Abstract
Filoviruses such as Ebola virus (EBOV), Marburg virus (MARV), and Sudan virus (SUDV) cause deadly viral hemorrhagic fever in humans, with high case-fatality rates; however, no licensed therapeutic agent or vaccine has been clinically approved to treat or prevent infection. T-705 (favipiravir) is a novel antiviral drug that has been approved for the treatment of influenza in Japan. T-705 exhibits broad-spectrum antiviral activity against different viruses, including MARV and EBOV, and here, we are the first to report the in vitro and in vivo antiviral activity of T-705 against SUDV. T-705 treatment reduced SUDV replication in Vero E6 cells. Subcutaneous administration of T-705, beginning 1–4 days after infection and continuing for 7 days, significantly protected SUDV-infected guinea pigs, with a survival rate of 83%–100%. Viral RNA replication and infectious virus production were also significantly reduced in the blood, spleen, liver, lungs, and kidney. Moreover, early administration of low-dose T-705 and late administration (at 5 days after infection) of higher-dose T-705 also showed partial protection. Overall, our study is the first to demonstrate the antiviral activity of T-705 against SUDV, suggesting that T-705 may be a potential drug candidate for use during outbreaks.
Keywords: T-705, guinea pig–adapted Sudan virus, guinea pig, subcutaneous administration
The Filoviridae family comprises 3 genera and multiple species, each represented by at least 1 virus: Ebola virus (EBOV), Sudan virus (SUDV), Bundibugyo virus, Taï Forest virus, and Reston virus, belong to the Ebolavirus genus; Marburg virus (MARV) and Ravn virus, belong to the Marburgvirus genus; and Lloviu virus, belonging to the Cuevavirus genus [1–3]. In humans and nonhuman primates, all filoviruses except Reston virus and Lloviu virus are capable of causing severe hemorrhagic fevers known as Ebola virus disease or Marburg virus disease, depending on the genus of the causative agent [3, 4]. EBOV, in particular, is responsible for numerous deadly outbreaks throughout Africa, including the 2014–2016 West African outbreak that resulted in >28000 cases and >11000 deaths [5], and for importation of virus into Europe and North America via infected travelers [6]. SUDV, which is about 58% identical to EBOV at the nucleotide level [7], has also caused several devastating outbreaks since its discovery in 1976, with cumulative case-fatality rates of >50%. Nevertheless, despite the global public health threat posed by these viruses, no vaccine or therapeutic agent has so far been clinically approved for the treatment of Ebola virus disease or Marburg virus disease. Moreover, although several candidate vaccines and therapeutic agents have been identified, the majority of these have been developed and tested to treat disease caused by EBOV or MARV [8, 9].
Previous studies have identified various small molecules and inhibitors that are effective against EBOV in vitro; however, only few of these have been assessed in animal models [10–13]. One such small molecule, known as T-705 (favipiravir; 6-fluoro-3-hydroxy-2-pyrazinecarboxamide), is a novel antiviral agent recently approved in Japan to treat influenza virus infection [14]. T-705 is a nucleoside analogue that, following phosphoribosylation by host enzymes, is recognized as a substrate by the viral RNA–dependent RNA polymerase and is thought to either directly inhibit polymerase activity or result in a catastrophic genetic mutation [10, 14–16]. Previous in vitro and in vivo studies have demonstrated that T-705 exhibits antiviral activity against a wide range of viruses, including EBOV, MARV, and other RNA viruses, such as alphaviruses, arenaviruses, paramyxoviruses, picornaviruses, flaviviruses, and bunyaviruses [14, 17–19]. Recently, we and other groups demonstrated the antiviral activity of T-705 against MARV and EBOV in vitro and in vivo [19–22]. However, whether T-705 is effective against other filoviruses, like SUDV, is unclear. Indeed, there has been a relative lack of research into SUDV countermeasures, owing at least in part to the absence of suitable experimental animal model for this virus. Recently, we developed a guinea pig–adapted SUDV (GA-SUDV) variant that causes uniformly lethal disease in guinea pigs and therefore permits the evaluation of antiviral countermeasures [23]. In the present study, we used the guinea pig model of SUDV infection to investigate the antiviral effect of T-705 and became the first group to demonstrate that this drug effectively inhibits SUDV infection both in vitro and in vivo.
MATERIALS AND METHODS
Ethics Statement
The animal study procedure was approved by the Animal Care Committee at the Canadian Science Centre for Human and Animal Health according to the guidelines of the Canadian Council on Animal Care. The animals were acclimatized for 7 days before starting the experiment, given food and water ad libitum, and monitored every day. All work with infectious SUDV and potential infectious materials derived from the infected animals was performed in the containment level 4 laboratory at the National Microbiology Laboratory (Winnipeg, Canada).
Cells, Animals, Viruses, and T-705
Vero E6 and monkey kidney CV-1 cells were obtained from the American Type Culture Collection and maintained at 37oC and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) and minimum essential medium (MEM), respectively, supplemented with 1% penicillin-streptomycin and 10% heat-inactivated fetal bovine serum (FBS). Wild-type SUDV variant Boneface (Sudan virus/C.porcellus-lab/SSD/1976/Nzara-Boneface) was used for the in vitro study and GA-SUDV (Sudan virus/NML/C.porcellus-lab/SSD/1976/Nzara-Boneface-GP) was used for all in vivo studies [23]. The T-705 compound (favipiravir; Chemical Abstract Service no. 259793-96-9) was purchased from BOC Science (Shirley, NY). For the in vitro study, T-705 was dissolved in dimethyl sulfoxide at a concentration of about 10 mg/mL and stored at −20°C. For the in vivo studies, T-705 was dissolved in sterile water with 74.6 mg/mL meglumine excipient according to the protocol of Safronetz et al [17]. The absence of cellular toxicity caused by T-705 at concentrations of <2 mg/mL [20] was confirmed by treating Vero E6 cells in triplicate with 1 mg/mL T-705 and observing them for cytopathic effect over 4 days.
In Vitro SUDV Infection and T-705 Efficacy
A series of diluted T-705 solutions was prepared in DMEM, starting at 100 µg/mL and followed by 1:3 dilutions. Vero E6 cells (95% confluent) were pretreated with T-705 for 1 hour. After pretreatment, the medium containing T-705 was removed, and the cells were infected with SUDV at a multiplicity of infection (MOI) of 0.01 in the presence of T-705. One hour after infection, the inoculum was removed and replaced with fresh DMEM with 2% FBS and T-705. The control cells were treated with DMEM and infected with virus at the same MOI in the absence of T-705. Cell supernatants were harvested at 72 hours after infection, and SUDV RNA levels were analyzed by reverse transcription–quantitative polymerase chain reaction analysis (RT-qPCR), as described below. The viral RNA level in medium containing control cells was considered indicative of 0% inhibition. Therefore, the inhibitory effects of T-705–treated cells were determined by measuring the ratio of the viral RNA yield from treated and control cells. The percentage inhibition of infection was calculated as 100% – [(Viral RNA in treated cells/viral RNA in control cells) x 100]. The half maximal effective concentration (EC50) of inhibitory function and the 90% effective inhibitory concentration (EC90) were determined according to the Reed-Muench method.
In Vivo SUDV Challenge and T-705 Efficacy
The in vivo efficacy of T-705 was tested in different independent studies. Female guinea pigs (strain Hartley; Charles River Laboratories) aged 6–8 weeks were challenged with GA-SUDV at 1000 times the median lethal dose (ie, fifty-three 50% tissue culture infectious doses [TCID50]/animal) by intraperitoneal injection. Guinea pigs were subsequently treated once per day by subcutaneous injection with either 150 mg/kg or 300 mg/kg of body weight of T-705, starting 1, 2, 3, 4, or 5 days after infection and continuing daily for 7 consecutive days. Control animals were treated with meglumine only in the same manner. All animals were observed daily for disease symptoms, and 5–18 guinea pigs in each treatment group were assessed for survival and weight changes. Additionally, to determine viral RNA levels and infectious virus titers, as well as blood cell counts, 2 groups of 3 guinea pigs were infected with GA-SUDV, as described above, and treated daily with 150 mg/kg or 300 mg/kg of body weight of T-705, starting on day 2 and continuing for 7 consecutive days, as described above. Control animals were uninfected (n = 3) or infected but treated with meglumine (n = 3), as described above. Blood samples were collected 5 and 9 days after infection, to assess viral RNA levels, infectious virus titers, and blood cell counts. Tissue samples were collected upon euthanasia 9 days after infection, to determine viral RNA levels and infectious virus titers.
Detection of Viral RNA by RT-qPCR Analysis
Viral RNA was extracted from guinea pig blood and tissue samples, using the QIAamp viral RNA mini kit (Qiagen, Germany) and the RNeasy mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. Viral RNA levels were measured by RT-qPCR analysis, using the Light Cycler 480 RNA Master Hydrolysis Probes kit (Roche, Germany) and the Light Cycler 480 thermal cycler (Roche, Germany). The primers and probe used in this study have been previously described [23]. Cycling conditions were as follows: 63°C for 3 minutes and 95°C for 30 seconds, followed by 45 cycles of 95°C for 15 seconds and 60°C for 30 seconds.
Titration of Infectious Virus
Titration of infectious GA-SUDV from blood specimens and different tissue homogenates was done by the TCID50 assay. In brief, samples were serially diluted 10-fold in MEM. CV-1 cells (80% confluent) in a 96-well plate (Corning) were then infected with 100 µL of each diluted sample. After 1 hour of adsorption at 37°C, the inoculum was removed, and cells were overlaid with 100 µL of fresh MEM containing 2% FBS. Infected cells were then incubated for 14 days, observed for cytopathic effect, and scored. Viral titers were calculated by the Reed and Muench method.
Blood Cell Counts and Data Analysis
Complete blood counts of white blood cells (WBCs), lymphocytes, neutrophils, monocytes, and platelets were performed by using the VetScan HM5 hematology system (Abaxis, Germany). Statistical analyses of viral RNA, TCID50, and blood cell count data were done by means of 1-way analysis of variance with the Bonferroni multiple-comparison test, using Graph Pad Prism 5 software. The survival curves were analyzed with the Mantel-Cox test.
RESULTS
In Vitro Antiviral Effect of T-705 Against SUDV
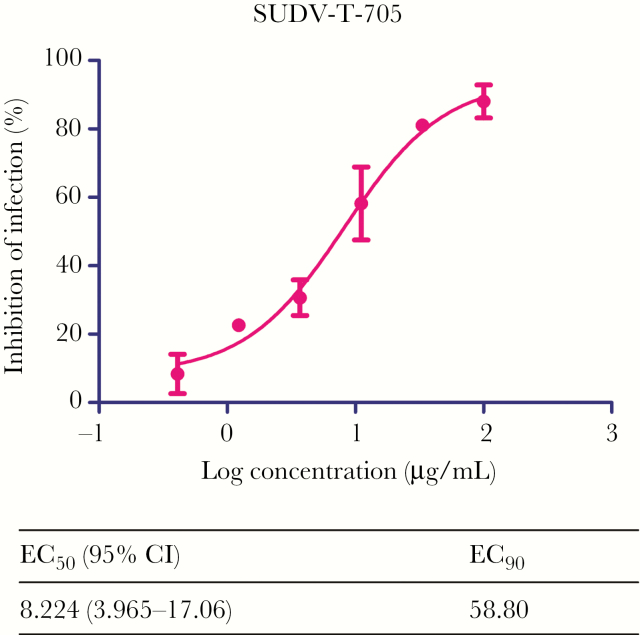
Vero E6 cells were infected with SUDV at a MOI of 0.01 and treated with different concentrations of T-705, ranging from 0.41 to 100 μg/mL. A total of 72 hours after infection the supernatants were harvested to detect the viral load. T-705 treatment reduced viral RNA levels in a dose-dependent manner. Overall, approximately 90% of viral RNA replication was inhibited during T-705 treatment, which resulted in EC50 and EC90 values of 8.224 µg/mL and 58.80 µg/mL, respectively (Figure 1). Consistent with findings from a previous study [20], no cytotoxic effect was observed when Vero cells were treated with T-705 at 1 mg/mL, a concentration 10 times higher than the highest concentration used in this study (data not shown).
Figure 1.
In vitro antiviral effect of T-705 against Sudan virus (SUDV). Vero E6 cells were pre-treated with a dilution series of T-705 (starting at 100 µg/mL followed by 1:3 dilutions) for 1 hour prior to infection with wild type SUDV at MOI 0.01. After 72 hours post infection, SUDV RNA in the supernatant was measured by reverse transcription–quantitative polymerase chain reaction (RT-qPCR). The half maximal effective concentration (EC50) and 90% effective inhibitory concentration (EC90) values for T-705 were calculated from the sigmoidal function. Abbreviation: CI, confidence interval..
In Vivo Efficacy of T-705 Treatment in GA-SUDV–Infected Guinea Pigs
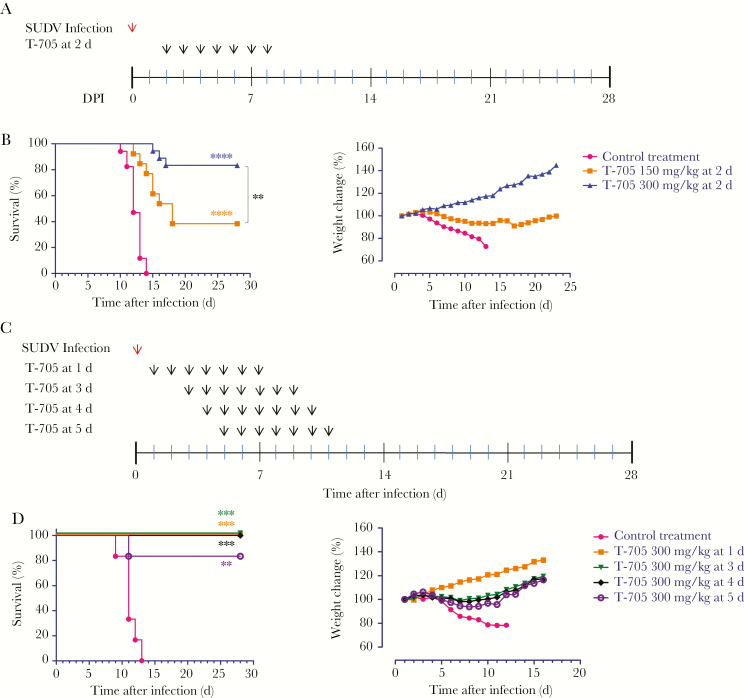
Initially, we sought to identify the antiviral activity and effective dose of T-705 in GA-SUDV–infected guinea pigs. Based on other studies investigating the effect of T-705 against arenaviruses in the guinea pig model [17, 24, 25], we chose 150 and 300 mg/kg/day doses to begin. Following infection with 1000 times the median lethal dose of GA-SUDV, guinea pigs were treated with control or by subcutaneous administration of T-705, at either 150 or 300 mg/kg/day, starting on day 2 after infection and continuing every day up to day 8 (Figure 2A). The control-treated animals started losing weight on days 4–5 after infection, and the entire control group (n = 17) was euthanized within 10–14 days (Figure 2B). Conversely, animals treated with 150 mg/kg/day or 300 mg/kg/day showed partial protection, with approximately 38% (5 of 13) and approximately 83% (15 of 18) of animals surviving, respectively (Figure 2B). Most animals that received 150 mg/kg/day began to lose weight on days 5–6 after infection, and 8 died 12–18 days after infection (Figure 2B). In contrast, most animals treated with 300 mg/kg/day of T-705 gained weight after treatment, with the exception of 3 animals, which lost significant weight during days 6–11 after infection and ultimately died 15–17 days after infection (Figure 2B). Overall, although both doses of T-705 offered some protection against GA-SUDV infection, the 300-mg/kg/day dose was clearly the most efficacious.
Figure 2.
Survival rate and relative weight change in guinea pig–adapted Sudan virus (GA-SUDV)–infected guinea pigs after T-705 treatment. A, Two groups of guinea pigs (n = 18 and 13, respectively) were challenged with GA-SUDV via the intraperitoneal route and treated subcutaneously with 300 mg/kg/day and 150 mg/kg/day of T-705, beginning 2 days after infection and continuing for 7 consecutive days. Seventeen control guinea pigs were treated with meglumine instead of study drug, according to the same schedule. All guinea pigs were monitored daily for survival and weight change. B, Kaplan-Meier survival curves and relative body weight changes in GA-SUDV–infected guinea pigs treated with 300 and 150 mg/kg/day of T-705, beginning 2 days after infection. C, Four groups of guinea pigs were challenged with GA-SUDV followed by daily T-705 treatment with 300 mg/kg/day, starting 1 (n = 5), 3 (n = 5), 4 (n = 6), and 5 (n = 6) days after infection and continuing for 7 consecutive days. Control animals were treated daily with meglumine, starting 1 day after infection and continuing for 7 days. D, Kaplan-Meier survival curves and relative body weight changes in GA-SUDV–infected guinea pigs after treatments. Relative weight changes are shown as the mean value of all guinea pig weights in each group. Statistical comparisons between control- and T-705-treated animals were done using the Mantel-Cox (log-rank) test:. **P < .01, ***P < .001, and ****P < .0001.
To further assess the 300-mg/kg/day dose, we performed a follow-up efficacy experiment to determine the protective efficacy of T-705 when administered early or late after infection. To this end, 4 groups of guinea pigs were infected with GA-SUDV and treated with T-705 at 300 mg/kg/day, starting 1, 3, 4, or 5 days after infection and continuing every day for a total of 7 consecutive days (Figure 2C). The control group was treated with meglumine for 7 days, beginning 1 day after infection. All animals treated daily with 300 mg/kg/day, starting 1 (n = 5), 3 (n = 5), and 4 (n = 6) days after infection, were protected against GP-SUDV infection (Figure 2D). All animals in which treatment began 1 day after infection remained healthy and gained weight throughout the experiment, while 1 and 2 animals in which treatment began 3 and 4 days after infection, respectively, lost some weight but recovered (Figure 2D). Guinea pigs treated with 300 mg/kg/day starting 5 days after infection (n = 6) were partially protected (83.3%), with all animals losing weight and 5 of 6 recovering (Figure 2D). Collectively, the in vivo findings suggest that T-705 is an effective treatment for SUDV infection that can provide complete protection at high and early doses (beginning 1, 3, and 4 days after infection) and partial protection when administrated beginning 5 days after infection. It is unclear why initiation of T-705 treatment on day 2 after infection offered only partial protection.
Viral RNA and Infectious Virus in GA-SUDV–Infected Guinea Pigs
To investigate the systemic spread of SUDV in T-705–treated and untreated guinea pigs, 2 groups of 3 guinea pigs were infected with GA-SUDV and treated with control or with 150 or 300 mg/kg/day of T-705, beginning 2 days after infection. Whole-blood specimens were collected 5 and 9 days after infection to analyze the viral RNA load by RT-qPCR, and the animals were euthanized 9 days after infection to harvest the liver, lung, kidney, and spleen for quantification of viral RNA and infectious virus. Treatment with 150 or 300 mg/kg/day of T-705 significantly reduced viral RNA levels in the blood by 1–1.5 logs 5 and 9 days after infection (Figure 3B). However, viral RNA levels in the liver, spleen, kidney, and lungs were significantly reduced only following treatment with 300 mg/kg/day of T-705 (Figure 3B). Accordingly, infectious virus titers were significantly reduced in the blood, liver, spleen, and kidney following treatment with 300 mg/kg/day but not 150 mg/kg/day, while both doses significantly reduced virus titers in the lung (Figure 3A).
Figure 3.
Infectious virus titers and RNA in blood and tissue specimens from guinea pig–adapted Sudan virus (GA-SUDV)–infected guinea pigs. A, Whole blood and tissue specimens were collected 9 days after infection from GA-SUDV–infected guinea pigs (n = 3 for each treatment group) treated with control or with 150 mg/kg/day or 300 mg/kg/day of T-705, starting 2 days after infection and continuing every day up to day 8. Infectious virus titers in blood specimens and tissue homogenates of liver, spleen, kidney, and lungs were determined by a 50% tissue culture infective dose (TCID50) assay. B, Two groups of 3 guinea pigs were challenged with GA-SUDV and treated with 150 and 300 mg/kg/day of T-705, starting 2 days after infection and continuing every day up to day 8. An additional group of 3 animals were treated with meglumine instead of study drug. Blood samples were collected 5 and 9 days after infection. Animals were euthanized and tissue samples were harvested 9 days after infection. SUDV genome equivalent (GEq) RNA levels were detected by reverse transcription–quantitative polymerase chain reaction analysis in blood, liver, spleen, kidney, and lungs. Statistical comparisons between control-treated and T-705–treated guinea pigs were done by 1-way analysis of variance with the Bonferroni multiple comparison correction. *P < .05 and **P < .01.
Blood Cell Count in SUDV-Infected Guinea Pigs
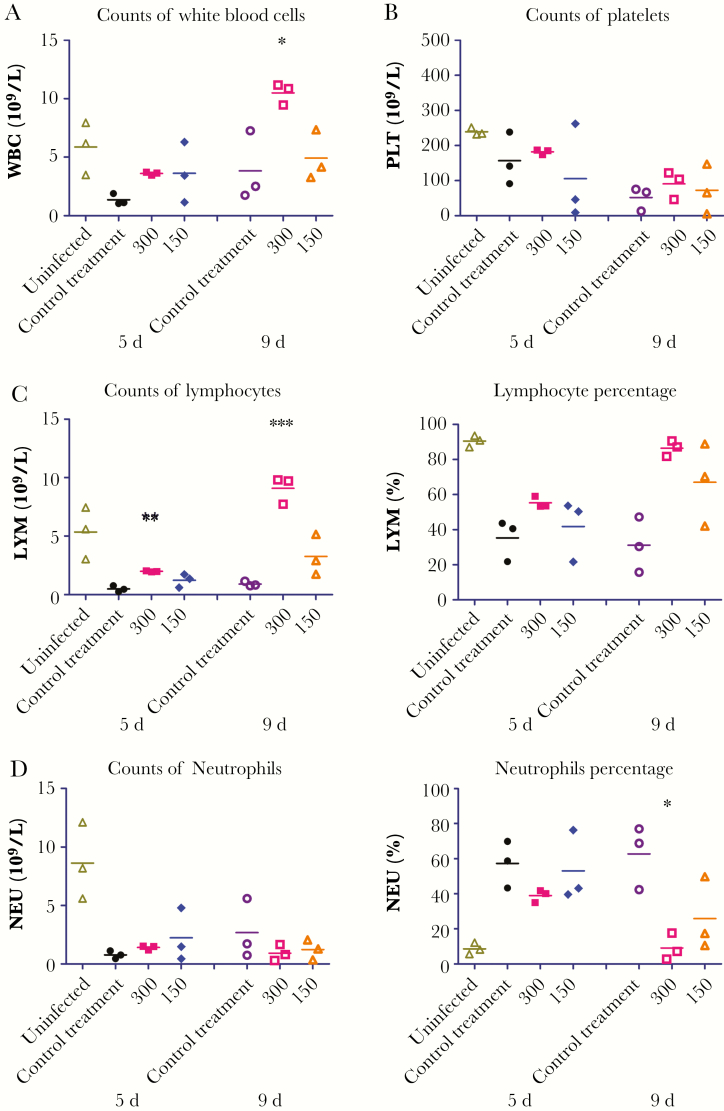
To assess the immune response to GA-SUDV infection in T-705–treated and untreated guinea pigs, we quantified the WBCs, lymphocytes, neutrophils, and platelets 5 and 9 days after infection. We also quantified the blood cells of uninfected animals. Nine days after infection, the WBC count was significantly higher in animals treated with 300 mg/kg/day of T-705, compared with those treated with control (Figure 4A). Although the lymphocyte population was reduced in most of the infected animals as compared to the uninfected animals, the count was significantly higher among animals treated with 300 mg/kg/day of T-705, compared with control-treated animals, at both 5 and 9 days after infection (Figure 4C), and this was reflected in the percentage of WBCs that were lymphocytes (Figure 4C). Nine days after infection, the percentage of neutrophils in animals treated with 300 mg/kg/day was similar to that in the uninfected animals but significantly lower than in the other T-705– and control-treated animals, although these differences were not as obvious in the absolute neutrophil counts (Figure 4D). There were no significant differences in platelet levels among the treated and untreated animals; however, platelet counts in all infected animals were lower than counts in mock-infected animals (Figure 4B).
Figure 4.
Blood cell counts in guinea pig–adapted Sudan virus (GA-SUDV)–infected guinea pigs. Two groups of 3 guinea pigs were challenged with GA-SUDV and treated with 150 and 300 mg/kg/day of T-705, starting 2 days after infection and continuing every day up to day 8. One group of uninfected animals and 1 group of infected, control-treated animals were also included. Blood samples were collected 5 and 9 days after infection. White blood cell (WBC) counts (A), platelet (PLT) counts (B), lymphocyte (LYM) counts and percentages (C), and neutrophil (NEU) counts and percentages (D) were calculated in whole-blood specimens, using the Abaxis HM5 system. Statistical comparisons between control-treated and T-705–treated infected animals were performed using 1-way analysis of variance with the Bonferroni multiple comparison correction. *P < .05, **P < .01, and ***P < .001.
DISCUSSION
Currently no clinically approved antifilovirus drugs or vaccines are available, and given the global public health threat posed by these viruses, the development of cross-protective therapeutics targeting all filoviruses is an urgent priority. T-705 (favipiravir) is an antiviral agent that is well tolerated in humans and currently approved for influenza treatment in Japan [14]. T-705 has also been shown to potently inhibit other RNA viruses, including EBOV and MARV [14, 19, 21, 22]. Like EBOV and MARV, SUDV is capable of causing severe disease and large outbreaks, yet relatively few countermeasures have been developed specifically against this virus [8, 9]. In this study, we have demonstrated that T-705 has a strong antiviral effect against SUDV both in vitro and in vivo, indicating that this drug may represent an effective pan-filovirus treatment.
Our in vitro data revealed that T-705 effectively inhibited SUDV infection in tissue culture, with efficacy similar to that seen when T-705 was evaluated against EBOV and MARV infection [19, 21]. Indeed, the EC50 for T-705 against SUDV was 8.224 µg/mL, which is within the same range as the EC50 values calculated for MARV and EBOV treatment—8.406 and 10.5 µg/mL, respectively [19, 21]. Moreover, previous investigations have shown that T-705 is only toxic to cells when used at a concentration of ≥2 mg/mL [20], which is much greater than the concentration of T-705 used in our study. Collectively, our results and those of previous reports indicate that SUDV, MARV, and EBOV are uniformly susceptible to similar concentrations of T-705 in vitro.
To evaluate the in vivo efficacy of T-705, we used our recently developed lethal guinea pig model of SUDV infection [23]. Initially, we noticed that the 300-mg/kg/day dose was more protective than the 150-mg/kg/day dose when treatment was administered beginning 2 days after infection, which is similar to what was observed in guinea pigs infected with Pichinde and Lassa viruses [17, 25]. Although the 150-mg/kg/day treatment limited weight loss, at least initially, in most animals—indicating some protection from disease—later during infection most animals began to lose weight, and only 5 of 13 animals ultimately recovered and survived. These data suggest that T-705 was partially effective but that the dose of 150 mg/kg/day was likely too low for effective viral inhibition. Accordingly, although 150 mg/kg/day appeared to reduce the levels of viral RNA and infectious virus detected in the blood and various tissues, the majority of these decreases were not statistically significant. Unlike the 150-mg/kg/day T-705 treatment, the 300-mg/kg/day treatment starting 2 days after infection significantly protected approximately 83% of animals. Although complete protection was not observed, most survivors were healthy and exhibited no weight loss after treatment was initiated, indicating that T-705 effectively prevented disease. Indeed, the effectiveness of this dose of T-705 was corroborated by the fact that levels of viral RNA and infectious virus were significantly reduced in all samples examined. Hematological analyses revealed high levels of lymphocytes in these treated animals (compared with the typically observed lymphopenia in untreated animals), which may suggest that T-705–mediated control of viral replication permitted the host to mount an effective immune response. The neutrophil percentage also decreased significantly by 9 days after infection (in contrast to the neutrophilia observed in the untreated animals), perhaps indicating a more controlled immune response to infection. Together these data suggest that 300 mg/kg/day of T-705, starting 2 days after infection, was highly effective at inhibiting SUDV infection, ameliorating disease presentation, and facilitating the development of an immune response, although future work, including cytokine and anti-SUDV antibody profiling, will be necessary to further characterize the host response following T-705 treatment.
Remarkably, 300-mg/kg/day T-705 treatment that began earlier or later than 2 days after infection resulted in complete protection of GA-SUDV-infected guinea pigs. This was not an unexpected result for treatment beginning 1 day after infection; however, we were surprised to find that treatment beginning 3 and 4 days after infection appeared to be more effective than treatment beginning 2 days after infection. It is possible that the relatively protracted disease course of SUDV infection (compared with that of EBOV infection, for example) [26–28] permitted an extended treatment window [29], and, indeed, even T-705 treatment begun 5 days after infection offered partial protection. However, why treatment beginning 2 days after infection also offered only partial protection remains unclear. One possible explanation may be related to the fact that, because the Hartley guinea pigs used in this study are outbred, variations among the animals may mean that larger sample sizes are required. Regardless, these data are promising because they suggest that a broad treatment window is available following SUDV infection, although more research is required.
While T-705 has demonstrated remarkable efficacy against multiple filoviruses, it is somewhat difficult to draw direct comparisons between different studies, owing not only to differences in the challenge viruses themselves but also to differences in drug dose, administration, and timing, as well as virus dose, inoculation route, and animal model system [19, 21, 30, 31]. For EBOV, administration of 300 mg/kg/day of T-705, beginning 2 days after infection and lasting for 8 days, resulted in 100% survival of infected mice, and doses as low as 30 mg/kg/day, beginning 2 days after infection, also offered complete protection from EBOV challenge [21]. A separate study demonstrated that an extended 11-day course of T-705 treatment at 37.5 mg/kg/day, beginning 1 day after infection, resulted in 100% survival following EBOV challenge but that treatment with 8 mg/kg/day, beginning at the time of infection, resulted in 0% survival [31]. These data demonstrate a large treatment window for T-705 against EBOV, suggesting that EBOV may be particular susceptible this drug; however, a nonhuman primate study failed to show significant efficacy despite an extended treatment course with high T-705 doses [30]. In the case of MARV infection, 300 mg/kg/day delivered for 8 consecutive days beginning 2 days after infection resulted in 100% survival of infected mice, while lower doses of T-705 (75–150 mg/kg/day) were only moderately effective [19]. Similarly, treatment with higher doses (300 mg/kg/day), beginning 3 or 4 days after infection, also demonstrated moderate efficacy [19]. Unlike findings for EBOV, however, T-705 was considerably more efficacious in the nonhuman primate model, although this may have been related to differences in drug administration [30]. Thus, based on available data, there appears to be no obvious treatment window or drug dose that is commonly effective against disparate filoviruses, although direct and controlled comparisons in a common animal model, using a common infection and treatment protocol, have yet to be performed. Overall, our study suggests that T-705 is able to effectively inhibit SUDV replication, prevent lethal disease in guinea pigs, and increase survival rates. Furthermore, these data, together with previous publications [19–22], suggest that T-705 may be a useful pan-filovirus treatment that could be deployed during outbreaks, which occur sporadically and can be caused by any one of a number of different filoviruses, including SUDV. Notably, during the West African EBOV outbreak, T-705 was used as a treatment, and, although efficacy was inconclusive, the drug appeared to be well tolerated [32]. Indeed, it is worth noting that, using the body surface area conversion calculation [33], the human dose of T-705 equivalent to the 300 mg/kg guinea pig dose used in this study is 65 mg/kg, which is similar to the loading dose used in the West African clinical trial [32]. Although further work is required, including characterization of the mechanism of action of T-705 against filoviruses, as well as evaluation of T-705 against SUDV in the nonhuman primate model, the data presented here suggest that T-705 may be an effective pan-filovirus therapeutic agent.
Notes
Acknowledgments. This work was supported by the Public Health Agency of Canada, the National Institutes of Health (grant U19 AI109762-1), and the Canadian Institutes of Health Research (grant IER-143487 to X. Q.). X. Q. conceived and designed the study. S. H., Z. Z., M. N. R., and X. Q. performed the experiments and analyzed the results. W. Z. prepared the T-705 (favipiravir). M. N. R., L. B., and X. Q. wrote and edited the manuscript. D. S. provided critical technical support and edited the manuscript. All authors read and commented on the manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sanchez A, Geisbert T, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, eds. Fields virology. Philadelphia: Lippincott Williams and Williams, 2007. [Google Scholar]
- 2. Kuhn JH, Andersen KG, Bào Y, et al. . Filovirus RefSeq entries: evaluation and selection of filovirus type variants, type sequences, and names. Viruses 2014; 6:3663–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuhn JH, Becker S, Ebihara H, et al. . Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol 2010; 155:2083–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miranda ME, Miranda NL. Reston ebolavirus in humans and animals in the Philippines: a review. J Infect Dis 2011; 204(Suppl 3):S757–60. [DOI] [PubMed] [Google Scholar]
- 5. Agua-Agum J, Allegranzi B, Ariyarajah A, et al. . After Ebola in West Africa--unpredictable risks, preventable epidemics. N Engl J Med 2016; 375:587–96. [DOI] [PubMed] [Google Scholar]
- 6. Uyeki TM, Mehta AK, Davey RT Jr, et al. ; Working Group of the U.S.–European Clinical Network on Clinical Management of Ebola Virus Disease Patients in the U.S. and Europe Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med 2016; 374:636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Towner JS, Sealy TK, Khristova ML, et al. . Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog 2008; 4:e1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cross RW, Mire CE, Feldmann H, Geisbert TW. Post-exposure treatments for Ebola and Marburg virus infections. Nat Rev Drug Discov 2018. doi: 10.1038/nrd.2017.251. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Reynolds P, Marzi A. Ebola and Marburg virus vaccines. Virus Genes 2017; 53:501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013; 100:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Warren TK, Wells J, Panchal RG, et al. . Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 2014; 508:402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warren TK, Jordan R, Lo MK, et al. . Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016; 531:381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Picazo E, Giordanetto F. Small molecule inhibitors of ebola virus infection. Drug Discov Today 2015; 20:277–86. [DOI] [PubMed] [Google Scholar]
- 14. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 2017; 93:449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baranovich T, Wong SS, Armstrong J, et al. . T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol 2013; 87:3741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5’-triphosphate towards influenza A virus polymerase. PLoS One 2013; 8:e68347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Safronetz D, Rosenke K, Westover JB, et al. . The broad-spectrum antiviral favipiravir protects guinea pigs from lethal Lassa virus infection post-disease onset. Sci Rep 2015; 5:14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furuta Y, Takahashi K, Shiraki K, et al. . T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res 2009; 82:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu W, Zhang Z, He S, Wong G, Banadyga L, Qiu X. Successful treatment of Marburg virus with orally administrated T-705 (Favipiravir) in a mouse model. Antiviral Res 2018; 151:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smither SJ, Eastaugh LS, Steward JA, Nelson M, Lenk RP, Lever MS. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antiviral Res 2014; 104:153–5. [DOI] [PubMed] [Google Scholar]
- 21. Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 2014; 105:17–21. [DOI] [PubMed] [Google Scholar]
- 22. Bixler SL, Bocan TM, Wells J, et al. . Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antiviral Res 2018; 151:97–104. [DOI] [PubMed] [Google Scholar]
- 23. Wong G, He S, Wei H, et al. . Development and Characterization of a Guinea Pig-Adapted Sudan Virus. J Virol 2016; 90:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gowen BB, Juelich TL, Sefing EJ, et al. . Favipiravir (T-705) inhibits Junín virus infection and reduces mortality in a guinea pig model of Argentine hemorrhagic fever. PLoS Negl Trop Dis 2013; 7:e2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mendenhall M, Russell A, Smee DF, et al. . Effective oral favipiravir (T-705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic Fever. PLoS Negl Trop Dis 2011; 5:e1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banadyga L, Wong G, Qiu X. Small animal models for evaluating filovirus countermeasures. ACS Infect Dis 2018; 4:673–85. [DOI] [PubMed] [Google Scholar]
- 27. Fisher-Hoch SP, Brammer TL, Trappier SG, et al. . Pathogenic potential of filoviruses: role of geographic origin of primate host and virus strain. J Infect Dis 1992; 166:753–63. [DOI] [PubMed] [Google Scholar]
- 28. Bowen ET, Platt GS, Lloyd G, Raymond RT, Simpson DI. A comparative study of strains of Ebola virus isolated from southern Sudan and northern Zaire in 1976. J Med Virol 1980; 6:129–38. [DOI] [PubMed] [Google Scholar]
- 29. Geisbert TW, Daddario-DiCaprio KM, Williams KJ, et al. . Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol 2008; 82:5664–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bixler SL, Bocan TM, Wells J, et al. . Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antiviral Res 2018; 151:97–104. [DOI] [PubMed] [Google Scholar]
- 31. Bixler SL, Bocan TM, Wells J, et al. . Intracellular conversion and in vivo dose response of favipiravir (T-705) in rodents infected with Ebola virus. Antiviral Res 2018; 151:50–4. [DOI] [PubMed] [Google Scholar]
- 32. Sissoko D, Laouenan C, Folkesson E, et al. ; JIKI Study Group Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in guinea. PLoS Med 2016; 13:e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22:659–61. [DOI] [PubMed] [Google Scholar]