The study demonstrates that treatment of monocytic cells with Ebola virus shed glycoprotein promotes their differentiation resulting in increased infection and cell death. The effects were inhibited by blocking Toll-like receptor 4 pathway.
Keywords: differentiation, Ebola virus, infection, shed GP, Toll-like receptor
Abstract
A better understanding of the mechanisms used by Ebola virus to disable the host immune system and spread the infection are of great importance for development of new therapeutic strategies. We demonstrate that treatment of monocytic cells with Ebola virus shed glycoprotein (GP) promotes their differentiation resulting in increased infection and cell death. The effects were inhibited by blocking Toll-like receptor 4 pathway. In addition, high levels of shed GP were detected in supernatants of cells treated with Ebola vaccines. This study highlights the role of shed GP in Ebola pathogenesis and also in adverse effects associated with Ebola vaccines.
The 2013–2016 Ebola virus (EBOV) epidemic in Western Africa resulted in 28616 infections including more than 11310 fatalities [1]. Despite the recent progress in characterization of pathogenesis of EBOV infection [2], there are still no approved vaccine or treatments available. Comparison between survivors and fatal human cases of EBOV infections demonstrated lower inflammation and viremia in survivors [3], indicating that inflammation may contribute to mortality. Ebola virus infects multiple types of cells including dendritic cells and macrophages [4], which represent major populations of antigen-presenting cells. More importantly, EBOV infection of dendritic cells leads to their abnormal maturation and subsequent death [5–7]. These effects contribute the deficiency in induction of innate and adaptive immune responses. A better understanding of the mechanisms by which the virus induces death of immune cells, disables the immune system, and causes uncontrolled inflammatory responses is needed to identify treatments of EBOV infection.
The only EBOV envelope glycoprotein (GP) is a type I transmembrane protein [8] that is anchored in infected cells. Ebola virus infections are accompanied by secretion of soluble shed GP resulting from proteolysis of full-length GP by cellular tumor necrosis factor α-converting enzyme (TACE) [9]. Shed GP was detected in the blood of infected guinea pigs and nonhuman primates [9, 10]. Shed GP, along with full-length GP, was demonstrated to bind Toll-like receptor (TLR)4 and activate its signaling pathway, leading to expression of proinflammatory cytokines, maturation of dendritic cells and macrophages, as well as increase in endothelial permeability [11, 12]. These effects were demonstrated to be dependent on glycosylation of shed GP and its capacity to bind TLR4 in a way similar to lipopolysaccharides (LPS) [12]. The goal of this work was to identify a possible role of shed GP in activation and infection of monocytes. We show that shed GP causes a significant increase in expression of monocyte activation markers in a manner partially dependent on TLR4, and the resulting increase in EBOV infection and cell death may contribute to “immune paralysis” observed during EBOV disease.
METHODS
Cells
Human monocytic cells THP-1 were obtained from the American Type Culture Collection ([ATCC] Manassas, Virginia) and cultured in Roswell Park Memorial Institute 1640 medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS) (GE Hyclone, Pittsburgh, PA) and 1% HEPES (Corning, Corning, New York). Vero-E6 cell lines were obtained from the ATCC and cultured in Dulbecco’s modified Eagle’s medium, supplemented with 10% HI-FBS (Thermo Fisher Scientific), 1% HEPES (Corning), 1% nonessential amino acids (Sigma-Aldrich, St. Louis, MO), 1% sodium pyruvate (Sigma-Aldrich), and 2% penicillin-streptomycin mix (Thermo Fisher Scientific).
Viruses and Virus-Like Particles
The recombinant EBOV, strain Mayinga, expressing green fluorescent protein (EBOV-GFP) was generated and amplified as described previously [5, 13]. All work with EBOV was performed in biosafety level 4 facilities of the Galveston National Laboratory. The vaccine construct VSV-ΔG-ZEBOVGP [14] was kindly provided by Dr. H. Feldmann (Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases). The vaccine construct HPIV3/ΔF-HN/EboGP was generated and amplified as previously described [15]. Ebola virus virus-like particles (VLPs) were generated as in our previous study [16] using mammalian cell codon optimized plasmids expressing EBOV GP pWRG7077:64755-2010-233-1_GP_optVP40 (codop-EBO-GP), EBOV VP40 pWRG7077:64759-2010-233-1-4_VP40_optVP40 provided by Dr. Sina Bavari (US Army Medical Research Institute of Infectious Diseases) and EBOV NP pCEZ-NP provided by Dr. Yoshihiro Kawaoka (University of Wisconsin).
Recombinant Shed Glycoprotein
Recombinant shed GP, which represents ectodomain of EBOV GP, was obtained by 2 methods. The first method involved transfection of codop-EBO-GP plasmid in 293T cells using TransIT-LT1 Mirus reagent (Mirus Bio, Madison, WI) for 72 hours. Transfected cells were collected and centrifuged at 10000 ×g for 10 minutes to remove cell debris, and supernatants containing shed GP were concentrated using Centricon-Plus 70 centrifugal filter units (EMD Millipore, Billerica, MA) following manufacturer’s recommendations. The second method involved infection of Vero-E6 cells with vaccine constructs VSV-ΔG-ZEBOVGP [14] and HPIV3/ΔF-HN/EboGP [15] at multiplicity of infection (MOI) 1 plaque-forming units (PFU)/cell for 72 hours. Then, supernatants were centrifuged for 10 minutes at 10000 ×g to remove cell debris and purified using 0.1-μm filter syringes (EMD Millipore). Because both vaccine constructs quickly replicate and abundantly produce shed GP, supernatants of vaccine-infected cells were not concentrated. Shed GP preparations were analyzed by Western blotting.
Confocal Microscopy
THP-1 cells were plated in 12-well plates at 1 × 106 cells per well and cultured with EBOV VLPs at MOI 3 particles/cell, quantitated using Virocyt Virus Counter 3100 (Sartorius Stedim Biotech) or shed GP from supernatants of plasmid-transfected cells at 20 μg/mL. Cells were incubated for 2 hours on ice, washed with phosphate-buffered saline (PBS) (Corning) with 2% heat-inactivated fetal bovine serum, fixed with 4% formaldehyde (Polysciences, Warrington, PA), loaded on positively charged slides (Thermo Fisher Scientific), and dried overnight. Immune serum raised against EBOV VLPs (Integrated BioTherapeutics, Rockville, MD) was diluted in PBS containing 1% bovine serum albumin (Thermo Fisher Scientific) and 0.1% Triton X-100 (Alfa Aesar) (PBS-BSA-TX100) at 1:100 and put on the slides for 1 hour. Then, slides were washed 3 times with PBS with 0.1% Triton X-100 and incubated with secondary donkey anti-rabbit antibodies (Cell Signaling Technology, Danvers, MA) conjugated with Alexa Fluor 647 (Thermo Fisher Scientific) diluted at 1:200 in PBS-BSA-TX100 for an additional 1 hour and washed as described above. Next, cells were incubated with 4’,6-diamidino-2-phenylindole (Thermo Fisher Scientific) at 1 µg/mL for 2 minutes and washed with PBS. The coverslips were mounted onto microscope slides using PermaFluor mounting medium (Thermo Fisher Scientific).
Analysis of THP-1 Cell Activation Markers Expression
THP-1 cells were plated in 24-well plates at 1 × 106 cells/well, treated or mock-treated with CLI-095 (InvivoGen, San Diego, CA) at 100 ng/mL for 1 hour, and cultured for 24 or 96 hour with LPS (500 ng/mL) (InvivoGen), EBOV-VLP, or shed GP in supernatants of plasmid-transfected cells supernatants of 293T cells. For analysis of monocyte activation, cells were harvested, fixed, and permeabilized with Cytofix/Cytoperm reagent (BD Biosciences, San Jose, CA) following manufacturer’s instructions and stained with the following antibodies: CD14-BUV395 (BD Biosciences no. 563561), CD11b-FITC (BD Biosciences no. 562793), and CD68-PE/Cy7 (BD Biosciences no. 565595).
Analysis of Ebola Virus Infectivity and Cell Death
Cells incubated with shed GP were centrifuged for 5 minutes at 250 ×g, supernatants were removed, and cells were infected with EBOV-GFP at a MOI 3 PFU/cell for 48 hours, and treated or mock-treated with CLI-095 at 100 ng/mL for 48 hours. Cells were harvested, fixed with 4% formaldehyde for 24 hours at 4°C, and analyzed for GFP expression by flow cytometry. To analyze cell death, cells were harvested, fixed with 4% formaldehyde for 24 hours at 4°C, and stained with annexin V antibody labeled with phycoerythrin (BD Biosciences no. 559763) and Live/Dead-Aqua blue dye (Thermo Fisher Scientific).
Statistical Analysis
Statistical methods are described in figure legends; the calculations were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA).
RESULTS
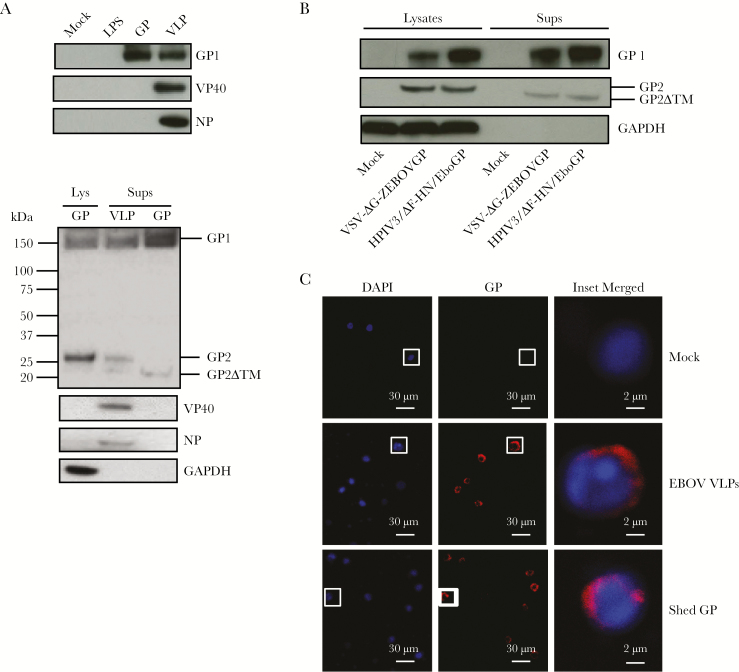
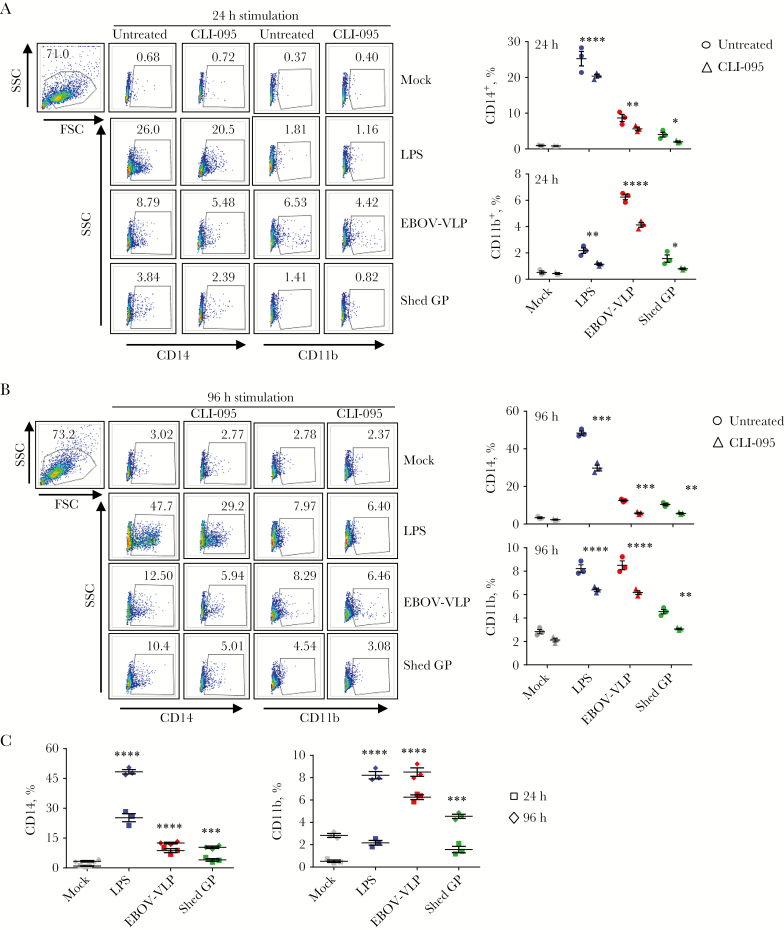
Previous reports demonstrated that monocyte activation and differentiation increases their susceptibility to EBOV [17]. Furthermore, recent studies showed that EBOV itself is involved in activation and differentiation of uninfected monocytes when spreading from infected cells [16]. We hypothesized that binding of shed GP to TLR4 leads to activation and differentiation of monocytes, resulting in their increased susceptibility to EBOV. To demonstrate the effect of shed GP, we used a recombinant form of this protein along with EBOV VLPs. We first confirmed the presence of shed GP in the supernatants of GP-transfected cells by Western blotting. In VLP supernatants, GP1 and full-length GP2 were detected, whereas in shed GP supernatants, GP1 and the truncated GP2ΔTM, which together compose shed GP [12], were detected (Figure 1A). The detection of the truncated GP2ΔTM form but not full-length GP2 in shed GP preparations suggests the absence of GP released from exosomes or vesicles budding from the plasma membrane. Because GP is the principal component of all EBOV vaccines, we also analyzed supernatants of cells infected with the vaccine constructs based on human parainfluenza type 3 (HPIV3) [15] and vesicular stomatitis virus (VSV) [14] vectors; in both cases, shed GP was readily detected (Figure 1B). We used THP-1 human monocytic cell line, which was analyzed using markers of activation and differentiation CD14, CD11b, and CD68. “Classic monocytes” in human blood have high expression of CD14 and CD11b and low expression of CD68, whereas less abundant “nonclassic” monocytes have low expression of CD14 and CD11b [18–20]. THP-1 cells, which are widely used as a model of EBOV infection [17, 21, 22], have low expression of CD14, CD11b, or CD68 under normal conditions but increased during activation and differentiation [23–26]. Binding of shed GP to THP-1 cells was confirmed by confocal microscopy (Figure 1C). Because stimulation with TLR4 promotes differentiation of monocytes to macrophages [27], THP-1 cells were pulsed with the TLR4 ligand LPS along with EBOV VLPs or shed GP in the presence or absence of the TLR4 inhibitor CLI-095 for 24 or 96 hours. Stimulation with shed GP, as well as VLPs and LPS, resulted in a significant increase in the levels of markers of differentiation CD14, CD11b, and CD68 at both 24 and 96 hours poststimulation (Figure 2A and B and Supplementary Figure 1A and B). The levels of markers of activation and differentiation were higher at 96 hours compared with 24 hours postinfection (Figure 2C and Supplementary Figure 1C). CLI-095 treatment significantly reduced the expression of all 3 differentiation markers, which was more pronounced at 96 hours, demonstrating the role of TLR4 in shed GP-induced differentiation of THP-1 (Figure 2A and B and Supplementary Figure 1A and B).
Figure 1.
Shed glycoprotein (GP) is produced by Ebola virus (EBOV) vaccine constructs and binds THP-1 cells. (A) Western blot analysis of supernatants containing lipopolysaccharides (LPS), shed GP, and EBOV virus-like particles (VLPs) used for stimulation of THP-1 cells (top panel) and of lysates and supernatants of GP-transfected cells and supernatants containing EBOV VLPs for various forms of GP (bottom panel). (B) Western blot analysis of GP1 and GP2 in cell lysates and supernatants after infection of Vero-E6 cells with the VSV-ΔG-ZEBOVGP and HPIV3/ΔF-HN/EboGP vaccine constructs. (C) Confocal microscopy of EBOV VLPs or exogenously added shed GP bound to THP-1 cells. Blue represents nuclei stained by 4’,6-diamidino-2-phenylindole (DAPI), and red represents GP. Puncti in merged images indicate GP bound to THP-1 cells.
Figure 2.
Treatment of THP-1 cells with shed glycoprotein (GP) promotes their Toll-like receptor 4-dependent differentiation. THP-1 cells were treated or mock-treated with CLI-095 and cultured with shed GP, Ebola virus (EBOV) virus-like particle (VLP), or lipopolysaccharides (LPS) for 24 hours or 96 hours. Cells were harvested and analyzed for expression of activation markers CD14 and CD11b by flow cytometry. (A) Primary data (left panel) and percentages of cells positive for CD14 (top right panel) and CD11b (bottom right panel) at 24 hours. (B) Primary data (left panel) and percentages of cells positive for CD14 (top right panel) and CD11b (bottom right panel) at 96 hours. (C) Percentages of THP-1 cells positive CD14 and CD11b after stimulation with LPS, VLP, or shed GP for 24 hours or 96 hours in the absence of CLI-095. Mean values based on triplicate samples ± standard error: *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001 (two-way analysis of variance followed by a Tukey’s multiple comparison test). Representative data from 2 independent experiments. Abbreviations: FSC, forward scatter; SSC, side scatter.
We next tested how shed GP-mediated differentiation affects the rate of EBOV infection. THP-1 cells were cultured in the presence of shed GP, VLPs, or LPS with or without CLI-095 for 24 hours or 96 hours and infected with EBOV-GFP for 48 hours. Presence of shed GP, as well as VLPs and LPS resulted in a significant increase in the percentages of infected cells (Figure 3A and B); the effect was more pronounced at 96 hours (Figure 3C). Again, adding of CLI-095 reduced the percentages of infected cells at both 24 and 96 hours (Figure 3A and B). Overall, these results demonstrate that TLR4-dependent activation and differentiation of THP-1 cells after shed GP binding increases their susceptibility to EBOV infection.
Figure 3.
Shed glycoprotein (GP) promotes Toll-like receptor 4-dependent THP-1 cells susceptibility to Ebola virus (EBOV) infection. THP-1 cells were stimulated with lipopolysaccharides (LPS), virus-like particles (VLPs), or shed GP with or without CLI-095 and infected with EBOV-green fluorescent protein (GFP). (A) Primary flow cytometry data (left panel) and percentages of cells positive for EBOV-GFP (right panel) at 24 hours. (B) Primary flow cytometry data (left panel) and percentages of cells positive for EBOV-GFP (right panel) at 96 hours. (C) Percentages of GFP-positive (infected) THP-1 cells prestimulated with shed GP, VLP, or LPS for 24 or 96 hours in the absence of CLI-095. Mean values based on triplicate samples ± standard error: ****, P < .0001 (two-way analysis of variance followed by a Tukey’s multiple comparison test). Representative data from 2 independent experiments. Abbreviations: FSC, forward scatter; SSC, side scatter.
We next examined the effect of shed GP on cell death (Figure 4A and B). THP-1 cells were cultured in the presence of shed GP or LPS for 24 or 96 hours with or without CLI-095, and cell death was analyzed by flow cytometry. Consistent with our data on the effects of shed GP on cell differentiation, shed GP-mediated activation significantly increased the percentages of dead cells compared with mock treatment (Figure 4A and B), and this effect was more pronounced at 96 hours poststimulation (Figure 4C). Furthermore, adding of CLI-095 reduced the rates of cell death at 96 hours (Figure 4A and B) further confirming the role of TLR4-dependent stimulation in the death of THP-1 cells. Overall, these results demonstrate that engagement of TLR4 by shed GP triggers cell death, which subsequently leads to impaired immune response and increased level of EBOV replication.
Figure 4.
Shed glycoprotein (GP) triggers Toll-like receptor 4-dependent death of THP-1 cells. THP-1 cells were stimulated with lipopolysaccharides (LPS), virus-like particles (VLPs), or shed GP with or without CLI-095, stained with LIVE/DEAD and annexin V and analyzed by flow cytometry. (A) Primary flow cytometry data. (B) Percentages of LIVE/DEAD+ cells. (C) Percentages of LIVE/DEAD-positive cells after 24- or 96-hour-long stimulation in the absence of CLI-095. Mean values based on triplicate samples ± standard error: ***, P < .001; ****, P < .0001, based on one-way analysis of variance (ANOVA) followed by a Dunnett’s multiple comparisons test (top graph panel B) and two-way ANOVA followed by a Tukey’s multiple comparison test (bottom graph panel B). Representative data from 2 independent experiments. Abbreviations: FSC, forward scatter; SSC, side scatter.
DISCUSSION
A previous study by Escudero-Pérez et al [12] described how shed GP activates dendritic cells and macrophages and modulates endothelial cells function through TLR4. Moreover, the study by Escudero-Pérez et al [12] demonstrated that release of pro- and anti-inflammatory cytokines modulates the inflammatory response during EBOV infection. Our study extends and complements the study by Escudero-Pérez et al [12] and demonstrates a new biological role for shed GP. We show that a direct binding of shed GP to monocytes promotes their differentiation that leads to their increased susceptibility to EBOV infection and cell death. Consistent with previous data demonstrating that monocyte differentiation promotes EBOV infection [17], our data confirm an increased infection of THP-1 cells associated with differentiation. Moreover, we convincingly demonstrate the role of TLR4 signaling in these effects, because a specific TLR4 inhibitor significantly reduced THP-1 differentiation, infection, and death associated with TLR4 stimulation by shed GP, as well as EBOV VLP or LPS (Figure 5).
Figure 5.
Shed glycoprotein (GP) triggers infection and death of THP-1 cells through Toll-like receptor 4 activation. A model representing mechanisms involved in infection and death of monocytes after their activation by shed GP. Abbreviations: DC, dendritic cells; EBOV, Ebola virus.
All EBOV vaccine candidates use GP as the sole antigen inducing a protective antibody response [28]. We demonstrated abundant presence of shed GP in supernatants of cells infected with HPIV3- and VSV-vectored EBOV vaccines (Figure 1B). Because the VSV-vectored vaccine replicates very efficiently, the amount of shed GP released by susceptible cells in circulation of vaccinees should be high. A recent clinical testing of VSV-ZEBOVGP vaccine demonstrated that vaccination at doses expected to be protective (5 × 107 PFU) induced adverse side effects including oligoarthritis, maculopapular dermatitis, vesicular dermatitis, and dermal vasculitis, which were clearly associated with GP and not the VSV vector [29]. The present study suggests that, at least in part, these effects are likely to be associated with shed GP, which spread systemically and leads to activation of TLR4 pathway. It also suggests that similar effects can be expected from any EBOV vaccine, if administered at doses leading to expression of GP at high levels.
CONCLUSIONS
Taken together, these results demonstrate the proinflammatory effect of shed GP through stimulation of TLR4 pathway, which results in increased differentiation of monocytes leading to amplified EBOV infection. We recently demonstrated that inhibition of TLR4 signaling reduces inflammation caused by filovirus infections and promotes survival [30]. Our findings identify the role of shed GP in spread of infection, suggesting that targeting of circulating shed GP may be another way to treat filovirus infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. I. designed and performed most of experiments in this study. R. I. S. and M. I. performed confocal microscopy experiments. N. M. L. contributed to experiments involving EBOV infections. M. I. and A. B. initiated the project and A. B. led the project. M. I. and A. B. composed the paper with input from all authors.
Acknowledgments. The authors are grateful to Dr. H. Feldmann for providing the vaccine construct VSV-ΔG-ZEBOVGP, Dr. Sina Bavari for providing the mammalian codon-optimizing plasmids encoding Ebola virus (EBOV) EBOV glycoprotein and EBOV VP40, Dr. Yoshihiro Kawaoka for providing plasmid encoding EBOV nucleoprotein, and Dr. Abhishek Prasad for the careful reading and opinions shared on the study.
Financial support. This study was funded by the National Institutes of Health (NIH) grant U19 AI109945-01 Project 2 (to A. B.) and NIH grant 1R01AI102887-01A1 (to A. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Centers for Disease Control and Prevention. 2014 Ebola Outbreak in West Africa - Case Counts 2016 Available at: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Accessed 22 January 2018.
- 2. Prescott JB, Marzi A, Safronetz D, Robertson SJ, Feldmann H, Best SM. Immunobiology of Ebola and Lassa virus infections. Nat Rev Immunol. 2017; 17:195–207. [DOI] [PubMed] [Google Scholar]
- 3. Baize S, Leroy EM, Georges AJ, et al. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol 2002; 128:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003; 163:2347–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lubaki NM, Ilinykh P, Pietzsch C, et al. The lack of maturation of Ebola virus-infected dendritic cells results from the cooperative effect of at least two viral domains. J Virol 2013; 87:7471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol 2003; 170:2797–801. [DOI] [PubMed] [Google Scholar]
- 7. Bosio CM, Aman MJ, Grogan C, et al. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis 2003; 188:1630–8. [DOI] [PubMed] [Google Scholar]
- 8. Sanchez A, Kiley MP, Holloway BP, Auperin DD. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res 1993; 29:215–40. [DOI] [PubMed] [Google Scholar]
- 9. Dolnik O, Volchkova V, Garten W, et al. Ectodomain shedding of the glycoprotein GP of Ebola virus. EMBO J 2004; 23:2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubins KH, Hensley LE, Wahl-Jensen V, et al. The temporal program of peripheral blood gene expression in the response of nonhuman primates to Ebola hemorrhagic fever. Genome Biol 2007; 8:R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okumura A, Pitha PM, Yoshimura A, Harty RN. Interaction between Ebola virus glycoprotein and host Toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J Virol 2010; 84:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Escudero-Pérez B, Volchkova VA, Dolnik O, Lawrence P, Volchkov VE. Shed GP of Ebola virus triggers immune activation and increased vascular permeability. PLoS Pathog 2014; 10:e1004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Towner JS, Paragas J, Dover JE, et al. Generation of eGFP expressing recombinant Zaire ebolavirus for analysis of early pathogenesis events and high-throughput antiviral drug screening. Virology 2005; 332:20–7. [DOI] [PubMed] [Google Scholar]
- 14. Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol 2004; 78:5458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bukreyev A, Marzi A, Feldmann F, et al. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology 2009; 383:348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iampietro M, Younan P, Nishida A, et al. Ebola virus glycoprotein directly triggers T lymphocyte death despite of the lack of infection. PLoS Pathog 2017; 13:e1006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinez O, Johnson JC, Honko A, et al. Ebola virus exploits a monocyte differentiation program to promote its entry. J Virol 2013; 87:3801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep 2015; 5:13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Betjes MG, Haks MC, Tuk CW, Beelen RH. Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-term culture. Immunobiology 1991; 183:79–87. [DOI] [PubMed] [Google Scholar]
- 20. Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV. CD68/macrosialin: not just a histochemical marker. Lab Invest 2017; 97:4–13. [DOI] [PubMed] [Google Scholar]
- 21. Dahlmann F, Biedenkopf N, Babler A, Jahnen-Dechent W, Karsten CB, Gnirss K, et al. Analysis of Ebola virus entry into macrophages. J Infect Dis 2015; 212(Suppl 2):S247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edwards MR, Liu G, Mire CE, et al. Differential regulation of interferon responses by Ebola and Marburg virus VP35 Proteinsp Cell Rep 2016; 14:1632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aldo PB, Craveiro V, Guller S, Mor G. Effect of culture conditions on the phenotype of THP-1 monocyte cell line. Am J Reprod Immunol 2013; 70:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bosshart H, Heinzelmann M. Lipopolysaccharide-mediated cell activation without rapid mobilization of cytosolic free calcium. Mol Immunol 2004; 41:1023–8. [DOI] [PubMed] [Google Scholar]
- 25. Yuan A, Hsiao YJ, Chen HY, et al. Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci Rep 2015; 5:14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015; 15:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takashiba S, Van Dyke TE, Amar S, Murayama Y, Soskolne AW, Shapira L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infect Immun 1999; 67:5573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marzi A, Feldmann H. Ebola virus vaccines: an overview of current approaches. Expert Rev Vaccines 2014; 13:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huttner A, Dayer JA, Yerly S, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase ½ trial. Lancet Infect Dis 2015; 15:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Younan P, Ramanathan P, Graber J, Gusovsky F, Bukreyev A. The Toll-like receptor 4 antagonist eritoran protects mice from lethal filovirus challenge. MBio 2017; 8:e00226–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.