Abstract
Aim
The aim of this study was to confirm whether patients with sacral chordoma benefit from adjuvant radiotherapy and to determine the optimal photon radiotherapy module for comprehensive treatment.
Background
Chordoma is a rare slow-growing neoplasm arisen from cellular remnants of the notochord. About 50% occur in the sacrococcygeal region. Surgical resection and adjuvant radiation therapy are recommended treatment due to the improving local control rate.
Materials and methods
118 patients treated by surgery and adjuvant radiotherapy from August 2003 to May 2015 were retrospectively analyzed. All patients received surgical resection after diagnosis. Among these patients, 44 were treated by exclusive surgery, and 48 were treated with adjuvant image-guided, intensity-modulated radiation therapy (IG-IMRT). In addition, 26 patients were treated with gamma knife surgery (GKS) after surgical resection. The median follow-up was 54 months for all patients. Kaplan–Meier analysis was used to calculate recurrence-free survival (RFS) overall survival (OS).
Results
Patients treated with adjuvant radiotherapy had better RFS (p = 0.014) than those treated exclusively by surgery. The patients in the IG-IMRT group exhibited better recurrence-free survival (p = 0.01) than the GKS group. Moreover, in the IG-IMRT group, patients treated by higher dose were associated with better RFS (p = 0.04). No significant difference in OS was found. No grade 3 late toxicity was found.
Conclusions
We confirmed that adjuvant radiotherapy improved RFS but not OS in sacral chordoma patients after surgery. Furthermore, favorable RFS and low adverse event rates were observed following IG-IMRT. Our results suggest that high dose IG-IMRT is an appropriate module of adjuvant radiotherapy for sacral chordoma patients.
Keywords: Sacral chordoma, Adjuvant radiotherapy, Image-guided intensity modulated radiotherapy, Gamma knife surgery, Survival
1. Introduction
Chordoma is a rare low-grade malignant bone neoplasm that originates from the notochordal remnants and accounts for 1–4% of all malignant bone neoplasms. In addition, 40–50% of chordomas are located in the sacrococcygeal region.1 {Jo, 2014, WHO classification of soft tissue tumors: an update based on the 2013 (4th) edition}. The standard of care for treatment of sacral chordoma is surgical resection.2 However, extended resection with negative margins is difficult to achieve because of the adjacent anatomical structures. Additionally, various complications are often noted, including wound infection, vascular injury, massive bleeding, damage to the intestine, and neurologic deficits.3
The local recurrence rate is approximately 40–65% in primary chordomas treated with surgery alone.4 Hence, postoperative adjuvant radiotherapy is important to optimize the treatment outcome.5, 6 Recent studies demonstrated that high-dose radiation therapy using protons and heavy ions performed as adjunct radiotherapy on sacral chordoma patients exhibits a promising outcome given the depth-dose characteristics that are able to achieve significant dose reductions in organs at risk and increased doses to the target volume.7, 8 Unfortunately, due to the limited resources for proton and heavy ion techniques in China,9 these procedures are not readily available for most sacral chordoma patients. Therefore, the exploration of more suitable photon therapy technology for chordomas is particularly important. Intensity modulated radiotherapy (IMRT), image-guided radiation therapy (IGRT) and gamma knife surgery (GKS) are the main photon therapy techniques.9 Previous studies have reported that IMRT improves the RFS of sacral chordoma patients as a combined radiotherapy method,10 whereas the outcome of GKS implemented in sacral chordoma patients has not been reported. A recent study demonstrated that outcomes after GKS were favorable for patients with skull base chordomas.11 To date, it is unknown which of these two widely applicable radiotherapy techniques in China is more favorable for sacral chordoma patients after operation.
2. Aim
This report is a retrospective study of surgery and IG-IMRT or GKS treatment in sacral chordoma patients. We analyzed one hundred and eighteen patients with sacral chordomas to confirm whether these patients benefit from adjuvant radiotherapy and to determine the optimal photon radiotherapy module for patients.
3. Materials and methods
3.1. Patient selection
One hundred and eighteen sacral chordomas patients treated with operation were included in our study for retrospective analysis. All patients were pathologically diagnosed as non-metastatic sacral chordomas at two hospitals from Aug. 2003 to May 2015. All participants gave informed consent to the use of their data for research purpose. The study was approved by the ethical committees of the participating institutes.
3.2. Surgery
Among these patients, 44 patients were treated by gross total resection only, while 48 patients with subsequent adjuvant Image-Guided, Intensity-Modulated Radiation Therapy (IG-IMRT), and other 26 patients with subsequent Gamma Knife Surgery (GKS) after surgical resection. A total of 182 operations were performed, including 118 initial operations and 64 repeat operations. All initial operations were completed within one month after diagnosis, comprising gross total resection (GTR) in 62 patients, subtotal resection (STR) in 56 patients (Table 1).
Table 1.
Characteristics and outcome of all 118 investigated patients.
| Characteristics | Total | Surgery only | Adjvuant radiotherapy |
|
|---|---|---|---|---|
| GKS | IG-IMRT | |||
| No. of patients | 118 | 44 | 26 | 48 |
| Gender | ||||
| Female | 58 | 14 | 16 | 28 |
| Male | 60 | 30 | 15 | 20 |
| Mean age, y | 49.1(17–75) | 51(25–73) | 47.6(17–69) | 49.3(22–75) |
| Surgery result | ||||
| GTR | 62 | 44 | 6 | 12 |
| STR | 56 | 0 | 20 | 36 |
| Median Follow-up, mo(range) | 54(24–140) | 62(24–111) | 44(26–94) | 52(24–140) |
| OS, %(95%CI) | ||||
| 2 y | 100 | 100 | 100 | 100 |
| 5 y | 80.1(70.8–89.4)* | 90.9(83.2–99.6) | 59.3(34.1–84.5) | 87.5(78.9–96.1) |
| RFS, %(95%CI) | ||||
| 2 y | 89.8(85.8–93.8) | 72.2(62.5–81.9) | 91.7(83.7–98.7) | 95.7(91.4–100) |
| 5 y | 44.5(36.7.9–52.3) | 29.8(18.3–41.3) | 18.3(3.0–33.6) | 70.9(59.6–82.2) |
| Recurrences | 64 | 28 | 14 | 22 |
GTR: gross total resection; STR: subtotal resection; OS: over survival; 95% CI: 95% confidence interval; RFS: recurrence-free survival.
3.3. Radiation therapy
3.3.1. Image-guided, intensity-modulated radiation therapy
Forty-eight patients were treated with postoperative Image-Guided, Intensity-Modulated Radiation Therapy. Treatment planning employ 1.5 mm thin-slice computed tomography (CT) simulation and 1.5T/3.0T magnetic resonance imaging (MRI) including T1WI fast spin-echo contrast-enhancement and T2WI fluid attenuated inversion recovery (FLAIR) MR sequence. CT-MRI fusion was used to guiding define the outline of a target area. Isodose treatment plans were made with Oncentra MasterPlan (Nucletron, Holland), Brainlab iPlan software (Westchester,Illinois, United States) and MIM Maestro (MIM Software, United States). Cone-beam CT was used for image guidance in IGRT. Elekta Synergy IGRT System (United Kingdom) and VARIAN Clinac-23EX IMRT system (United States) were used to deliver the radiation plan. All patients were immobilized using a thermoplastic mask for both CT simulation and RT procedures. By reference to preoperative and postoperative CT/MRI imaging data and operation record, we confirm the primary cancer area and the operation area. The boost volume (PTV2) was defined as a contrast enhancement region with a safety margin of 5 mm and the clinical target volume (CTV) as a visible tumor plus a margin for potential spread/residual tumor cells. In case of the patients with complete resection, the CTV encompassed the tumor bed including post-operative changes visible on MRI/CT. The planning target volume (PTV1) was defined as the CTV with a margin of 5 mm. Median total dose to the boost volume (PTV2) was 71.4 Gy (range, 70–74 Gy) in 2 Gy median per fraction using an integrated boost concept. Median dose to target volume (PTV1) was 59.4 Gy (range, 56–64 Gy) in 1.8 Gy median per fraction over 6–7 weeks.
3.4. Gamma knife surgery
Twenty-six patients were treated with postoperative gamma knife surgery performed using Leksell Gamma Knife Model C (Elekta, Stockholm, Sweden). Patients were fixed in Leksell stereotactic frame, 3.0 T MRI was employed to create a suitable fiducial system including T1WI fast spin-echo contrast enhancement and T2WI FLAIR MR sequence. The stereotactic frame was applied under local anesthesia, following administration of oral benzodiazepine. Stereotactic MRI was used for dose planning in all patients; all Gamma Knife procedures were performed with Leksell Gamma Plan® (Elekta, Stockholm, Sweden). The median target volume was 6.8 cm3 (range 1.5–17.3 cm3). Patients in the GKS group underwent a total of 2 sessions of treatment, the mean prescription dose applied to the tumor margin was 22.6 Gy, and the mean maximal dose was 29.3 Gy. The average BED prescription dose applied to the tumor margin was 61.5 Gy, and the mean maximal BED was 81.5 Gy.
3.5. Data collection and statistical analysis
Clinical information and follow-up data were obtained from the oncology department and telephone interviews of the patients or family members were conducted, the clinical follow-up data including the patient's age, sex, surgical outcome, radiotherapy techniques, radiation dose, recurrences, disease-free survival and overall survival. All patients were followed for at least 2 years or until death. Kaplan–Meier analysis was utilized to calculate recurrence-free survival (RFS), overall survival (OS). Survival curves for overall and RFS were derived by the Kaplan–Meier method (IBM SPSS statistics 21) and compared using a log-rank test. The relative risk of death was estimated as a hazard ratio (HR) using Cox regression (IBM SPSS statistics 21).
4. Results
4.1. Patient characteristics
Overall, the mean age of all 118 patients was 49.1 years (range, 17–75 years). In total, 64 patients presented recurrence, all of them were local recurrence, 44 patients were within exclusive the surgery group, 26 patients within the GKS group while other 48 patients in the IG-IMRT group. Details of the patient characteristics are summarized in Table 1.
4.2. Survival
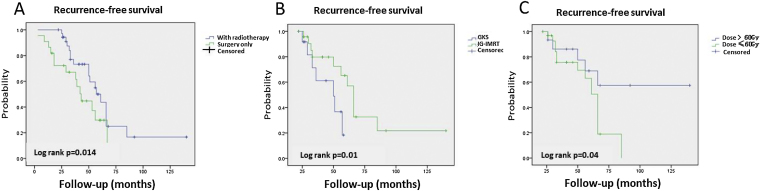
The median follow-up period was 54 months (range, 24–140 months) for all investigated patients, 62 months (range, 24–111 months) for the exclusive surgery group, 44 months (range, 26–94 months) and 52 months (range, 24–140 months) for the GKS group and the IG-IMRT group, respectively. The 2-year and 5-year RFS rates were 72.2% and 29.8%, respectively, for patients within the exclusive surgery group. 2-year and 5-year RFS rates were 91.7% and 18.3%, respectively, for patients within the GKS group. Moreover, 2-year and 5-year RFS rates were 95.7% and 70.9%, respectively, for patients within the IG-IMRT group. Interestingly, patients with sacral chordomas treated with adjuvant radiotherapy have a better RFS than those treated by exclusive surgery (p = 0.014) (Fig. 1), but no difference was found in OS between these two groups. Additionally, compared to GKS group, patients treated by IG-IMRT after surgery exhibited a significant higher RFS rates (p = 0.01). The Kaplan–Meier survival curves representing RFS of the patients according to their radiotherapy technique are presented in Figures (Fig. 1).
Fig. 1.
(A) Kaplan–Meier analysis of recurrence-free survival in 118 patients treated with exclusive surgery or adjuvant radiotherapy, (B) recurrence-free survival in 74 patients treated with Image-guided, Intensity-modulated Radiation Therapy or gamma knife surgery for sacral chordoma. (C) Recurrence-free survival in 48 patients received higher dose and lower dose with Image-guided, Intensity-modulated Radiation Therapy (IG-IMRT).
For patients treated by IG-IMRT as adjuvant radiotherapy, PTV1 received higher dose (>60 Gy) radiotherapy have a better RFS than lower dose (≤60 Gy) (p = 0.04) (Fig. 1). There was no significant difference was found in OS between these two subgroups.
Furthermore, the 2-year and 5-year OS rates were 100% and 59.3%, respectively, for patients within the GKS group,; while 2-year and 5-year OS rates were 100% and 87.5%, respectively, for patients within the IG-IMRT group. However, no significant difference was found for OS of patients with sacral chordomas between those treated by GKS and IG-IMRT (p = 0.409). Interestingly, stratified analysis did not find any significant difference for either RFS or OS between patients underwent a GTR or STR followed by adjuvant radiotherapy. Additionally, there was no significant prognostic factor for RFS by Cox regression analysis.
4.3. Toxicity and side reaction
Radiation treatment toxicities are summarized in Table 2. There were thirty-eight instances of acute Grade 1 and 2 cutaneous and digestive toxicities, thirty cases within the IG-IMRT group and other four within the GKS group. Eight early Grade 3 toxicities were observed, Six of them were cutaneous and one was digestive, all of them within the IG-IMRT group. Two late grade 1 cutaneous toxicity cutaneous was reported. No Grade 2 or 3 toxicities were observed. Statistical analysis did not show any difference of acute toxicities or late toxicities between IG-IMRT group and the GKS group.
Table 2.
Radiation treatment toxicities.
| Acute toxicities(IG-IMRT/GKS) |
Late toxicities(IG-IMRT/GKS) |
|||||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| Skin | 8/2 | 8/2 | 6/0 | 2/0 | 0/0 | 0/0 |
| Gastro-intestinal | 8/2 | 6/2 | 2/0 | 0/0 | 0/0 | 0/0 |
| Urinary | 6/2 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Neurological | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
5. Discussion
Here, we retrospectively analyzed 118 consecutive sacral chordoma patients undergoing adjuvant radiotherapy after surgery. In total, 44 patients were treated by exclusive surgery, and 26 patients underwent GSK for adjuvant radiotherapy. The remaining 48 patients underwent post-operative IG-IMRT. We sought to confirm whether patients with sacral chordoma benefit from adjuvant radiotherapy and to determine the optimal photon radiotherapy module after surgery for these patients.
The standard of care treatment for sacral chordoma is en bloc resection plus adjuvant radiotherapy.12 Surgical margin extension was considered to be the most important prognostic factor;13, 14, 15 however, sufficient margins are only achieved in 50% of cases, based on previous studies.14, 16 In the present study, GTR and STR were achieved in 52.5% and 47.5% of primary tumors, respectively, in all investigated patients. Our results indicated that patients with sacral chordomas treated with adjuvant radiotherapy exhibit better RFS than those treated exclusively by surgery (p = 0.014). Moreover, no significant difference in RFS or OS was found between patients who underwent GTR compared with STR with concomitant radiotherapy. Previous studies reported that the RFS after surgery for sacral chordomas was approximately 35–50%.14, 17, 18 Our data revealed a similar RFS for patients who had undergone surgery alone. Eid AS19 and colleagues demonstrated no difference in RFS between resection with wide excision and subtotal resection after adjuvant radiotherapy. Additionally, for unresectable sacral chordomas, radiotherapy alone has achieved a favorable RFS.20 Based on the evidence above, we strongly suggest that adjuvant radiotherapy should be considered as an important part of comprehensive treatment for sacral chordoma patients. Moreover, surgical margin extension must be carefully pursued after comprehensive consideration of adjacent vital organs. Our results confirmed that sacral chordoma patients benefited from adjuvant radiotherapy and suggested that gross total resection was not a reliable predictor for patients undergoing surgery followed by adjuvant radiotherapy.
Furthermore, our data revealed that patients subjected to IG-IMRT as a postoperative radiotherapy technique exhibited a significantly increased RFS compared with patients who underwent GKS. Given the low α/β values2 of chordomas, GKS was considered an appropriate module for treatment. However, our findings revealed contradictory results. A recent study demonstrated a similar RFS for patients with intracranial chordomas who had undergone GKS.11 Zabel-du Bois et al.21 reported that IMRT photon RT resulted in an RFS of 42% and an OS of 83% with a 4.5-year median follow-up, which is comparable to our results. Additionally, patients with skull base chordomas who underwent IG-IMRT had a better PFS than those who underwent GKS.22 In our study, patients within the IG-IMRT group received a lower dose of radiotherapy (median doses to the boost volume were 71.4 Gy and 76 Gy in our study and their study, respectively). Chordomas are radioresistant, and a high dose is needed for adjuvant radiotherapy, which may lead to a better RFS.23 As radiotherapy techniques have progressed, proton and heavy iron therapy achieved favorable outcomes for sacral chordoma patients, For proton and heavy iron, the 5-year local control was 58% and 77.2%, respectively.20, 24, 25, 26 However, given the limited availability of radiotherapy in developing countries and areas,9, 27 these techniques are not widely used at present. Hence, our results suggested that high-dose IG-IMRT was the most appropriate adjuvant radiotherapy module for sacral chordoma patients in undeveloped countries and areas.
Moreover, our results demonstrated no significant difference in OS between patients undergoing IG-IMRT or GKS as postoperative radiotherapy. All eighteen patients with local tumor recurrence received secondary surgery12 or radiotherapy.6 Although the outcome was better in patients treated for primary tumors compared with locally recurring sacral chordoma,21, 28 both surgery and radiotherapy are effective methods for prolonging the OS of patients within recurrence sacral chordoma.29 The follow-up time in our study may not be sufficiently long to observe a difference. Additionally, only two deaths were observed in each group before the endpoint of our study. The small sample size may lack power for statistical analysis.30
In summary, we report favorable recurrence-free survival rates and adverse event rates following IG-IMRT and suggest that IG-IMRT is an appropriate module of adjuvant radiotherapy for sacral chordoma patients. Ultimately, future prospective clinical trials are needed to confirm the optimal radiotherapy module for treatment of sacral chordoma patients.
Conflict of Interest statements
None declared.
References
- 1.Jo V.Y. Fletcher CDM: WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95–104. doi: 10.1097/PAT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 2.Biermann J.S., Chow W., Reed D.R. NCCN Guidelines Insights: Bone Cancer, Version 2. J. Natl. Comprehen. Cancer Network. 2017;155 doi: 10.6004/jnccn.2017.0017. 2017, Jnccn 15. [DOI] [PubMed] [Google Scholar]
- 3.Bergh P., Kindblom L.G., Gunterberg B., Remotti F., Ryd W., Meis-Kindblom JM: Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122–2134. doi: 10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Ruosi C., Colella G., Di Donato S.L., Granata F., Di Salvatore M.G., Fazioli F: Surgical treatment of sacral chordoma: survival and prognostic factors. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. Eur Spine J. 2015;24(Suppl 7):912–917. doi: 10.1007/s00586-015-4276-4. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein K.D., Goldberg S., Liebsch N.J. Treatment outcomes of patients with primary chordomas treated with preoperative radiation (alone) followed by surgery. Int J Radiat Oncol Biol Phys. 2016;96:E711–E712. [Google Scholar]
- 6.Kabolizadeh P., Chen Y.L., Liebsch N. Updated outcome and analysis of tumor response in mobile spine and sacral chordoma treated with definitive high-dose photon/proton radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97:254–262. doi: 10.1016/j.ijrobp.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Mohan R., Grosshans D: Proton therapy - present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai R., Kamada T., Araki N., Bone Soft T: Carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 cases. Int J Radiat Oncol Biol Phys. 2016;95:322–327. doi: 10.1016/j.ijrobp.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Wang L., Lu J.J., Yin W., Lang J: Perspectives on patient access to radiation oncology facilities and services in Mainland China. Semin Radiat Oncol. 2017;27:164–168. doi: 10.1016/j.semradonc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y., Laufer I., Cox B.W. Preliminary results of high-dose single-fraction radiotherapy for the management of chordomas of the spine and sacrum. Neurosurgery. 2013;73:673–680. doi: 10.1227/NEU.0000000000000083. discussion 680. [DOI] [PubMed] [Google Scholar]
- 11.Kim J.H., Jung H.H., Chang J.H., Chang J.W., Park Y.G., Chang WS: Gamma Knife surgery for intracranial chordoma and chondrosarcoma: radiosurgical perspectives and treatment outcomes. J Neurosurg. 2014;121(Suppl):188–197. doi: 10.3171/2014.7.GKS141213. [DOI] [PubMed] [Google Scholar]
- 12.Stacchiotti S., Sommer J., Chordoma Global Consensus G: Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16:e71–e83. doi: 10.1016/S1470-2045(14)71190-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen K.W., Yang H.L., Lu J., Liu J.Y., Chen XQ: Prognostic factors of sacral chordoma after surgical therapy: a study of 36 patients. Spinal Cord. 2010;48:166–171. doi: 10.1038/sc.2009.95. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs B., Dickey I.D., Yaszemski M.J., Inwards C.Y., Sim FH: Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–2216. doi: 10.2106/JBJS.D.02693. [DOI] [PubMed] [Google Scholar]
- 15.Osaka S., Kodoh O., Sugita H., Osaka E., Yoshida Y., Ryu J: Clinical significance of a wide excision policy for sacrococcygeal chordoma. J Cancer Res Clin Oncol. 2006;132:213–218. doi: 10.1007/s00432-005-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stacchiotti S., Casali P.G., Lo Vullo S. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol. 2010;17:211–219. doi: 10.1245/s10434-009-0740-x. [DOI] [PubMed] [Google Scholar]
- 17.Ruggieri P., Angelini A., Ussia G., Montalti M., Mercuri M: Surgical margins and local control in resection of sacral chordomas. Clin Orthop. 2010;468:2939–2947. doi: 10.1007/s11999-010-1472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulen C.A., Temple H.T., Fox W.P., Sama A.A., Green B.A., Eismont F.J. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am. 2006;88:1532–1539. doi: 10.2106/JBJS.D.02533. [DOI] [PubMed] [Google Scholar]
- 19.Eid A.S., Chang U.K., Lee S.Y., Jeon DG: The treatment outcome depending on the extent of resection in skull base and spinal chordomas. Acta Neurochir. (Wien) 2011;153:509–516. doi: 10.1007/s00701-010-0928-7. [DOI] [PubMed] [Google Scholar]
- 20.Imai R., Kamada T., Araki N. for B and Soft Tissue S: Carbon Ion Radiation Therapy for Unresectable Sacral Chordoma: An Analysis of 188 Cases. Int J Radiat Oncol Biol Phys. 2016;95:322–327. doi: 10.1016/j.ijrobp.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Zabel-du Bois A., Nikoghosyan A., Schwahofer A. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiotherapy and oncology. J Eur Soc Therapeut Radiol Oncol. 2010;97:408–412. doi: 10.1016/j.radonc.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Sahgal A., Chan M.W., Atenafu E.G. Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: preliminary outcomes. Neuro-oncol. 2015;17:889–894. doi: 10.1093/neuonc/nou347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noel G., Habrand J.L., Jauffret E. Radiation therapy for chordoma and chondrosarcoma of the skull base and the cervical spine. Prognostic factors and patterns of failure. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2003;179:241–248. doi: 10.1007/s00066-003-1065-5. [DOI] [PubMed] [Google Scholar]
- 24.Nishida Y., Kamada T., Imai R. Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int J Radiat Oncol Biol Phys. 2011;79:110–116. doi: 10.1016/j.ijrobp.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 25.DeLaney T.F., Liebsch N.J., Pedlow F.X. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110:115–122. doi: 10.1002/jso.23617. [DOI] [PubMed] [Google Scholar]
- 26.Kabolizadeh P., Chen Y.L., Liebsch N. Updated outcome and analysis of tumor response in mobile spine and sacral chordoma treated with definitive high-dose photon/proton radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97:254–262. doi: 10.1016/j.ijrobp.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Datta N.R., Samiei M., Bodis S: Radiation therapy infrastructure and human resources in low- and middle-income countries: present status and projections for 2020. Int J Radiat Oncol Biol Phys. 2014;89:448–457. doi: 10.1016/j.ijrobp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Holliday E.B., Mitra H.S., Somerson J.S. Postoperative proton therapy for chordomas and chondrosarcomas of the spine: adjuvant versus salvage radiation therapy. Spine. 2015;40:544–549. doi: 10.1097/BRS.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 29.Yamada Y., Gounder M., Laufer I: Multidisciplinary management of recurrent chordomas. Curr Treat Options Oncol. 2013;14:442–453. doi: 10.1007/s11864-013-0247-3. [DOI] [PubMed] [Google Scholar]
- 30.Pak K., Uno H., Kim D.H. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]