Short abstract
Cooling temperatures and low pH have profound effects on somatosensory functions including nociception. The effects not only can be mediated by cooling temperature transducers and proton transducers expressed in subpopulations of somatosensory neurons but may also be mediated by ion channels involving membrane excitability such as voltage-dependent K+ channels in somatosensory neurons. In the present study, we performed the in situ patch-clamp recordings from nociceptive-like trigeminal ganglion neurons in ex vivo trigeminal ganglion preparations of adult rats. We determined effects of cooling temperatures and low pH on membrane properties and voltage-dependent currents in nociceptive-like trigeminal ganglion neurons. Action potential rheobase levels were decreased when nociceptive trigeminal ganglion neurons were cooled from 24°C down to 12°C or when extracellular pH levels were reduced from 7.3 to 6. This indicates that the excitability of nociceptive-like trigeminal ganglion neurons was increased at the cooling temperatures and low pH. The decreases of action potential rheobase levels were accompanied by increases of trigeminal ganglion neuron input resistances at cooling temperatures and low pH, suggesting a possible involvement of background K+ channels. Cooling temperatures and low pH suppressed voltage-activated inward Na+ currents and also voltage-dependent outward K+ currents in nociceptive-like trigeminal ganglion neurons. Voltage-dependent outward K+ currents in nociceptive-like trigeminal ganglion neurons consist of inactivating A-type K+ currents and non-inactivating type K+ currents, and the former were more sensitive to cooling temperatures and low pH. Collectively, suppressing multiple types of K+ channels may be associated with the enhanced excitability of nociceptive trigeminal ganglion neurons by cooling temperatures and low pH.
Keywords: Temperature, cold, low pH, pain, trigeminal ganglion neurons, K+ channels
Introduction
Cooling temperatures and low pH in tissues have profound effects on somatosensory functions and nociception under both physiological and pathological conditions. For example, a normal subject can experience painful sensations at cooling temperatures below 15°C but a patient with peripheral neuropathy may experience severe pain at an innocuous cooling temperature of 17°C. Low pH in muscles after strenuous exercise and in arthritic joints is an important biological factor contributing to hyper-excitability of nociceptive afferent nerve endings and pain in these tissues. Most of recent studies have focused on sensory transducers directly involving the detection of cooling temperatures and protons in tissues. Studies have shown that transient receptor potential cation channel subfamily M member 8 (TRPM8 channels)1–3 and acid-sensing ion channels (ASIC)4 are main cold-transducers and proton-transducers, respectively, in somatosensory nerves. Cooling temperatures and low pH (protons) activate these ion channels to excite somatosensory nerves leading to sensory signals including nociception. TRPM8 and ASIC channels are expressed on subpopulations of nociceptive somatosensory neurons, and their activation evokes excitatory currents that are not long lasting.1,2,5,6 The prolonged effects of cooling temperatures and low pH on somatosensory functions and pain may be mediated by other mechanisms independent of the transducers of cooling temperatures and protons.
Cooling temperatures and low pH may have prolonged effects on somatosensory neurons’ excitability via their effects on voltage-dependent ion channels since temperatures and protons may have tonic effects on the activity of these ion channels. For example, cooling temperatures could greatly suppress tetrodotoxin-senstive (TTX-sensitive) voltage-gated Na+ channels in somatosensory neurons to result in the suppression of their excitability at cooling temperatures.7 However, TTX-resistant voltage-gated Na+ channels are less effectively suppressed by cooling temperatures.7,8 TTX-resistant voltage-gated Na+ channels have been shown to be essential in nociception at noxious cold temperatures.8 Interestingly, somatosensory neurons with TTX-resistant voltage-gated Na+ channels became more excitable at cooling temperatures as shown in our previous studies using cultured dorsal root ganglion (DRG) neurons.7 We have found that the enhanced excitability of somatosensory neurons by cooling temperatures are partially due to the suppression of Kv7.2-mediated M-type K+ currents by cooling temperatures.9 Kv7.2 channels are voltage-gated K+ channels that can be activated by voltages at low thresholds and these channels are involved in controlling neuronal excitability. In addition to M-type K+ channels, A-type K+ channels (IA channels) formed by Kv1.4, Kv4.1, and Kv4.3 channels10,11 also are activated by voltages at low thresholds.12 IA channels in nociceptive neurons serve as a brake to oppose membrane depolarization and limit action potential (AP) firing. Our previous study in cultured DRG neurons have suggested that suppression of IA channels may underlie the enhanced DRG neuron excitability at cooling temperatures (IA).7 However, effects of cooling temperatures and low pH on IA currents of nociceptive-like trigeminal ganglion (TG) neurons and their excitability have not been studied.
Somatosensory neurons’ excitability is also controlled by background K+ currents mediated by two-pore domain K+ channels (K2P channels, also known as background or leak K+ channels),13 and the activity of these channels plays a major role in basic membrane properties including input resistance.13 K2P channels include 15 members, and several K2P members have been found to be expressed in somatosensory neurons including TG neurons.14 Some K2P channels such as channels of TREK subfamily are highly temperature sensitive,14 and others such as channels of TASK subfamily are sensitivity to acidic pH.14 Thus, effects of cooling temperatures and low pH on input resistances of nociceptive-like TG neurons may provide insights into a potential role of temperature-sensitive and acid-sensitive K2P channels in nociceptive-like TG neurons’ excitability.
In the present study, we used ex vivo TG preparation and in situ patch-clamp recordings from nociceptive-like TG neurons to determine the effects of cooling temperatures and low pH on membrane properties and voltage-dependent currents of nociceptive-like TG neurons
Materials and methods
Ex vivo TG preparations
Sprague–Dawley rats at ages of 5–7 weeks were used in all experiments. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Animal care and use conformed to the National Institutes of Health guidelines for care and use of experimental animals. Rats were euthanized by overdose of isoflurane, and TGs were immediately dissected out. Connective tissues on the surface of TGs were removed carefully by a pair of forceps under a dissection microscope. The TGs were affixed in a 0.5-ml recording chamber by a tissue anchor and submerged in a Krebs solution. The Krebs solution contained (in mM): 117 NaCl, 3.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3 and 11 glucose, and osmolarity was adjusted to 325 mOsm with sucrose. The solution was saturated with 95% O2 and 5% CO2. Unless otherwise indicated, the bath solution was maintained at 24°C and pH at 7.3. The recording chamber was mounted on the stage of an Olympus IX50 microscope that was equipped with an infrared-differential interference contrast optical system. To facilitate electrode penetration through connective tissues, the TGs were briefly exposed to 0.05% dispase II plus 0.05% collagenase in the Krebs solution for 5–6 min and the enzymes were then washed off with the Krebs solution. Under a 40× objective, individual TG neurons were visualized for patch-clamp recordings.
In situ patch-clamp recordings
Patch-clamp recordings were performed in situ on TG neurons of ex vivo TG preparations using electrodes whose resistances were ∼6 MΩ after filling with recording internal solution. Recording internal solution contained (in mM): 135 K-gluconate, 0.5 CaCl2, 2.4 MgCl2, 5 EGTA, 10 HEPES, 5 Na2ATP and 0.33 NaGTP-TRIS salt; the pH of the solution was adjusted to 7.3 with KOH. To achieve a good membrane seal with a targeted TG neuron in the ex vivo TG preparation, a positive pressure was applied into a patch-clamp recording electrode while approaching the targeted TG neuron. The positive pressure was produced by compressing 5–8 ml air in a 25-ml syringe that was connected to the holder of the recording electrode. After the electrode penetrated through satellite cell membranes and approached the membrane of the targeted TG neuron, a negative pressure was applied into the electrode through the syringe until gigaohm seal was achieved. Signals were recorded and amplified using a MultiClamp 700B amplifier, filtered at 2 kHz and sampled at 5 kHz using pCLAMP 10 software (Molecular Devices).
Measuring electrophysiological properties of TG neurons
Under the whole-cell current-clamp mode, step current pulses were injected into cells through patch-clamp electrodes. The step currents were applied from −50 pA to 2000 pA in an increment of 25 pA each step and each step had a duration of 250 ms. To determine voltage-activated currents, TG neurons were held at −70 mV under the whole-cell patch-clamp configuration and whole-cell currents were evoked by a series of voltage steps ranging from −100 to 50 mV with a 10 mV increment each step. The duration of each step was 500 ms. Unless otherwise indicated, membrane voltages mentioned in the texts have been corrected for calculated junction potentials at 24°C.
Patch-clamp recordings were performed at bath solution temperatures of 24°C, 17°C, and 12°C and bath solution pH of 7.3 and 6. The temperatures of bath solutions were controlled by a Peltier cooling device (Model TCM-1, Warner Instrument, CT, USA), which were delivered to patched cells from a short tube (0.2 cm L, 500 µm ID) with the outlet 2 mm away from the recorded cells. The temperatures at the recording sites were continuously recorded with a thermal probe that attached to the controller of the Peltier cooling device.
Data analysis
Clampfit 10 software was used to measure data of whole-cell recordings from voltage- and current-clamp experiments. Unless otherwise indicated, data are presented as mean ± SEM, *P < 0.05, **P < 0.01, and ***P < 0.001, paired Student’s t-test.
Results
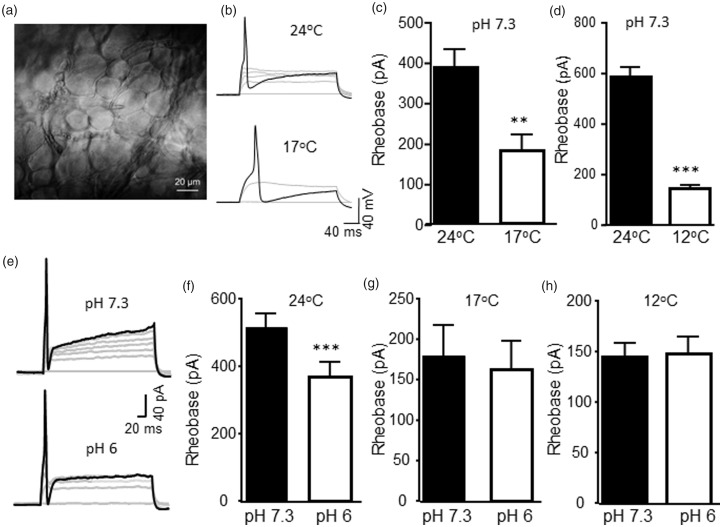
We used ex vivo TG preparations and made in situ patch-clamp recordings from TG neurons (Figure 1(a)). Under this recording condition, TG neurons better represent their intact conditions in comparison with cultured or acutely dissociated TG neurons. All recordings were performed from small-sized TG neurons with their diameters <35 μm. The AP of every TG neuron included in the present study had a shoulder in its repolarization phase. Therefore, we consider these TG neurons as nociceptive-like neurons. All the nociceptive-like TG neurons included in the present study showed no detectable inward currents under the voltage-clamp mode when cooling temperature ramp from 24°C to 12°C or low pH of 6 Krebs solutions were bath applied to these neurons. The lack of detectable inward currents at the cooling temperatures and low pH in these cells may be due to slow applications of solutions to cause TRPM8 channel and ASIC desensitization before evoking detectable macroscopic currents. We determined nociceptive-like TG neurons’ excitability at 24°C and cooling temperatures of 17°C and 12°C (Figure 1(b) to (d)). This was done by injecting depolarizing currents via recording electrodes to elicit APs and measuring the threshold currents required for AP firing (AP rheobase). In these experiments, the bath solution was maintained at pH 7.3 during all three testing temperatures. In nociceptive-like TG neurons tested at both 24°C and 17°C, AP rheobase levels were significantly decreased from 388 ± 49 pA at 24°C to 179 ± 38 pA at 17°C (n = 6, P < 0.01, Figure 1(b) and (c)). In a different set of TG neurons tested at both 24°C and 12°C, AP rheobase levels were significantly reduced from 588 ± 38 pA at 24°C to 145 ± 14 pA at 12°C (n = 10, P < 0.001, Figure 1(d)). We determined effects of low pH (pH 6 Krebs solution) on AP rheobase levels at different temperatures. At 24°C, AP rheobase levels were 515 ± 41 pA at pH 7.3, and significantly reduced to 368 ± 44 pA at pH 6 (n = 15, P < 0.001, Figure 1(e) and (f)). However, at 17°C or 12°C, AP rheobase levels were not significantly affected by low pH of 6 (Figure 1(g) and (h)). The AP rheobase levels at 17°C were 179 ± 38 pA at pH 7.3 (n = 6) and 162 ± 35 pA at pH 6 (n = 6, P = 0.1, Figure 1G). At 12°C, the AP rheobase levels were 145 ± 14 pA at pH 7.3 (n = 10) and 148 ± 18 pA at pH 6 (n = 10, P = 0.78, Figure 1(h)). Effects of cooling temperatures and low pH on other parameters of membrane and actions potentials in nociceptive-like TG neurons are shown in Table 1. The most significant changes were the broadening of AP width at cooling temperatures and low pH.
Figure 1.
Effects of cooling temperatures and low pH on the excitability of nociceptive-like trigeminal ganglion neurons. (a) Image taken under a 40× objective shows TG neurons in an ex vivo trigeminal ganglion preparation. An asterisk indicates a small-sized TG neuron being recorded. (b) Two sets of sample traces show action potential firing in a nociceptive TG neuron at 24°C (top panel) and 17°C (bottom panel). AP firing was elicited by injections of depolarizing currents. Black traces are membrane responses with APs at rheobase levels of 475 pA (top) and 150 pA (bottom). (c) Bar graph shows rheobase levels measured at 24°C (closed bar, n = 6) and 17°C (open bar, n = 6). (d) Rheobase levels measured at 24°C (closed bar, n = 10) and 12°C (open bar, n = 10). In both (b) and (c), the Krebs bath solution for cell perfusion had pH of 7.3. (e) Two sets of sample traces show action potential firing in a TG neuron at pH 7.3 (top panel) and pH 6 (bottom panel). Black traces are membrane responses with APs at rheobase levels of 575 pA (top) and 150 pA (bottom). (f) Bar graph shows rheobase levels measured at pH 7.3 (closed bar, n = 15) and pH 6 (open bar, n = 15). In both (e) and (f), the Krebs bath solution was maintained at 24°C. (g) Rheobase levels measured at 17°C at pH 7.3 (closed bar, n = 6) and pH 6 (open bar, n = 6). (h) Rheobase levels measured at 12°C at pH 7.3 (closed bar, n = 10) and pH 6 (open bar, n = 10). Data represent mean ± SEM, *P < 0.05; ***P < 0.001.
Table 1.
Membrane and action potential parameters of nociceptive-like TG neurons at cooling temperatures and low pH.
| Membrane capacitance (pF) | Resting membrane potential(mV) | AP threshold (mV) | AP width (ms) | AP amplitude (mV) | AP numbers at 2× rheobase | ||
|---|---|---|---|---|---|---|---|
| pH 7.3 | 24°C (n=15) | 33±2 | −73±2 | −30±2 | 3.3±0.3 | 111±2 | 1.8±0.4 |
| 17°C (n=6) | 26±3 | −75±3 | −33±4 | 6.9±0.9*** | 112±4 | 1.3±0.3 | |
| 12°C (n=10) | 34±3 | −73±2 | −34±2 | 12.1±0. 7*** | 104±4 | 1.0±0 | |
| pH 6.0 | 24°C (n=15) | 30±2 | −72±3 | −31±2 | 4.0±0.3 | 102±3## | 1.5±0.3 |
| 17°C (n=6) | 25±3 | −72±4# | −32±4 | 7.9±1.2## | 106±4## | 1.4±0.4 | |
| 12°C (n=10) | 34±4 | −74±3 | −34±3 | 13.0±0.7## | 100±4 | 1.1±0.1 |
Note: Data represent mean ± SEM. ***P < 0.001, one-way ANOVA, comparing to the measures at 24°C within the pH 7.3 group; #P < 0.05, ##P < 0.01, paired t test, comparing between measures at pH 7.3 and pH 6 in the same temperature groups. TG: trigeminal ganglion.
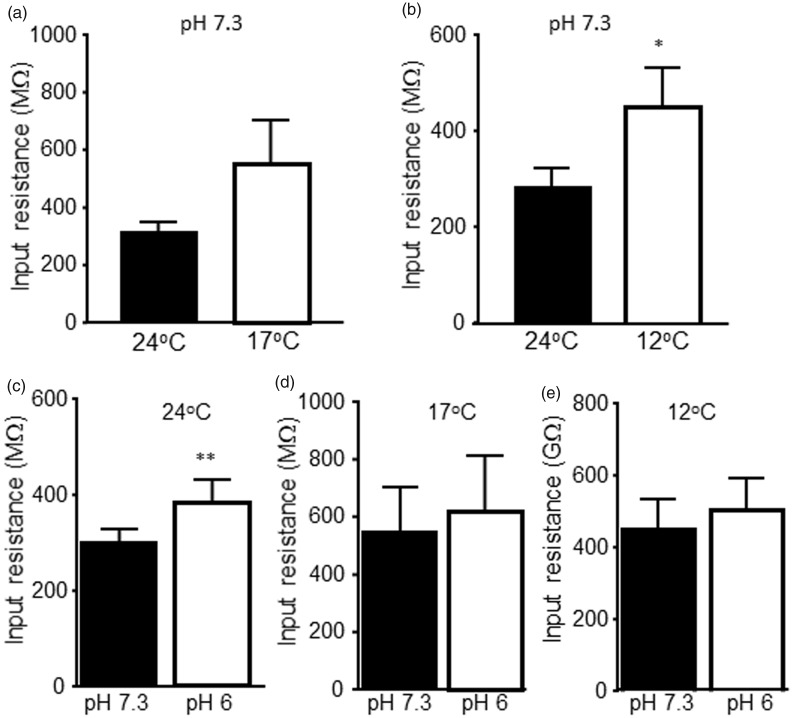
We examined effects of cooling temperatures and low pH on membrane input resistance of nociceptive-like TG neurons. In nociceptive-like TG neurons tested at pH 7.3, input resistances were 313 ± 34 MΩ at 24°C and were 555 ± 153 MΩ at 17°C (n = 6 P = 0.11, Figure 2(a)). In 10 other nociceptive-like TG neurons tested at pH 7.3, input resistances were 283 ± 40 MΩ at 24°C and were significantly increased to 450 ± 82 MΩ at 12°C (n = 10, P < 0.05, Figure 2(b)). We tested effects of low pH on input resistance of nociceptive-like TG neurons. At 24°C, input resistances were significantly increased from 303 ± 28 MΩ at pH 7.3 to 387 ± 46 MΩ at pH 6 (n = 15, P < 0.01, Figure 2(c)). At 17°C, input resistances were 555 ± 153 MΩ at pH 7.3 and 622 ± 190 MΩ at pH 6 (n = 6), and the input resistances were not significantly different at the two pH levels tested (Figure 2(d)). At 12°C, input resistances were 450 ± 82 MΩ (n = 10) at pH 7.3 and 503 ± 91 MΩ at pH 6 (n = 10) and were also not significantly different at the two pH levels tested (Figure 2(e)).
Figure 2.
Effects of cooling temperatures and low pH on input resistances of nociceptive-like trigeminal ganglion neurons. (a) Summary data of membrane input resistances of nociceptive-like TG neurons at 24°C (closed bar, n = 6) and 17°C (open bar, n = 6). (b) Summary data of membrane input resistances of nociceptive-like TG neurons at 24°C (closed bar, n = 10) and 12°C (open bar, n = 10). In both (a) and (b), the Krebs bath solution for cell perfusion had pH of 7.3. (c) Summary data of membrane input resistances of nociceptive-like TG neurons at pH 7.3 (closed bar, n = 15) and pH 6 (open bar, n = 15). The Krebs bath solution was maintained at 24°C. (d) Summary data of membrane input resistances of nociceptive-like TG neurons at pH 7.3 (closed bar, n = 6) and pH 6 (open bar, n = 6). The Krebs bath solution was maintained at 17°C. (e) Summary data of membrane input resistances of nociceptive-like TG neurons at pH 7.3 (closed bar, n = 10) and pH 6 (open bar, n = 10). The Krebs bath solution was maintained at 12°C. Data represent mean ± SEM, *P < 0.05; **P < 0.01.
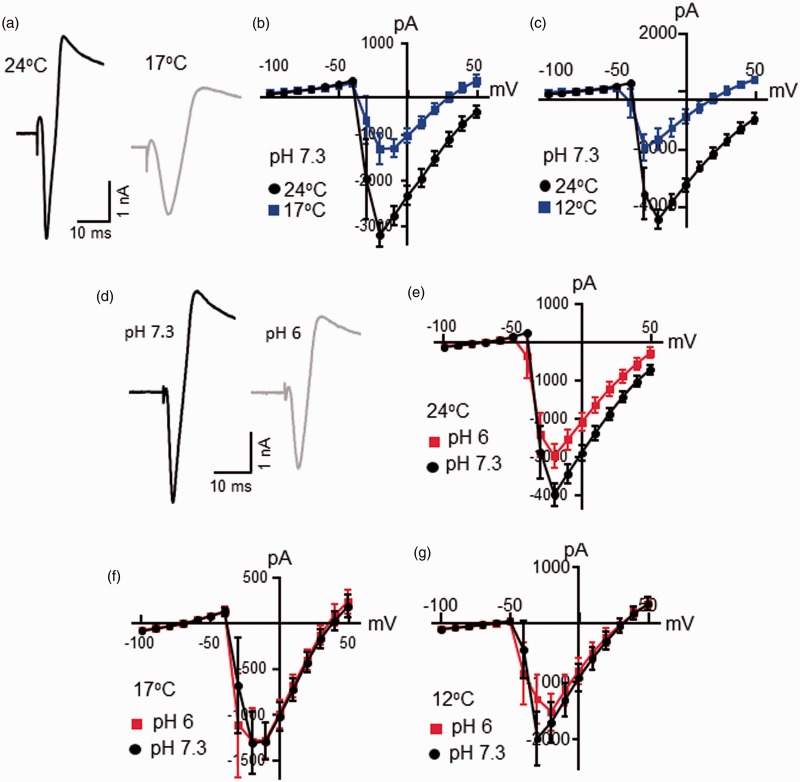
Under the voltage-clamp configuration, voltage steps applied to nociceptive-like TG neurons evoked inward currents at initial time, and the inward currents were mainly carried by voltage-gated Na+ channels. We examined voltage-activated inward currents in nociceptive-like TG neurons at 24°C, 17°C, and 12°C (Figure 3(a) to (c)). This set of experiments was performed at pH 7.3. Comparing the amplitude of voltage-activate inward currents recorded at 24°C, voltage-activated inward currents were smaller at cooling temperatures of 17°C and 12°C (Figure 3(a) to (c)). For example, in a set of six cells tested, the peak amplitudes of voltage-activated inward currents evoked at −20 mV were −3175 ± 270 pA at 24°C and reduced to −1303 ± 343 pA at 17°C (n = 6, P < 0.001, Figure 3(b)). In a different set of 10 cells tested, the peak amplitudes of voltage-activated inward currents evoked at −20 mV were −4502 ± 339 pA at 24°C and reduced to −1709 ± 360 pA at 12°C (n = 10, P < 0.001, Figure 3(c)). We determined the effects of low pH on voltage-activated inward currents in nociceptive-like TG neurons. At 24°C, voltage-activated inward currents were smaller at low pH of 6.0 in comparison with the voltage-activated inward currents measured at pH 7.3 (n = 15, Figure 3(d) and (e)). The peak amplitudes of voltage-activated inward currents evoked at −20 mV were −3985 ± 301 pA at pH 7.3 and reduced to −2985 ± 325 pA at pH 6.0 (n = 15, P < 0.05, Figure 3(e)). However, at cooling temperatures of 17°C, amplitudes of voltage-activated Na+ currents measured at pH 7 were not different from those measured at pH 6 (n = 6, Figure 3(f)). Similarly, at 12°C, amplitudes of voltage-activated Na+ currents measured at pH 7 were not different from those measured at pH 6 (n = 10, Figure 3(g)).
Figure 3.
Effects of cooling temperatures and low pH on voltage-activated inward currents in nociceptive-like trigeminal ganglion neurons. (a) Sample traces of voltage-activated inward currents in a nociceptive-like TG neuron at 24°C (black) and 17°C (gray). The voltage step was from −100 mV to 50 mV. (b) I-V curve of voltage-activated inward currents of nociceptive-like TG neurons at 24°C (black, n = 6) and 17°C (blue, n = 6). (c) I-V curve of voltage-activated inward currents of nociceptive-like TG neurons at 24°C (black, n = 10) and 12°C (blue, n = 10). In (a) to (c), the Krebs bath solution for cell perfusion had pH of 7.3. (d) Sample traces of voltage-activated inward currents in a nociceptive-like TG neuron at pH 7.3 (black) and pH 6 (gray). The voltage step was from −100 mV to 50 mV. (e) I-V curve of voltage-activated inward currents of nociceptive-like TG neurons at pH 7.3 (black, n = 15) and pH 6 (red, n = 15). In both (d) and (e), the Krebs bath solution was maintained at 24°C. (f) I-V curve of voltage-activated inward currents of nociceptive-like TG neurons at pH 7.3 (closed bar, n = 6) and pH 6 (open bar, n = 6). The Krebs bath solution was maintained at 17°C. (g) I-V curve of voltage-activated inward currents of nociceptive-like TG neurons at pH 7.3 (closed bar, n = 10) and pH 6 (open bar, n = 10). The Krebs bath solution was maintained at 12°C. Data represent mean ± SEM.
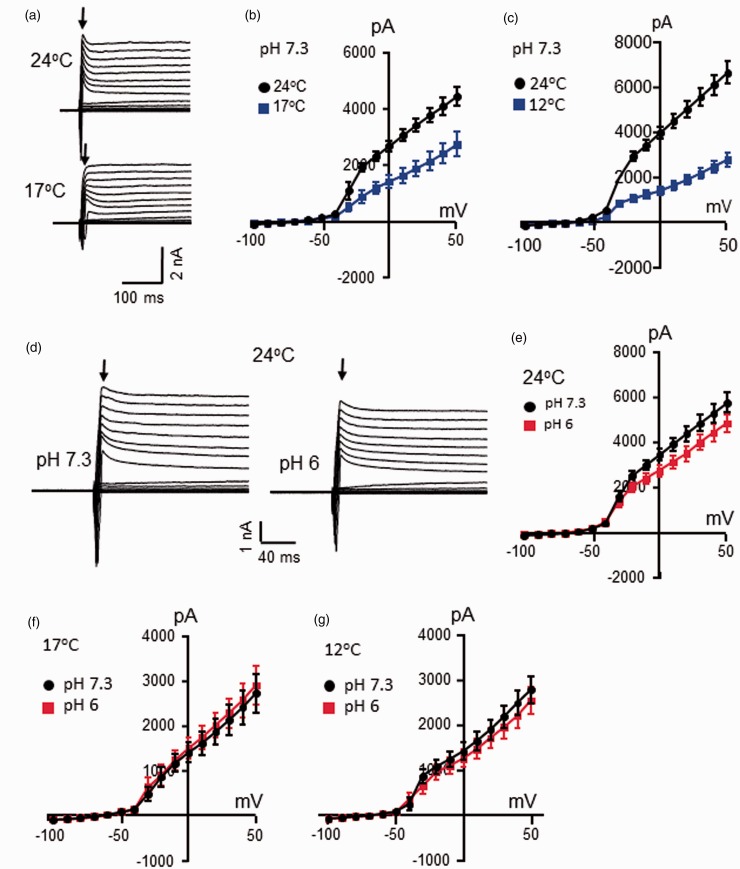
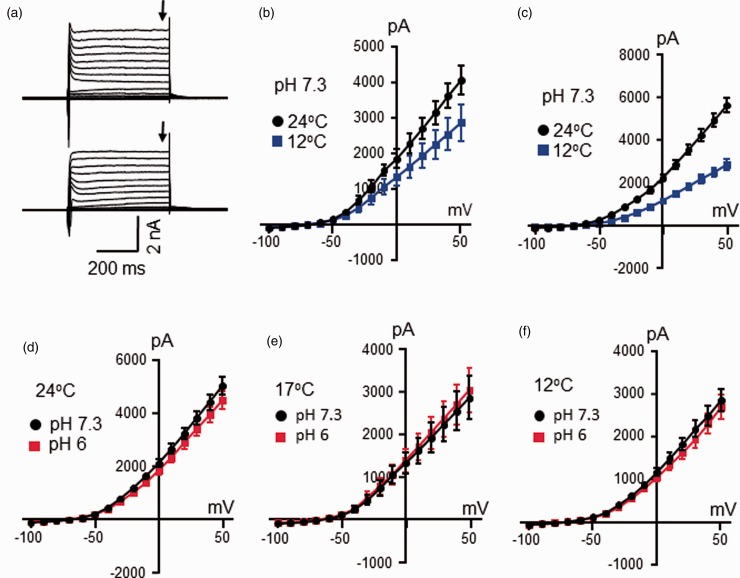
Voltage steps applied to nociceptive-like TG neurons evoked outward currents consisting of inactivating current component at initial phase (Figure 4) and non-inactivating current component in the late phase of the outward currents (Figure 5). The voltage-dependent inactivating current component was mainly carried by IA currents.7 We determined effects of temperatures and low pH on IA current components of voltage-dependent outward currents. As shown in Figure 4(a) to (c), IA current components of the voltage-dependent outward currents were suppressed when temperatures were decreased from 24°C to 17°C or from 24°C to 12°C (Figure 4(a) to (c)). Measured at 0 mV, the amplitudes of IA current components were 2676 ± 184 at 24°C and reduced to 1414 ± 225 at 17°C (n = 6, P < 0.001, Figure 4(b)). In a different set of cells, the amplitudes of IA current components measured at 0 mV were 3979 ± 267 at 24°C and reduced to 1423 ± 195 at 12°C (n = 10, P < 0.001, Figure 4(c)). These temperature-dependent changes of IA current components corresponded to Q10 values of 2.7 ± 0.2 (n = 16). We tested effects of pH 6 on IA current components in nociceptive-like TG neurons (Figure 4(d) to (g)). At 24°C, IA current components were smaller at pH 6 in comparison with the IA current components measured at pH 7.3. Measured at the voltage of 0 mV, the amplitudes of IA current components were 3466 ± 255 at pH 7.3 and reduced to 2756 ± 261 at pH 6 (n = 15, P < 0.001, Figure 4(d) and (e)). However, at cooling temperatures of 17°C or 12°C, the amplitudes of IA current components at pH 6 were similar to those at pH 7.3 (Figure 4(f) and (g)).
Figure 4.
Effects of cooling temperatures and low pH on voltage-dependent inactivating outward current components in nociceptive-like trigeminal ganglion neurons. (a) Sample traces show voltage-dependent inactivating outward current components (arrows indicated) in a nociceptive-like TG neuron at 24°C (top) and 17°C (bottom). The voltage step was from −100 mV to 50 mV. (b) I-V curve of voltage-dependent inactivating outward current components in nociceptive-like TG neurons at 24°C (black, n = 6) and 17°C (blue, n = 6). (c) I-V curve of voltage-dependent inactivating outward current components of nociceptive-like TG neurons at 24°C (black, n = 10) and 12°C (blue, n = 10). In (a) to (c), the Krebs bath solution for cell perfusion had pH of 7.3. (d) Sample traces of voltage-dependent inactivating outward current components of a nociceptive-like TG neuron at pH 7.3 (left) and pH 6 (right). The voltage step was from −100 mV to 50 mV. (e) I-V curve of voltage-dependent inactivating outward current components in nociceptive-like TG neurons at pH 7.3 (black, n = 15) and pH 6 (red, n = 15). In both (d) and (e), the Krebs bath solution was maintained at 24°C. (f) I-V curve of voltage-dependent inactivating outward current components of nociceptive-like TG neurons at pH 7.3 (black, n = 6) and pH 6 (red, n = 6). The Krebs bath solution was maintained at 17°C. (g) I-V curve of voltage-dependent inactivating outward current components of nociceptive-like TG neurons at pH 7.3 (black, n = 10) and pH 6 (red, n = 10). The Krebs bath solution was maintained at 12°C. Data represent mean ± SEM.
Figure 5.
Effects of cooling temperatures and low pH on voltage-dependent non-inactivating outward current components in nociceptive-like trigeminal ganglion neurons. (a) Sample traces show voltage-dependent non-inactivating outward current components (arrows indicated) in a nociceptive-like TG neuron at 24°C (top) and 17°C (bottom). The voltage step was from −100 mV to 50 mV. (b) I-V curve of voltage-dependent non-inactivating outward current components of nociceptive-like TG neurons at 24°C (black, n = 6) and 17°C (blue, n = 6). (c) I-V curve of voltage-dependent non-inactivating outward current components in nociceptive-like TG neurons at 24°C (black, n = 10) and 12°C (blue, n = 10). In (a) to (c), the Krebs bath solution for cell perfusion had pH of 7.3. (d) I-V curve of voltage-dependent non-inactivating outward current components in nociceptive-like TG neurons at pH 7.3 (black, n = 15) and pH 6 (red, n = 15). The Krebs bath solution was maintained at 24°C. (e) I-V curve of voltage-dependent non-inactivating outward current components of nociceptive-like TG neurons at pH 7.3 (black, n = 6) and pH 6 (read, n = 6). The Krebs bath solution was maintained at 17°C. (f) I-V curve of voltage-dependent non-inactivating outward current components of nociceptive-like TG neurons at pH 7.3 (black, n = 10) and pH 6 (red, n = 10). The Krebs bath solution was maintained at 12°C. Data represent mean ± SEM.
We examined effects of cooling temperatures and low pH on voltage-dependent non-inactivating outward current components of nociceptive-like TG neurons. As shown in Figure 5(a) to (c), cooling temperatures from 24°C to 17°C showed a tendency of decreases of non-inactivating outward current components, and significant decreases of non-inactivating outward current components were observed with cooling temperatures from 24°C to 12°C. For example, at 0 mV, the amplitude of non-inactivating outward current components was 1868 ± 254 pA at 24°C and was 1344 ± 250 pA at 17°C (n = 6, P = 0.11). In a different set of TG neurons and measured at 0 mV, the amplitude of non-inactivating outward current components was 2257 ± 173 pA at 24°C and reduced to 1163 ± 90 pA at 12°C (n = 10, P < 0.001). The calculated Q10 values based on the current changes at 0 mV at these temperatures were 1.8 ± 0.15 (n = 15). We tested effects of pH 6 on non-inactivating outward current components at different temperatures (Figure 5(d) to (f)). At all three temperatures, 24°C, 17°C, and 12°C, non-inactivating outward current components were not different between the tests at pH 7.3 and pH 6 (Figure 5(d) to (f)).
Discussion
Using the in situ patch-clamp recordings from TG neurons in the ex vivo TG preparations, this study shows that nociceptive-like TG neurons are more excitable at cooling temperatures and low pH levels. The changes in the excitability is accompanied by the increases of membrane input resistance and decreases of voltage-dependent outward K+ currents. The effects of low pH on the excitability, membrane properties, and voltage-dependent outward K+ currents depend on temperatures.
Increases of membrane input resistances by cooling temperatures and low pH may be a mechanism underlying the reduction of AP rheobase levels of nociceptive-like TG neurons since depolarizing currents would be more efficient to excite the TG neurons with increased membrane input resistance. Membrane input resistance is mainly controlled by K2P channels in somatosensory neurons. A decrease of K2P channel activity would lead to an increase of membrane input resistance in a cell. K2P channels show variable temperature sensitivity from temperature insensitive such as TASK-315 to highly temperature sensitive such as TREK channels.16,17 For temperature-sensitive K2P channels, their channel activity can be largely suppressed by cooling temperatures. Therefore, the effects of cooling temperatures on the input resistance of nociceptive-like TG neurons were likely due to their suppression of temperature-sensitive K2P channels. Consistently, we have previously shown that temperature-sensitive K2P channels including TREK-1, TREK-2, and TRAAK are expressed in TG neurons, and most of these neurons are small-sized, potentially nociceptive TG neurons.18 K2P channels also show variable sensitivity to acidic pH, some are insensitive to low pH such as TRAAK channels and others are highly sensitive to low pH such as TASK channels.14 For the K2P channels that are highly sensitive to acidic pH, their channel activity is suppressed at low pH.13 Therefore, increases of input resistance of nociceptive-like TG neurons at low pH may be due to the inhibition of acid-sensitive K2P channels at acidic pH in our study. Acid-sensitive K2P channels including TASK-3 are found to be expressed in DRG neurons.19 They may also be expressed in nociceptive-like TG neurons to respond to pH changes in tissues. This may contribute to the changes of membrane properties and excitability of nociceptive-like TG neurons in acidic tissue conditions. Further experiments are needed to use electrophysiological and pharmacological approaches to isolate temperature-sensitive and acid-sensitive K2P currents and to fully study their roles in the excitability of nociceptive TG neurons at cooling temperatures and low pH conditions.
We show that cooling temperatures and low pH suppressed both voltage-activated inward Na+ currents and voltage-dependent outward K+ currents in nociceptive-like TG neurons. The effects of cooling temperatures on voltage-activated Na+ currents in nociceptive-like TG neurons are consistent with our previous studies in cultured DRG neurons.7 One would expect that the suppression of voltage-gated Na+ channels by cooling temperatures and low pH would reduce neuronal excitability. However, the excitability of a cell is controlled by multiple factors including cell membrane input resistance as well as voltage-dependent K+ channels. We show that both inactivating components and non-inactivating components of voltage-dependent outward K+ currents were suppressed at cooling temperatures. Previously we have shown that IA currents were suppressed by cooling temperatures in cultured DRG neurons, and this cooling effect on IA currents was suggested to partially contribute to the enhanced excitability of some cultured DRG neurons at cooling temperatures. IA currents are thought to be mediated by Kv1.4, Kv3.4, Kv4.2, and Kv4.3 channels in DRG neurons.11 In TG neurons, we have recently shown that Kv4.3 channels are highly expressed in small-sized and nociceptive-like TG neurons.20 Thus, IA current components recorded in our nociceptive TG neurons are likely to be mediated by Kv4.3 channels and possibly also other inactivating voltage-gated K+ channels. We show that IA current components were more sensitive to cooling temperatures in comparison with the non-inactivating outward current components. The Q10 was 3 for IA current components and was about 2 for non-inactivating outward current components in nociceptive-like TG neurons. Since IA currents play an important role in controlling neurons excitability,7 suppressing IA currents by cooling temperatures in nociceptive-like TG neurons would increase excitability of these neurons at cooling temperatures. We also observed suppression of IA current components at low pH in nociceptive-like TG neurons at 24°C, which may also contribute to the increased excitability of these neurons at low pH levels. However, effects of low pH on IA current components were not observed at cooling temperatures of 17°C and 12°C, likely due to the overwhelmingly inhibitory effects on IA currents by cooling temperatures that occluded the effect of low pH.
Enhanced excitability of nociceptive-like TG neurons by cooling temperatures and low pH may contribute to peripheral nociceptor hypersensitivity leading to hyperalgesia under pathological conditions. We propose that IA K+ channels and temperature- and acid-sensitive K2P channels may be important in nociception associated with cooling temperatures and low pH under both physiological and pathological conditions.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health grants DE018661 and DE023090 to JGG.
References
- 1.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416: 52–58. [DOI] [PubMed] [Google Scholar]
- 2.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108: 705–715. [DOI] [PubMed] [Google Scholar]
- 3.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain 2005; 1: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boscardin E, Alijevic O, Hummler E, Frateschi S, Kellenberger S. The function and regulation of acid-sensing ion channels (ASICs) and the epithelial Na(+) channel (ENaC): IUPHAR Review 19. Br J Pharmacol 2016; 173: 2671–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol (Lond) 2006; 576: 215–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarria I, Ling J, Zhu MX, Gu JG. TRPM8 acute desensitization is mediated by calmodulin and requires PIP(2): distinction from tachyphylaxis. J Neurophysiol 2011; 106: 3056–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarria I, Ling J, Gu JG. Thermal sensitivity of voltage-gated Na+ channels and A-type K+ channels contributes to somatosensory neuron excitability at cooling temperatures. J Neurochem 2012; 122: 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 2007; 447: 855–858. [DOI] [PubMed] [Google Scholar]
- 9.Kanda H, Gu JG. Effects of cold temperatures on the excitability of rat trigeminal ganglion neurons that are not for cold sensing. J Neurochem 2017; 141: 532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 2001; 98: 13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phuket TR, Covarrubias M. Kv4 channels underlie the subthreshold-operating A-type K-current in nociceptive dorsal root ganglion neurons. Front Mol Neurosci 2009; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston J, Forsythe ID, Kopp-Scheinpflug C. Going native: voltage-gated potassium channels controlling neuronal excitability. J Physiol (Lond) 2010; 588: 3187–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 2010; 90: 559–605. [DOI] [PubMed] [Google Scholar]
- 14.Lotshaw DP. Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys 2007; 47: 209–256. [DOI] [PubMed] [Google Scholar]
- 15.Kang D, Hogan JO, Kim D. THIK-1 (K2P13.1) is a small-conductance background K(+) channel in rat trigeminal ganglion neurons. Pflugers Arch 2014; 466: 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E. TREK-1 is a heat-activated background K(+) channel. Embo J 2000; 19: 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol (Lond) 2005; 564: 103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viatchenko-Karpinski V, Ling J, Gu JG. Characterization of temperature-sensitive leak K(+) currents and expression of TRAAK, TREK-1, and TREK2 channels in dorsal root ganglion neurons of rats. Mol Brain 2018; 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GT, Cho YW, Tak HM, Lee JS, Kim EJ, Han J, Kang D. Age-related changes in two-pore domain acid-sensitive K(+) channel expression in rat dorsal root ganglion neurons. Clin Exp Pharmacol Physiol 2012; 39: 43–48. [DOI] [PubMed] [Google Scholar]
- 20.Viatchenko-Karpinski V, Ling J, Gu JG. Down-regulation of Kv4.3 channels and a-type K(+) currents in V2 trigeminal ganglion neurons of rats following oxaliplatin treatment. Mol Pain 2018; 14: 174480691775099. [DOI] [PMC free article] [PubMed] [Google Scholar]