Abstract
Background
A need to develop therapeutics to treat Ebola virus disease patients in remote and resource-challenged settings remains in the wake of the 2013–2016 epidemic in West Africa. Toward this goal, we screened drugs under consideration as treatment options and other drugs of interest, most being small molecules approved by the Food and Drug Administration. Drugs demonstrating in vitro antiviral activity were advanced for evaluation in combinations because of advantages often provided by drug cocktails.
Methods
Drugs were screened for blockade of Ebola virus infection in cultured cells. Twelve drugs were tested in all (78 pair-wise) combinations, and 3 were tested in a subset of combinations.
Results
Multiple synergistic drug pairs emerged, with the majority comprising 2 entry inhibitors. For the pairs of entry inhibitors studied, synergy was demonstrated at the level of virus entry into host cells. Highly synergistic pairs included aripiprazole/piperacetazine, sertraline/toremifene, sertraline/bepridil, and amodiaquine/clomiphene.
Conclusions
Our study shows the feasibility of identifying pairs of approved drugs that synergistically block Ebola virus infection in cell cultures. We discuss our findings in terms of the theoretic ability of these or alternate combinations to reach therapeutic levels. Future research will assess selected combinations in small-animal models of Ebola virus disease.
Keywords: Ebola virus, filovirus, hemorrhagic fever viruses, anti-viral, drug cocktails, prophylactic, Marburg virus
No approved drugs were available for patients during the 2013–2016 outbreak of Ebola virus (EBOV) infection in West Africa, and since then none have been approved. Late in the epidemic, a number of therapeutic options were tested by using various clinical trial designs, including the ZMapp monoclonal antibody cocktail, several small molecules, and a vesicular stomatitis virus–based vaccine [1, 2]. The vaccine proved efficacious in preventing Ebola virus disease (EVD) [3]. ZMapp trended toward protection in patients with EVD from the West African outbreak [4], and since then, other antibodies have been identified that block multiple ebolaviruses [5, 6]. Despite these advances, a need to develop small-molecule therapeutics with which to rapidly respond to an outbreak of an ebolavirus remains. Because they are often stable at room temperature and orally available, low-molecular-weight drugs could be easily deployed and administered at the first sign of an outbreak, as prophylactic therapy, and/or during development of immune responses to a vaccine.
Toward this goal and with appreciation of potential advantages of a repurposing strategy, several groups screened orally available approved drugs for anti-EBOV activity [7–9]. A major advantage of a repurposing strategy would be the relative speed with which a drug could be deployed, compared with the time needed to develop, test, manufacture, and deliver a novel drug. Additional advantages include knowledge of the pharmacokinetic and safety profiles of the drug and, since many approved drugs target host pathways, the increased likelihood that identified compounds will be useful against multiple filoviruses. A challenge in any repurposing strategy is attaining therapeutic drug levels for the new indication, which, in this case, involves preventing or ameliorating symptoms in patients with EVD. Toward that challenge, a further approach is to identify synergistic pairs of approved drugs, thus lowering the dose of each drug needed and thereby improving the chances of reaching therapeutic levels and minimizing adverse effects [10]. Here we present results of a rescreen of candidate drugs against EBOV and the identification of pairs of drugs that block the virus synergistically in cell cultures.
METHODS
Cells, Virus, and Virus Infections
Vero E6 and Huh7 cells were maintained at the Integrated Research Facility, National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; Frederick, MD), following cell source instructions. HEK293T/17 cells were maintained at the University of Virginia (Charlottesville) as previously described [11]. A stock of the Makona isolate, Ebola virus/H.sapiens-tc/GIN/14/WPG-C05 (EBOV/Mak), a gift of Dr Gary Kobinger (Public Health Agency of Canada, Winnipeg), was generated and titered as previously described [12]. All procedures using live EBOV were performed under biosafety level 4 conditions. See Supplementary Methods for details.
Analysis of Single Agents for Activity Against EBOV
Efficacy and cytotoxicity studies were performed in parallel as previously described [12]. In brief, Huh7 cells were seeded in black, clear-bottomed, 96-well plates. Drugs in 50-µL of Dulbecco’s modified Eagle’s medium (shipped frozen by the National Center for Advancing Technology and Sciences [NCATS], NIH [Bethesda, MD], or prepared fresh at the Integrated Research Facility) were transferred to the cells 1 hour prior to inoculation with EBOV/Mak. After 48 hours, plates were fixed, and EBOV/Mak was detected with a mouse anti-EBOV VP40 antibody. Half-maximal inhibitory concentrations (IC50) were calculated as described previously [12]. Cytotoxicity was measured on non–virus-infected plates, using Cell Titer Glo. The screen was performed with 3 replicates for each drug concentration, and the assay was repeated at least twice for confirmation. See Supplementary Methods for details.
Analysis of Drug Combinations for Synergy Against EBOV
Drug combination plates in a 6 × 6 matrix with 1:2 dilutions (shipped frozen by the NCATS or prepared fresh at the Integrated Research Facility) were transferred to triplicate cell plates for efficacy and cytotoxicity assays. The cells were infected and assayed as described above. The data sets for the drug combinations were analyzed by NCATS in-house software, which determines a panel of synergy metrics [10, 13–15]. Plots from parallel tests for cytotoxicity were scrutinized to ensure that toxicity was not contributing to observed antiviral synergy. All anti-EBOV efficacy and toxicity synergy data are publically available at: https://tripod.nih.gov/matrix-client/. Drug interactions were also characterized by MacSynergy II [16, 17]. Both methods of synergy data analysis are described in detail in the Supplementary Methods.
Preparation and Assay of Transcription and Replication–Competent Virus-Like Particles (VLPs) and Entry-Reporter VLPs
Transcription and replication–competent VLPs corresponding to the Mayinga isolate of EBOV were prepared, and 293T/17 cells were transfected to prepare transcription and replication–competent VLP target cells as previously described [11, 18] with plasmids obtained from Drs Heinz Feldmann and Thomas Hoenen (Rocky Mountain Laboratories, NIAID, Hamilton, MT). Single-agent drug tests were conducted in triplicate, using drugs shipped frozen from the NCATS or freshly prepared at the University of Virginia essentially as described previously [11] and in detail in the Supplementary Methods. Entry-reporter VLPs were prepared (with EBOV glycoprotein from the Mayinga isolate) and VLP entry assays performed as described previously [11] and in detail in the Supplementary Methods.
RESULTS
Identification of Drugs With Anti-EBOV Activity
We first selected 23 approved and 5 additional drugs and tested them for activity as single agents against EBOV in cell cultures. Ten drugs were on the World Health Organization (WHO) list of candidate EBOV therapeutics [19], 16 had been reported active against EBOV in cells, and 2 had recently been described as active against other viruses (Supplementary Data Set 1, Tab 1). Fifteen of 28 drugs were previously tested for protection of mice against EBOV lethality (Supplementary Data Set 1, Tab 1; Bixler et al [2] provide an excellent review of drug efficacies against EBOV in animal models).
Based on findings of independent cell-based tests against EBOV (Supplementary Data Set 1, Tab 2) and EBOV transcription and replication–competent VLPs (Supplementary Data Set 1, Tab 3, Test 1), 13 compounds were designated as hits because they demonstrated an IC50 of ≤8.5 μM and maximal inhibition of ≥70% in cell cultures (Supplementary Data Set 1, Tab 5). Rescreening with freshly plated drugs (Test 2) confirmed the initial 13 drugs and revealed 4 others that met the IC50 and maximal inhibition criteria (Supplementary Data Set 1, Tabs 3 and 4), yielding 17 drugs designated as initial hits (Supplementary Dataset 1, Tab 5).
Identification of Synergistic Drug Pairs with Activity Against EBOV
For the first synergy test (Supplementary Data Set 2, Tab 1, Matrix 1), 12 drugs were tested in all possible combinations (composed of 78 unique drug pairs) for blockade of EBOV infection. Nine were hits against EBOV in single-agent test 1. Aripiprazole and piperacetazine were added for mechanistic reasons (piperacetazine was also a hit in VLP test 1, and both drugs were hits in the second antivirus single-agent test; Table 1). Favipiravir was added to expand the number of postentry inhibitors and because it was undergoing a clinical trial in patients with EVD [20, 21]; favipiravir was recently shown to enhance survival in cynomolgus macaques infected with EBOV [22]. Although strophanthin and emetine had initial hits, they were not included in pair-wise analyses because they were deemed toxic (in Huh7 cells) and contraindicated for a disease associated with hypovolemia, respectively [23, 24].
Table 1.
Drugs Advanced to Synergy Tests Against Ebola Virus Infection of Huh7 Cells
| Druga | Anti-EBOV Activity Detected, by Testb | Stage Blocked | Step Blockedc | Status | Clinical Used |
|---|---|---|---|---|---|
| 3-Deazaneplanocin A | 1, 2 | Postentry | RNA synthesis | Preclinical study | NA |
| Amodiaquine e | 2 | Entry | Acidification | FDA approved | Antimalarial |
| Apilimod | 1, 2 | Entry | Traffic | Phase 2 study in US | NA |
| Aripiprazole | 2 | Entry | Internalizationf | FDA approved | Psychosis |
| Azithromycin e | No | Entry | Fusionf | FDA approved | Antibacterial |
| Bepridil e | 2 | Entry | Fusion | FDA approvedg | Hypertension |
| Clomiphene | 1, 2 | Entry | Fusion | FDA approved | Female infertility |
| Colchicine | 1, 2 | Entry | Traffic | FDA approved | Gout |
| Favipiravir | No | Postentry | RNA synthesis | JMHLW approved, phase 3 study in US | Antiinfluenza |
| Mycophenolate | 1, 2 | Postentry | RNA Synthesis | FDA approved | Immunosuppression |
| Omacetaxine | 1, 2 | Postentry | Protein synthesis | FDA approved | Hematologic tumors |
| Piperacetazine | 2 | Entry | Acidificationf | FDA approvedg | Schizophrenia |
| Sertraline | 1 | Entry | Fusion | FDA approved | Depression |
| Sunitinib | 1, 2 | Entry | Traffich | FDA approved | Renal and GI tumors |
| Toremifene | 1, 2 | Entry | Fusion | FDA approved | Breast cancer |
Abbreviations: FDA, Food and Drug Administration; GI, gastrointestinal; JMHLW, Japanese Ministry of Health, Labor and Welfare; US, United States.
aDrugs tested in combinations against Ebola virus in matrix synergy tests (Supplementary Data Set 2), unless otherwise indicated.
bDrug activity against Ebola virus in single-agent test 1 (with frozen chemical plates) or 2 (with freshly plated drugs). Positive drug activity in test 1 or 2 is indicated by test number.
c“Acidification” denotes endosome acidification, “traffic” denotes traffic to late endosomes, “internalization” denotes internalization from the cell surface, and ”fusion“ denotes fusion with late endosome.
dInformation on clinical use was obtained from DrugBank (available at: https://www.drugbank.ca/).
eDrugs tested in combinations against Ebola virus in subsequent tests (Supplementary Data Set 3).
fE. A. Nelson and J. M. White, unpublished data.
gCurrently discontinued.
hAction of sunitinib against EBOV is postulated to be similar to action against dengue virus [33].
The 78 pairs (all combinations of 12 drugs) were tested at all combinations of 6 doses of each drug for inhibition of EBOV infection and, in parallel, for cytotoxicity. The resulting data were then analyzed using software that determined a panel of synergy metrics (Supplementary Data Set 2, Tab 1, Matrix 1). Four parameters, Excess HSA (the excess over the highest single-agent response), Beta (a parameter that minimizes the difference between the observed combination effect and the effect obtained from the Bliss independence model), Gamma (a parameter that minimizes the difference between the observed combination effect and Gaddums noninteraction model), and DBSumNeg (the summation of the Δ Bliss negative values) were used to rank the 78 combinations. Table 2 shows data for the 9 pairs that met all 4 synergy criteria imposed. The drugs comprising these 9 pairs were the entry inhibitors apilimod, clomiphene, colchicine, sertraline, sunitinib, and toremifene and the postentry inhibitor, 3-deazaneplanocin A. Synergy plots for 4 of these pairs (clomiphene/sertraline, clomiphene/apilimod, sertraline/toremifene, and toremifene apilimod) are shown in Figure 1. Twelve of the 78 pairs were retested under similar conditions (Supplementary Data Set 2, Tab 2, Matrix 2). For the majority of these pairs, the results were highly similar. The exceptions were rationalized as being due to a change in chemical source (apilimod [25]) and to changes in dosing (Supplementary Data Set 2, Tab 3).
Table 2.
Drug Pairs Meeting Synergy Criteria in the First Synergy Test (Matrix 1) Against Ebola Virus Infection in Vitro
| Drug 1 | Drug 2 | Synergy Metric | |||
|---|---|---|---|---|---|
| Excess HSA | Beta | Gamma | DBSumNeg | ||
| Clomiphene | Sertraline | -624.87914 | 0.72461 | 0.70293 | -5.47307 |
| Clomiphene | Apilimod | -611.45525 | 0.81777 | 0.7437 | -4.90124 |
| Sertraline | Toremifene | -508.27371 | 0.71123 | 0.67734 | -4.40112 |
| Toremifene | Apilimod | -534.71687 | 0.7834 | 0.72715 | -4.35384 |
| Colchicine | Sertraline | -650.30131 | 0.81128 | 0.68203 | -4.14049 |
| Clomiphene | Colchicine | -517.9869 | 0.83442 | 0.74282 | -3.52601 |
| Colchicine | Sunitinib | -432.75556 | 0.85566 | 0.75889 | -2.92912 |
| Colchicine | 3-Deazaneplanocin A | -634.77105 | 0.87178 | 0.62148 | -2.71511 |
| Colchicine | Toremifene | -465.88398 | 0.84175 | 0.71685 | -2.61726 |
The synergy criteria imposed were as follows: Excess HAS, less than -400; Beta, <0.9; Gamma, <0.8; and DBSumNeg, less than -2. See Results for a description of the 4 synergy metrics and the Supplementary Methods for details on experimental design and synergy metrics.
Figure 1.
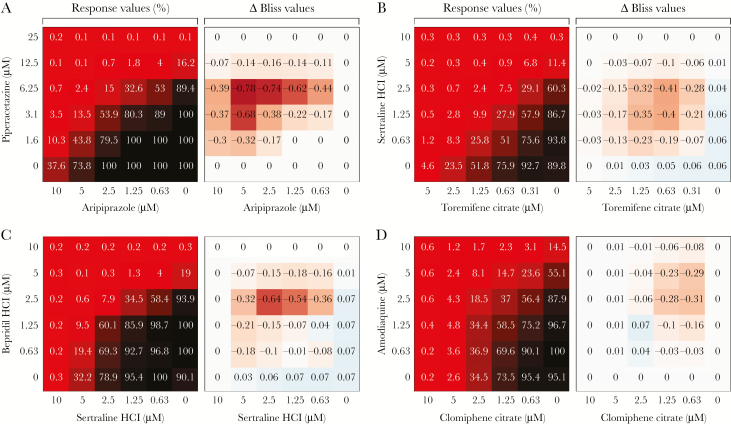
Four drug combinations with synergistic activity against Ebola virus. Huh7 cells were pretreated with drug combinations (shipped frozen) for 1 hour, inoculated at a multiplicity of infection of 0.5 for 48 hours, and assessed by fluorescence assay. Shown are findings of dose matrix (6 × 6) evaluation of 4 drug combinations from Matrix 1: clomiphene citrate/sertraline HCl (A), clomiphene citrate/apilimod (B), sertraline HCl/toremifene citrate (C), and toremifene citrate/apilimod (D). A, The heat map of the percentage response shows the antiviral activity of each combination (100% corresponds to no activity), and the Δ Bliss plot indicates how much a combination effect differs from the additive effect as determined by the Bliss model. The experiment was run once or twice with triplicate wells per dose. A Δ Bliss of 0 indicates an additive effect, a Δ Bliss of <0 indicates a synergistic effect, and a Δ Bliss of >0 indicates an antagonistic effect.
Tests of Additional Drug Pairs
We next tested 16 additional pairs for blockade of EBOV infection, using freshly plated compounds. Combinations with bepridil were tested because bepridil was a hit in a published screen [7] and in screens with freshly plated drugs (Supplementary Data Set 1, Tabs 3 and 4) and because it protects well in the mouse model of EBOV infection ([7]; L. DeWald, unpublished data). One combination with tetrandrine was tested because tetrandrine is considered to use the same mechanism as bepridil, but with greater potency [26]. Combinations with amodiaquine were tested because amodiaquine was on the WHO list of candidate anti-EBOV therapeutics [19, 27] and is an antimalarial that was given with some reported benefit [28] to patients with EVD. Azithromycin was tested in 6 pairs (Supplementary Data Set 3) because it was also on the WHO list, was a hit in published screens [8, 9], was a near hit or hit in our rescreens (Supplementary Data Set 1, Tabs 3 and 4), and because antibiotic therapy is often administered to patients with EVD [24, 27]. A last additional pair was aripiprazole/piperacetazine. This combination was added because neither drug functioned optimally in the first pair-wise test (Supplementary Data Set 2, Tab 1), because of the steps in the entry process they target (Table 1), and because aripiprazole/piperacetazine was independently found to synergistically block EBOV infections (L. Johansen, unpublished data). Four pairs that showed high synergy in the first synergy tests were retested for comparison (Supplementary Data Set 3). Two drugs that were hits against EBOV in the antivirus single-agent rescreen, amiodarone and chloroquine, were not tested in pairs. These drugs had a potency and mode of action similar to those of other drugs in the set: bepridil (similar to amiodarone) and amodiaquine and piperacetazine (similar to chloroquine).
The results of the additional pair-wise tests are presented in Supplementary Data Set 3. Aripiprazole/piperacetazine showed synergy above that seen for the reference pairs (sertraline/toremifene and clomiphene/sertraline) analyzed in the same manner. Experimental differences, noted in Supplementary Data Set 3, Tab 2, likely account for the differences in absolute values for the reference pairs between Supplementary Data Sets 2 and 3. Other pairs (bepridil/sertraline, amodiaquine/clomiphene, and azithromycin/apilimod) showed synergy comparable to that seen for the reference pairs. Synergy plots for 1 reference and 3 synergistic pairs from the additional tests are shown in Figure 2. The plot for an additional synergistic pair, azithromycin/apilimod, is shown in Supplementary Figure 1.
Figure 2.
Additional drug combinations with synergistic activity against Ebola virus. Shown are findings of a dose matrix (6 × 6) evaluation of piperacetazine/aripiprazole (A), sertraline HCl/toremifene citrate (B), bepridil HCl/sertraline HCl (C), and amodiaquine/clomiphene citrate (D). Huh7 cells were pretreated with drug combinations (freshly prepared) for 1 hour, inoculated at a multiplicity of infection (MOI) of 0.21 (A and D) or a MOI of 0.5 (B and C) for 48 hours, and assessed by chemiluminescence assay. Data are displayed as in Figure 1. The experiment was run twice with triplicate wells per dose. Heat maps from 1 of 2 experiments are shown. A Δ Bliss of 0 indicates an additive effect, a Δ Bliss of <0 indicates a synergistic effect, and a Δ Bliss of >0 indicates an antagonistic effect.
Synergy at the Level of EBOV Cell Entry
Most of the synergistic pairs identified are composed of 2 drugs classified as entry inhibitors (Table 1). We tested 4 of these pairs to assess whether they are synergistic at the level of entry, using VLPs that monitor EBOV entry into the host cell cytoplasm [29]. Two pairs, sertraline/toremifene and toremifene/apilimod, were depicted for anti-EBOV synergy in Figure 1; apilimod/bepridil and bepridil/toremifene composed the other 2 pairs. As seen in Table 3, synergy at the level of EBOV VLP entry into the cytoplasm was seen for all 4 pairs tested.
Table 3.
Detection of Synergy in Blocking Cell Entry of Ebola Virus–Like Particles
| Drug 1 | Top Concentration, μM | Drug 2 | Top Concentration, μM | Log Synergy Volumea | Experiments, No.b |
|---|---|---|---|---|---|
| Apilimod | 0.1 | Bepridil | 10 | 11.98 | 2 |
| Sertraline | 10 | Toremifene | 5 | 6.57 | 2 |
| Apilimod | 0.1 | Toremifene | 5 | 6.00 | 2 |
| Bepridil | 10 | Toremifene | 5 | 3.58 | 2 |
| Clomiphene | 5 | Clomiphenec | 5 | 0.63 | 3 |
Four drug combinations were tested for ability to block Ebola virus–like particle entry, and synergistic effects were determined using MacSynergy software, as described in the Supplementary Methods.
aMacSynergy scores are given at the 99.9% confidence interval. Log synergy volume is averaged over the number of experiments.
bTwo or 3 plates were analyzed in each experiment.
cThe self-cross pair clomiphene/clomiphene was used as an expected negative control.
DISCUSSION
This study identified pairs of drugs that synergistically inhibit EBOV infection in cell cultures. Most of the synergistic combinations comprise 2 entry inhibitors, highlighting the potential benefit of synergistically targeting the earliest stage of the EBOV life cycle. From initial tests of 78 pairs, drugs that partnered well with multiple other drugs were clomiphene, colchicine, sertraline, and toremifene; synergies were also seen with apilimod and sunitinib. Subsequent tests revealed synergies with amodiaquine, azithromycin, and bepridil and for the pair aripiprazole/piperacetazine.
Our findings have caveats. First, we did not examine all possible combinations of all single-agent hits. Amiodarone and chloroquine were not tested in pairs, as they were deemed similar in potency and mechanism to bepridil and amodiaquine (and piperacetazine), respectively. Three hits (Table 1) were only tested in a subset of possible combinations. Second, some drugs did not inhibit optimally in the first synergy tests (Supplementary Data Set 2, Tabs 1–3). Third, had the single-agent threshold criteria been lower, additional drugs would have been tested for synergy. Fourth, our set was limited to only 4 postentry inhibitors. Hence, additional drug pairs with synergistic anti-EBOV activity will almost certainly be identified. For example, further evaluation of pairs of entry and postentry inhibitors will be informative, including additional tests with favipiravir [20–22] (Supplementary Data Set 2, Tab 1) or other postentry inhibitors under development.
We can rationalize the ability of certain drug pairs to synergistically inhibit EBOV infection because the components inhibit distinct stages of the entry process. For example, aripiprazole and piperacetazine block EBOV particle internalization from the cell surface and endosome acidification (required for EBOV entry), respectively (Table 1). Apilimod and colchicine block EBOV trafficking to endolysosomes, and both synergize with multiple EBOV fusion inhibitors (eg, clomiphene, sertraline, and toremifene). For other pairs (eg, clomiphene/sertraline and sertraline/toremifene), the mechanism of synergy is not clear, as both partners are currently classified as fusion inhibitors. In this respect, it is interesting that 3 drugs found in synergistic pairs (bepridil, sertraline, and toremifene) have recently been shown to bind to a common pocket in the EBOV glycoprotein [30, 31].
Other studies have assessed synergy among pairs or triplets of anti-EBOV drugs. McCarthy et al [32] reported synergies between combinations of interferon β, nucleoside analogues, and toremifene. We did not test interferons and, on the basis of their low activity as single agents (Supplementary Data Set 1) [[12]), did not advance the antiretroviral nucleoside analogues tested by McCarthy et al. We did, however, test favipiravir (in 12 combinations) and found modest synergies with 4 other drugs (Supplementary Data Set 2, Tab 1). In a second study, Bekerman et al reported synergy for the combination sunitinib/erlotinib [33]. Because erlotinib did not meet the single-agent threshold criteria imposed here, we did not test this pair. We did, however, identify synergistic combinations of sunitinib and other drugs (Table 2 and Supplementary Data Set 2, Tab 1). Last, Sun et al explored triplets of anti-EBOV entry inhibitors [34]. Using combinations containing either toremifene or chloroquine, they found that toremifene/clarithromycin/posaconazole and toremifene/mefloquine/posaconazole synergistically blocked EBOV infections. Consistently, we found that toremifene/azithromycin and toremifene/amodiaquine synergistically inhibited EBOV (Supplementary Data Set 3).
A key question is whether any of the identified synergistic pairs have therapeutic potential. Among the first set (Figure 1, Table 2, and Supplementary Data Set 2, Tab 1), we currently exclude pairs with colchicine (associated with high toxicity) and apilimod. In a first test, apilimod did not protect EBOV-challenged mice (L. DeWald et al, unpublished data), perhaps because of inhibition of interleukin 12 production [35]. Despite being a strong synergizer, the low plasma level (ie, maximum concentration [Cmax]) of clomiphene will likely limit its clinical use, except perhaps in EBOV survivors; clomiphene’s ocular and male reproductive tract tissue penetration may be of interest for use in EVD survivors in whom EBOV persistence in ocular fluid and semen has been documented [36, 37]. From the first synergy set, a pair under consideration is sertraline/toremifene. This combination reduces the IC50 for each drug by approximately 3–6-fold (Figures 1 and 2), bringing them within range of their Cmax. Potential pairs from follow-up sets include bepridil/sertraline, azithromycin/sertraline, and amodiaquine/toremifene. As discussed above, the reduction in IC50 in each pair brings the partner drug in range of its Cmax.
However, the IC50 obtained for the individual drugs within each candidate pair are not significantly below their individual Cmax. Hence, as suggested by Sun et al, a cocktail of 3 approved drugs may be needed to provide protection [34]. In this respect, an interesting third drug to consider is aripiprazole (Abilify; Otsuka Pharmaceutical, Tokyo, Japan). Aripiprazole is highly synergistic with both piperacetazine (this study) and vinorelbine (Johansen et al, unpublished data), which may relate to its unique mode of action (Table 1). In the aforementioned pairs, the IC50 for aripiprazole is in range of attainable steady-state Cmax. Hence, a triplet of aripiprazole (which blocks EBOV internalization), amodiaquine (which blocks endosome acidification [ie, it functions like piperacetazine]), plus a fusion inhibitor (sertraline, toremifene, bepridil [or amiodarone], or azithromycin) might constitute an effective 3-drug cocktail. As mentioned above, the inclusion of a strong postentry inhibitor could have an enhancing effect. We are currently exploring these possibilities. A further top priority is testing selected combinations in small-animal models of EBOV infection, as anti-EBOV activity in cell cultures may not translate to in vivo protection [38].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Laura Bollinger, for technical writing services in preparation of this manuscript; Jiro Wada, for figure preparation; and Pam Strausberg, for formatting assistance.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views or policies of the Department of Health and Human Services or of the institutions and companies affiliated with the authors.
Financial support. This work (including that supporting Supplemental Materials) was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID); the Integrated Research Facility, Division of Clinical Research, NIAID; Battelle Memorial Institute (contract HHSN272200700016I); the National Institutes of Health (grant RO1 AI114776); and the Intramural Program, National Center for Advancing Translational Sciences.
Potential conflicts of interest. J. L., R. G., L. E. D., R. S. B., and M. R. H. performed this work as employees of Battelle Memorial Institute. Subcontractors to Battelle Memorial Institute who performed this work are J. D., B. J. H., and E. P., as employees of Tunnell Consulting; H. Z., as an employee of Loveless Commercial Contracting; W. M. V., J. M., N. D., and G. G. O. Jr, as employees of MRIGlobal; and I. C. as an employee of Leidos Biomedical Research. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 9th International Symposium on Filoviruses, Marburg, Germany, 13–16 September 2017
References
- 1. Cross RW, Mire CE, Feldmann H, Geisbert TW. Post-exposure treatments for Ebola and Marburg virus infections. Nat Rev Drug Discov 2018. [DOI] [PubMed] [Google Scholar]
- 2. Bixler SL, Duplantier AJ, Bavari S. Discovering drugs for the treatment of Ebola virus. Curr Treat Options Infect Dis 2017; 9:299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davey RT Jr, Dodd L, Proschan MA, et al. A randomized, controlled trial of ZMapp for Ebola virus Infection. N Engl J Med 2016; 375:1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wec AZ, Herbert AS, Murin CD, et al. Antibodies from a human survivor define sites of vulnerability for broad protection against ebolaviruses. Cell 2017; 169:878–90.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao X, Howell KA, He S, et al. Immunization-elicited broadly protective antibody reveals ebolavirus fusion loop as a site of vulnerability. Cell 2017; 169:891–904.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johansen LM, DeWald LE, Shoemaker CJ, et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med 2015; 7:290ra89. [DOI] [PubMed] [Google Scholar]
- 8. Kouznetsova J, Sun W, Martínez-Romero C, et al. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect 2014; 3:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madrid PB, Chopra S, Manger ID, et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One 2013; 8:e60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehár J, Krueger AS, Avery W, et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol 2009; 27:659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson EA, Barnes AB, Wiehle RD, Fontenot GK, Hoenen T, White JM. Clomiphene and its isomers block Ebola virus particle entry and iInfection with similar potency: potential therapeutic implications. Viruses 2016; 8:E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cong Y, Dyall J, Hart BJ, et al. Evaluation of the activity of lamivudine and zidovudine against Ebola virus. PLoS One 2016; 11:e0166318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berenbaum MC. What is synergy?Pharmacol Rev 1989; 41:93–141. [PubMed] [Google Scholar]
- 14. Cokol M, Chua HN, Tasan M, et al. Systematic exploration of synergistic drug pairs. Mol Syst Biol 2011; 7:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 1995; 47:331–85. [PubMed] [Google Scholar]
- 16. Prichard M, Aseltine K, Shipman C Jr. MacSynergyTM II. Ann Arbor: Univeristy of Michigan, 1992. [Google Scholar]
- 17. Prichard MN, Shipman C Jr. A three-dimensional model to analyze drug-drug interactions. Antiviral Res 1990; 14:181–205. [DOI] [PubMed] [Google Scholar]
- 18. Watt A, Moukambi F, Banadyga L, et al. A novel life cycle modeling system for Ebola virus shows a genome length-dependent role of VP24 in virus infectivity. J Virol 2014; 88:10511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola http://www.who.int/medicines/ebola-treatment/2015_0703TablesofEbolaDrugs.pdf?ua=1 Accessed 23 February 2018.
- 20. Bai CQ, Mu JS, Kargbo D, et al. Clinical and virological characteristics of Ebola virus disease patients treated with favipiravir (T-705)-Sierra Leone, 2014. Clin Infect Dis 2016; 63:1288–94. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen TH, Guedj J, Anglaret X, et al. ; JIKI study group Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis 2017; 11:e0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guedj J, Piorkowski G, Jacquot F, et al. Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques. PLoS Med 2018; 15:e1002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manno BR, Manno JE. Toxicology of ipecac: a review. Clin Toxicol 1977; 10:221–42. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization. Clinical management of patients with viral haemorrhagic fever: a pocket guide for front-line health workers. Interim emergency guidance for country adaptation http://apps.who.int/iris/bitstream/10665/205570/1/9789241549608_eng.pdf?ua=1 Accessed 26 February 2018.
- 25. Morris P, Moore C, Thomas C. Apilimod. IUCRData 2017; 2:x170693. [Google Scholar]
- 26. Sakurai Y, Kolokoltsov AA, Chen CC, et al. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015; 347:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. Interim list of WHO essential medicines necessary to treat Ebola cases based on existing guidelines http://www.who.int/medicines/ebola-treatment/medicines_ebola_17nov.pdf?ua=1 Accessed 26 February 2018.
- 28. Gignoux E, Azman AS, de Smet M, et al. Effect of artesunate-amodiaquine on mortality related to Ebola virus disease. N Engl J Med 2016; 374:23–32. [DOI] [PubMed] [Google Scholar]
- 29. Shoemaker CJ, Schornberg KL, Delos SE, et al. Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection. PLoS One 2013; 8:e56265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao Y, Ren J, Harlos K, et al. Toremifene interacts with and destabilizes the Ebola virus glycoprotein. Nature 2016; 535:169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ren J, Zhao Y, Fry EE, Stuart DI. Target identification and mode of action of four chemically divergent drugs against Ebolavirus infection. J Med Chem 2018; 61:724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCarthy SD, Majchrzak-Kita B, Racine T, et al. A rapid screening assay identifies monotherapy with interferon-ß and combination therapies with nucleoside analogs as effective inhibitors of Ebola virus. PLoS Negl Trop Dis 2016; 10:e0004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bekerman E, Neveu G, Shulla A, et al. Anticancer kinase inhibitors impair intracellular viral trafficking and exert broad-spectrum antiviral effects. J Clin Invest 2017; 127:1338–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun W, He S, Martínez-Romero C, et al. Synergistic drug combination effectively blocks Ebola virus infection. Antiviral Res 2017; 137:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai X, Xu Y, Kim YM, Loureiro J, Huang Q. PIKfyve, a class III lipid kinase, is required for TLR-induced type I IFN production via modulation of ATF3. J Immunol 2014; 192:3383–9. [DOI] [PubMed] [Google Scholar]
- 36. Deen GF, Broutet N, Xu W, et al. Ebola RNA persistence in semen of Ebola virus disease survivors - final report. N Engl J Med 2017; 377:1428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 2015; 372:2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Falzarano D, Safronetz D, Prescott J, Marzi A, Feldmann F, Feldmann H. Lack of protection against ebola virus from chloroquine in mice and hamsters. Emerg Infect Dis 2015; 21:1065–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.