Abstract
Background
The pathophysiology of Ebola virus disease (EVD) is still poorly understood. This study aimed at identifying soluble biomarkers that inform on disease mechanisms.
Methods
Fifty-four soluble mediators of the immune, coagulation, and endothelial system were measured in baseline and follow-up samples from hospitalized patients with EVD, using Luminex technology. Cross-sectional expression levels and changes over time were correlated with outcome.
Results
Levels of circulating proinflammatory cytokines and chemokines, as well as markers of endothelial dysfunction and coagulopathy, were elevated on admission to hospital in patients who died from EVD as compared to survivors. These markers further increased in patients who died and/or decreased over time in survivors. In contrast, markers of gut integrity and T-cell response were higher in survivors and increased until discharge.
Conclusions
Inflammatory response, endothelial integrity, gastric tissue protection, and T cell immunity play a role in EVD pathophysiology.
Keywords: Ebola virus disease, cytokines, soluble mediators
Ebola virus (EBOV) is the prototype filovirus causing Ebola virus disease (EVD), a severe infection characterized by strong inflammation, profound disruption of host immune responses, and a multiorgan syndrome that resembles septic shock [1, 2]. One of the most common findings in EVD is an increased level of circulating proinflammatory cytokines, which has been associated to high viral load [3]. Previous studies have correlated the expression of proinflammatory mediators in plasma from patients with acute EVD to disease severity, thereby pointing out that cytokine expression might be used as a predictor of EVD outcome [4–6].
However, most studies do not consider the kinetics of soluble mediators. With the exception of 2 studies conducted in small cohorts of patients with EVD [4, 7], there are no longitudinal data reflecting the kinetics of a natural infection [8].
Here, we provide cross-sectional and longitudinal data on the expression of soluble mediators (cytokines and chemokines) in the blood of patients with EVD diagnosed at the European Mobile Laboratory (EMLab) units deployed to the Ebola treatment centers (ETCs) of Guéckédou and Coyah in Guinea in 2014 and 2015. Our data suggest that endothelial integrity, gastric tissue protection, and T-cell immunity may be important correlates of EVD survival.
METHODS
Ethics Statement
The National Committee of Ethics in Medical Research of Guinea, as well as the Ethics Committee of the Medical Association of Hamburg, approved the use of diagnostic leftover samples and the corresponding patient data for this study (permits 11/CNERS/14 and PV4910). As the samples had been collected as part of the public health response to contain the outbreak in Guinea, informed consent was not obtained from patients. For the purpose of this study, the samples were anonymized. We had a waiver to work with anonymized material and data.
Patients and Samples
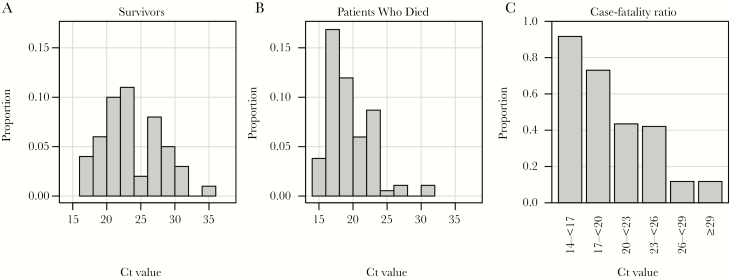
Plasma samples collected in ethylenediaminetetraacetic acid–containing tubes were obtained from hospitalized patients with EVD treated at the ETCs in Guéckédou and Coyah. Clinical EVD diagnoses were confirmed by the EMLab, using reverse transcription polymerase chain reaction (RT-PCR) as described elsewhere [9]. Frozen plasma samples were stored on site at –20°C, shipped on dry ice to the Bernhard Nocht Institute for Tropical Medicine (BNITM; Hamburg, Germany), and stored at –80°C in the biosafety level 4 laboratory. Patient data were obtained from EMLab and World Health Organization (WHO) databases. To increase the power of statistical analysis, study patients were retrospectively selected from the database according to the following criteria: similar numbers of patients in the groups of survivors and patients who died and comparable distributions of age and sex in both groups. In total, 180 patients with RT-PCR–confirmed EVD (33 patients from Guèckèdou, who were also included in a previous study [9], and 147 patients from Coyah), accounting for 388 plasma samples, were included in the study. The median number of samples per patient was 2 (range, 1–8 samples). According to the selection criteria, 52% of the study subjects had a fatal outcome, and 54% were female. Mean age (±SD) was 32.2 ± 17.3 years. Patients who died were older (mean age [±SD], 35.9 ± 17.8 years) than survivors (mean age [±SD], 28.1 ± 15.8 years). In addition to standard of care, 66 patients (37%) received favipiravir on a compassionate use basis. Fatalities had lower cycle threshold (Ct) values from the EBOV RT-PCR on admission (median, 18.7; interquartile range [IQR], 17.1–21.0), compared with survivors (median, 23.0; IQR, 20.4–27.3; Figure 1A and 1B). The case-fatality rate was inversely correlated with the Ct, indicating an association between poor outcome and high virus load (Figure 1C), as has been described previously [9].
Figure 1.
Association of Ebola virus disease outcome with the cycle threshold (Ct) value obtained by Ebola virus reverse transcription–polymerase chain reaction analysis of the first sample from 180 patients. Note that 33 of 180 patients were previously described in a similar analysis [9]. A, Histogram of Ct values from survivors. B, Histogram of Ct values from patients who died. C, Case-fatality ratio depending on Ct value.
Luminex-Based Assays for Soluble Mediators
Infectious specimens were analyzed in the biosafety level 4 laboratory at the BNITM. Soluble mediators were quantified using commercial immunoassays on the Luminex 200 system (Luminex). The following assays were obtained from Merck Millipore (Schwalbach, Germany) and performed according to manufacturer’s instructions: a 26-plex assay for epidermal growth factor (EGF), granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, fractalkine, growth-regulated oncogene α (GROα), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, tumor necrosis factor α (TNF-α), monocyte chemoattractant protein 1 (MCP-1), MCP-3, soluble CD40 ligand, vascular endothelial growth factor (VEGF), interferon γ (IFN-γ), interferon α2 (IFN-α2), IFN-γ–inducible protein 10 (IP-10), interleukin 2 (IL-2), IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p40, IL-12p70, IL-1 receptor antagonist (IL-1RA), IL-1α, and IL-1β; a 6-plex assay for soluble IL-1 receptor type II (sIL-1RII), soluble TNF receptor I (sTNFRI), sTNFRII, soluble VEGF receptor 1 (sVEGFR1), sVEGFR2, and sVEGFR3; a 5-plex assay for C-reactive protein (CRP), fibrinogen, sL-selectin, platelet factor 4, and von Willebrand factor (vWF); 4-plex assays for soluble intracellular adhesion molecule 1 (sICAM-1), regulated on activation, normal T-cell expressed and secreted (RANTES), soluble vascular cell adhesion molecule 1 (sVCAM-1), plasminogen activator inhibitor 1, a disintegrin and metalloproteinase with a thrombospondin type 1 motif 13, D-dimer, serum amyloid antigen (SAA), sP-selectin, sE-selectin, platelet endothelial cell adhesion molecule 1 (Pecam-1), tissue factor (TF), and thrombomodulin; 3-plex assays for granzyme B (GrzB), soluble Fas (sFas), sFas ligand (sFasL), MCP-2, thrombopoietin, and TNF-related apoptosis-inducing ligand; 2-plex assays for antithrombin III and complement factor H, IL-29, and macrophage colony-stimulating factor (M-CSF); and a single-plex assay for ferritin. Assay for tissue plasminogen activator (TPA), IFN-β, and B-cell activating factor (BAFF) was obtained from Affymetrix and performed as a 3-plex assay according to the manufacturer’s instructions. Multiplex assay analysis was done with the Milliplex Analyst 5.1 software. Values outside the upper or lower end of the standard curve (ie, out-of-range values) were considered as maximum or minimum values, respectively.
To establish a reference range for the concentration of soluble mediators in blood, samples from 33 healthy human subjects (reference controls) were measured.
Cross-sectional Analysis of Soluble Mediators
Measurements of soluble mediators in the initial specimens from 71 patients (48%) who survived EVD and 76 patients (52%) who died from EVD were included in the cross-sectional analysis. BAFF, ferritin, fibrinogen, IL-1, IL-1β, IL-2, IL-4, IL-5, and IL-12p70 were excluded because >75% of measurements in initial samples were out of range. Median ratios (median expression in fatalities over median expression in survivors) were calculated for mediators with <50% of values out of range (n = 50). The nonparametric Mann-Whitney U test was used to determine the statistical differences between group medians. If >50% of measurements of a mediator were out of range (n = 4), parameters were dichotomized into high and low expression, where the out-of range values constituted one group and the remaining values the other group. These categorical expressions were compared between patients who died and survivors by performing χ2 analysis and calculating relative risks (RRs) with 95% confidence intervals (CIs).
Receiver operating characteristic (ROC) curves were calculated to evaluate the ability of a soluble mediator in the initial specimens to discriminate survivors from patients who died. Only mediators with <50% values out of range were included in the analysis. The area under the ROC curve (AUC) is a measure of the classification performance. AUCs of <0.7 and 0.7–0.8 were classified as having poor and fair, respectively, discrimination ability. AUCs >0.8 were not observed in our study.
Principal component analysis (PCA) transforms correlated measurements into a set of uncorrelated values (so called principal components) by summarizing covariance measured in different dimensions, hence simplifying the underlying data structure. PCA was performed using soluble mediators with a Mann-Whitney U test P value of <.05 in the differential analysis of survivors as compared to patients who died. The PCA’s first 2 components were presented in a diagram to identify patient clusters characterized by common expressions of soluble mediators.
Longitudinal Analysis of Soluble Mediators
Patients with at least 2 serial samples were included in the analysis. The median hospital stay of the patients was 10 days (IQR, 6–14 days). All available EBOV RT-PCR–positive samples of a patient were analyzed. For survivors, levels of soluble mediators in at least 1 RT-PCR–negative sample, collected in the recovery phase, were also included. BAFF, ferritin, fibrinogen, GROα, IL-12p70, IL-1β, IL-2, IL-29, IL-4, IL-5, L-selectin, and sFasL were excluded from the analysis because >50% of the longitudinal measurements were out of range. The final analysis included 51 soluble mediators determined in 268 samples from 71 patients, of whom 26 (37%) died and 45 (63%) survived. To focus analysis on the change over time, soluble mediator values for a patient were normalized by the respective mediator value of the first specimen from that patient (the relative value was calculated as the value for the follow-up specimen divided by the value for the initial specimen). The association between expression kinetics of soluble mediators and patient outcome was analyzed using linear regression. The normalized (relative) soluble mediator values were log2 transformed before analysis because they represent a ratio between 2 measurements. An interaction term for “days after infection” and “death” was included in the models to test whether the kinetics of mediator expression differs between patients who died and those who survived.
Statistical Analysis
Normally distributed continuous variables were described with their means and SDs and nonnormally distributed variables with their medians and IQRs. Categorical variables were reported using the frequency and percentage. Observations with missing values were excluded from the calculations. All analyses were performed with R, version 3.4.3, using the packages pROC (version 1.12.1) to calculate ROC curves, epitools (version 0.5–10) to calculate RRs, and multcomp (version 1.4–8) for linear combination of regression coefficients.
RESULTS
Cross-sectional Analysis of Soluble Mediator Expression
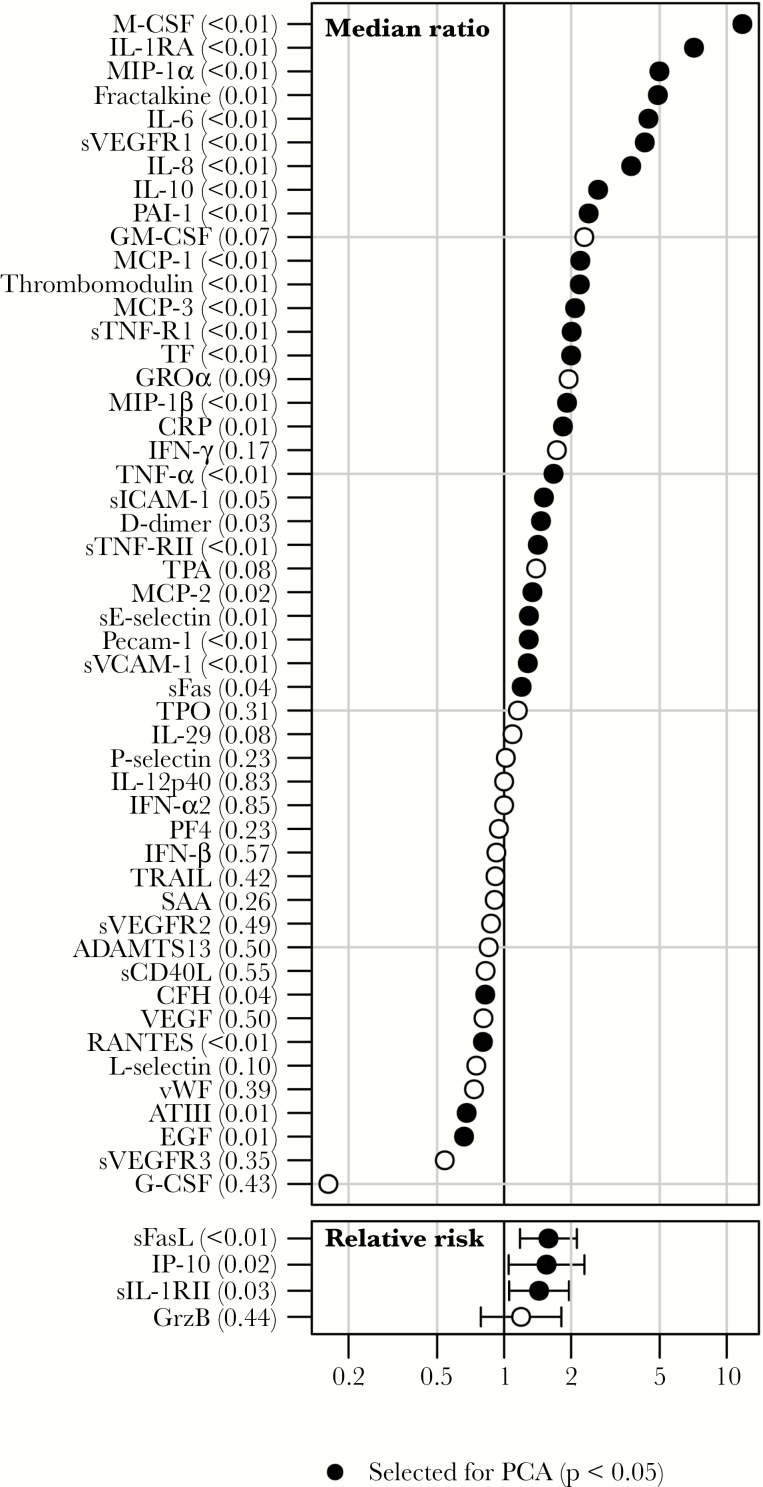
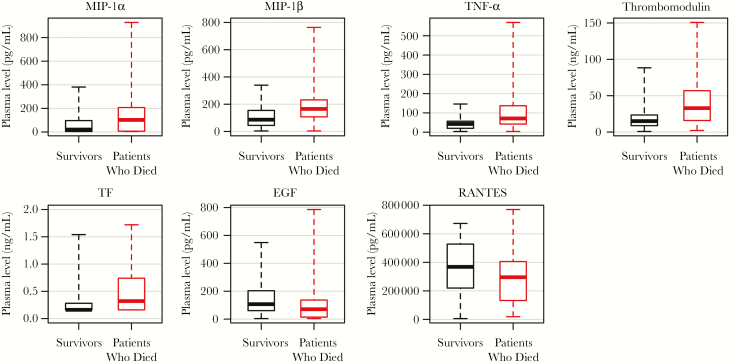
A statistically significant difference in the first sample between patients who died and those who survived was observed for 32 of 54 soluble mediators (59%), of which 28 (88%) showed higher expression in patients who died (Figure 2 and Supplementary Figure 1). Box plots for selected mediators are shown in Figure 3. Fatal outcome was associated with high expression of proinflammatory chemokines (MIP-1α, MIP-1β, and TNF-α), as well as markers of endothelial dysfunction (thrombomodulin) and TF (Figure 3). Since several of these soluble mediators are produced mainly by monocytes/macrophages [10, 11], these findings suggest an involvement of activated myeloid cells in inflammation and endothelial disruption during EVD. Interestingly, survivors showed higher expression of EGF associated with gut integrity (Figure 3), suggesting that the activation of compensatory mechanisms for endothelial dysfunction and gut disruption may be important host responses to overcome EVD. In addition, survivors showed higher expression of RANTES, suggesting activation of T-cell function and recruitment of T cells to sites of infection [12] (Figure 3).
Figure 2.
Association of expression of soluble mediators with outcome. The median concentration of a soluble mediator in patients who died was divided by the median concentration in survivors for mediators with <50% of values out of range. Statistical significance was tested with the Mann-Whitney U test. Mediators with >50% out-of-range measurements were separated into 2 groups, with one group composing the out-of range values and the other group composing the remaining values. Relative risks with 95% confidence intervals were calculated, and differences between patients who died and survivors were tested for statistical significance, using the χ2 test.
Figure 3.
Box plots of selected soluble mediators differently expressed in patients who died and survivors on admission to hospital. Only values measured in the initial sample collected for Ebola virus disease diagnostic assays were considered in the analysis. The boxes represent medians with interquartile ranges; the whiskers depict minimum and maximum values (range). The differences between survivors and patients who died were evaluated via the nonparametric Mann–Whitney U test. All parameters shown in the figure showed P values of ≤.01.
ROC curves were calculated for 50 soluble mediators (Supplementary Figure 2). The estimated AUCs ranged from 0.49 to 0.76 (median, 0.61; IQR, 0.53–0.64). Six mediators showed a fair discrimination (defined as an AUC of 0.7–0.8), namely MIP-1β, TNF-α, M-CSF, IL-10, IL-8, and IL-1RA. The predictive performance of the remaining markers was poor.
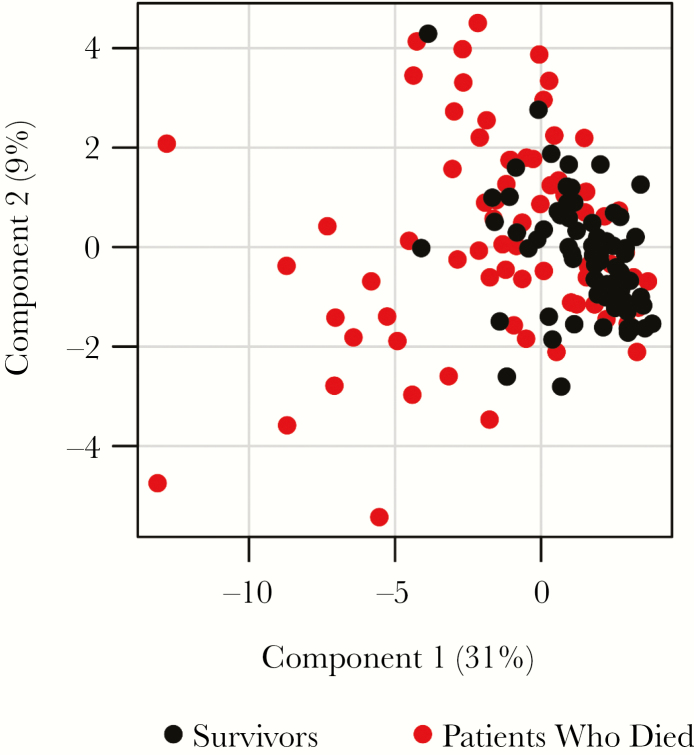
The PCA was calculated, with the 32 mediators showing a statistically significant association with outcome. The first 2 principal components accounted for only 40% of the total model variance, highlighting the diversity of expression patterns of soluble mediators in EVD (Figure 4). Survivors showed rather similar expression profiles, while patients who died were much more diverse. The profiles of survivors and patients who died overlapped, suggesting that the outcome is not associated with a specific expression pattern of soluble mediators.
Figure 4.
Principal component analysis (PCA) biplot for soluble mediator expression in patients who died as compared to survivors on admission to hospital. PCA was performed on 32 soluble mediators with a P value < .05 that were differently expressed between survivors and patients who died. Only values measured in the initial sample collected for Ebola virus disease diagnostic analysis were considered in the analysis.
Longitudinal Expression of Soluble Mediators
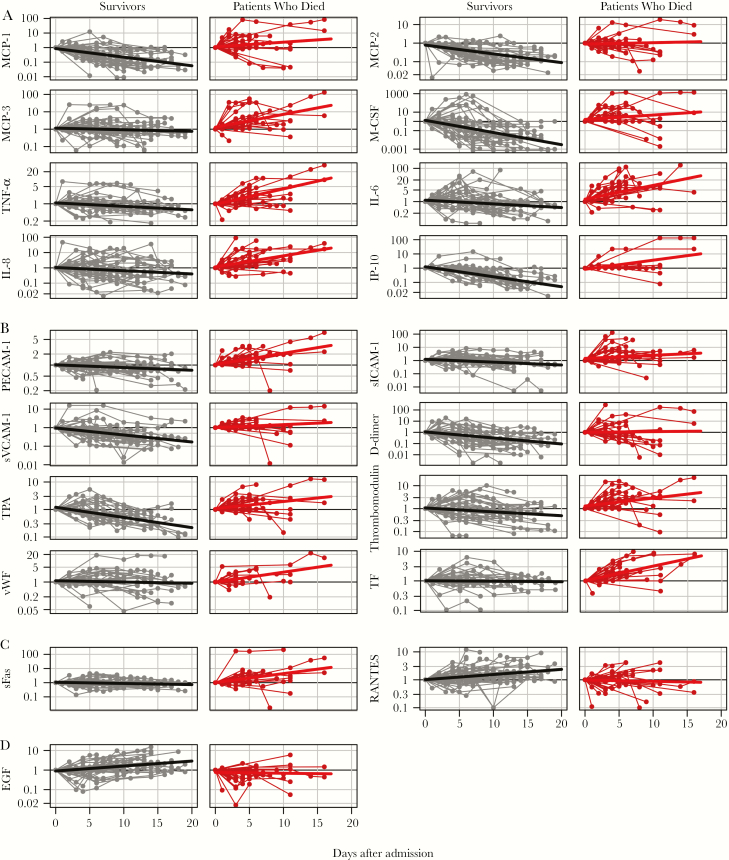
Longitudinal data are shown in Supplementary Figure 2; selected soluble mediators are shown in Figure 5. Thirty-two mediators (63%) showed statistically significantly different (P < .05) expression kinetics between survivors and patients who died (Supplementary Table 1). Four patterns were observed. GrzB, IL-10, IL-1RA, IL-6, IL-8, IP-10, M-CSF, MCP-1, PECAM-1, sICAM-1, sTNF-RII, sVEGFR1, thrombomodulin, TNF-α, and TPA expression increased in patients who died but decreased in survivors. CRP, MCP-3, MIP-1β, sE-selectin, sFas, sIL-1RII, sTNFRI, TF, and vWF expression increased in patients who died but remained unchanged in survivors. In contrast, EGF and RANTES increased in survivors but remained unchanged in patients who died. D-dimer, IFN-γ, IL-1, MCP-2, SAA, and sVCAM-1 expression decreased in survivors and remained unchanged in patients who died. In particular, the trends in the expression of proinflammatory cytokines and chemokines MCP-1, MCP-2, MCP-3, M-CSF, TNF-α, IL-6, IL-8, and IP-10, which increased in patients who died and/or decreased in survivors over time (Figure 5A), are in agreement with previous studies [4–6] and support the association between high levels of virus replication, poor outcome, and inflammation in EVD. Also in agreement with previous studies [4, 6], we observed an increasing expression of IL-10 in patients who died, which probably reflects efforts of the host to control immune homeostasis (Supplementary Figure 3).
Figure 5.
Expression of selected soluble mediators during the course of Ebola virus disease. A, Proinflammatory cytokines and chemokines. B, Markers of endothelial function and coagulation. C, Markers of T-cell function. D, Markers of gastric tissue integrity. Note the logarithmic scale of the y-axis.
Similarly, levels of PECAM-1, sICAM-1, and sVCAM-1 increased in patients who died and/or decreased in survivors (Figure 5B). In addition, patients who died showed increased expression of TPA, TF, and thrombomodulin, while survivors showed progressive reduction in the expression of D-dimers, TPA, and thrombomodulin, suggesting control of endothelial integrity and coagulation. These kinetics are in agreement with the reported association between endothelial dysfunction and disseminated intravascular coagulation in EVD [5, 13]. Fatal EVD was also associated with increasing levels of vWF, a protein that promotes adhesion to injured endothelium, which supports cross-sectional data obtained in patients with EVD infected with Sudan virus [14] (Figure 5B).
Our data also indicated trends regarding expression markers of T-cell function (Figure 5C). Survivors showed increasing expression of RANTES, which is related to recruitment of T cells to peripheral tissues, as well as T-cell activation. However, expression of sFAS, which marks T-cell cytotoxicity, was higher in fatal cases of EVD and increased to the point of death. These results suggest controlled T-cell function in surviving patients and exacerbated cytotoxicity in patients who died.
Finally, we observed opposite trends in the expression of EGF, which plays a role in the maintenance of gastric tissue integrity (Figure 5D). Whereas survivors showed increasing expression of EGF until recovery, the levels decreased over time in patients who died.
DISCUSSION
A strategy to gain insight into the pathophysiology of EVD is the identification of biomarkers that correlate with disease severity. The potential applications of biomarker data include clinical management and therapy design. Our results are in agreement with previous studies indicating that endothelial dysfunction is an important predictor of EVD severity in humans and nonhuman primates [2, 4, 15, 16]. The endothelial activation might be a consequence of the high levels of circulating inflammatory cytokines, in particular TNF-α [12, 17]. This is also consistent with our observation of high circulating levels of TNF-α and other proinflammatory cytokines and chemokines in fatal cases. Several of these mediators (ie, MCP-1, MCP-2, MCP-3, and MIP-1α/β) are produced mainly by myeloid cells [10], suggesting that infected antigen-presenting cells may be an important source of inflammatory mediators during EVD. Clinically, it would be important to determine the involvement of endothelium activation in the hypovolemic shock that is often associated with EVD [3] and to design measures to control inflammatory homeostasis in patients with EVD. The observed concomitant production of antiinflammatory cytokines such as IL-10 is consistent with the notion that EVD is associated with immune dysregulation and with host efforts to control immune homeostasis [6].
An important consequence of endothelium activation is the concomitant activation of coagulation [18, 19]. Thus, endothelial dysfunction is probably associated with DIC, and in fact, our data show elevated levels of coagulation markers also in fatal cases of EVD, including D-dimers, TF, and thrombomodulin. These findings are similar to those described in previous studies of Ebola virus, Sudan virus, and Marburg virus infection in humans and nonhuman primates [19–21]. Interestingly, however, hemorrhage was described less frequently during the West African EVD outbreak, compared with previous outbreaks [1, 22, 23]. It would be interesting to determine which factors are responsible for these differences.
A prominent feature of the EVD outbreak in West Africa was the involvement of the gastrointestinal tract, with vomiting and diarrhea as prominent symptoms [1, 21, 24]. Our data indicated that surviving patients had significantly higher levels of circulating EGF, a secreted protein involved in gastric protection [25]. Previously, downregulation of the EGF receptor by EBOV glycoprotein was proposed as a mechanism to explain glycoprotein-induced cell rounding and detachment in vitro [26, 27]. It is therefore possible that EGF may support cell adhesion in vivo and protect the gastric tissue from glycoprotein-mediated effects. This hypothesis needs to be further investigated in relevant animal models.
Finally, we have observed significant differences in the expression of markers related to T-cell function. T-cell immunity is important for EBOV clearance and EVD recovery, and several previous studies have indicated that proper T-cell activation and control of T-cell homeostasis correlate with a positive outcome [6, 28–30]. Our data argue in favor of T-cell recruitment to peripheral tissues as a correlate of recovery. However, the expression of T-cell molecules related to cytotoxicity showed the opposite trend. In a parallel submission (see the report by Speranza et al in this issue), we provide evidence that our cytokine data are consistent with transcriptomics data showing that survival was associated with high levels of expression of RANTES and low levels of cytotoxic mediators. Further functional studies shall provide insight into the mechanisms by which T cells influence EVD outcome.
Two limitations of our study should be mentioned. First, while we found several mediators that are differentially expressed between patients who died and survivors and inform the pathophysiology of the disease, none of them showed strong power in the ROC analysis to predict the outcome, and PCA did not reveal a specific pattern of mediators associated with outcome. In addition, we do not have data on the expression of these mediators in other diseases, such as other viral hemorrhagic fevers or bacterial sepsis. We therefore would not consider these mediators as biomarkers of EVD in a strict sense. Second, a portion of the patients has received favipiravir on a compassionate use basis in addition to standard of care. In the longitudinal analysis, we compared the trend of soluble mediators between treated and untreated patients who died and survivors, respectively. However, no significant differences were found, although we cannot exclude that effects were not detected, owing to the low number of patients in these 4 subgroups.
In summary, our findings indicate that control of endothelial and gastric integrity as well as T-cell immunity are correlated with EVD survival and may assist in the design of novel treatment strategies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the World Health Organization (WHO) field teams, Medicines sans Frontier teams, and the medical teams deployed by the Cuban government in Coyah; and all volunteers who participated in the activities of EMLab (a technical partner of the WHO Emerging and Dangerous Pathogens Laboratory Network and the Global Outbreak Alert and Response Network) in West Africa.
Financial support. This work was supported in the context of the projects EVIDENT, REACTION!, and EVAg that received funding from the European Union’s Horizon 2020 Research and Innovation Program (grants 666100, 666092, and 653316, respectively) and in the context of service contract IFS/2011/272–372 funded by the Directorate-General for International Cooperation and Development. Further financial support was provided by the German Research Foundation (MU3565/3-0 to C. M.-F. and GU883/5-1 to S. G.) and the German Center for Infection Research (TTU 01.702).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schieffelin JS, Shaffer JG, Goba A, et al. ; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014; 371:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet 2011; 377:849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muñoz-Fontela C, McElroy AK. Ebola Virus Disease in Humans: Pathophysiology and Immunity. Curr Top Microbiol Immunol 2017; 411:141–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McElroy AK, Harmon JR, Flietstra TD, et al. Kinetic Analysis of Biomarkers in a Cohort of US Patients With Ebola Virus Disease. Clin Infect Dis 2016; 63:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McElroy AK, Erickson BR, Flietstra TD, et al. Ebola hemorrhagic Fever: novel biomarker correlates of clinical outcome. J Infect Dis 2014; 210:558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruibal P, Oestereich L, Lüdtke A, et al. Unique human immune signature of Ebola virus disease in Guinea. Nature 2016; 533:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisfeld AJ, Halfmann PJ, Wendler JP, et al. Multi-platform ‘Omics analysis of human Ebola virus disease pathogenesis. Cell Host Microbe 2017; 22:817–29 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bixler SL, Goff AJ. The role of cytokines and chemokines in Filovirus infection. Viruses 2015; 7:5489–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kerber R, Krumkamp R, Diallo B, et al. Analysis of diagnostic findings from the european mobile laboratory in Gueckedou, Guinea, March 2014 through March 2015. J Infect Dis 2016; 214:S250–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 2014; 5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutchinson KL, Rollin PE. Cytokine and chemokine expression in humans infected with Sudan Ebola virus. J Infect Dis 2007; 196 (Suppl 2):S357–63. [DOI] [PubMed] [Google Scholar]
- 13. Geisbert TW, Hensley LE, Larsen T, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol 2003; 163:2347–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McElroy AK, Erickson BR, Flietstra TD, et al. Von Willebrand factor is elevated in individuals infected with Sudan virus and is associated with adverse clinical outcomes. Viral Immunol 2015; 28:71–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol 2007; 7:556–67. [DOI] [PubMed] [Google Scholar]
- 16. McElroy AK, Erickson BR, Flietstra TD, et al. Biomarker correlates of survival in pediatric patients with Ebola virus disease. Emerg Infect Dis 2014; 20:1683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hensley LE, Young HA, Jahrling PB, Geisbert TW. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett 2002; 80:169–79. [DOI] [PubMed] [Google Scholar]
- 18. Fisher-Hoch SP, Platt GS, Neild GH, et al. Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola). J Infect Dis 1985; 152:887–94. [DOI] [PubMed] [Google Scholar]
- 19. Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis 2003; 188:1618–29. [DOI] [PubMed] [Google Scholar]
- 20. van Paassen J, Bauer MP, Arbous MS, et al. Acute liver failure, multiorgan failure, cerebral oedema, and activation of proangiogenic and antiangiogenic factors in a case of Marburg haemorrhagic fever. Lancet Infect Dis 2012; 12:635–42. [DOI] [PubMed] [Google Scholar]
- 21. Lyon GM, Mehta AK, Varkey JB, et al. ; Emory Serious Communicable Diseases Unit Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med 2014; 371:2402–9. [DOI] [PubMed] [Google Scholar]
- 22. Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ 1978; 56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 23. Ksiazek TG, Rollin PE, Williams AJ, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 1999; 179(Suppl 1):S177–87. [DOI] [PubMed] [Google Scholar]
- 24. Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis 2015; 15:1292–9. [DOI] [PubMed] [Google Scholar]
- 25. Skov Olsen P. Role of epidermal growth factor in gastroduodenal mucosal protection. J Clin Gastroenterol 1988; 10 (Suppl 1):S146–51. [DOI] [PubMed] [Google Scholar]
- 26. Simmons G, Wool-Lewis RJ, Baribaud F, Netter RC, Bates P. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J Virol 2002; 76:2518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zampieri CA, Fortin JF, Nolan GP, Nabel GJ. The ERK mitogen-activated protein kinase pathway contributes to Ebola virus glycoprotein-induced cytotoxicity. J Virol 2007; 81:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McElroy AK, Akondy RS, Davis CW, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci U S A 2015; 112:4719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cimini E, Viola D, Cabeza-Cabrerizo M, et al. Different features of Vδ2 T and NK cells in fatal and non-fatal human Ebola infections. PLoS Negl Trop Dis 2017; 11:e0005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dahlke C, Lunemann S, Kasonta R, et al. Comprehensive characterization of cellular immune responses following Ebola virus infection. J Infect Dis 2017; 215:287–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.