ABSTRACT
Cataract is the leading cause of blindness globally with surgery being the only form of treatment. But cataract surgery is accompanied by complications, chiefly intra-ocular infections. Hence, preventive nanoformulations may be extremely beneficial. In the present study, novel chitosan-coated liposomal formulations encapsulating a combination of drugs, lanosterol and hesperetin were prepared and characterized. The combinatorial liposomes were prepared by thin film evaporation active extrusion method. The characterization of liposomes was done by transmission electron microscopy, zeta potential, encapsulation efficiency, stability, cytotoxicity and in vitro release studies. The main difference between the chitosan-coated and uncoated combinatorial liposomes is the release of drugs as indicated by the in vitro release studies. The slow and sustained release of the drugs from chitosan-coated ones as against the burst release from uncoated indicates an increased retention time for combinatorial drugs in cornea. This leads to a delay in progression of cataract as seen from in vivo studies. Cytotoxicity studies indicate no cell toxicity of the coating of chitosan or the combination of drugs. Stability studies indicate that there were almost no changes in size, zeta potential and polydispersity index values of the combinatorial liposomes upon storage at room temperature for 60 days. Another important study is the estimation of antioxidant defense system. The estimated values of glutathione reductase, malondialdehyde and chief antioxidant enzymes point toward an upregulation of antioxidant defense system. From the results, it may be concluded that novel chitosan-coated combinatorial liposomes are effective in delaying or preventing of cataract.
KEYWORDS: Cataract, chitosan, liposomes, combinatorial, antioxidant, rat
1. Introduction
More than half the cases of blindness across the world are a result of cataract which is a serious condition of opacification of lenses caused by protein aggregation [1,2]. Transparency and refraction of lens is a cumulative effect of the crystalline protein present in high concentrations [3]. The chief method of treatment available today for cataract is surgery which, although is very successful, is associated with significant cost and several other morbidities. The major disadvantage of the eye drops for quick treatment or prevention of cataract is corneal impermeability as a result of electrostatic repulsion on the surface of eye, which also causes unavailability of ocular drug. Therefore in order to prevent cataract altogether, novel nanoformulations of drugs may prove beneficial. Zhao in his landmark research discussed the efficiency of a drug named lanosterol which reduces aggregation of protein in lenses thereby reducing formation of cataract [1]. This discovery comes as tremendous relief because of the disadvantages associated with surgery. However, recent research by Shangumam et al. [4] refutes the study by Zhao et al., indicating that lanosterol may not be completely active in curing cataract as effectively and fast as indicated. Hence, this in itself points to us the shortfalls of a single drug therapy for cataract impediment.
Combinatorial drug therapy along with nanotechnology has unlocked a huge box of prospects. These stable, biocompatible, biodegradable and non-imunogenic nanocarriers are able to encapsulate multiple drugs, leading to synergistic effects of these therapeutic agents on the affected lenses. These nanocarriers may be in the form of liposomes [5], cubosomes, niosomes, cyclodextrins, dendrimers, micelles, solid lipid nanoparticles, core shell nanoassemblies and silica particles [6]. Lipid components of the nanocarrier may interact with the lipid part of tear drop thereby enabling the drug to stay in the conjunctival area for an increased time where they may perform as drug storehouse [7–9].
The main challenge that lies in our study is in the modification of the combination of drugs for increased residence time in ocular surface, and increased bioavailability of the drugs [10–12]. It is also of greatest importance that the preparation and characterization of the combinatorial drug-loaded lipid nanocarrier may be carried out with utmost precision. The aims of the study were to prepare and characterize combinatorial drug-loaded liposomes for cataract prevention and treatment. The combination of drugs chosen was lanosterol and hesperetin, since lanosterol, a tetracyclic triterpenoid compound was already proposed as a cataract impediment drug by Zhao et al. [1] although refuted by Shangumam [4] for not being quick enough. Hesperetin, 4ʹmethoxy derivative of eriodictyol and a flavonoid, may perfectly synergize with lanosterol being a natural flavonoid and also is proven to be capable of inhibiting reactive oxygen species by activating the antioxidant enzymes, namely, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and glutathione (GSH) reductase [13–15]. These two drugs were encapsulated in a liposomal formulation and noted that with liposomal encapsulation there occur substantial changes in the drug functional properties like ability to invade the immune system and increased half-life. The surfaces of the liposome were modified to make them more stable and prolong retention in corneal region. The surfaces of the liposome were coated with N-trimethyl chitosan which is a derivative of chitosan. It has already been proved in studies that chitosan with low molecular weight possess advantages like enhanced time of retention and improved bioavailability [16–18]. Secondly, the liposomes were also characterized of their size, cytotoxicity, in vitro release studies and zeta potential measurements to note any significant changes in their properties as a result of the coating. The size of the liposome is a crucial parameter which aided in characterization of the same. The charge, particle size and surface hydration of the liposome are critical for their clearance from the blood stream. The nanocarriers were delivered at the site of induced cataract in Sprague Dawley albino rats and the changes in the lenses were photographed.
2. Materials and methods
Lanosterol, hesperetin, distearoylphosphatidylethanolamine (DSPE)-Polyethylene Glycol 2000, distearoylphosphatidylcholine (DSPC), cholesterol (CHOL) were supplied by Avanti Polar Lipids (Beijing, China). 4–2-Hydroxyethyl-1-piperazineethanesulfonic acid (HEPES) was obtained from Thermo Fisher Scientific, China.
Human ocular epithelial cell line was procured from China. Dulbecco’s minimum essential medium (DMEM), 2.5% fetal bovine serum, L-glutamine, penicillin and streptomycin were obtained from GibcoTM (Beijing, China). Live/dead cell assay kit was procured from Thermo Fisher Scientific, China. The chemicals utilized in this experiment were obtained from Sigma (Shanghai, China). N-trimethyl chitosan was obtained from Chitolife Co., Korea. The substances utilized in the study are of premier analytical standard. The substances were utilized as acquired without added refinement. Milli-Q water was used for all experiments.
2.1. Animals
Sprague Dawley albino rats (12 days old weighing 26–29 g) were procured from Sankyo Labo Service Corporation, Tokyo, Japan. The animals were housed in temperature controlled cages at 25ºC ±5ºC. Commercial rat feed was fed to the rats with water ad libitum. The Affiliated Hospital of Dali University small animals’ research committee approved all the procedures carried out in the study. The ethical clearance was obtained in March 2018.
2.2. Preparation of combinatorial liposome
The combinatorial liposomes were prepared by thin film evaporation method as per the description by Dhule et al. [19], Banerjee et al. [20], Gong et al. [21] and Pulford et al. [22] with suitable modifications. Active extrusion method was utilized where drugs are suspended with phospholipids in aqueous solution [6]. 1:1:10 (w/w) ratios were used to mix the hydrophilic drugs lanosterol and hesperetin and phospholipids. Lanosterol (0.011 g), hesperetin (0.011g) was dissolved in 0.5 ml phosphate buffered saline (PBS) followed by heating for 3 h at 50°C. Centrifugation at 2000 g for 10 min led to separation of supernatant drug mixture. The two phospholipids, DSPC, DSPE and cholesterol were then assorted in the ratio 2:2:1 (w/w). This benefit of this particular process lies in prolonging the circulation time of the liposome. DSPC/CHOL/DSPE liposomal formulation were synthesized by suspending 13.75 mg of lipid in 2 ml of chloroform and subsequently re-suspending in 2 ml of 400 mM citrate/5 mM phosphate buffer (pH 4.0). A weight of 0.05 g each of supernatant drug mixture and phospholipid were liquefied in 10 ml of 2:1 v/v mixture of chloroform and methanol. A rotary evaporator was utilized for 3 h to evaporate the solution and for 1 h with 5 ml of 1X PBS at 50°C and 125 rpm, hydration of the acquired dry lipid-co-drug films was carried out with suitable volume of strained HEPES buffered saline (10 mM HEPES and 150 mM NaCl, pH 7.0). Freezing and thawing was carried out for five cycles of and subsequently nine times emission was done through a 100 nm membrane at 65°C. Thereafter, the lipid suspension was obtained. Preparation of empty liposomes was carried out following the same procedure but without the liposomal preparation to be used as control to understand the consequence of phospholipids on cell lines.
2.3. Coating the combinatorial liposomes
The procedure was amended from Li et al. [23] with proper modifications as needed. Low molecular weight chitosan at the weight ratio of 1% was added slowly to 2% acetic acid solution. Agitation of the solution was carried for over 12 h and strained through paper filter to remove undissolved oversized particles. Chitosan coating was carried out by mixing 10 ml of combinatorial drug-loaded and empty liposomes into 10 ml chitosan solution under unceasing agitation for 12 h. Ultrasonication procedure for 5 min at 75 W was done to reduce the size alongside breaking chitosan bridges may form between liposomal droplets and chitosan. Uncoated combinatorial liposomes were also stored as positive control.
2.4. Zeta potential analysis
The size of the chitosan-coated combinatorial liposomes and their zeta potential was measured in Malvern ZetaSizer (Malvern Instruments Ltd., India). About 1 ml each of the chitosan-coated and uncoated combinatorial liposomes was assigned to the zeta cell and measurements were recorded. The experiment was performed at 25°C.
2.5. Transmission electron microscopy (TEM) studies for morphological analysis
TEM procedure was implemented from Li, Joung et al. [23], Li, Lee et al. [24] and Li, Shing et al. [25] with slight modifications. The investigations were conducted with a high resolution transmission electron microscopy (TEM, JEM-2010HR). Briefly, 1 ml each of the chitosan-coated and uncoated combinatorial liposomes was diluted with PBS for 10 times which was placed on the carbon-coated copper grid and kept for 2 min. These were then stained with phosphotungstic acid (2% diluted in distilled water). Additionally, the samples were air dried and incubated overnight before viewing the TEM captures. The protocol was modified from Huang et al. [26].
2.6. Storage stability and shelf life studies
Chitosan-coated and uncoated combinatorial liposomes were stored at room temperature around 30°C for 2 months (60 days). At the end of the 60 days, polydispersity index (PDI), zeta potential and size were calculated again to evaluate the stability and shelf life of both chitosan-coated and uncoated combinatorial liposomes.
2.7. In vitro release studies
Dialysis bags (pore size 5 nm) each were loaded separately with chitosan-coated and uncoated combinatorial liposomes at concentration of 7 mg in simulated tear fluid (150 ml). The release investigation began by insertion of the end-sealed dialysis bag into 150.0 ml of prepared simulation of tear fluid (pH – 7.4) at 37ºC with persistent pulsating. Controlled conditions were set under continuous stirring inside the water bath set at 37°C. One milliliter of solution was withdrawn at definite time interims of 2, 4, 6, 12, 24, 36, 48, 60 and 72 h. The medium was renewed each time after removal. Drug concentration in the samples was determined by UV-Vis spectroscopy at 419 nm as compared to standard as described by Anuchapreeda et al. [27].
2.7.1. Statistical analysis
Complete data in this research work were articulated as means and standard deviation (mean ±SD) and processed by Origin 8. Assessments were completed by means of the one-way analysis of variance (ANOVA). P value of <0.05 was deliberated as statistically significant difference.
2.8. Encapsulation efficiency of combinatorial drug-loaded liposomes
For determination of encapsulation efficiency of the combinatorial drugs-loaded liposomes, to a centrifuge tube of capacity 4 ml, 1 ml of solution of uncoated combinatorial liposomes and chitosan-coated liposomes was supplemented. For 1.5 h, samples were rotated at the rate of 100,000 g on ultracentrifuge. Drugs in supernatant and pellet were calculated utilizing UV spectroscopy. The absorbance of the drugs was calculated at different concentrations via UV spectroscopy. A standard curve for the combination of drugs was made by plotting absorbance and concentration. Using this curve, the concentration of non-encapsulated drugs in supernatant was determined.
The encapsulation efficiency defined as % encapsulation = (L/T) × 100, where L – concentration of drugs in liposome, T – total concentration of drugs in the liposome formulation, was calculated [28].
2.9. Cell culture and cytotoxicity test
2.9.1. Cell culture
Human ocular epithelial cell line was procured from China. Culturing of the cells was completed in DMEM in the prescribed conditions of 37° with 5% CO2 in a moist environment. 2.5% fetal bovine serum was added to the DMEM for cytotoxicity assays along with 2 mM L-glutamine, 50 UI/ml penicillin and 50 UI/ml streptomycin [20,29]. After every 3 days, media was renewed. Trypsin incubation was done to remove the cultures which attended confluency and the cells were seeded @300 μl per well into 96-well plates at a concentration of 106 cells/ml. Before exposed to the liposomal preparation, the cultures were retained at room temperature (37°C) for 1 day to permit the cells to attain confluency and attach to the well plates.
2.9.2. Cytotoxicity test
Cytotoxicity of chitosan-coated and uncoated combinatorial liposomes was evaluated according to Banerjee et al. [20] and Dou et al. [29] with appropriate modifications. Confluent cell cultures were obtained as mentioned in the procedure above. MTT assay was being used for the evaluation of in vitro cytotoxicity. Cells were harvested at 5 × 103 cells/well in 96-well plates and gestated for 72 h to make sure that cells are viable when the follow-ing were added; 0.1 ml of PBS (control), empty (0.1 mg/ml), uncoated combinatorial liposomes (0.1 mg/ml) and chitosan-coated combinatorial liposomes (0.1 mg/ml) were supplemented and incubation was done for 24 h. 0.1 mg/ml was chosen as the dosage since it is frequently used for ophthalmic formulations. Then, 20 μl of MTT solution (5 mg/ml) was supplemented in every well and incubation of the plate was done at 37°C for 4 h. The absorbance was calculated at 492 nm after the incubation by means of a microplate reader.
Cytocompatibility of PBS, empty liposomes, uncoated combinatorial liposomes and chitosan-coated combinatorial liposomes were also assessed by live/dead cell viability assay. Kit for live/dead cell assay was procured from Thermo Fisher Scientific, China. The procedure is followed as mentioned by the manufacturer.
2.10. In vivo studies: selenite-induced cataract and treatment with liposomes
Thirty rats (13 days old weighing 26–29 g) were divided in three groups of three rats each. Suckling rats 13 days old were seen to develop rapid bilateral nuclear cataracts upon injection of sodium selenite.
rats treated with empty liposomes (group 1)
rats treated with uncoated combinatorial liposomes (group 2)
rats treated with chitosan-coated combinatorial liposomes (group 3)
Group 1 was administered empty liposomes, group 2 was administered uncoated combinatorial liposomes and group 3 was administered chitosan-coated combinatorial liposomes at the rate of 2.5 µl/g bodyweight of the animal for 3 days intraperitoneally. The dosage was determined as per the protocol of Nakazawa [13] and Nahomi et al. [30]. Sodium selenite (Na2SeO3) 20 μmol/g bodyweight was introduced subcutaneously in all three groups on the first day after 4 h of liposome administration. On the sixth day, the rats (18 days old) lenses were observed through slit lamp microscopy when they first opened their eyes. The cataracts were graded on a scale of 1–6 as reported by Hiraoka et al. [31]. They were euthanized following an inhalation of overdose of 5% isoflurane.
The anterior part of the eyes (both) of the rat was cut just above the limbus using a scalpel and operating microscope for magnification. Very carefully the lens was excised after removing suspensory ligaments; extreme care was meted out to elude infection from external sources. Immediately, the lenses were enclosed in filter paper to eliminate the surrounding vitreous fluid.
The lenses isolated were immersed in 24-well culture plate containing 2 ml of DMEM supplemented with 20% fetal bovine serum, 100 μg/ml of streptomycin and 100 IU/ml penicillin. Incubation of the lenses were done at 37ºC under 90% moisture, 95% air and 5% CO2 gas atmosphere for 2 h. Thereafter, lenses were washed, weighed and processed for assessment of biochemical parameters. Homogenization of each lens was done in 1 ml of 0.1 M-phosphate buffer (pH 7). Six equal parts of the homogenate were made for estimation of GSH, malondialdehyde (MDA), SOD, CAT, GPX and GSH transferase. The process followed above was modified from Gupta et al. [32].
2.10.1. Estimation of GSH
The procedure was followed of Moron et al. [33] with suitable modifications. Centrifugation of the homogenate was carried out at 4ºC @ 5000 rpm for 15 min. To the supernatant, 0.5 ml of 10% trichloroacetic acid was added and again centrifuged. The supernatant thus attained was protein-free and further countered with 4 ml of 0.3 M of Na2HPO4 (pH 8.0) and 0.5 ml of 0.04% (w/v) 5,5ʹ-dithiobis-2-nitrobenzoic acid. The consequential yellowish colored substance was analyzed further by measuring its absorbance in a spectrophotometer at 412 nm. An equivalent standard was also retained.
2.10.2. Estimation of MDA
The procedure followed was that of Kei et al. [34] with minor modifications. Homogenate was mixed with 0.15M KCl and at 10,000 rpm, centrifugation was carried out for 10 min. 0.2 ml of 8.1% of SDS, 1.5 ml of 20% acetic acid (pH 3.5) and 1.5 ml of TBA were reacted with 0.2 ml of the supernatant. Heating of the samples was carried out for 60 min in hot water bath. 5 ml of n-butanol pyridine mixture was added to each sample after cooling. The solution forcefully vortexed for intense shaking and centrifugation was carried out @ 5000 rpm for 10 min. Separation was carried out of the organic layer and absorbance was noted in the spectrophotometer at 515 nm. For calculation of unknown MDA in the samples, concurrently several quantities of 1,1ʹ3, 3ʹ-tetra ethoxy propane were utilized to achieve standard curves.
2.10.3. Enzyme assay
SOD, CAT, GPX and GSH-S-transferase (GST) are the main antioxidant enzymes whose activities were measured following the mentioned protocol. Lens homogenate (10% w/v) was made in 50 mM of phosphate buffer (pH 7.0) at 4°C after centrifuging @ 5000 rpm for 15 min, and the supernatant utilized for calculation of enzyme up or down regulation.
2.10.3.1. SOD
Monitoring of the capacity of the enzyme to impede the oxidation of epinephrine was measured at 480 nm spectrophotometrically [35]. One unit of SOD activity is defined as the quantity of enzyme requisite for 50% inhibition of auto-oxidation of epinephrine. The protocol was modified from Misra et al. [36].
2.10.3.2. CAT
Activity of CAT was calculated at 240 nm by following the decay of H2O2 at 240 nm spectrophotometrically. One unit of CAT activity is defined as nmol of H2O2 decayed per min/mg protein. The protocol was modified from Aebi et al. [37].0
2.10.3.3. GPX
Observation of activity of enzyme was done at 340 nm. One unit of enzyme activity is described as 1 nmol of NADPH used per minute at 37°C. The protocol was modified from Paglia et al. [38].
2.10.3.4. GST
For calculation of GST activity, conjugation of GSH with 1 chloro, 2-4 dinitro benzene (CDNB), a hydrophilic substrate, was seen at 340 nm spectrophotometrically. One unit of GST is described as the quantity of enzyme required to conjugate 1 μmol of CDNB with GSH/min. Assessment of protein content in each sample was done as per Habig [39] and Lowry [40].
3. Results
3.1. Liposome characterization
The chitosan-coated and uncoated combinatorial liposomes are clearly depicted in Figure 1. The liposomes are generally spherical in shapes as can be clearly seen. It is also being observed that the coated liposomes in comparison with the uncoated are not perfectly spherical but a little roughed out around the edges. The sizes of the coated liposomes are bigger as obvious. The coated liposomes are around 180–260 nm whereas the uncoated ones are 110–155 nm.
Figure 1.
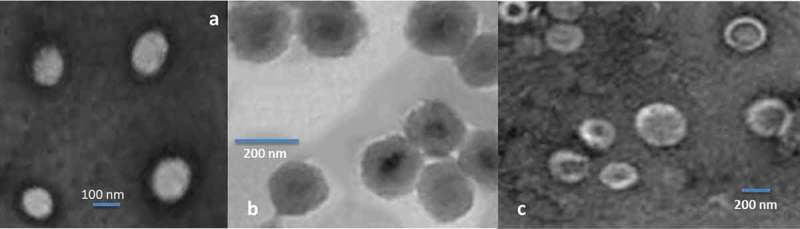
Transmission electron micrographs: (a) empty liposomes, (b) uncoated combinatorial liposomes and (c) chitosan-coated combinatorial liposomes.
3.2. Zeta potential measurement
Zeta potential measurements provide an indication of size and charge of the nanocarrier systems which are extremely significant for the functional performance of the nanoliposomes [41,42] The size distribution of the nanocarrier helps improvement of appropriate nanocarriers for the particular therapeutic commitments as in this case, the prevention of cataract. Size also helps in estivation of in vivo drug release behavior, biological fate, toxicity and the specific directing of drugs co-encapsulated in liposomes after administration. Also it may impact the loading of combinatorial drugs, their release and stability of combinatorial drugs inside nanocarriers [43].
The particle size distribution is depicted in Table 1. The measurements were done in triplicate and the results given as mean ±SD. The average size of the chitosan-coated combinatorial liposomes range from 210 to 240 nm whereas the uncoated ones range from 140 to 185 nm along with empty ones which were in the range of 100–105 nm. The zeta potential of the chitosan-coated combinatorial liposomes are in the range of −22.6 ± 2.14 as against the uncoated ones which are in the range of −19.6 ± 3 also depicted in Table 1. The zeta potential of empty liposomes was noted at −11.1. The zeta potential can greatly influence the stability of the nanosystems.
Table 1.
Size, polydispersity index (PDI) and zeta potential of empty, chitosan-coated and uncoated combinatorial liposomes.
| Size (nm) | PDI | Zeta potential (mV) | |
|---|---|---|---|
| Empty liposomes | 100.12 ± 2.32 | 0.29 ± 0.03 | −11.1 ± 4.22 |
| Chitosan-coated combinatorial liposomes | 224 ± 10.34 | 0.25 ± 0.06 | −22.6 ± 2.14 |
| Uncoated liposomes | 162.23 ± 9.21 | 0.22 ± 0.02 | −19..6 ± 3.01 |
Polydispersity index of the empty liposomes was noted at 0.29, uncoated liposomes at 0.22 and chitosan-coated combinatorial liposomes at 0.25. This depicted monodispersed liposomes and a narrow scale of size distribution.
3.3. Storage stability and shelf life studies
Measurement of size, PDI and zeta potential was done after storing the empty liposomes, chitosan-coated and uncoated combinatorial liposomes for 60 days at room temperature. This indicated the shelf life of these combinatorial liposomes. It was seen that there is a slight decrease in size of the uncoated liposomes whereas the size of chitosan-coated combinatorial liposome increased with a decrease in PDI and zeta potential. The empty liposomes indicated a decrease in size and zeta potential. The results are indicated in Table 2.
Table 2.
Size, polydispersity index (PDI) and zeta potential of empty, chitosan-coated and uncoated combinatorial liposomes after 60 days of storage at room temperature.
| Size (nm) | PDI | Zeta potential (mV) | |
|---|---|---|---|
| Empty liposomes | 90.22 ± 2.32 | 0.11 ± 0.02 | −10.1 ± 2.23 |
| Chitosan-coated combinatorial liposomes | 240 ± 9.34 | 0.23 ± 0.04 | −21.6 ± 2.35 |
| Uncoated liposomes | 158.23 ± 7.21 | 0.21 ± 0.04 | −20.6 ± 1.05 |
3.4. In vitro release studies
The in vitro release studies depicted in Figure 2 reveal an interesting trend. The uncoated combinatorial liposomes depicted an immediate burst release in which about 60% of the combinatorial drug was released and rest of the drug was released slowly over a period of 72 h. The coated liposomes released about 40% drug in initial stage and rest slowly over a period of 1 week. This confirms that providing a chitosan coating would result in slow and sustained release of the combination of drug.
Figure 2.
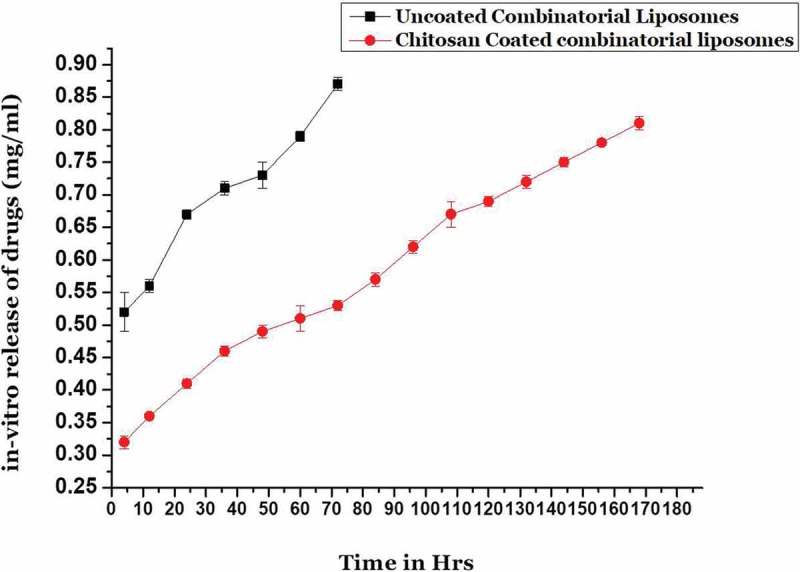
In vitro release kinetics of uncoated combinatorial liposomes and chitosan-coated combinatorial liposomes.
3.5. Encapsulation efficiency
The pharmacokinetic efficiency of two lipophilic drugs may be increased by co-encapsulation of both drugs in a nanocarrier like a liposome. The coating of chitosan does not prove to be a hindrance in encapsulation of the drugs. Instead it may act as a barrier against leaching out of the drugs. This may be proved by the encapsulation efficiency of the chitosan-coated combinatorial liposomes. The total amount of drugs loaded per ml of liposomes was 1.4 mg and total amount of drugs encapsulated was 0.81 mg/ml (combinatorial drug-loaded liposomal solution) whereas in uncoated liposomes it was 0.88 mg/ml. Therefore, % of encapsulation efficiency is 58% in case of chitosan-coated combinatorial liposomes and 62% in case of uncoated liposomes. The values are shown in Figure 3.
Figure 3.
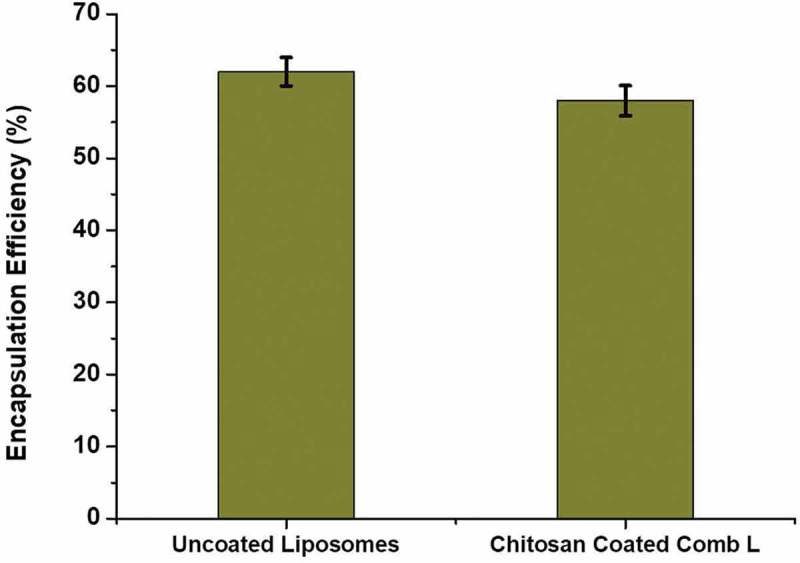
Encapsulation efficiency (%) of uncoated combinatorial liposomes and chitosan-coated combinatorial liposomes.
3.5.1. Statistics
The encapsulation efficiencies of chitosan-coated combinatorial liposomes along with uncoated liposomes were evaluated by one factorial ANOVA followed by the Scheffe’s F-test.
3.6. Cytotoxicity test
The cytocompatibility or the cell toxicity of the chitosan-coated and uncoated combinatorial liposomes and the empty liposomes may be clearly seen in Figure 4. There is a notable difference between the cytotoxicity of chitosan-coated combinatorial liposomes and uncoated liposomes. The empty liposomes as the control do not possess cell toxicity whereas the chitosan-coated combinatorial liposomes possess lower cytotoxicity when compared with the uncoated ones. However, there is no significant difference between the chitosan-coated combinatorial liposomesand uncoated ones.
Figure 4.
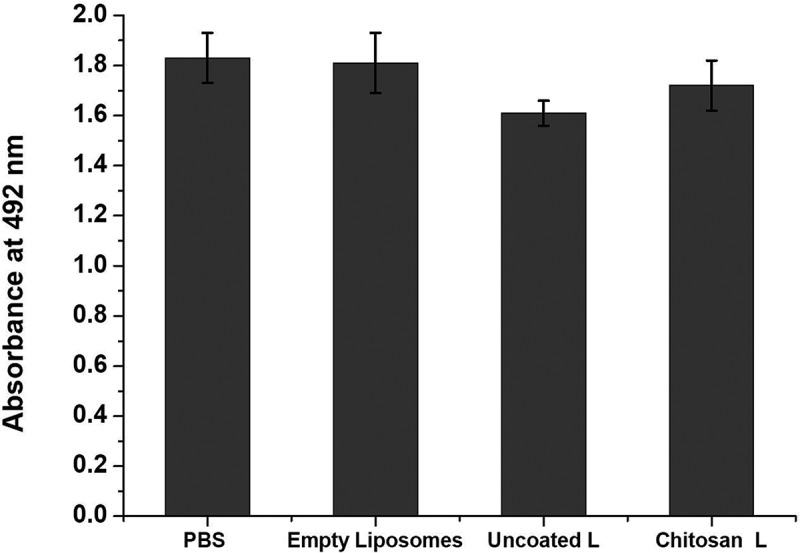
Cytocompatibility of cells treated with PBS (control), empty liposomes, uncoated combinatorial liposomes and chitosan-coated combinatorial liposomes.
Cytocompatibility was also indicated by the live/dead cell assay kit. The increased densities of live cells staining green treated with chitosan-coated combinatorial liposomes have a similar morphology with that of the cells treated with control and empty liposomes clearly illustrated in Figure 5. This indicates less toxicity of the chitosan-coated combinatorial liposomes than uncoated ones since cell densities in case of uncoated ones are lower.
Figure 5.
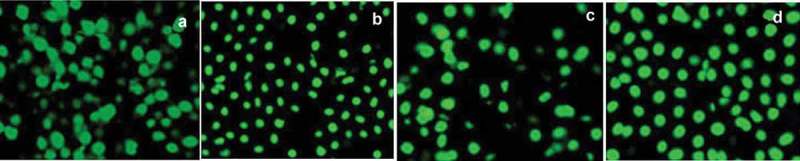
Cytocompatibility of cells as shown by live/dead cell assay. (a) Dense cell growth treated with PBS (control), (b) cells treated with empty liposomes, (c) cells treated with uncoated combinatorial liposomes and (d) cells treated with chitosan-coated combinatorial liposomes.
3.7. In vivo studies
The slit lamp microscopy images are illustrated in Figure 6. They clearly indicate that group 1 after 6 days shows full development of cataract or stage 5 cataract. Group 2 in the beginning displayed stage 0 cataract and by the end of day 6 shows stage 1 cataract or it may be termed between stages 0 and 1. Group 3 rats display stage 0 cataract clearly. This is in accordance with the anticipated results.
Figure 6.
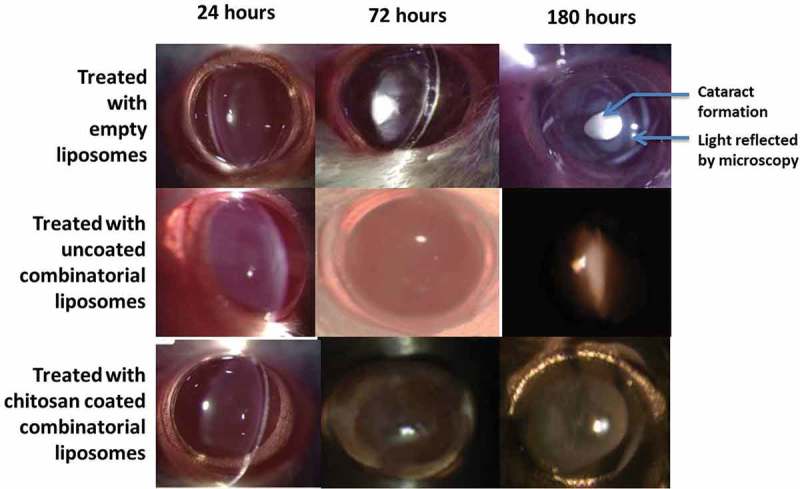
Slit-lamp microscopy Images seen after treatment with empty liposomes, uncoated combinatorial liposomes and chitosan-coated combinatorial liposomes for up to 180 h.
3.7.1. Effect on GSH and MDA
The changes in levels of GSH and MDA were notable between group 1 and groups 2 and 3. Changes between groups 2 and 3 were not significant. GSH was noted at 0.07 ± 0.006 µmol/g in group 1 and 0.74 ± 0.03 and 0.88 ± 0.02 µmol/g in groups 2 and 3, respectively. MDA levels were increased to 41.33 ± 1.23 nmol/g in group 1 in the absence of combinatorial drugs in liposomes. On treatment with uncoated liposomes and chitosan-coated combinatorial liposomes in groups 2 and 3, the MDA levels fell to 20.32 ± 1.02 and 17.88 ± 1.02 nmol/g, respectively.
3.7.2. Enzyme assays
Major enzymes in the antioxidant defense system, such as SOD, CAT, GPX and GST, were evaluated for their concentration in the lens homogenate as shown in Figure 8. It was found that the concentrations of these enzymes were extremely less in group 1 and increased in groups 2 and 3. SOD was found to be 0.88 ± 0.03 IU/mg protein in group 1 and in group 2 it was found to be 1.28 ± 0.03 IU/mg protein and 2.2 ± 0.01 IU/mg protein in group 3. CAT was found to be 0.18 ± 0.01 IU/mg protein in group 1, 0.61 ± 0.03 IU/mg protein in group 2 and 0.77 ± 0.02 IU/mg protein in group 3. GPX was found as 3.82 ± 0.8 IU/mg protein in group 1, 6.62 ± 0.3 IU/mg protein in group 2 and 7.12 ± 0.7 IU/mg protein in group 3, respectively. GST was found as 1.89 ± 0.5 IU/mg protein in group 1, 3.72 ± 0.6 IU/mg protein in group 2 and 4.12 ± 0.7 IU/mg protein in group 3, respectively. Values are noted in Table 3 and illustrated in Figure 7.
Figure 8.
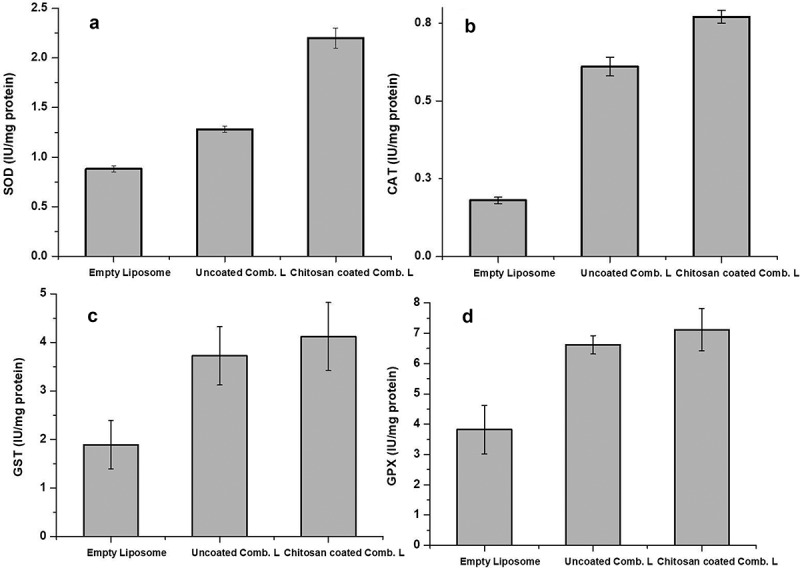
Estimation of chief antioxidant enzymes: (a) SOD, (b) CAT, (c) GST and (d) GPX in rat lenses.
Table 3.
Activities of antioxidant enzymes in rat lenses.
| Treatment groups (n = 6) |
SOD (IU/mg protein) | CAT(IU/mg protein) | GPX(IU/mg protein) | GST(IU/mg protein) |
|---|---|---|---|---|
| Group 1(treated with empty liposomes) | 0.88 ± 0.03 | 0.18 ± 0.01 | 3.82 ± 0.08 | 1.89 ± 0.05 |
| Group 2 (treated with uncoated combinatorial liposomes) | 1.28 ± 0.03 | 0.61 ± 0.03 | 6.62 ± 0.03 | 3.72 ± 0.06 |
| Group 3 (treated with chitosan-coated combinatorial liposomes) | 2.2 ± 0.01 | 0.77 ± 0.02 | 7.12 ± 0.07 | 4.12 ± 0.07 |
Figure 7.
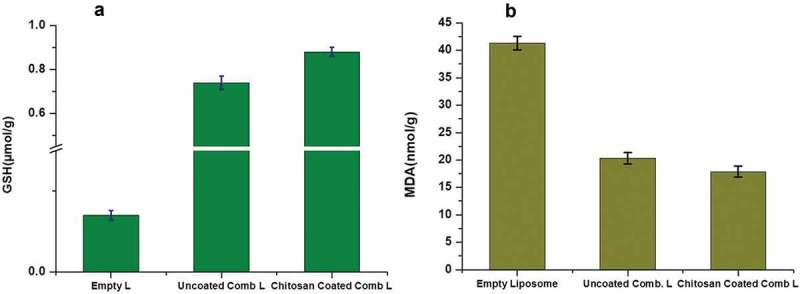
Estimation of GSH (a) and malondialdehyde lipid peroxidation (b) of rat lenses.
3.7.3. Statistics
Values are depicted as mean ±SD. p < 0.001 (group 1 or control vs. group 2) and p ˂ 0.005 (group 1 or control vs. group 3) as compared to control. Here in the study, n = 6, i.e. no. of rats = 3 but 2 eyes of each rat = 6 lenses.
4. Discussion
Globally approximately few million lens extraction are done annually because of cataract. These consume significant costs in health care with a steady increase. It is due to these reasons that alternative therapeutics, such as prevention of cataract, is being investigated thoroughly. Nanocarriers, such as liposomes, encompass several properties which makes absolutely suitable for ocular drug delivery. The chief focus has always been to improve the adhesion of these nanoformulations to cornea by utilizing several mucoadhesives [44]. In order to ensure that liposomes carry out the exact functions that we may expect them to, several modifications of the liposomes need to be carried out. In our study, we similarly carry out a modification of the liposomes to retain them in the corneal area for an increased period of time and preventing the burst release of the drugs that it encapsulates. We have very innovatively incorporated two different lipophilic drugs in the liposomes for better prevention of cataract. The co-encapsulation or co-loading of the two drugs in liposomes was challenging since both are lipophilic in nature and would be easy to pass the phospholipid bilayers and difficult to enter the aqueous core of the liposome. It would have been equally easy for these two lipophilic drugs to emerge out of the liposome at the first chance. Hence, a layer of chitosan coating was provided to ensure slow and sustained release of drugs. Additionally, chitosan coating provides for membrane stabilization and antioxidant functions. The combinatorial theory helped the two drugs to make up for the insufficiencies of each other. Hence what originates is an improved synergism. It was observed that coated liposomes are complex formulations, however, easy preparation without any complex steps makes it an extremely beneficial option. Another exciting observation is the fact that the uncoated combinatorial liposomes are equally potent in prevention of cataract, the only fact that these lipophilic drugs embedded on the surface instead of the aqueous core are immediately released making the replenishment of liposomal formulation essential within a day or two. The chitosan-coated combinatorial liposomes retention time in the cornea is more than a week. The increased encapsulation efficiency of uncoated liposomes may be attributed to the absence of coating since chitosan coating is generally formed by electrostatic interaction between chitosan and phospholipid bilayers of liposome. Such a coating is generally very stable and may prevent drug encapsulation in greater quantities [45]. However as can be seen from the results, there is not a significant difference between the uncoated and chitosan-coated combinatorial liposomes. This is because, the slow and sustained release of drug for a week from the chitosan-coated combinatorial liposomes compensates for the relative lower encapsulation of drug. The increased diameter and highest zeta potential of the chitosan-coated liposomes is a result of the coating around it. The PDI values reveal that the liposomes distribution is regular and unified. The size of the liposomes indicates that they are big enough to avoid being taken up in the circulation and small enough to avoid sedimentation. There is no significant difference between the cell toxicities in control, empty liposomes and combinatorial drug-loaded ones. PBS (control) and empty liposomes are not toxic to the cells as the results show. Chitosan coating over the drug-loaded liposomes make them cytocompatible too. Measurements of variations in size, PDI and zeta potential were done after 2 months of storage of nanoemulsions at room temperature. Slight decrease in size of the empty and uncoated liposomes may be accounted by the fact that the phospholipid bilayer of the liposome may have rearranged itself during storage result in constriction of size. The increased size of chitosan-coated liposomes may be due to the rearrangement of the chitosan coating on the surface. Another noteworthy fact is that, the chitosan-coated liposomes can be stored for a longer period that the uncoated ones since possible antifungal or microbial effect on the liposomes may be ruled out owing to the fact that chitosan has established antimicrobial properties [46,47].
Apart from physical characterization and modification of these liposomes, the in vivo efficacy of these nanocarriers is of extreme significance. The present study clearly indicates no progression of cataract on being treated with the chitosan-coated combinatorial liposomes and delay in progression in cataract on treatment with uncoated liposomes. This is of course attributed to the slow and sustained release as against burst release. Moreover, the antioxidant property of chitosan may synergistically act with combination of drugs for upregulation of antioxidant status for prevention of cataract. One of the important reasons for development of cataract is oxidative stress. We have observed that exposure to selenite had damaged the complete antioxidant status of the lens. Lens level of GSH had decreased and lipid peroxidation had increased as evident from the level of MDA in the group treated with empty liposomes. The antioxidant enzymes level had also been depleted severely on exposure to selenite as pro-oxidant. But with treatment on uncoated and coated combinatorial liposomes, there is an upregulation in the antioxidant status with SOD enzyme levels going up followed by CAT, GPX and GST. Combinatorial liposomes exhibited anticataract activity with diminished amount of lipid peroxide and increased amount of GSH and antioxidant enzymes as against selenite-induced oxidative stress.
5. Conclusion
It must be remembered that formation of cataract or cataractogenesis as it is called, is a multifactorial pathogenesis all of which has not been clarified as yet. It is yet to be investigated whether the combinatorial drugs in nanocarriers are helpful in delaying or prevention of the condition. It is also known that a favorable redox balance is essential for prevention of cataracts. This is what we tried to achieve by combinatorial drug therapy within a coated liposome. It is a clear cut observation from our study that a combination of drugs like hesperetin and lanosterol in liposomes as a nanocarrier helps in prevention of cataract. Layer of chitosan coating over the liposomes improves the retention time of liposomes within the cornea and reduces its burst release of drug. Slow and sustained release of drug may enhance the chances of renewal of drug. It also helps in counteracting cataract by improving the levels of antioxidant enzymes against induced oxidative stress.
Author’s contribution
Aikelaimu Aierken, Paerheti Muhemaiti and Adiya Aximu performed the experiments jointly and wrote the manuscript partially. Zulipiya analyzed the results and was a major contributor in writing the results and discussion. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgement
This study was supported by Supported by the National Natural Science Foundation of China (No.81400420).
References
- [1].Zhao L, Chen . J, Zhu J, et al. Lanosterol reverses protein aggregation in cataracts. Nature. 2015;523(7562):607–611. [DOI] [PubMed] [Google Scholar]
- [2].Moreau KL, King JA.. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med. 2012;18(5):273–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bloemendal H, de Jong W, Jaenicke R, et al. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86(3):407–485. [DOI] [PubMed] [Google Scholar]
- [4].Shanmugam PM, Barigali A, Kadaskar J, et al. Effect of lanosterol on human cataract nucleus. Indian J Ophthalmol. 2015;63(12):888–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu C, Lan Q, He W, et al. Octa-arginine modified lipid emulsions as a potential ocular delivery system for disulfiram: a study of the corneal permeation, transcorneal mechanism and anti-cataract effect. Colloids Surf B Biointerfaces. 2017;160:305–314. [DOI] [PubMed] [Google Scholar]
- [6].Hu C-M, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83(8):1104–1111. [DOI] [PubMed] [Google Scholar]
- [7].Gan L, Wang J, Jiang M, et al. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov Today. 2013;18(5–6):290–297. [DOI] [PubMed] [Google Scholar]
- [8].Aoki S, Mizote H, Minamoto A, et al. Systemic FK506 improved tear secretion in dry eye associated with chronic graft versus host disease. Br J Ophthalmol. 2005;89(2):243–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alany RG, Rades T, Nicoll J, et al. W/O microemulsions for ocular delivery: evaluation of ocular irritation and precorneal retention. J Control Release. 2006;111(1–2):145–152. [DOI] [PubMed] [Google Scholar]
- [10].Patel A. Ocular drug delivery systems: an overview. World J Pharm. 2013;2(2):47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dubald M, Bourgeois S, Andrieu V, et al. Ophthalmic drug delivery systems for antibiotherapy—a review. Pharmaceutics. 2018;10(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yellepeddi VK, Palakurthi S. Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther. 2016;32:67–82. [DOI] [PubMed] [Google Scholar]
- [13].Nakazawa Y, Oka M, Bando M, et al. Hesperetin prevents selenite-induced cataract in rats. Mol Vis. 2015;21:804–810. [PMC free article] [PubMed] [Google Scholar]
- [14].Emim JA, Oliveira AB, Lapa AJ. Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J Pharm Pharmacol. 1994;46(2):118–122. [DOI] [PubMed] [Google Scholar]
- [15].Hwang SL, Yen GC. Modulation of Akt, JNK, and p38 activation is involved in citrus flavonoid-mediated cytoprotection of PC12 cells challenged by hydrogen peroxide. J Agric Food Chem. 2009;57(6):2576–2582. [DOI] [PubMed] [Google Scholar]
- [16].Abdelbary G. Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm Dev Technol. 2011;16(1):44–56. [DOI] [PubMed] [Google Scholar]
- [17].Mehanna MM, Elmaradny HA, Samaha MW. Mucoadhesive liposomes as ocular delivery system: physical, microbiological, and in vivo assessment. Drug Dev Ind Pharm. 2010;36(1):108–118. [DOI] [PubMed] [Google Scholar]
- [18].Li N, Zhuang C, Wang M, et al. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int J Pharm. 2009;379(1):131–138. [DOI] [PubMed] [Google Scholar]
- [19].Dhule SS, Penfornis P, Frazier T, et al. Curcumin-loaded gamma-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8(4):440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Banerjee S, Sen K, Pal TK, et al. Poly(styrene-co-maleic acid)-based pH-sensitive liposomes mediate cytosolic delivery of drugs for enhanced cancer chemotherapy. Int J Pharm. 2012;436(1–2):786–797. [DOI] [PubMed] [Google Scholar]
- [21].Gong T, Su XT, Xia Q, et al. Biodegradable combinatorial drug loaded pH-sensitive liposomes for enhanced osteosarcoma therapeutics. J Biomater Tissue Engg. 2017;7(10):952–961. [Google Scholar]
- [22].Pulford B, Reim N, Bell A, et al. Liposome-siRNA-peptide complexes cross the blood-brain barrier and significantly decrease PrP on neuronal cells and PrP in infected cell cultures. PloS one. 2010;5(6):11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li J, Hwang I-C, Chen X, et al. Effects of chitosan coating on curcumin loaded nano-emulsion: study on stability and in vitro digestibility. Food Hydrocoll. 2016;60:138–147. [Google Scholar]
- [24].Li J, Lee IW, Shin GH, et al. Curcumin-Eudragit® E PO solid dispersion: a simple and potent method to solve the problems of curcumin. Eur J Pharm Biopharm. 2015;94:322–332. [DOI] [PubMed] [Google Scholar]
- [25].Li J, Shin GH, Chen X, et al. Modified curcumin with hyaluronic acid: combination of pro-drug and nano-micelle strategy to address the curcumin challenge. Food Res Int. 2015;69:202–208. [Google Scholar]
- [26].Huang Z, Wang H, Gao C, et al. Drug loaded gold nano-particulates for therapeutics of myocardial infarction in rat model. J Biomater Tissue Engg. 2018;8(2):197–205. [Google Scholar]
- [27].Anuchapreeda S, Fukumori Y, Okonogi S, et al. Preparation of lipid nanoemulsions incorporating curcumin for cancer therapy. J nanotechnol. 2012;11:2012. [Google Scholar]
- [28].Cholkar K, Gunda S, Earla R, et al. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS PharmSciTech. 2015;16(3):610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dou QL, Wei YY, Gu YN, et al. Investigating the therapeutic effects of N-acetylcysteine decorated poly (L-lactic acid) nanoparticles on transfusion induced acute lung injury. J Biomater Tissue Engg. 2017;7(1):69–76. [Google Scholar]
- [30].Nahomi RB, Wang B, Raghavan CT, et al. Chaperone peptides of α-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem. 2013;288(18):13022–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hiraoka T, Clark JI. Inhibition of lens opacification during the early stages of cataract formation. Invest Ophthalmol Vis Sci. 1995;36(12):2550–2555. [PubMed] [Google Scholar]
- [32].Gupta SK, Kalaiselvan V, Srivastava S, et al. Evaluation of anticataract potential of Triphala in selenite-induced cataract: in vitro and in vivo studies. J Ayurveda Integr Med. 2010;1(4):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582(1):67–78. [DOI] [PubMed] [Google Scholar]
- [34].Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90(1):37–43. [DOI] [PubMed] [Google Scholar]
- [35].Srikumar R, Parthasarathy NJ, Manikandan S, et al. Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol Cell Biochem. 2006;283(1–2):67–74. [DOI] [PubMed] [Google Scholar]
- [36].Misra HP, Fridovich I. The oxidation of phenylhydrazine: superoxide and mechanism. Biochemistry. 1976;15(3):681–687. [DOI] [PubMed] [Google Scholar]
- [37].Aebi H. Catalase In: Methods of enzymatic analysis, 2nd ed. Vol. 2, Weinheim/New York: Elsevier; 1974. p. 673–684. [Google Scholar]
- [38].Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- [39].Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- [40].Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- [41].Ahsan F, Rivas IP, Khan MA, et al. Targeting to macrophages: role of physicochemical properties of particulate carriers–liposomes and microspheres–on the phagocytosis by macrophages. J Control Release. 2002;79(1–3):29–40. [DOI] [PubMed] [Google Scholar]
- [42].Galindo-Rodríguez S, Allémann E, Doelker E, et al. Versatility of three techniques for preparing ibuprofen-loaded methacrylic acid copolymer nanoparticles of controlled sizes. J Drug Deliv Sci Technol. 2005;15(5):347–354. [Google Scholar]
- [43].Mohanraj V, Chen Y. Nanoparticles-a review. Trop J Pharm Res. 2006;5(1):561–573. [Google Scholar]
- [44].Iezhitsa I, Agarwal R, Saad SDB, et al. Mechanism of the anticataract effect of liposomal magnesium taurate in galactose-fed rats. Mol Vis. 2016;22:734. [PMC free article] [PubMed] [Google Scholar]
- [45].Guo J, Ping Q, Jiang G, et al. Chitosan-coated liposomes: characterization and interaction with leuprolide. Int J Pharm. 2003;260:167–173. [DOI] [PubMed] [Google Scholar]
- [46].Knapczyk J, Macura AB, Shtslsaw Pawlik B. Simple tests demonstrating the antimycotic effect of chitosan. Int J Pharm. 1992;80(1–3):33–38. [Google Scholar]
- [47].Zorofchian Moghadamtousi S, Abdul Kadir H, Hassandarvish P, et al. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:186864. [DOI] [PMC free article] [PubMed] [Google Scholar]


