Abstract
Background
Several vaccine platforms have been successfully evaluated for prevention of Ebola virus (EBOV) disease (EVD) in nonhuman primates and humans. Despite remarkable efficacy by multiple vaccines, the immunological correlates of protection against EVD are incompletely understood.
Methods
We systematically evaluated the antibody response to various EBOV proteins in 79 nonhuman primates vaccinated with various EBOV vaccine platforms. We evaluated the serum immunoglobulin (Ig)G titers against EBOV glycoprotein (GP), the ability of the vaccine-induced antibodies to bind GP at acidic pH or to displace ZMapp, and virus neutralization titers. The correlation of these outcomes with survival from EVD was evaluated by appropriate statistical methods.
Results
Irrespective of the vaccine platform, protection from EVD strongly correlated with anti-GP IgG titers. The GP-directed antibody levels required for protection in animals vaccinated with virus-like particles (VLPs) lacking nucleoprotein (NP) was significantly higher than animals immunized with NP-containing VLPs or adenovirus-expressed GP, platforms that induce strong T-cell responses. Furthermore, protective immune responses correlated with anti-GP antibody binding strength at acidic pH, neutralization of GP-expressing pseudovirions, and the ability to displace ZMapp components from GP.
Conclusions
These findings suggest key quantitative and qualitative attributes of antibody response to EVD vaccines as potential correlates of protection.
Keywords: adenovirus, Ebola virus, immune correlate, vaccine, virus-like particles
Over the past 50 years, filoviruses, primarily Ebola virus (EBOV), have caused multiple human outbreaks with high fatality rates. The 2014–2016 EBOV disease (EVD) epidemic in West Africa, caused by 2 new isolates of Zaire EBOV (Mak-1 [GenBank accession no. KP178538] and Mak-2 [GenBank accession no. KP240932]), led to 28 616 infections and 11 310 deaths (http://www.who.int/csr/disease/ebola/en/). During the 2014–2016 EVD outbreak, a vaccine based on replication-competent vesicular stomatitis virus (VSV) lacking G protein and expressing EBOV glycoprotein (rVSVΔG-ZEBOV-GP) was tested in a ring vaccination phase III efficacy trial. This trial was reported to be 100% efficacious in preventing transmission of EVD among vaccinated adults, indicating the prospect of an effective prophylactic EBOV vaccine [1]. Other virus vector-based vaccines, including chimpanzee adenovirus vector [2, 3], and a prime boost regimen of adenovirus-vectored vaccine with recombinant modified vaccinia Ankara (MVA) [4] expressing full-length EBOV GP have also been tested for safety and immunogenicity in healthy individuals. Furthermore, other vaccine platforms such as virus-like particles (VLPs) expressing EBOV GP, matrix protein VP40, and the nucleoprotein (NP) [5, 6], a rabies-based inactivated vaccine expressing EBOV GP [7], a GP-expressing Venezuelan equine encephalitis virus-based replicon [8], and replication-competent VSV-based vaccines [9, 10] have shown remarkable efficacy in preclinical challenge studies in nonhuman primates (NHPs).
Despite these advances, the mechanisms of vaccine-mediated protection and correlates of protective immunity against EVD remain poorly understood. Vector-based vaccines such as adenovirus [3] and VSV [11] induce both strong GP-specific CD4 and CD8 T-cell and antibody responses. Virus-like particle vaccination induces dominant NP-specific T-cell and GP-targeted antibody responses [6, 12]. Recent advances in immunotherapy of EVD with polyclonal convalescent macaque immunoglobulin (Ig)G [13] or monoclonal antibody (mAb) cocktails such as ZMapp [14], ZMab [15], MB-003 [16] indicate that antibodies can protect against filoviruses, supporting a vaccine approach focused on generation of antibody responses. However, it is not known which attributes of vaccine-elicited antibody response are a reliable predictor of survival in EVD.
Studies in knockout mice indicate that CD8 T cells are absolutely required, whereas both CD4 and B cells partially contribute to protective efficacy of EBOV VLPs expressing GP, NP, and matrix protein (VP40) (referred to hereafter as triple VLP) [17]. Several studies in rodents [18, 19] and NHPs demonstrated the protective efficacy of triple VLPs (reviewed in [12]). Virus-like particle-immunized NHPs exhibit strong T-cell responses to NP and antibody responses to GP, whereas the T-cell response to GP is less pronounced [6]. However, the requirement for NP-directed responses in the efficacy of EBOV VLPs remains unknown.
In this study, we generated VLPs expressing only GP and VP40 (referred to as double VLP hereafter) to simplify the VLP vaccine and evaluated its efficacy in comparison to triple VLPs. We found that vaccination with the double VLPs, despite their induction of higher antibody titers, provided less protection than vaccination with triple VLPs. Protection in double VLP-vaccinated NHPs was strictly dependent on anti-GP antibody titer, and a clear cutoff for protective IgG enzyme-linked immunosorbent assay (ELISA) titer could be defined for this vaccine platform. Further examination of a larger number of NHPs vaccinated with either VLPs or adenovirus-vectored GP showed highly significant correlation between survival and anti-GP antibody titers, neutralization, and the ability of the serum antibodies to bind to GP at acidic pH. In contrast, antibody titers against NP or VP40 exhibited no correlation with protection similar to previous observations in mice [20] and guinea pigs [21]. These findings strongly suggest that anti-GP antibodies are reliable predictors of protection in NHPs; however, the pattern of antibody response and its correlation with protection varies depending on the vaccine platform.
MATERIALS AND METHODS
Production of Ebola and Marburg Virus-Like Particles
Two types of VLPs were produced using baculovirus expression system in insect cells: (1) VLPs expressing the glycoprotein, the matrix protein, and (2) the NP (triple VLPs) and VLPs expressing only GP and VP40 (double VLPs), as we have described previously in detail [18, 22, 23]. Double and triple VLPs were characterized using a battery of assays including total protein (BCA), identity (Western blotting using mouse monoclonal or epitope-specific rabbit antibodies recognizing EBOV GP, VP40, and NP), electron microscopy, and endotoxin content, as previously described [18, 22, 23].
Vaccination of Nonhuman Primates
Vaccination of cynomolgus macaques (4 to <9 kg; Worldwide Primates, Miami, FL) was performed at US Army Medical Research Institute of Infectious Diseases ([USAMRIID] Frederick, MD) or Covance (Denver, PA). The animals were found to be antibody-negative for filovirus, simian T-lymphotropic virus, simian immunodeficiency virus, and herpes B virus before study initiation. For the NHP study described here, cohorts of 2–5 NHPs (Table 1) were vaccinated via intramuscular (i.m.) injection with EBOV “double” or “triple” VLPs, supplemented with Alhydrogel, RIBI (Corixa, Hamilton, MT) or QS-21 adjuvant (Antigenics, Lexington, MA), or no VLP (QS-21 only) on study days 0 and 42. All injection sites were observed daily for 7 days after each dose and weekly thereafter. Each site was scored for redness and swelling according to the method of Draize et al [24]. Blood samples were obtained under anesthesia from the femoral veins of monkeys on study days −1, 14, 42, 56, and 63 and processed within 4–6 hours. Plasma or serum samples were aliquoted and frozen until analysis.
Table 1.
Summary of the Study Designs and Antibody Titers of NHPs Vaccinated With Triple VLPs (GP/VP40/NP) or Double VLPs (GP/VP40) (Study ID: IBT_01220)a
| Group No., Vaccine, Dose | NHP ID No. | Time of Death (dpi) | Peak Viremia (dpi; pfu/mL) | Peak Viremia (dpi; GE/mL) | Antibody Titers (AU/mL) | ||
|---|---|---|---|---|---|---|---|
| GP∆TM | GP∆Muc | VP40 | |||||
| 1 Triple VLP, 3 mg |
AR791 | Survived | <LOD | Day 7; 1.0E+04 | 1553 | 1057 | 2842 |
| AP229 | Survived | <LOD | <LOD | 412 | 770 | 3461 | |
| AR920 | Survived | <LOD | <LOD | 1324 | 1267 | 2258 | |
| 2 Double VLP, 3 mg |
AT233 | 7 | Day 7; 4.25E+07 | Day7; 2.6E+10 | 645 | 732 | 2802 |
| AR280 | 8 | Day 7; 2.95E+06 | Day 7; 6.6E+09 | 573 | 1011 | 757 | |
| SZ77 | Survived | <LOD | Day 7; 4.2E+05 | 970 | 1218 | 1382 | |
| AR960 | 10 | Day 10; 6.80E+06 | Day 10; 1.2E+07 | 938 | 1551 | 3545 | |
| BM669 | Survived | <LOD | Day 7; 9.8E+06 | 2067 | 2149 | 6606 | |
| 3 Double VLP, 200 µg |
AP601 | 7 | Day 7; 3.50E+07 | Day 7; 7.9E+08 | 634 | 672 | 5150 |
| AP360 | Survived | <LOD | <LOD | 3069 | 2428 | 1412 | |
| AR546 | 10 | Day 10; 5.50E+01 | Day 10; 1.2E+07 | 1392 | 1815 | 5113 | |
| AT164 | Survived | <LOD | <LOD | 2218 | 2764 | 4332 | |
| 4 Double VLP, 75 µg |
YS87 | 8 | Day 8; 4.80E+04 | Day 7; 3.2E+06 | 575 | 613 | 350 |
| AP633 | 9 | Day 7; 7.50E+06 | Day 7; 2.3E+10 | 612 | 569 | 2470 | |
| AR965 | 10 | Day 7; 3.60E+01 | Day 7; 7.5E+05 | 1170 | 1045 | 3593 | |
| 5 Double VLP, 25 µg |
AT105 | Survived | <LOD | <LOD | 1476 | 2543 | 2525 |
| AT3 | Survived | <LOD | <LOD | 1882 | 2024 | 4950 | |
| AT237 | 9 | Day 7; 5.45E+06 | Day 7; 1.1E+09 | 835 | 635 | 8039 | |
| 6 Control |
AP355 | 6 | Day 6; 1.45E+07 | Day 6; 1.5E+10 | 1.2 | 5.8 | 44.1 |
| AR919 | 6 | Day 6; 4.50E+07 | Day 6; 6.3E+10 | 1.2 | 5.8 | 92.6 | |
Abbreviations: dpi, day postinfection; GE, genome equivalent; LOD, limit of detection; NHP, nonhuman primates; pfu, plaque-forming units; qRTPCR; quantitative reverse-transcription polymerase chain reaction; VLP, virus-like particles.
aAll vaccinations were performed with QS-21 as adjuvant on days 0 and 42. Antibody titers are from the last bleed before challenge (3–4 weeks after the last vaccine). Peak viremia levels from plaque assay and qRTPCR analysis are shown.
The vaccination portions of the studies were conducted in compliance with the current standard operating procedures (SOPs) of Covance Research Products, Inc. (Denver, PA and Alice, TX) and with any applicable amendments. All planned changes or revisions of the study protocols at Covance were written in the form of a protocol amendment, signed by the Study Director and the Sponsor, dated and maintained with the protocol. All procedures were in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Office of Laboratory Animal Welfare. In the opinion of the Sponsor and Study Director, the studies conducted at Covance did not unnecessarily duplicate any previous work (US Department of Agriculture [USDA] Regulation: Animal Welfare Regulations 9 Code of Federal Regulations [CFR], Subchapter A). Both Covance facilities are Association for the Assessment and Accreditation of Laboratory and Care International (AAALAC) accredited.
Certain portions of this animal research were conducted according to research protocols approved by the USAMRIID Institutional Animal Care and Use Committee (IACUC). Work at USAMRIID was performed in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011. The USAMRIID is fully accredited by AAALAC. All challenge studies and necropsies were conducted under maximum containment in an animal biosafety level (BSL)-4 facility at USAMRIID.
Studies performed at University of Texas Medical Branch (UTMB) were conducted in compliance with the approved study protocol approved by the institutional IACUC, as well as applicable UTMB SOPs and any applicable amendments. All planned changes or revisions of the study protocol were documented. All procedures in this study were conducted in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Office of Laboratory. The UTMB Animal Resource Center is AAALAC accredited, and UTMB operates as follows: to comply with the USDA Animal Welfare Act (Public Law 89–544) as amended by PL91-579 (1970), PL94-279 (1976), and 45 CFR37618 (6-30-80); to comply with Health Research Extension Act of 1985 (Public Law 99–158); follows the Public Health Service Policy on Humane Care and Use of Laboratory Animals (revised September 1986); and follows the Guide for the Care and Use of Laboratory Animals, Department of Health, Education, and Welfare (National Institutes of Health) 85-23. The UTMB is a registered Research Facility under the Animal Welfare Act.
Protein Production and Determination of Imunnoglobulin G Antibody Titers Against Glycoprotein and Matrix Protein VP40
Ebola virus GP with the transmembrane domain deleted (GPΔTM) or the mucin-like domain (MLD) and transmembrane domain-deleted ectodomain (GP∆Muc) were produced in insect cells and purified by chromatography as previously described [5]. Production of VP40 in Escherichia coli was previously described [25]. Details of production of NP in E coli is provided in Supplemental Methods. A serology ELISA method was developed to determine the serum IgG titers against GPΔTM, GP-∆Muc, VP40, and NP, as detailed in the Supplemental Methods. To determine the ability of serum antibodies to bind GP at acidic pH and competition with ZMapp, the ELISAs were performed in acidic buffer or in presence of ZMApp components as detailed the Supplemental Methods.
Ebola Virus Seed and Nonhuman Primate Challenge Study
The strain of EBOV used in this study was isolated from an infected patient in the 1995 outbreak in Kikwit, Zaire at the Centers for Disease Control and Prevention (Atlanta, GA). The virus stock used at USAMRIID was propagated 2 passages in Vero cells and 4 passages in VeroE6 cells and had a titer of 1.4 × 108 plaque-forming units (pfu)/mL. The virus stock used at UTMB was propagated 2 passages on VeroE6 cells and had a titer of 5.25 × 105 pfu/mL. For studies performed at USAMRIID, animals were transferred from BSL-2 to BSL-4 ≥48 hours before challenge. In the remaining studies, animals were transferred from Covance to the UTMB BSL-4 facility 7 days before challenge. Animals were challenged via i.m. injection with EBOV on study day 70 (referred to as 0 days postinfection [dpi]) with 1000 pfu in 0.5 mL phosphate-buffered saline i.m. in the thigh. The leg in which the virus was injected was recorded for observation purposes. A scoring sheet was used for assisting in determining the time of euthanasia depending on clinical signs (eg, respiratory distress, weakness, inability to move when prodded, hemorrhage, macular rash, etc). Animals were euthanized after deep anesthesia (~9 mg/kg i.m. injection of Telazol) by intracardiac administration of Euthasol (~1 mL/4.5 kg) in accordance with the 2007 American Veterinary Medical Association Guidelines on Euthanasia. Euthanasia was performed by qualified personnel. Death was verified by the absence of a palpable heartbeat at no less than 5 minutes post-exsanguination. All procedures were approved by USAMRIID, Covance, or UTMB IACUC. Blood samples were collected from animals on 0, 3, 5, 7, 21, and 28 dpi and used for blood chemistry, hematology, and viral load analysis as described in Supplemental Methods.
Historical Serum Samples
Serum samples from previous vaccination studies performed at USAMRIID and Public Health Agency of Canada were obtained. All of these animal studies were performed under approval of the local IACUC committees. Historical samples were stored at −80°C until use in the serology assays.
Vesicular Stomatitis Virus-Pseutotype Neutralization Assay
Neutralizing potency of the sera was tested in a VSV-pseudotype system expressing EBOV GP as described previously [26] and detailed in the Supplemental Methods.
Statistical Methods
The data analyses were from NHP studies in which animals received different vaccine types. The sera from the last time point before challenge with the virus were used for analysis, as well as survival outcome and day of death. For survivors, the day of death is censored at 28 dpi (study termination). The parameters for analysis were as follows: (1) serum antibody titer against Ebola GPΔTM; (2) serum antibody titer against Ebola GPΔMuc; (3) serum antibody titer against Ebola VP40 (double and triple VLP groups only); (4) serum antibody titer against Ebola NP (triple VLP group only); (5) ratio of the binding of each serum diluted at 1:100 to GP at pH 4.5 divided by the binding of the same serum sample to GP at pH 7.4; (6) ratio of the binding of each serum to GP at pH 5.5 divided by the binding of the same serum sample to GP at pH 7.4; (7) percentage of displacement of ZMapp by each sample measured at a single dilution (NOTE: negative values indicate that the serum increased the binding of ZMapp to GP instead of competing with it, positive values show competition); (8) percentage of neutralization of a pseudotype virus carrying Ebola GP at a 1:25 dilution of the immune serum.
The number and percentage of NHPs were tabulated by survival outcome at 28 dpi. Each of the above-listed parameters was summarized descriptively by survival outcome (dead vs alive) and compared statistically using 2-sided hypothesis tests without adjustment for multiplicity. Nominal P values are presented.
Before the above analyses, the data were assessed for normality on both the original and log-transformed scale. If deviations from the normality assumption were detected, the comparison of study parameters by survival outcome was performed using Wilcoxon rank-sum tests, with the 2-sample 2-sided t test performed on log-transformed data as a sensitivity analysis. It was noted, based on visual inspection of the normal quantile plots, that the titer endpoints deviate from normality inconsistently on both the original and log scales. For percentage of displacement of ZMapp and percentage of neutralization, the original scale includes negative values; therefore, the log-transformation was not appropriate. However, the normality assumption appears to be appropriate on the original scale for these 2 endpoints (Supplementary Table S2). As a result, comparisons for each of the endpoints by survival outcome were performed using Wilcoxon rank-sum text as well as by 2-sample, 2-sided t test on the log scale as a sensitivity analysis, with the exception of the percentage of displacement of ZMapp components and percentage of neutralization endpoints, for which the t test was performed on the original scale.
The time to death (in days) was analyzed to assess the relationship of each parameter listed above with survival outcomes. Estimates of median survival time along with corresponding 95% confidence intervals were summarized by vaccine type where calculable (ie, at least 50% of NHPs with outcome of death required for calculation of median), and Kaplan-Meier survival curves were plotted. Day of death was censored at the time of last assessment for NHPs who survived the challenge (day 28 postinfection). Separate Cox proportional hazards models were fitted to the time to death data, with each parameter listed above included as a continuous covariate. Hazard ratios and 95% confidence intervals were presented.
RESULTS
Comparative Efficacy of Ebola Virus Virus-Like Particles Vaccine in Presence or Absence of the Nucleoprotein
We have previously demonstrated that administration of 2 or 3 doses of triple VLP vaccine along with RIBI adjuvant in cynomolgus macaques provides full protection against lethal challenge with EBOV [5, 6, 12]. Nucleoprotein is not required for the formation of EBOV VLPs [22], but it can increase the yield of VLP [27, 28] and provide additional CD8 T-cell epitopes for enhanced cell-mediated immunity (CMI) [6, 29–31]. To examine whether double VLPs are as immunogenic and induce similar levels of protection as triple VLPs, NHPs were randomized into 6 groups and vaccinated twice at days 0 and 42 (Table 1). Group 1 received triple VLPs at 3-mg dose and groups 2–5 received a range of double VLP doses from 25 µg to 3 mg, along with 100 µg of QS-21 as adjuvant on days 0 and 42. Immunizations did not affect the body weight, leukocyte counts, hematological parameters, or serum chemistry, except for mild and transient erythema and swelling at the injection site, presumably related to the adjuvant (data not shown).
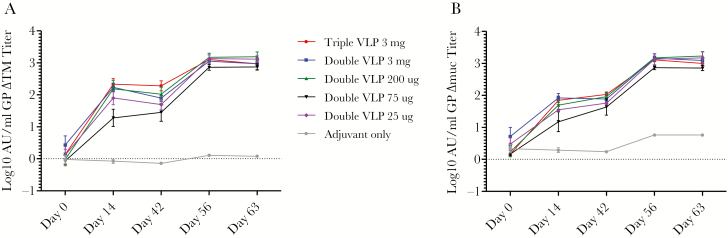
Animals were bled on days 0, 14, 42, 56, and 63 for serological analysis before being challenged on day 70, and the antibody response was measured by ELISA against GP∆TM, GP∆Muc, and VP40 (Table 1). As shown in Figure 1, immunization with 1 dose of triple and double VLPs induced a moderate antibody response to GP, and this response was boosted by approximately 10-fold upon second vaccination. Unexpectedly, no correlation was observed between the double VLP vaccine dose administered and the resulting magnitude of the antibody response.
Figure 1.
Antibody response to double and triple virus-like particle (VLP) vaccination. Total immunoglobulin G titers was determined against GP∆TM (A) and GP∆Muc (B) in cynomolgus macaques vaccinated with the indicated doses of Ebola virus VLPs along with QS-21 as adjuvant.
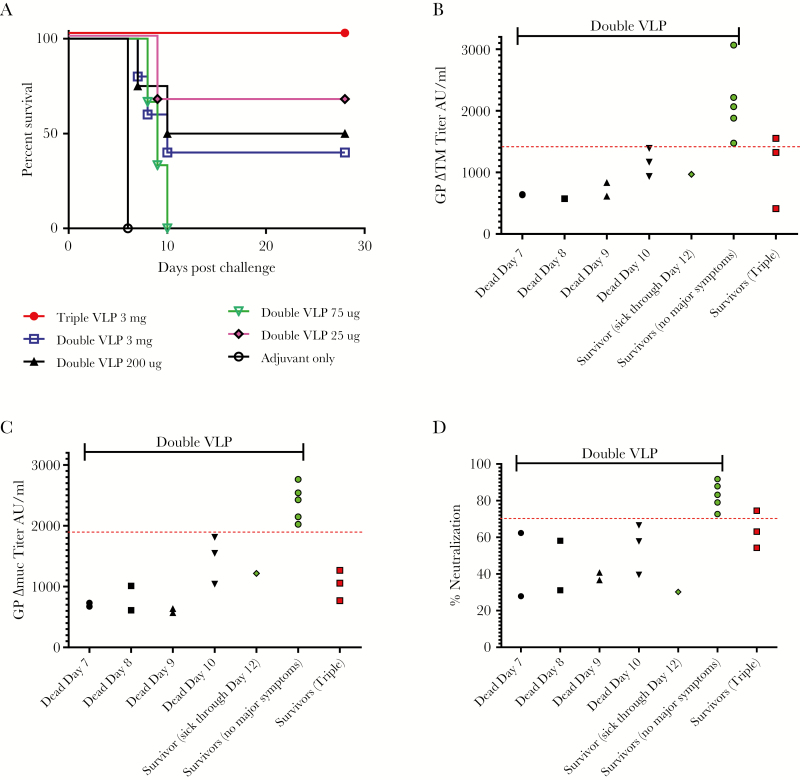
Animals were challenged on study day 70 with 1000 pfu of EBOV (913 pfu based on back-titering). Consistent with our previous reports [5, 6], all 3 triple VLP-vaccinated monkeys survived the challenge with no abnormal clinical symptoms; in contrast, 9 of the 15 NHPs vaccinated with double VLP succumbed to infection.
Viremia was measured by quantitative reverse-transcription polymerase chain reaction (qRTPCR) and standard plaque assays. Control animals became viremic on day 3 (based on qRTPCR) or day 4 (based on plaque assay) with peak levels of 1.45E+07 and 4.50E+07 pfu/mL or 1.5E10 and 6.3E10 GE/mL before death at 6 dpi (Table 1). None of the surviving animals showed any viremia detectable by plaque assay, and all fatal cases showed onset of viremia at 5 or 6 dpi and peaking on the day of death (Table 1). However, virus was transiently detectable by qRTPCR in 2 surviving animals in double VLP groups and 1 animal in the triple VLP group (Table 1).
Blood chemistry data are shown in Supplementary Figure S1. Glucose levels were generally maintained in surviving animals but decreased in fatal cases, likely due to anorexia. Nonsurvivors displayed unchanged or moderately increased blood urea nitrogen and creatinine in blood after infection. Only a single animal (AT233) showed increased uric acid. Levels of calcium, albumin, and total protein remained unchanged in survivors, whereas most nonsurvivors showed slightly reduced levels. Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and gamma glutamyltransferase levels remained stable in survivors except for SZ77 that showed slight elevation and was sick through day 12, whereas the levels for these enzymes were elevated in most nonsurvivors. Amylase remained stable among survivors, but it varied in nonsurvivors, with 5 animals showing 2- to 7-fold increase and 3 animals showing 30%–50% decreased levels. All surviving animals had normal levels of C-reactive proteins (CRP) except for SZ77, whereas all nonsurvivors had greatly increased levels of CRP. Most animals exhibited a spike in white blood cell count after challenge, most notably among fatal cases, whereas hematocrit and hemoglobin levels remained largely stable. Percentage of lymphocytes was significantly decreased among nonsurvivors. Platelet counts decreased in most animals and was more pronounced among nonsurvivors.
Although survival in animals vaccinated with double VLPs did not correlate with the vaccine dose (Figure 2A), it did correlate directly with antibody titers against GP. All double VLP-vaccinated, surviving animals had an anti-GP∆TM antibody titer of higher than 1400 AU/mL, except for 1 animal (SZ77) with an antibody titer of 970 AU/mL, and this animal was sick through day 12 before recovering (Figure 2B). All animals that succumbed to EVD had an anti-GP∆TM titer of less than 1400 AU/mL, and the antibody titer appeared to correlate with the day of death (Figure 2B). This correlation was more pronounced for antibodies to GP∆Muc with a cutoff of ~1900 AU/mL separating survivors from nonsurvivors except SZ77, which was sick through day 12 (Figure 2C).
Figure 2.
Efficacy of double and triple virus-like particles (VLPs) against Ebola virus (EBOV) challenge in cynomolgus macaques. Survival of macaques vaccinated with VLPs after EBOV challenge was monitored for 28 days (A). Antibody titers against were GP∆TM (B) and GP∆Muc (C) as well as percentage of neutralization (D) are shown for individual animals with the day of death or survivorship indicated on the x-axis. Black symbols signify dead animals and colored symbols indicate the survivors. The red line separates the double VLP-vaccinated survivors from lethal cases with a single exception.
The GP∆TM antibody titers of all 3 animals vaccinated with triple VLP were similar to fatal cases of double VLP-vaccinated animals or close to the cutoff level, although all 3 animals survived with no clinical symptoms (Figure 2B). The GP∆Muc titers of the triple VLP-vaccinated animals were far below the apparent cutoff for survival of double VLP-vaccinated animals (Figure 2C). A similar pattern was observed with respect to virus neutralization and fatality using the rVSV-GP pseudotype assay at a 1:200 dilution of the sera. (Figure 2D).
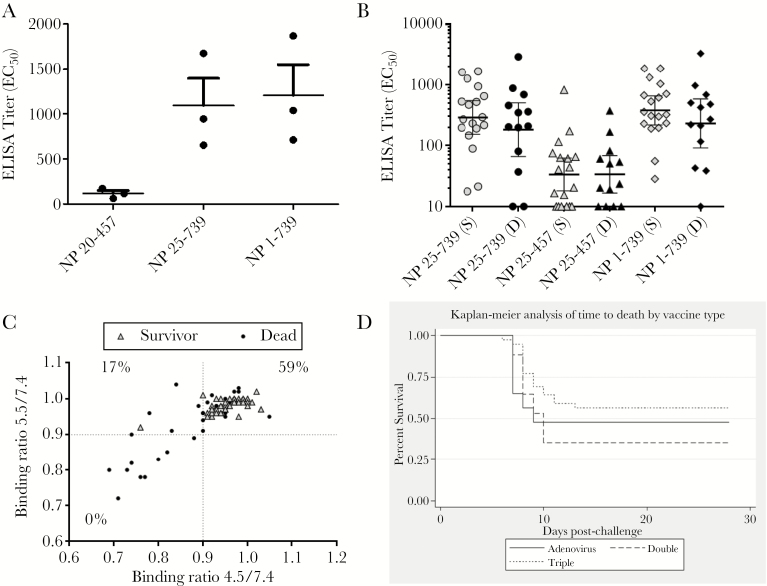
There was no correlation between survival and antibody titer to VP40 (P = .8186, unpaired t test) (Table 1). The requirement for higher anti-GP titer for survival in double VLP-vaccinated animals suggested that, in the triple VLP-vaccinated animals, antibody or T-cell response to NP may be contributing to the protection. Due to unavailability of peripheral blood mononuclear cells from these animals, we were unable to evaluate T-cell responses. However, we tested the antibody responses to NP in the sera from the 3 triple VLP-vaccinated NHPs using an NP ELISA assay. For this purpose, we used 3 variations of NP proteins fused to maltose binding protein as coating antigen: NP25-457, NP25-739, and full-length NP1-739. Deletion of the first 24 residues in Ebola NP decreases the ability of NP to oligomerize [32], whereas constructs ending at residue 457 represent the shared domain organization of negative sense ribonucleic acid virus-specific region, and the NP458-739 is the filoviral-specific region [33]. As shown in Figure 3A, the animals showed high antibody titers to the full-length and NP25-739 but very low titers against NP25-457, suggesting that the response is primarily directed against the filoviral specific C-terminal domain of NP.
Figure 3.
(A) The immunoglobulin G response to 3 nucleoprotein (NP) constructs from the 3 animals in study IBT_01220 vaccinated with triple virus-like particles (VLPs). (B) Antibody response of all triple VLP-vaccinated animals listed in Table 2, stratified by survivors (S) and dead (D). (C) Binding of the sera was determined at pH 4.5 or 5.5 relative to binding at pH 7.4 by enzyme-linked immunosorbent assay (ELISA) and plotted against each other. Sera are stratified based on survival from challenge. The numbers in quadrants represent percentage of survival for each quadrant. (D) Kaplan–Meier survival curve of the animals in the 3 vaccine groups.
Serological Analysis of Historical Nonhuman Primate Sera
To further evaluate the protective role of GP-specific antibody responses against EVD, we sought to determine the antibody titers in a larger number of sera from vaccinated NHPs in previous studies. Sera from several studies using triple VLP vaccines (Table 2) and sera from a vaccine study using GP-expressing adenovirus along with adenovirus-expressed interferon alpha, a vaccine known to induce strong T-cell responses [34] (Supplementary Table S.3), were collected for this analysis. We determined the antibody titers to GP∆TM, GP∆Muc, VP40, and NP (for triple VLP samples) in these sera (Table 2 and Supplementary Table S.3). Furthermore, we tested these sera for neutralization of rVSV-GP pseudotype assay. Due to limited amount of sera available, we were unable to perform neutralization on samples from the adenovirus vaccine study, as well as 3 NHP samples from VLP studies (A0023, AP690, and 201033).
Table 2.
Summary of the Study Designs and Antibody Titers of NHP Vaccinated With Tripe VLP (GP/VP40/NP) Used for Statistical Analysisa
| Study IDb | NHP ID | Day of Death | eVLP | mVLP | Antibody Titers (AU/mL) | VLP Source |
Adjuvant | No. of Vaccinations | Vaccination Schedule (Week) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GP∆TM | GP∆Muc | VP40 | NP 1–739 |
NP 25–457 |
NP 25–739 |
||||||||||
| VLP No. 2 | C250B | Survived | x | 920 | 2147 | 355 | 670 | 10 | 525 | Mam. | RIBI | Two | Week 0 and 6 | Unpublished | |
| 305B | 9 | x | 427 | 720 | 102 | 686 | 10 | 692 | |||||||
| E358A | 7 | x | 219 | 452 | 531 | 10 | 10 | 10 | |||||||
| VLP No. 3 | C573 | Survived | x | 573 | 858 | 433 | NT | NT | NT | Mam. | RIBI | Three | Week 0, 6, 12 | Unpublished | |
| 91–495 | Survived | x | 702 | 706 | 884 | NT | NT | NT | |||||||
| 120–334 | 11 | x | 281 | 382 | 173 | NT | NT | NT | |||||||
| VLP No. 5 | C0167 | Survived | x | x | 815 | 1322 | 1897 | 234 | 44 | 147 | Mam. | RIBI | Three | Week 0, 6, 12 | [19] |
| C0219 | Survived | x | x | 1245 | 2052 | 1476 | 196 | 20 | 191 | ||||||
| C0379 | Survived | x | x | 896 | 1004 | 2345 | 308 | 17 | 217 | ||||||
| C0548 | Survived | x | x | 370 | 451 | 626 | 323 | 10 | 270 | ||||||
| C1019 | Survived | x | x | 738 | 1262 | 1081 | 228 | 62 | 197 | ||||||
| VLP No. 7 | 304021 | Survived | x | x | 229 | 1803 | 3663 | 357 | 40 | 231 | BV/IC | RIBI | Three | Week 0, 6, 12 | Unpublished |
| 204005 | Survived | x | x | 249 | 1530 | 4515 | NT | NT | NT | ||||||
| 201033 | 13 | x | x | 424 | 2105 | 4644 | NT | NT | NT | ||||||
| 104003 | Survived | x | x | 593 | 2653 | 4201 | NT | NT | NT | BV/IC | QS-21 | ||||
| 201007 | 10 | x | x | 351 | 1506 | 3202 | 969 | 373 | 876 | ||||||
| 201013 | 8 | x | x | 424 | 465 | 4402 | 271 | 10 | 202 | ||||||
| VLP No. 9 | 502090 | Survived | x | x | 93 | 441 | 1379 | 28 | 10 | 21 | BV/IC | QS-21 | One | [5] | |
| 502084 | 6 | x | x | 19 | 108 | 3244 | 43 | 19 | 37 | ||||||
| 504060 | Survived | x | x | 117 | 332 | 3082 | 1321 | 10 | 1267 | ||||||
| J16 | Survived | x | x | 387 | 1761 | 7373 | 616 | 75 | 531 | BV/IC | QS-21 | Two | Week 0, 6 | ||
| 112713 | Survived | x | x | 320 | 1279 | 9077 | 299 | 10 | 293 | ||||||
| A0023 | Survived | x | x | 358 | 2515 | 7337 | 1872 | 824 | 1616 | ||||||
| A0024 | Survived | x | x | 798 | 1991 | 9760 | 56 | 10 | 18 | BV/IC | QS-21 | Three | Week 0, 6, 12 | ||
| 302557 | Survived | x | x | 356 | 1483 | 13 684 | 532 | 65 | 470 | ||||||
| 206345 | 11 | x | x | 847 | 2207 | 5276 | 3293 | 168 | 2906 | ||||||
| 208411 | 9 | x | x | 256 | 1130 | 17 577 | 39 | 82 | 10 | BV/IC | Alum | Two | Week 0, 6 | Unpublished | |
| 204225 | Survived | x | x | 281 | 983 | 18 895 | 190 | 15 | 89 | ||||||
| 107309 | 9 | x | x | 124 | 387 | 18 250 | 117 | 54 | 80 | ||||||
| 206517 | 8 | x | x | 102 | 437 | 14 305 | NT | NT | NT | BV/IC | Alum | Three | Week 0, 6, 12 | ||
| 202419 | 8 | x | x | 501 | 987 | 4734 | NT | NT | NT | ||||||
| 204535 | 8 | x | x | 405 | 1320 | 15 036 | 424 | 23 | 349 | ||||||
| IBT_00534 | GA759 | 8 | x | x | 55 | 91 | 1376 | 503 | 20 | 455 | BV/IC | QS-21 | Two | Week 0, 3 | Unpublished |
| AR601 | 8 | x | x | 43 | 99 | 2537 | 221 | 10 | 198 | ||||||
| GA689 | 10 | x | x | 21 | 35 | 1418 | 212 | 61 | 208 | ||||||
| AP690 | 8 | x | x | 4 | 10 | 2710 | 478 | 50 | 360 | ||||||
| IBT_01220 | AR791 | Survived | x | 1553 | 1057 | 2842 | 1038 | 172 | 944 | BV/IC | QS-21 | Two | Week 0, 6 | This report | |
| AP229 | Survived | x | 412 | 770 | 3461 | 1869 | 63 | 1675 | |||||||
| AR920 | Survived | x | 1324 | 1267 | 2258 | 711 | 116 | 652 | |||||||
Abbreviations: BV, baculovirus; eVLP, Ebola virus-like particles; IC, insect cell expression system; Mam., mammalian expression system; mVLP, Marburg virus-like particles; NT, not tested; USAMRIID, US Army Medical Research Institute of Infectious Diseases; UTMB, University of Texas Medical Branch; VLP, virus-like particles.
aAntibody titers are from the last bleed before challenge.
bStudies with VLP designation were entirely performed at USAMRIID. Studies with IBT designation were performed at Covance (vaccination) and UTMB (challenge).
Because the GP-receptor interactions occur in the endosomes [35–37], the ability of antibodies to effectively neutralize the virus may relate to the stability of the GP-antibody interaction at acidic pH. To address this question, we also examined the ability of the sera to bind to GP at pH 5.5 and 4.5.
A cocktail of 3 mAbs known as ZMapp has shown remarkable efficacy in NHPs [14]. Induction of antibodies that target the epitopes recognized by ZMapp [38] may be critical for vaccine efficacy. To examine this hypothesis, we also performed a competition ELISA to determine the relative presence of the murine versions of ZMapp component antibodies in the vaccinated NHP sera. These data were subjected to statistical analysis to examine potential correlation with protection from lethal challenge as described below.
Analysis of Survival Outcome
The summary of the survival outcomes by vaccine type is shown in Supplementary Table S.4. As shown in Table 3, overall, titers for GP∆TM and GP∆Muc were significantly higher (P < .001) in surviving NHPs compared with NHPs that died; no significant difference in VP40 titers was observed (P > .05). Analysis of sera from triple VLP-vaccinated animals showed no significant difference in NP titers between survivors and dead animals (Figure 3B). Results from the t test were consistent with those from the non-parametric Wilcoxon rank-sum test for titer endpoints. Binding ratio at pH 4.5/7.4 was significantly higher (P < .001) in surviving NHPs compared with NHPs who died; however, the binding ratio at pH 5.5/7.4 was only marginally higher in the surviving NHPs (P = .05, Wilcoxon rank sum) (Table 3). As shown in Figure 3C, sera from all but 1 surviving animal (38 of 39) maintained more than 90% of binding to GP at pH of 5.5 or less, whereas 26 of 40 fatal cases showed the same property. Overall, 59% of sera that maintained over 90% binding at both pH 4.5 and 5.5 were from survivors. In contrast, only 17% of the animals with >10% loss of binding at pH 4.5 despite >90% binding at pH 5.5 survived the challenge. None of the 10 animals whose sera lost more than 10% binding at both acidic pH values survived the challenge. These data suggest that the ability of the antibodies to bind GP at low pH is important for protection. Percentage of displacement of ZMapp and percentage of neutralization was significantly higher in surviving NHPs compared with NHPs who died (P < .05 and P < .001, respectively) (Table 3). Of note, some serum samples increased ZMapp binding resulting in negative displacement values.
Table 3.
Summary Statistics by Survival Outcome
| Variable | Survival Outcome | N | Mean (SD) | Median | Range |
P Value |
|---|---|---|---|---|---|---|
| Ebola GP-∆TM titer | Alive | 39 | 1062.34 (881.00) | 798.15 | 93.21–3879.08 | <.001a <.001b |
| Dead | 39 | 337.49 (343.62) | 202.51 | 4.45–1391.59 | ||
| Overall | 78 | 699.91 (757.87) | 425.47 | 4.45–3879.08 | ||
| Ebola GP-∆Muc titer | Alive | 39 | 1253.00 (790.20) | 1218.49 | 28.56–2764.40 | <.001a <.001b |
| Dead | 39 | 582.80 (622.40) | 437.11 | 2.29–2207.26 | ||
| Overall | 78 | 917.90 (782.99) | 751.01 | 2.29–2764.40 | ||
| Ebola VP40 titer | Alive | 28 | 4351.16 (4299.68) | 2961.99 | 354.90–18 895.37 | .904a .544b |
| Dead | 28 | 5202.59 (5168.83) | 3568.98 | 101.79–18 250.06 | ||
| Overall | 56 | 4776.87 (4730.29) | 3352.22 | 101.79–18 895.37 | ||
| Binding ratio pH 4.5/7.4 | Alive | 39 | 0.95 (0.044) | 0.95 | 0.76–1.03 | <.001a <.001b |
| Dead | 40 | 0.89 (0.091) | 0.92 | 0.69–1.05 | ||
| Overall | 79 | 0.92 (0.078) | 0.94 | 0.69–1.05 | ||
| Binding ratio pH 5.5/7.4 | Alive | 39 | 0.98 (0.020) | 0.98 | 0.92–1.02 | .003a .050b |
| Dead | 40 | 0.94 (0.080) | 0.96 | 0.72–1.04 | ||
| Overall | 79 | 0.96 (0.062) | 0.98 | 0.72–1.04 | ||
| %Displacement of ZMapp | Alive | 39 | 8.17 (16.70) | 7.49 | −42.95 to 42.74 | .014c .015b |
| Dead | 40 | −1.44 (17.13) | −3.50 | −47.27 to 30.41 | ||
| Overall | 79 | 3.30 (17.50) | 5.75 | −47.27 to 42.74 | ||
| %Neutralization | Alive | 27 | 64.95 (20.06) | 71.3 | 27.3–95.2 | <.001c <.001b |
| Dead | 26 | 38.29 (25.86) | 37.35 | −27.4 to 85.7 | ||
| Overall | 53 | 51.87 (26.53) | 56.4 | −27.4 to 95.2 |
Abbreviation: SD, standard deviation.
aTwo-sample, 2-sided t test for log-transformed values.
bWilcoxon rank-sum test.
cThe t test is performed on untransformed values.
For each vaccine type, titers for GP∆TM and GP∆Muc were significantly higher in surviving NHPs compared with NHPs who succumbed to infection (P < .01) (Table 4). Overall, the antibody response to GP tends to be higher in double VLP-vaccinated animals. Among surviving animals vaccinated with adenovirus-based vaccine, the anti-GP∆TM titers were significantly higher than the response to GP∆Muc (P < .05) (Supplementary Table S.5). In surviving animals vaccinated with double VLP, the anti-GP∆TM response was slightly but not significantly lower (P > .05); in triple VLP-vaccinated survivors, response was significantly lower (P < .001) than GP∆Muc. No significant difference in VP40 or NP titers was observed (P > .05) (Figure 3B and Table 4).
Table 4.
Summary Statistics by Vaccine Type and Survival Outcome
| Vaccine Type | Variable | Survival Outcome | N | Mean (SD) | Median | Range |
P Value |
|---|---|---|---|---|---|---|---|
| Adenovirus | Ebola GP-∆TM | Alive | 11 | 1492.76 (1109.60) | 1251.5 | 275.64–3879.08 | <.001a <.001b |
| Dead | 11 | 101.62 (64.35) | 96.01 | 16.66–202.51 | |||
| Overall | 22 | 797.19 (1046.48) | 239.08 | 16.66–3879.08 | |||
| Ebola GP-∆Muc | Alive | 11 | 551.95 (484.64) | 408.44 | 28.56–1551.47 | <.001a <.001b |
|
| Dead | 11 | 41.50 (28.09) | 35.84 | 2.29–94.1 | |||
| Overall | 22 | 296.73 (424.81) | 80.48 | 2.29–1551.47 | |||
| Double | Ebola GP-∆TM | Alive | 6 | 1947.14 (711.02) | 1974.40 | 970.10–3069.28 | .003a .002b |
| Dead | 11 | 685.53 (397.83) | 634.29 | 34.27–1391.59 | |||
| Overall | 17 | 1130.80 (801.94) | 938.10 | 34.27–3069.28 | |||
| Ebola GP-∆Muc | Alive | 6 | 2187.98 (544.87) | 2288.68 | 1218.49–2764.40 | .002a .002b |
|
| Dead | 11 | 893.67 (461.94) | 732.49 | 199.23–1814.64 | |||
| Overall | 17 | 1350.48 (795.38) | 1044.53 | 199.23–2764.40 | |||
| Ebola VP40 | Alive | 6 | 3534.51 (2108.72) | 3428.44 | 1381.94–6606.422 | .958a .763b |
|
| Dead | 11 | 4196.16 (2878.17) | 3592.58 | 350.36–10 024.99 | |||
| Overall | 17 | 3962.64 (2583.26) | 3592.58 | 350.36–10 024.99 | |||
| %Neutralization | Alive | 6 | 0.74 (0.225) | 0.81 | 0.30–0.92 | .011c .012b |
|
| Dead | 11 | 0.39 (0.223) | 0.40 | 0.01–0.67 | |||
| Overall | 17 | 0.51 (0.279) | 0.58 | 0.01–0.92 | |||
| Triple | Ebola GP-∆TM | Alive | 22 | 605.82 (398.47) | 492.5 | 93.21–1552.93 | .004a .006b |
| Dead | 17 | 264.90 (227.58) | 256.22 | 4.45–847.34 | |||
| Overall | 39 | 457.22 (372.67) | 370.28 | 4.45–1552.93 | |||
| Ebola GP-∆Muc | Alive | 22 | 1348.521 (656.19) | 1273.30 | 331.79–2652.72 | .002a .008b |
|
| Dead | 17 | 731.90 (703.32) | 452.36 | 10.28–2207.26 | |||
| Overall | 39 | 1079.74 (736.34) | 1003.85 | 10.28–2652.72 | |||
| Ebola VP40 | Alive | 22 | 4573.88 (4740.05) | 2961.99 | 354.90–18 895.37 | .956a .630b |
|
| Dead | 17 | 5853.80 (6225.77) | 3243.74 | 101.79–18 250.06 | |||
| Overall | 39 | 5131.79 (5399.08) | 3201.5 | 101.79–18 895.37 | |||
| NP | Alive | 18 | 602.66 (568.80) | 340.3 | 28.4–1872 | .335a .337b |
|
| Dead | 13 | 558.70 (867.89) | 270.5 | 10–3293 | |||
| Overall | 31 | 584.23 (696.50) | 323.2 | 10–3293 | |||
| %Neutralization | Alive | 21 | 0.62 (0.191) | 0.64 | 0.27–0.95 | .010c .010b |
|
| Dead | 15 | 0.38 (0.289) | 0.36 | −0.27 to 0.86 | |||
| Overall | 36 | 0.52 (0.263) | 0.55 | −0.27 to 0.95 |
Abbreviations: NP, nucleoprotein; SD, standard deviation.
aTwo-sample, 2-sided t test for log-transformed values.
bWilcoxon rank-sum test.
cThe t test is performed on untransformed values.
Overall, the time to death was shorter for NHPs immunized with adenovirus or double VLP vaccine, with longer times to death observed in NHPs treated with triple VLP vaccine, as illustrated in Figure 3D. Median times to death and corresponding 95% confidence intervals are shown in Supplementary Table S.6; however, due to the low number of NHPs with observed deaths in the follow-up period, the median is not calculable for triple VLP vaccine, and upper 95% confidence intervals are not calculable overall and for all vaccine types.
Cox-proportional hazards models were fitted to the time to death data for each vaccine type and titer endpoint separately, as well as by titer endpoint overall (Table 5). In general, the risk of death is significantly reduced for each 1-unit increase in log GP∆TM or log GP∆Muc titer (P < .01). The risk of death does not appear to be impacted by changes in VP40 or NP titer (P > .05).
Table 5.
Survival Analysis Results by Vaccine Type: Cox Proportional Hazards Model
| Vaccine Type | Variable | Hazard Ratioa | 95% Confidence Interval | P Valueb |
|---|---|---|---|---|
| Adenovirus | Ebola GP-∆TM | 0.39 | 0.22–0.68 | .001 |
| Ebola GP-∆Muc | 0.35 | 0.17–0.74 | .006 | |
| Double | Ebola GP-∆TM | 0.51 | 0.31–0.84 | .008 |
| Ebola GP-∆Muc | 0.05 | 0.01–0.34 | .003 | |
| Ebola VP40 | 0.89 | 0.14–5.47 | .897 | |
| Triple | Ebola GP-∆TM | 0.57 | 0.42–0.78 | <.001 |
| Ebola GP-∆Muc | 0.30 | 0.15–0.61 | .001 | |
| Ebola VP40 | 1.04 | 0.42–2.56 | .928 | |
| Ebola NP | 0.71 | 0.46–1.11 | .132 | |
| Overall | Ebola GP-∆TM | 0.57 | 0.47–0.70 | <.001 |
| Ebola GP-∆Muc | 0.43 | 0.30–0.62 | <.001 | |
| Ebola VP40 | 1.05 | 0.49–2.27 | .894 |
aHazard ratio per 1 unit increase in the log-transformed variable.
bLog-rank test.
DISCUSSION
Several lines of evidence suggest that antibodies against EBOV GP play a critical role in protection against EVD. Multiple mAbs and antibody cocktails have been reported to protect against EBOV as well as other filoviruses [14, 16, 39–42]. A previous analysis of 54 macaques vaccinated with various adenovirus constructs expressing GP or GP plus NP showed a strong correlation between total anti-GP IgG titer and survival [30]. However, this study does not define a clear cutoff separating survivors from fatal cases, and, despite statistically significant correlations, the spectrum of IgG response among survivors and fatal cases are largely overlapping, suggesting that other factors, such as CMI, play a role in protection. It is also likely that qualitative attributes of the antibody response that cannot be captured in a total antibody ELISA are important for protection.
Here, we report a comparative study using a VLP-based vaccine known to induce high NP-directed T-cell responses (triple VLP) [6] with double VLPs lacking NP. In the absence of NP, the vaccine induced higher levels of anti-GP antibody, and higher IgG levels were needed for protection compared with the triple VLP. It remains to be determined why VLPs lacking NP induced higher anti-GP titers. A previous studies indicated that although expression of NP increases the rate of VLP release [27], the overall morphology of VLPs is not affected by coexpression of NP [43]; however, to our knowledge, it is not known whether NP affects the density of GP spikes on the surface of particles. Because the antibody titers are lower in triple VLP-vaccinated NHPs (Table 4 and Supplementary Table S5), the increased efficacy is most likely related to NP-mediated T-cell responses. In a larger cohort of macaques vaccinated with VLPs or adenovirus-expressed GP, we confirmed significant correlation between anti-GP titer and survival irrespective of the platform, whereas survival did not correlate with IgG titers for VP40 or NP. Although a cutoff for protection based on anti-GP titers could not be defined for triple VLP and adenovirus vaccines, all of the double VLP-vaccinated survivors had an antibody titer of >1900 AU/mL against GP∆Muc except for a single animal, which was very sick through day 12 but eventually survived.
The current studies expand beyond any other published studies to date by examining qualitative attributes of the antibody response that correlate with protection. Our data show that neutralization of rVSV-GP, as well as the ability of the antibodies to bind to GP at acidic pH, correlate with survival. Filoviruses use macropinocytosis to enter the acidic environment of the endosomes, where GP is proteolytically cleaved by cathepsins, exposing the receptor-binding site of GP to interact with its endosomal receptor Nieman Pick C 1 (NPC-1). Triggering the productive fusion of viral and endosomal membranes is dependent on this cleavage and additional low pH-dependent events [44, 45]. Therefore, the ability to bind at acidic pH is an important attribute of neutralizing antibodies, specifically those targeting the viral fusion mechanism, as we and others have recently reported [42, 46]. Our current data show that vaccine induced antibodies from the surviving macaques bind GP much more strongly at acidic pH compared with animals that succumb to infection.
ZMapp, a cocktail of 3 mAbs that target the glycan cap and the base of EBOV GP, protects NHPs from lethal EBOV challenge. To evaluate whether vaccine-mediated protection correlates with the ability of the vaccine to induce ZMapp-like antibodies, we evaluated the ability of the immune sera to displace components of ZMapp. These data showed that survivors had a higher titer of antibodies that compete with ZMapp. Some of the sera increased binding of ZMapp components to GP. Among survivors, only sera from 1 of 39 animals showed >20% increase in ZMapp binding, whereas this was observed in 4 of 40 sera from lethal cases. It is possible that binding of certain classes of antibodies may have an allosteric effect on binding to these epitopes. This would be consistent with our recent report showing cooperative binding between 2 classes of anti-EBOV mAbs [41].
Our findings suggest that the quality of antibody response is critically important. In a recent study, Khurana et al [47] performed a comprehensive analysis of the antibody responses to the rVSVΔG-ZEBOV-GP vaccine tested in a phase I clinical trial. These data showed that the response was dominated by antibodies against MLD, a highly glycosylated domain believed to mask key neutralizing epitopes [48]. In contrast, the study shows poor to moderate response among vaccinees to the regions of GP2 encompassing the internal fusion loop and the N terminus of GP1 that forms the base of GP trimer along with GP2. Nonetheless, the success of VSV-based EBOV vaccines suggests that sufficient protective antibodies are elicited and/or a major involvement of CMI responses. These protective antibodies could include antibodies binding to MLD that can activate antiviral effector functions as previously proposed [49]. In the current study, we evaluated antibody responses to both full-length GP∆TM and MLD-deleted GP (GP∆Muc). Antibody titers to GP∆TM was significantly higher than GP∆Muc in adenovirus-vaccinated animals, suggesting that a large portion of these antibodies may be MLD-specific. In contrast, VLP-vaccinated survivors showed higher titers against GP∆Muc than GP∆TM, suggesting that the antibody response was not skewed towards MLD.
CONCLUSIONS
In summary, although lack of data on T-cell responses of these animals remains a limitation of our study, our findings provide strong evidence that antibodies to GP can be used as a reliable correlate of protective immune response in NHPs. This study further emphasizes the importance of evaluating the qualitative attributes of the antibody response. As more protective epitopes are being identified, it is important that future studies focus on the analysis of the epitope-specific antibody profiles. Obtaining a comprehensive understanding of the correlates of protective antibody response in NHPs is critically important for development of EBOV vaccines under US Food and Drug Administration Animal Rule and will also support efforts to define immune correlates to predict efficacy in humans.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank D. Negley, S. VanTongeren, K. Stuthman, D. Reed and J. Wells for excellent technical assistance, Larry Zeitlin for provision of ZMapp; and D. Levey, C. Kensil, and S. Monks of Agneus, Inc. for provision of QS-21 adjuvant.
Financial support. This work was funded in part by National Institute of Allergy and Infectious Diseases contract no. HHSN272200800055C and grant nos. R01AI126587 and R01AI132204 (to M. J. A.) and PO1 AI120943 (to G. K. A.). This work was also partially funded by the Joint Science Technology/Defense Threat Reduction Agency and Medical Countermeasure Systems/Joint Program Executive Office (to S. B.).
Potential conflicts of interest. M. J. A. and F. W. H. have stocks or stock options in Integrated Biotherapeutics. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Henao-Restrepo AM, Camacho A, Longini IM, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Santis O, Audran R, Pothin E, et al. . Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 2016; 16:311–20. [DOI] [PubMed] [Google Scholar]
- 3. Ledgerwood JE, DeZure AD, Stanley DA, et al. . Chimpanzee adenovirus vector Ebola vaccine. N Engl J Med 2017; 376:928–38. [DOI] [PubMed] [Google Scholar]
- 4. Ewer K, Rampling T, Venkatraman N, et al. . A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med 2016; 374:1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warfield KL, Dye JM, Wells JB, et al. . Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PLoS One 2015; 10:e0118881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis 2007; 196(Suppl 2):S430–7. [DOI] [PubMed] [Google Scholar]
- 7. Johnson RF, Kurup D, Hagen KR, et al. . An inactivated rabies virus-based Ebola vaccine, FILORAB1, adjuvanted with glucopyranosyl lipid A in stable emulsion confers complete protection in nonhuman primate challenge models. J Infect Dis 2016; 214:342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herbert AS, Kuehne AI, Barth JF, et al. . Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J Virol 2013; 87:4952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mire CE, Matassov D, Geisbert JB, et al. . Single-dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature 2015; 520:688–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, et al. . Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine 2008; 26:6894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mire CE, Geisbert JB, Marzi A, Agans KN, Feldmann H, Geisbert TW. Vesicular stomatitis virus-based vaccines protect nonhuman primates against Bundibugyo ebolavirus. PLoS Negl Trop Dis 2013; 7:e2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warfield KL, Aman MJ. Advances in virus-like particle vaccines for filoviruses. J Infect Dis 2011; 204(Suppl 3):S1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dye JM, Herbert AS, Kuehne AI, et al. . Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A 2012; 109:5034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qiu X, Wong G, Audet J, et al. . Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014; 514:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qiu X, Audet J, Wong G, et al. . Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci Rep 2013; 3:3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pettitt J, Zeitlin L, Kim do H, et al. . Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med 2013; 5:199ra13. [DOI] [PubMed] [Google Scholar]
- 17. Warfield KL, Olinger G, Deal EM, et al. . Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol 2005; 175:1184–91. [DOI] [PubMed] [Google Scholar]
- 18. Warfield KL, Bosio CM, Welcher BC, et al. . Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A 2003; 100:15889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Warfield KL, Posten NA, Swenson DL, et al. . Filovirus-like particles produced in insect cells: immunogenicity and protection in rodents. J Infect Dis 2007; 196(Suppl 2):S421–9. [DOI] [PubMed] [Google Scholar]
- 20. Wilson JA, Bray M, Bakken R, Hart MK. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology 2001; 286:384–90. [DOI] [PubMed] [Google Scholar]
- 21. Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine 2005; 23:3033–42. [DOI] [PubMed] [Google Scholar]
- 22. Bavari S, Bosio CM, Wiegand E, et al. . Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med 2002; 195:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swenson DL, Warfield KL, Kuehl K, et al. . Generation of Marburg virus-like particles by co-expression of glycoprotein and matrix protein. FEMS Immunol Med Microbiol 2004; 40:27–31. [DOI] [PubMed] [Google Scholar]
- 24. Draize JH, Alvarez E, Whitesell MF. Toxicity and primary irritation of some chemical compounds following oral administration and skin application. Fed Proc 1946; 5:174. [PubMed] [Google Scholar]
- 25. Vu H, Shulenin S, Grolla A, et al. . Quantitative serology assays for determination of antibody responses to Ebola virus glycoprotein and matrix protein in nonhuman primates and humans. Antiviral Res 2016; 126:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Howell KA, Qiu X, Brannan JM, et al. . Antibody treatment of Ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep 2016; 15:1514–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kallstrom G, Warfield KL, Swenson DL, et al. . Analysis of Ebola virus and VLP release using an immunocapture assay. J Virol Methods 2005; 127:1–9. [DOI] [PubMed] [Google Scholar]
- 28. Licata JM, Johnson RF, Han Z, Harty RN. Contribution of ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J Virol 2004; 78:7344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sullivan NJ, Geisbert TW, Geisbert JB, et al. . Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 2003; 424:681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sullivan NJ, Martin JE, Graham BS, Nabel GJ. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol 2009; 7:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilson JA, Hart MK. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J Virol 2001; 75:2660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leung DW, Borek D, Luthra P, et al. . An intrinsically disordered peptide from Ebola virus VP35 controls viral RNA synthesis by modulating nucleoprotein-RNA interactions. Cell Rep 2015; 11:376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirchdoerfer RN, Wasserman H, Amarasinghe GK, et al. . Filovirus structural biology: the molecules in the machine. Curr Top Microbiol Immunol 2017; 411:381–417. [DOI] [PubMed] [Google Scholar]
- 34. Richardson JS, Yao MK, Tran KN, et al. . Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine. PLoS One 2009; 4:e5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hunt CL, Lennemann NJ, Maury W. Filovirus entry: a novelty in the viral fusion world. Viruses 2012; 4:258–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nanbo A, Imai M, Watanabe S, et al. . Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog 2010; 6:e1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saeed MF, Kolokoltsov AA, Albrecht T, et al. . Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog 2010; 6:e1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murin CD, Fusco ML, Bornholdt ZA, et al. . Structures of protective antibodies reveal sites of vulnerability on Ebola virus. Proc Natl Acad Sci U S A 2014; 111:17182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flyak AI, Ilinykh PA, Murin CD, et al. . Mechanism of human antibody-mediated neutralization of Marburg virus. Cell 2015; 160:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flyak AI, Shen X, Murin CD, et al. . Cross-reactive and potent neutralizing antibody responses in human survivors of natural Ebolavirus infection. Cell 2016; 164:392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Howell KA, Brannan JM, Bryan C, et al. . Cooperativity enables non-neutralizing antibodies to neutralize Ebolavirus. Cell Rep 2017; 19:413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao X, Howell KA, He S, et al. . Immunization-elicited broadly protective antibody reveals ebolavirus fusion loop as a site of vulnerability. Cell 2017; 169:891–904 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson RF, Bell P, Harty RN. Effect of Ebola virus proteins GP, NP and VP35 on VP40 VLP morphology. Virol J 2006; 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aman MJ. Chasing Ebola through the endosomal labyrinth. MBio 2016; 7:e00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spence JS, Krause TB, Mittler E, Jangra RK, Chandran K. Direct visualization of Ebola virus fusion triggering in the endocytic pathway. MBio 2016; 7:e01857–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wec AZ, Herbert AS, Murin CD, et al. . Antibodies from a human survivor define sites of vulnerability for broad protection against Ebolaviruses. Cell 2017; 169:878–90.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khurana S, Fuentes S, Coyle EM, Ravichandran S, Davey RT Jr, Beigel JH. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med 2016; 22:1439–47. [DOI] [PubMed] [Google Scholar]
- 48. Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 2008; 454:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilson JA, Hevey M, Bakken R, et al. . Epitopes involved in antibody-mediated protection from Ebola virus. Science 2000; 287:1664–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.