients in South Australia: a demonstration of the utility of population-based data linkage. J Eval Clin Pract. 2014;20:467–477. [DOI] [PubMed] [Google Scholar]
- [12].Charlton M, Schlichting J, Chioreso C, et al. Challenges of rural cancer care in the USA. Oncology (Williston Park). 2015;29:633–640. [PubMed] [Google Scholar]
- [13].Ahmed S, Shahid RK. Disparity in cancer care: a Canadian perspective. Curr Oncol. 2012;19:e376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Haggar F, Boushey R. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Naalakkersuisut Dept. of Health. Government of Greenland. Forslag til kræftplan (National Cancer plan); 2013. Avalilable from: http://www.peqqik.gl/Footerpages/.
- [16].WHO International Classification of Diseases for Oncology (ICD-O-3); 2018. Avalible from:http://codes.iarc.fr/.
- [17].Iversen LH, Green A, Ingeholm P, et al. Improved survival of colorectal cancer in Denmark during 2001–2012 – the efforts of several national initiatives. Acta Oncol (Madr). 2016;55:10–23. [DOI] [PubMed] [Google Scholar]
- [18].Kaplan EL. P M. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958. [Google Scholar]
- [19].The Danish Colorectal Cancer Group (DCCG) Annual report on Colorectal Cancer; 2012. Avalible from: http://www.dccg.dk.
- [20].Sorbye H, Pfeiffer P, Cavalli-Björkman N, et al. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer. 2009;115:4679–4687. [DOI] [PubMed] [Google Scholar]
- [21].Holmsted K, Nørring K, Laustrup LC, et al. Many unexpected abdominal findings on staging computed tomography in patients with colorectal cancer. Dan Med Bull. 2011;58:A4308. [PubMed] [Google Scholar]
- [22].McIntosh J, Sylvester P, Virjee J, et al. Pulmonary staging in colorectal cancer – is computerised tomography the answer? Ann R Coll Surg Engl. 2005;87:331–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shia J, Wang H, Nash GM, et al. Lymph node staging in colorectal cancer: revisiting the benchmark of at least 12 lymph nodes in R0 resection. J Am Coll Surg. 2012;214:348–355. [DOI] [PubMed] [Google Scholar]
- [24].Sjo OH, Merok MA, Svindland A, et al. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis Colon Rectum. 2012;55:307–315. [DOI] [PubMed] [Google Scholar]
- [25].The Danish Colorectal Cancer Group (DCCG) Annual report on Colorectal Cancer 2010; 2010. Avalible from:www.dccg.dk.
- [26].Gunderson LL, Jessup JM, Sargent DJ, et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].The Danish Colorectal Cancer Group (DCCG) Annual report on Colorectal Cancer 2007–2008; 2008. Avalible from:www.dccg.dk.
- [28].André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. [DOI] [PubMed] [Google Scholar]
- [29].Rahbari NN, Elbers H, Askoxylakis V, et al. Neoadjuvant radiotherapy for rectal cancer: meta-analysis of randomized controlled trials. Ann Surg Oncol. 2013;20:4169–4182. [DOI] [PubMed] [Google Scholar]
- [30].Chemnitz-Madsen R, Tolstrup J. Colorectal cancer in greenland. Unpubliched 2018. [Google Scholar]
- [31].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [32].Mairey I, Bjerregaard P, Brønnum-Hansen H. Gender difference in health expectancy trends in Greenland. Scand J Public Health. 2014;42:751–758. [DOI] [PubMed] [Google Scholar]
- [33].Jung K-W, Park S, Kong H-J, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Matsuda A, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2008: A study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44:388–396. [DOI] [PubMed] [Google Scholar]
- [35].Kim S-E, Paik HY, Yoon H, et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hansen IO, Jess P. Possible better long-term survival in left versus right-sided colon cancer - a systematic review. Dan Med J. 2012;59:A4444. [PubMed] [Google Scholar]
- [37].Amri R, Stronks K, Bordeianou LG, et al. Gender and ethnic disparities in colon cancer presentation and outcomes in a US universal health care setting. J Surg Oncol. 2014;109:645–651. [DOI] [PubMed] [Google Scholar]
- [38].Foster PA. Oestrogen and colorectal cancer: mechanisms and controversies. Int J Colorectal Dis. 2013;28:737–749. [DOI] [PubMed] [Google Scholar]
- [39].Nunatsinni nakorsaaneqarfik - Landslægeembedet Statistics Greenland; 2013. Avalible from:https://nun.gl/Emner/Udgivelser/Aars.
- [40].Zisman AL, Nickolov A, Brand RE, et al. Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med. 2006;166:629–634. [DOI] [PubMed] [Google Scholar]
- [41].Acott AA, Theus SA, Marchant-Miros KE, et al. Association of tobacco and alcohol use with earlier development of colorectal cancer: should we modify screening guidelines? Am J Surg. 2008;196:915–918. discussion 918–9. [DOI] [PubMed] [Google Scholar]
- [42].Aage H. Alcohol in Greenland 1951–2010: consumption, mortality, prices. Int J Circumpolar Health. 2012;71:18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jensen AB, Hounsgaard L. I only smoke when I have nothing to do: a qualitative study on how smoking is part of everyday life in a Greenlandic village. Int J Circumpolar Health. 2013;72:21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Janout V, Kollárová H. Epidemiology of colorectal cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2001;145:5–10. [DOI] [PubMed] [Google Scholar]


