Abstract
At the onset of the 2013–2016 epidemic of Ebola virus disease (EVD), no vaccine or antiviral medication was approved for treatment. Therefore, considerable efforts were directed towards the concept of drug repurposing or repositioning. Amiodarone, an approved multi-ion channel blocker for the treatment of cardiac arrhythmia, was reported to inhibit filovirus entry in vitro. Compassionate use of amiodarone in EVD patients indicated a possible survival benefit. In support of further clinical testing, we confirmed anti-Ebola virus activity of amiodarone in different cell types. Despite promising in vitro results, amiodarone failed to protect guinea pigs from a lethal dose of Ebola virus.
Keywords: amiodarone, antiviral, Ebola virus
Repurposing of clinically developed and approved drugs for the treatment of Ebola virus (EBOV) disease (EVD) has gained a lot of interest, most recently during the 2013–2016 EVD outbreak in Western Africa. Amiodarone is an inexpensive and well known drug for the treatment of cardiac arrhythmias. A recent report suggested that amiodarone inhibits EBOV replication in cell culture at concentrations corresponding to levels found in the sera of patients undergoing antiarrhythmic therapy with amiodarone [1]. Based on these findings, amiodarone was given under compassionate use to patients during the EVD outbreak in Sierra Leone. A physician who worked at an Ebola treatment unit in Lakka, Sierra Leone, contracted EVD and self-initiated treatment with 1 oral dose of 400 mg and 2 intravenous doses of 1200 mg [2]. The physician recovered, but the effect of amiodarone treatment is not clear because the patient subsequently received 2 other drugs, FX06 and favipiravir [2]. In addition to this single case, approximately 80 patients received up to 30 mg/kg per day amiodarone in Ebola treatment units in Freetown, Sierra Leone in December 2014 [3, 4]. A decrease in case fatality rate was reported when compared with local historical data [5, 6]. However, the study was not completed in the setting of a formal clinical trial, and the statistical significance of this result is not known. Amiodarone was categorized by the World Health Organization as a potential candidate for formal clinical trials on the condition that more detailed data on in vitro and in vivo activity are provided. In this study, we report results of testing the activity of amiodarone against EBOV in different cell types and in a study performed in a guinea pig model of EBOV infection.
METHODS
Cells and Virus
Vero E6 (CRL-1586; American Type Culture Collection [ATCC], Manassas, VA), HeLa (CCL-2; ATCC), and Huh 7 (human hepatocellular carcinoma) cells were maintained following recommended protocols. Human monocyte-derived macrophages (MDMs) were generated as previously described [7]. Ebola virus/H.sapiens-tc/GIN/2014/Makona-C05 (EBOV/Mak; GenBank accession no. KX000398.1) and guinea pig-adapted Ebola virus/UTMB/C.porcellus-lab/COD/1976/Mayinga (GenBank accession no. KY425630.1, obtained from Dr. Thomas Geisbert, University of Texas Medical Branch at Galveston, Galveston, TX) (EBOV/May-GPA) were propagated as previously described [7]. Virus stock and challenge inoculum titers were determined by plaque assay on Vero E6 cells as previously described [8].
Cell-Based Testing of Ebola Virus Antiviral Agents
The cell-based EBOV drug screen and cytotoxicity assays were performed as previously described [7]. In brief, Vero E6 and Huh 7 cells were seeded at 3–4 × 104 cells/well, and MDMs were seeded at 1 × 105 cells/well in 96-well plates. After 24 hours (h), cells were treated with Amiodarone Hydrochloride Injection (Hikma Farmaceutica, Portugal) at 3-fold dilutions starting from 40 µM. Cells were infected with EBOV/Mak 1 h after the addition of the drugs in biosafety level 4-containment at specified multiplicities of infection (MOIs). After 48 h, plates were fixed, and EBOV/Mak was detected with a mouse antibody specific for EBOV VP40 protein (no. B-MD04-BD07-AE11; US Army Medical Research Institute of Infectious Diseases) [9] followed by staining with Alexa Fluor 594 goat antimouse immunoglobulin (Ig)G (heavy + light chain) antibody (Life Technologies, Grand Island, NY) or with antimouse IgG-peroxidase labeled antibody (no. 074-1802; KPL, Gaithersburg, MD). Fluorescence or luminescence was quantified on a plate reader (Infinite M1000 Pro; Tecan US, Morrisville, NC). The signal of treated, infected wells was normalized to uninfected control wells and measured (in percent) relative to untreated infected wells. Nonlinear regression analysis was performed, and the 50% inhibitory concentrations (EC50s) were calculated from fitted curves (log [agonist] versus response [variable slope] with constraint to remain above 0%) (GraphPad Software, La Jolla, CA). The EBOV drug screen assay was carried out with 3 replicates for each drug concentration, and the assay was repeated at least twice for confirmation.
To evaluate cytotoxicity, cells were treated with amiodarone as described above in absence of virus. At 48 h after drug addition, cell viability was quantified using the CellTiter Glo luminescent cell viability assay kit (Promega, Madison, WI).
Pharmacokinetics of Amiodarone in Guinea Pigs
Jugular vein catheters were placed in Hartley guinea pigs (gender balanced; 6–7 weeks old; Charles River Laboratories, Stone Ridge, NY) by the vendor before shipment. Animals were singly housed in hanging polycarbonate solid-bottom microisolator cages with hardwood chip bedding and provided Harlan Teklad Certified Guinea Pig Chow (no. 2040C) and purified water ad libitum. Animals were administered a single dose of Amiodarone Hydrochloride Injection (50 mg/mL; APP Pharmaceuticals, LLC, Schaumburg, IL) via oral gavage at a dose of 160 mg/kg. Two female guinea pigs had a slight eschar formation on the ventral neck area near the site of jugular vein catheter surgery. All other animals appeared normal throughout the study.
Blood (~300 μL) was collected from the jugular vein catheter port at 0.25, 0.5, 1, 2, 3, 4, 6, 8, 24, 48, and 72 h postdose. Plasma concentrations of amiodarone and its metabolite, desethylamiodarone (des-AMI), were determined by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Amiodarone and des-AMI were extracted from plasma samples using plasma protein precipitation with acetonitrile, and stable isotope forms of amiodarone and des-AMI were used as internal standards. The lower limit of quantitation of the method was 0.5 ng/mL. The LC-MS/MS was performed using a Waters 2795 Alliance Integrated System (Waters Corporation, Milford, MA) in multiple reaction monitoring mode and a Gemini C6-Phenyl (Phenomenex, Torrance, CA), 50 × 2.1 mm, 5-μm column, using gradient elution with 0.1% formic acid in water and 0.1% formic acid in acetonitrile as the mobile phase. Pharmacokinetic (PK) parameters were analyzed using Phoenix WinNonlin software (version 6.4; Certara, Princeton, NJ) to perform noncompartmental modeling.
To determine whether amiodarone treatment provided survival benefit, a log-rank analysis comparing the amiodarone-treated group to the control group was performed using GraphPad Prism 6. For the purposes of comparison, the single animal that succumbed on day 2 was omitted from analysis due to presumptive aspiration secondary to treatment, based on observations at necropsy.
Efficacy of Amiodarone in Guinea Pigs
Male and female Hartley guinea pigs (gender balanced; 6–7 weeks old) were obtained from Charles River Laboratories. Animals were doubly housed in microisolator cages with CareFresh bedding and provided Teklad Global High Fiber Guinea Pig Diet (no. 2041) and purified (reverse osmosis) water ad libitum. Two groups (n = 15, 8 female and 7 male) were treated by oral gavage once daily with 160 mg/kg Amiodarone Hydrochloride Injection (Hikmara Farmaceutica, Portugal) under anesthesia beginning 3 days before virus exposure and continuing until end of study. Control animals received an equivalent volume of water by oral gavage. Both groups were challenged intraperitoneally with 3620 plaque-forming units of EBOV/May-GPA. Animals were observed and weighed daily.
All animal studies were conducted in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and were approved by either the Institute Animal Care and Use Committee of SRI International or National Institute of Allergy and Infectious Diseases, Division of Clinical Research, and were in compliance with the Animal Welfare Act regulations, Public Health Service policy, and the Guide for the Care and Use of Laboratory Animals recommendations [10].
RESULTS
In Vitro Activity of Amiodarone
Amiodarone was tested for activity against EBOV/Mak and EBOV/May-GPA in Vero E6 and Huh 7 cells and MDMs (Table 1, Supplementary Figure S1). At an MOI of 0.1 and an assay end point of 48 h, amiodarone inhibited EBOV/Mak in Vero E6 (EC50 = 15.9 µM) and Huh 7 (EC50 = 5.5 µM) cells and macrophages (EC50 = 6.6 µM). The selectivity index (SI = CC50/EC50) takes cytotoxicity into account and ranged from at least 2.5 to over 7.5 depending on cell type. To determine whether the drug performs comparably against EBOV/May-GPA in vitro before in vivo studies, the assays were repeated once in Vero E6 (EC50 = 14.9 µM, MOI 0.31) and Huh 7 (EC50 = 7.8 µM, MOI 0.08) cells using EBOV/May-GPA as the infectious agent (Table 1, Supplementary Figure S1).
Table 1.
Effects of Amiodarone on Replication of Ebola Virus (Makona) and Guinea Pig-adapted Ebola Virus
| Virus | Cell Type | MOI | CC50 (µM)a | EC50 (µM)a | SIb |
|---|---|---|---|---|---|
| EBOV/Mak | Vero E6 | 0.10 | >40.0 | 15.9 ± 1.0 | >2.5 |
| Huh 7 | 0.10 | >40.0 | 5.5 ± 0.7 | >7.2 | |
| MDM | 0.10 | 29.5 | 6.6 ± 2.3 | 4.5 | |
| EBOV/May-GPA | Vero E6 | 0.31 | >40.0 | 14.9 ± 3.7 | >8.2 |
| Huh 7 | 0.08 | 38.2 | 7.8 ± 0.05 | 4.9 |
Abbreviations: CC50, concentration with 50% cytotoxicity; EBOV/Mak, Ebola virus Makona variant; EC50, concentration with 50% efficacy; EBOV/May-GPA, guinea pig-adapted Mayinga variant of Ebola virus; MOI, multiplicity of infection; SI, selectivity index.
aCC50 and EC50 values are mean values ± standard deviation from 2 to 4 dose-response curves.
bSI = CC50/EC50
Pharmacokinetics of Amiodarone in Guinea Pigs
A PK study of amiodarone in the guinea pigs was performed to confirm that drug exposure in guinea pigs is similar as that in humans. Amiodarone dosing was modeled after the regimen given to EVD patients in the compassionate use study conducted in Sierra Leone [5]. Patients with EVD received 20 mg/kg (intravenously) or 30 mg/kg (orally). The human equivalent dose of amiodarone in guinea pigs was determined to be 160 mg/kg for oral dosing.
The mean maximum serum concentrations (Cmax) for amiodarone was 4653 ng/mL (6.83 µM) and 6193 ng/mL (9.08 µM) for male and female guinea pigs, respectively (Supplementary Table S1 and Supplementary Figure S2). These Cmax were in a similar range as those in human serum (27 mg/kg dose; Cmax = 4.4–20.9 µM) [11]. The drug was absorbed quickly with a mean time of maximum concentration (Tmax) of 2.7 h (males) and 2 h (females). The Cmax values for the metabolite des-AMI were approximately 7% of the amiodarone peak levels, with a mean Tmax of 5.3 h. Exposure to des-AMI, based on the AUCinf was approximately 17%–20% of the AUCinf for the parent drug. The elimination half-life (t1/2) for amiodarone was approximately 10–11 h, and for des-AMI, the mean t1/2 was longer, approximately 15–16 h. Mean apparent total clearance (Cl/F) was approximately 2900 and 2200 mL/kg per hour for males and females, respectively. Mean apparent volume of distribution during the terminal phase (Vz/F) values were above 30000 mL/kg corresponding to a high volume of distribution.
Efficacy of Amiodarone in Guinea Pigs
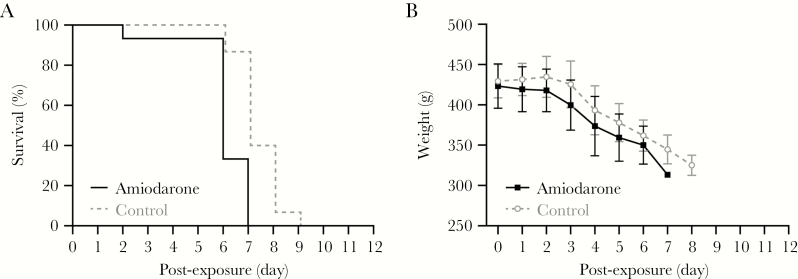
A study in the guinea pig model of EBOV infection was performed to evaluate a potential in vivo effect of amiodarone on EVD. Two groups of guinea pigs (n = 15, 7 males and 8 females) were treated once a day with an oral dose of 160 mg/kg amiodarone or vehicle starting 3 days before exposure to EBOV until study end (day 6–9). A comparison of survival and weight between males and females within each group did not reveal a significant difference (Supplementary Figure S3). Analysis of data of the 2 groups (n = 15, males and females combined) demonstrated that both the treated and control animals succumbed to disease with a median time-to-disposition of 6 and 7 days postexposure, respectively (Figure 1). Comparison of the survival curves of amiodarone and control groups by log-rank analysis indicated a significant difference (P = .0009, hazard ratio 4.15), suggesting that amiodarone treatment may have accelerated time to death. Clinical progression of both groups was similar including weight loss, scruffy appearance, and lethargy preceding death. A single animal in the amiodarone-treated group unexpectedly succumbed at day 2 postexposure. Pathological examination suggested this resulted from respiratory complications due to aspiration secondary to treatment given under anesthesia.
Figure 1.
Effect of amiodarone treatment in guinea pigs infected with guinea pig-adapted Mayinga variant of Ebola virus (EBOV/May-GPA). Guinea pigs (n = 15, 8 female and 7 male) received once-a-day oral treatment with 160 mg/kg amiodarone starting 3 days before virus challenge; the control group (n = 15, 8 female and 7 male) was treated with water in parallel. All animals were challenged on day 0 intraperitoneally with 3620 plaque-forming units of EBOV/May-GPA strain and monitored for survival (A) and weight (B).
DISCUSSION
Repurposing drugs that have already secured US Food and Drug Administration approval for treatment of disease is attractive because it has the potential to greatly reduce development costs and accelerate clinical availability by eliminating rigorous and extensive safety testing required for novel drugs. Amiodarone is widely used in cardiological practice with a wealth of information on pharmacology and potential side effects, both short- and long-term, in patients. Amiodarone is a multi-ion channel inhibitor that prolongs the phase 3 of the cardiac action potential, which is associated with a decrease in calcium permeability and an increase in potassium permeability [12]. Based on in vitro data demonstrating that amiodarone inhibits EBOV infection, the drug was given under compassionate use to 65 EVD patients in Sierra Leone [5]. The results indicating lower mortality were intriguing but lacked statistical significance, and it became a priority to generate additional, more detailed preclinical data on activity against EBOV.
In this study, we confirmed the activity of amiodarone against the Makona isolate of EBOV in several established cell lines. The EC50 values in our EBOV drug screen assay ranged from 5.5 to 15.9 µM in Huh 7 and Vero E6 cells and primary human MDMs. Similar activity was reported for amiodarone against the recombinant EBOV expressing the green fluorescent protein (EC50 = 7.6 µM) [13], whereas another study reported higher activity in vitro (0.37–2 µM) [1]. In part, this difference may be due to differences in assay methods, including that the cells were exposed to replicating EBOV for 1 h in the presence of the drug, after which the virus inoculum was removed and replaced with drug media for 20 h [1]. In contrast, the cells in our assay were exposed to replicating virus and drug for 48 h, possibly allowing for multiple rounds of virus replication that may account for the reduced activity of amiodarone in our assay.
In a previous report, results of 2 studies on amiodarone treatment (90 mg/kg) were conflicting with 40% or 0% survival in a mouse model of EVD [14]. It is important to determine whether drug effects in the guinea pig model for EVD correlate with drug exposure in the guinea pig. Therefore, we conducted a pharmacokinetics study in guinea pigs and observed that the concentrations of amiodarone increased rapidly and peaked within 2–4 h after the oral dose. The metabolite des-AMI peaked a few hours later. The des-AMI metabolite is active for the primary indication of amiodarone (cardiac arrhythmia), but it is unknown whether it has activity against EBOV. Both the parent drug and metabolite decreased steadily after the peak; at 72 h, the plasma concentrations of amiodarone and des-AMI were above the lower limit of quantification in all animals, indicating good exposure to the drug. Plasma drug concentrations of both the parent drug and metabolite tended to be higher in the female animals.
We further investigated in vivo efficacy in the guinea pig model for EVD. The PK study in healthy guinea pigs using the human equivalent dose 160 mg/kg (oral) resulted in serum levels (Cmax = 6.8–9.1 µM) similar to those found in arrhythmia patients treated with a single dose of 27 mg/kg (Cmax = 4.4–20.9 µM) [11]. The serum concentrations were within the range of the EC50 values found for EBOV inhibition in vitro. However, when treating guinea pigs with 160 mg/kg amiodarone, no protective benefit on EVD progression or survival was demonstrated, and there was an indication that amiodarone may have a negative impact on survival. Despite higher plasma concentrations of the drug in females, no obvious difference in survival was observed in females than in males.
A single 160 mg/kg dose of amiodarone was well tolerated in healthy animals used in the PK study; however, tolerability of amiodarone was not assessed for a duration similar to that used in the efficacy study, and either drug accumulation or repeat-dose drug-induced adverse effects may explain the lower tolerability seen in the efficacy study. It is possible that in EBOV-infected guinea pigs the drug is metabolized or distributed differently than in humans resulting in toxicity. Amiodarone is known to be associated with pulmonary and hepatic toxicity, and electrolyte levels and ECG changes need to be monitored carefully in patients during amiodarone treatment.
CONCLUSIONS
While in vitro anti-EBOV activity of amiodarone was confirmed in several different cell types, including human macrophages, no anti-EBOV activity was observed for amiodarone when tested in the guinea pig model of EVD. However, without additional PK and tolerability information, we cannot eliminate the possibility that a beneficial effect of amiodarone for treating EVD may be observed with further refinement of treatment regimens.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Disease online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Presented in part: Spring Research Festival 2015, May 6, 2015, Frederick, MD.
Acknowledgments. We thank Dr. William Eugene Dowling and Ann E. Eakin (Division of Microbiology and Infectious Diseases, National Institutes of Health) for providing resources for the pharmacokinetic (PK) study. We thank Robin Gross, James Logue, Tracey Burdette, and Nicole Murphy for outstanding assistance in performing the drug screen. We acknowledge Julie Nop for in-life activities for the PK study and Drs. Liang Tang and Rob Swezey for bioanalytical sample analysis. We acknowledge Laura Bollinger for technical writing services in preparation of this manuscript and Jiro Wada for figure preparation.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views or policies of the US Department of Health and Human Services or of the institutions and companies affiliated with the authors.
Financial support. This work was funded by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases (NIAID); Integrated Research Facility (NIAID, Division of Clinical Research); Battelle Memorial Institute’s prime contract with NIAID (Contract no. HHSN272200700016I); and SRI International’s prime contract with NIAID (Contract no. HHSN2722011000221).
Potential conflicts of interest. Y. C., L. E. D., J. C. J., and R. S. B. performed this work as employees of Battelle Memorial Institute (BMI). Subcontractors to BMI who performed this work are B. J. H., J. D., and E. P. as employees of Tunnell Consulting, Inc.; H. Z. as an employee of Loveless Commercial Contracting, Inc.; D. M. G., J. M., and G. G. O. as employees of Midwest Research Institute; and K. G. O., C. E. G., and J. C. M. as employees of SRI International. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gehring G, Rohrmann K, Atenchong N, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother 2014; 69:2123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf T, Kann G, Becker S, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet 2015; 385:1428–35. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Ebola vaccines, therapies, and diagnostics: questions and answers. Available at: http://www.who.int/medicines/emp_ebola_q_as/en/. Accessed January 3, 2018. [Google Scholar]
- 4. Emergency NGO Onlus. Clinical study to assess efficacy and safety of amiodarone in treating patients with Ebola virus disease (EVD) in Sierra Leone. Emergency amiodarone study against Ebola (EASE). Available at: http://www.clinicaltrials.gov/ct2/results?term=NCT02307591. Accessed January 3, 2018. [Google Scholar]
- 5. Sweiti H, Ekwunife O, Jaschinski T, Lhachimi SK. Repurposed therapeutic agents targeting the Ebola virus: a systematic review. Curr Ther Res Clin Exp 2017; 84:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola. Available at: http://www.who.int/medicines/ebola-treatment/en/. Accessed January 3, 2018. [Google Scholar]
- 7. Cong Y, Dyall J, Hart BJ, et al. Evaluation of the activity of lamivudine and zidovudine against Ebola virus. PLoS One 2016; 11:e0166318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Honko AN, Johnson JC, Marchand JS, et al. High dose sertraline monotherapy fails to protect rhesus macaques from lethal challenge with Ebola virus Makona. Sci Rep 2017; 7:5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vogel P, Connolly B, Abplanalp D, et al. Pathology of experimental Ebola-Zaire (Mayinga) virus infection transmitted to guinea pigs by oral, conjunctival and tonsillar routes. Cell Vis 1997; 4:298–307. [Google Scholar]
- 10. National Research Council(2011)Guide for the care and use of laboratory animals. 8 edn. The National Academies Press, Washington, DC. doi:10.17226/12910 [Google Scholar]
- 11. Kannan R, Nademanee K, Hendrickson JA, Rostami HJ, Singh BN. Amiodarone kinetics after oral doses. Clin Pharmacol Ther 1982; 31:438–44. [DOI] [PubMed] [Google Scholar]
- 12. Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol 1999; 84:20R–8R. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. Ebola drug test database. Available at: http://www.who.int/medicines/ebola-treatment/test-database/en/. Accessed January 3, 2018. [Google Scholar]
- 14. Madrid PB, Panchal RG, Warren TK, et al. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect Dis 2015; 1:317–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.