Abstract
No therapeutics are approved for the treatment of filovirus infections. Bepridil, a calcium channel blocker developed for treating angina, was identified as a potent inhibitor of filoviruses in vitro, including Ebola and Marburg viruses, and Ebola virus in vivo. We evaluated the efficacy of bepridil in a lethal mouse model of Marburg virus disease. A dose of 12 mg/kg bepridil once or twice daily resulted in 80% or 90% survival, respectively. These data confirm bepridil’s broad-spectrum anti-filovirus activity warranting further investigation of bepridil, or improved compounds with a similar mechanism, as a pan-filovirus therapeutic agent.
Keywords: antiviral, bepridil, Marburg virus
Filoviruses can cause severe hemorrhagic fever in humans and are often associated with a high fatality rate. No vaccines or therapeutics are currently approved for use to prevent or treat disease caused by these viruses. A screen of United States- and ex-United States-approved drugs identified the calcium channel blocker, bepridil, as an active inhibitor of multiple filoviruses, including Ebola virus (EBOV), Sudan virus (SUDV), Marburg virus (MARV), and Ravn virus in tissue culture [1]. Bepridil inhibits EBOV at a late stage of viral entry, possibly by binding to the viral glycoprotein (GP) and preventing fusion with the endosomal membrane [1, 2]. A 100% survival rate was obtained when mice infected with mouse-adapted EBOV were treated with bepridil (12 mg/kg) twice a day, beginning on the day of virus exposure [1]. In this study, we evaluated the efficacy of bepridil in the murine model of MARV disease to determine whether bepridil has broad anti-filovirus activity in vivo.
METHODS
Cells and Virus
Vero E6 (American Type Culture Collection 1586, Manassas, VA) cells were maintained in Dulbecco’s modified Eagle’s medium ([DMEM] Gibco, Thermo Fisher Scientific) with 10% heat-inactivated fetal bovine serum ([HI-FBS] Gibco, Thermo Fisher Scientific) following recommended protocols. Marburg virus/H.sapiens-tc/AGO/2005/Ang-1379v (MARV/Ang, BioSample accession no. SAMN05916381) was used for in vitro studies and was propagated from the master stock (BioSample accession no. SAMN05859702) in VERO C1008 (E6) cells, Working Cell Bank, NR-596 obtained through BEI Resources (National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health, Manassas, VA). The MARV working stock was propagated using Minimum Essential Medium-alpha, GlutaMAX, no nucleosides (Gibco, Thermo Fisher Scientific) supplemented with 2% HI-FBS. After harvest, HI-FBS was added at a final concentration of 10% before cryopreservation. For mouse studies, mouse-adapted MARV NML/M.musculus-lab/AGO/2005/Ang-MA-P2 (MARV/Ang-MA) was obtained from the Public Health Agency of Canada through Dr. Gary Kobinger. This material was passaged once in vivo through BALB/c mice, and spleens were collected at necropsy. A master stock was generated by infecting VERO C1008 (E6) cells.
In Vitro Cell-Based Assay
The cell-based MARV drug screen assay was performed as previously described [1, 3], with some modifications. In brief, Vero E6 cells were seeded at a density of 3 × 104 cells/well in black, clear bottom 96-well plates approximately 24 hours (h) before adding bepridil hydrochloride (HCl). Bepridil HCl (no. B5016, lot no. BCBQ7735V; Sigma-Aldrich, St. Louis, MO) was diluted in DMEM with 10% HI-FBS and added to the cell media to a final concentration ranging from 0.16 to 20 µM. Cells were infected with MARV (multiplicity of infection [MOI] = 1.0) in biosafety level 4-containment 1 h after the addition of the drug (final volume = 200 µL) and fixed with 10% neutral-buffered formalin after an additional 48 h. Cells were washed 3 times with phosphate-buffered saline (PBS), permeabilized with 0.25% Triton X-100 (no. H1429; Promega, Madison, WI) in PBS, and blocked with 3% bovine serum albumin (no. A7906; Sigma-Aldrich) in PBS. MARV was detected with a mouse antibody specific for the viral protein 40 (VP40) (no. 0203-012; IBT Bioservices, Rockville, MD). A horseradish peroxidase-conjugated goat antimouse secondary antibody (KPL no. 074-1802; SeraCare Life Sciences, Milford, MA) was used to detect the primary antibody, and the Pierce SuperSignal ELISA Pico Chemiluminescent Substrate Kit (no. 37069; Thermo Fisher Scientific, Waltham, MA) was used to amplify the signal, in accordance with manufacturer’s instructions. Luminescence was measured using a plate reader (Infinite M1000 Pro; Tecan US, Morrisville, NC).
A cell viability assay to measure cytotoxicity was performed as previously described [4]. Cells were plated in black, opaque 96-well plates and treated with bepridil HCl as described above. Cells were mock-infected with DMEM containing no virus. The cell viability assay was performed in parallel to fixing infected cells using the CellTiter Glo luminescent cell viability assay kit (Promega) in accordance with the manufacturer’s instructions, and luminescence was measured using an Infinite M1000 Pro plate reader.
For efficacy plates, the average background signal from uninfected control wells was subtracted from the signal obtained for all infected wells on each plate. For cell viability plates, the average background signal from wells containing no cells was subtracted from the signal obtained for all other wells. Infectivity and cell viability were measured as a percentage relative to untreated infected cells and untreated cells, respectively. The half-maximal inhibitory concentration (IC50) and half-maximal cytotoxicity concentration (CC50) were calculated from the nonlinear regression analysis fitted curve (log[agonist] vs response [variable slope]) using GraphPad Software (GraphPad Software Inc., La Jolla, CA). The experiment was repeated twice with a replicate of 3 on 1 (cell viability) or 2 (efficacy) 96-well plates.
Murine Marburg Virus Infection Model
Female BALB/c mice were procured from Charles River Laboratories (Wilmington, MA). Mice were housed 5 per cage in microisolator cages with CareFresh bedding and provided Teklad autoclavable rodent diet (no. 2018SX; Envigo, Indianapolis, IN) and purified (reverse osmosis) water ad libitum. Four groups (n = 10) were treated intraperitoneally (IP) once or twice daily with 12 mg/kg bepridil HCl (no. B5016; Sigma-Aldrich) in 1.1% dimethyl sulfoxide (no. D24387; Sigma-Aldrich) in saline (0.9% NaCl Injection; USP, Baxter Healthcare Corporation, Deerfield, IL), or with an equivalent volume (0.2 mL) of 1.1% dimethyl sulfoxide in saline without bepridil HCl. Treatment was initiated 4–5 h before virus exposure and mice were treated for up to 10 consecutive days. All mice were challenged IP with 195 plaque-forming units of MARV/Ang-MA. For mice receiving treatment twice a day, the second treatment was administered 4–5 h after virus exposure. Mice were anesthetized using 4%–5% isoflurane in an induction box for all virus and treatment administration procedures. Animals were observed and weighed (by cage) daily.
Ethics Statement
Animals were housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All experimental procedures were approved by the NIAID, Division of Clinical Research, Animal Care and Use Committee, and were in compliance with the Animal Welfare Act regulations, Public Health Service policy, and the Guide for the Care and Use of Laboratory Animals recommendations [5] .
RESULTS
The activity of bepridil HCl was tested against MARV in Vero E6 (African green monkey kidney) cells to confirm reproducibility of activity (published by Johansen et al [1]) using the compound source and lot number intended for use in the mouse MARV challenge study. Cells were infected at an MOI of 1 in the presence bepridil (concentration range of 0.16–20 µM) for 48 h. Cells were fixed, permeabilized, and stained with an antibody specific to the VP40 protein to assess viral inhibition. The cell-based assay confirmed that bepridil HCl inhibits MARV replication with an average maximum response of 95% ± 4.2% and an IC50 of 5.99 ± 1.05 µM (Figure 1).
Figure 1.
Antiviral activity of bepridil hydrochloride (HCl) against Marburg virus (MARV) in Vero E6 cells. Vero E6 cells were treated for 1 hour with the indicated concentrations of bepridil HCl and subsequently infected with MARV/Angola at a multiplicity of infection of 1 for 48 hours. Cells were fixed and stained with an antibody to the MARV viral protein 40 protein. Antiviral activity of bepridil HCl is shown in gray, and the inhibition of cell viability (cytotoxicity) is shown in black. The experiment was run on duplicate plates with triplicate wells per dose (mean ± standard deviation; n = 3). One representative graph from 2 independent experiments is shown.
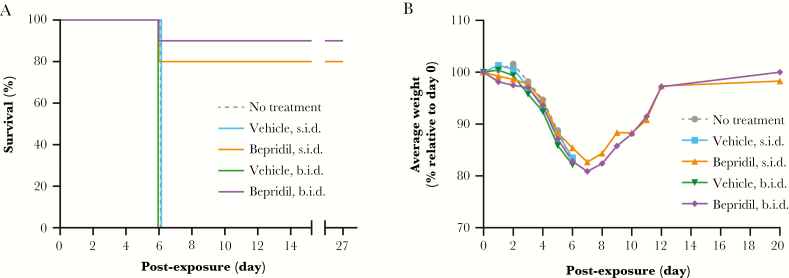
The in vivo efficacy of bepridil was evaluated against MARV in the murine model of disease. The experimental design, including the drug source (Sigma-Aldrich), dose (12 mg/kg), regimen (twice daily dosing, days 0–9 post-exposure), vehicle (dimethyl sulfoxide in saline), and route of administration (IP), was modeled after a previous study evaluating the efficacy of bepridil HCl against EBOV in the murine model of disease [1]. All mice in the vehicle control groups succumbed to disease on day 6 post-exposure (Figure 2A). However, 8 of 10 mice treated once a day and 9 of 10 mice treated twice a day recovered from MARV/Ang-MA infection and survived to the end of the study (day 27 post-exposure). Mice receiving bepridil HCl (12 mg/kg) once or twice a day developed mild-to-moderate signs of clinical disease (eg, decrease in activity, rapid breathing rate, head tilt, abnormal nesting behavior, unthrifty appearance) before recovering, with once-daily treated mice displaying milder clinical signs than those receiving treatments twice a day. The average group weight for control and drug-treated groups decreased by at least 10% by day 5 postexposure (Figure 2B), but bepridil HCl-treated mice who survived past day 6 postexposure gradually and continuously began to recover from the loss of body mass.
Figure 2.
Effect of bepridil hydrochloride (HCl) treatment in mice infected with Marburg virus (MARV). Female BALB/c mice were exposed intraperitoneally (IP) to 195 plaque-forming units of mouse-adapted Marburg virus (Angola) on day 0. Mice were untreated or treated IP with vehicle (1.1% dimethyl sulfoxide in saline) or vehicle containing 12 mg/kg bepridil HCl 4 hours before virus exposure. Treated mice received additional doses of test or control article once (s.i.d.) or twice (b.i.d.) a day for up to 10 days. (A) Survival was monitored after virus exposure to the end of the study (day 27). (B) The total group weight was recorded daily, and the average weight per group was calculated. The relative weight of surviving mice only is indicated from day 7 through day 20 postexposure for groups receiving bepridil HCl s.i.d. or b.i.d.
DISCUSSION
Bepridil was previously shown to inhibit EBOV in vitro and in vivo in a murine model of disease [1]. The anti-EBOV activity of bepridil may be mediated by directly interacting with GP and inhibiting a late step in the entry process (eg, prevention of fusion between the viral and endosome membranes) [2]. Multiple other compounds with anti-EBOV activity, including sertraline, benztropine, toremifene, and paroxetine, bind in the same large cavity of the viral GP and may inhibit viral entry through a similar mechanism [2, 6]. Alternatively, bepridil may inhibit calcium signaling necessary for endolysosomal fusion [7, 8], although a definitive mechanism of action of bepridil on inhibiting EBOV replication has yet to be established.
Most of these compounds have also been shown to inhibit other filoviruses, including SUDV, MARV, and Ravn viruses in cell culture [1, 3, 9]. Although the amino acids lining the viral GP-binding cavity are highly conserved between EBOV and SUDV [2], sequence identity of MARV GP to EBOV GP is only ~28% [10, 11]. Therefore, we wanted to replicate the in vivo efficacy studies performed for EBOV in the MARV mouse model of disease to determine whether bepridil treatment provides a similar survival benefit following MARV infection.
We confirmed the in vitro antiviral activity of bepridil against MARV in Vero E6 cells. An IC50 of 5.99 ± 1.05 µM obtained in this study was similar to the initial experiment (5.52 ± 0.28 µM) [1] and was within range of steady-state levels achieved in human plasma (Cmax of 2.48–6.3 µM) [1, 12, 13]. Furthermore, bepridil demonstrated similar efficacy (80%–90% survival) in the murine model of MARV disease as previously reported for EBOV (100% survival) [1]. Administration of 12 mg/kg bepridil HCl twice a day resulted in 90% survival (9 of 10), and when the same dose of bepridil was administered only once a day (half the total daily dose), treatment resulted in an 80% survival rate. However, the clinical signs of disease were more prevalent when the animals received twice a day dosing, which was likely the result of anesthesia required during animal manipulation procedures. These results suggest that other dosing regimens or drug delivery methods may improve efficacy.
Bepridil HCl was approved by the US Food and Drug Administration for treating angina pectoris, but it was subsequently discontinued in the United States due to cardiovascular side effects, specifically torsades de pointes, but it is still widely used in other countries [14, 15]. Adverse events were observed in angina patients who received long-term (4–12 weeks) treatment with bepridil (200–600 mg, once a day, orally) [13]. Short-term use of bepridil to treat patients with EBOV or MARV disease may result in fewer adverse effects and may be less problematic than that observed in angina patients requiring long-term treatment (>4 weeks). Direct clinical use of bepridil for treating filovirus infections would require careful consideration of the risk-to-benefit value to the patient and would have to be implemented with caution to account for the potential side effects.
CONCLUSIONS
These data support continued efforts to further evaluate the anti-filovirus properties of bepridil HCl and to design improved compounds with a similar pan-filovirus antiviral mechanism, which may include improved GP-binding properties. Further development could result in more efficacious broad-spectrum antivirals with reduced adverse effects for the treatment of filovirus infections.
Notes
Acknowledgments. We acknowledge Laura Bollinger for technical writing services in preparation of this manuscript and Jiro Wada for figure preparation. We thank Dr. Gary Kobinger for his generous gift of MARV/Ang-MA.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views or policies of the US Department of Health and Human Services or of the institutions and companies affiliated with the authors.
Financial support. This work was funded by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID); Integrated Research Facility (NIAID, Division of Clinical Research); and Battelle Memorial Institute’s prime contract with NIAID (Contract no. HHSN272200700016I).
Potential conflicts of interest. L. E. D., J. M. S., R. G., Y. C., and M. R. H. performed this work as employees of Battelle Memorial Institute (BMI). Subcontractors to BMI who performed this work are J. D. and E. P. as employees of Tunnell Consulting, Inc.; H. Z. as an employee of Loveless Commercial Contracting, Inc.; D. M. G. and G. G. O. as employees of MRI Global; and L. T., E. K., and I. A. as employees of Charles River. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Johansen LM, DeWald LE, Shoemaker CJ, et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med 2015; 7:290ra89. [DOI] [PubMed] [Google Scholar]
- 2. Ren J, Zhao Y, Fry EE, Stuart DI. Target identification and mode of action of four chemically divergent drugs against Ebola virus infection. J Med Chem 2018; 61:724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johansen LM, Brannan JM, Delos SE, et al. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med 2013; 5:190ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cong Y, Dyall J, Hart BJ, et al. Evaluation of the activity of lamivudine and zidovudine against Ebola virus. PLoS One 2016; 11:e0166318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Research Council Guide for the care and use of laboratory animals. 8 edn. Washington, DC: The National Academies Press, 2011. doi:10.17226/12910 [Google Scholar]
- 6. Zhao Y, Ren J, Harlos K, et al. Toremifene interacts with and destabilizes the Ebola virus glycoprotein. Nature 2016; 535:169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitterreiter S, Page RM, Kamp F, et al. Bepridil and amiodarone simultaneously target the Alzheimer’s disease beta- and gamma-secretase via distinct mechanisms. J Neurosci 2010; 30:8974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morgan AJ, Platt FM, Lloyd-Evans E, Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J 2011; 439:349–74. [DOI] [PubMed] [Google Scholar]
- 9. Cheng H, Lear-Rooney CM, Johansen L, et al. Inhibition of Ebola and Marburg virus entry by G protein-coupled receptor antagonists. J Virol 2015; 89:9932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manicassamy B, Wang J, Rumschlag E, et al. Characterization of Marburg virus glycoprotein in viral entry. Virology 2007; 358:79–88. [DOI] [PubMed] [Google Scholar]
- 11. Sanchez A, Trappier SG, Ströher U, Nichol ST, Bowen MD, Feldmann H. Variation in the glycoprotein and VP35 genes of Marburg virus strains. Virology 1998; 240:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bixler SL, Duplantier AJ, Bavari S. Discovering drugs for the treatment of Ebola virus. Curr Treat Options Infect Dis 2017; 9:299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hollingshead LM, Faulds D, Fitton A. Bepridil. A review of its pharmacological properties and therapeutic use in stable angina pectoris. Drugs 1992; 44:835–57. [DOI] [PubMed] [Google Scholar]
- 14. Perelman MS, McKenna WJ, Rowland E, Krikler DM. A comparison of bepridil with amiodarone in the treatment of established atrial fibrillation. Br Heart J 1987; 58:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakazato Y. The resurfacing of bepridil hydrochloride on the world stage as an antiarrhythmic drug for atrial fibrillation. J Arrhythmia 2009; 25:4–9. [Google Scholar]