Abstract
Urine drug testing by immunoassay is widely used to detect nonmedical drug use and to monitor patients prescribed controlled substances. A key attribute of urine drug testing immunoassays is cross-reactivity, namely the response of various compounds compared to the target of the assay. In this report, we analyzed the variability in how manufacturer cross-reactivity data are summarized in package inserts for commercially available amphetamines, benzodiazepines, and opiates immunoassays, 3 broad drug classes commonly included in routine drug testing panels. Specifically, we determined the number of compounds tested for cross-reactivity, manner in which cross-reactivity is measured, concentration units used, how often compounds known to be cross-reactive with marketed urine drug testing immunoassays prior to 2010 were tested, availability of the package insert online, and how often cross-reactivity on “designer drugs” was found in the package inserts. There was wide variability in the number of compounds tested (both positive and negative), with the highest number of tested compounds generally found in point-of-care urine drug testing applications. Most package inserts used ng/mL as the concentration units and expressed cross-reactivity in terms of equivalent concentrations to the assay calibrator. Approximately 50% of package inserts were directly available online. Cross-reactivity data were sparse with respect to “off-target” drugs known to be cross-reactive prior to 2010 (an example being quinolone antibiotics and opiates immunoassays) and designer drugs. The present study indicates lack of consistency in cross-reactivity information in package inserts, complicating the interpretation of urine drug testing results. We use 3 example clinical cases to illustrate practical challenges accessing and interpreting cross-reactivity data.
Keywords: amphetamines, benzodiazepines, designer drugs, false positives, immunoassay, opiates
Introduction
Substance abuse continues to be a significant medical and public health issue in the United States and other countries.1,2 An ongoing epidemic of nonmedical use of prescription drugs has added to the existing challenges associated with abuse of cocaine, heroin, methamphetamine, and other “street drugs.”3-6 Drug testing in patients serves as a diagnostic and monitoring tool in the management of substance abuse.7-11
In the clinical setting, urine drug testing (UDT) is commonly used to detect nonmedical drug use and to monitor adherence of patients prescribed controlled substances.7,9-13 Many drugs and drug metabolites are excreted in urine, allowing for windows of detection range from hours to days (or occasionally weeks) for commonly targeted drugs with standard testing techniques.12,13 Immunoassays (antibody-based assays) are currently the most common methodology for UDT, frequently used as “drug screens.”9,11 Clinical laboratories based in hospitals or other larger care facilities often perform UDT immunoassays on automated or semiautomated clinical chemistry platforms. Point-of-care (POC) devices (eg, urine cups or strips) for UDT allow for testing with rapid turnaround time without the need for laboratory equipment in a variety of settings.
Immunoassays used for UDT can be classified into 2 broad categories: those targeting a class of drugs with multiple clinically relevant compounds (eg, amphetamines, benzodiazepines, or opiates) and those narrowly targeted toward a single drug and/or its unique metabolites (eg, buprenorphine, fentanyl, methadone, or the cocaine metabolite benzoylecgonine).9,11,14 A false-negative result occurs when the immunoassay fails to detect a drug or drug metabolite within the targeted class. A common example is that many opiates screening assays poorly detect oxycodone and its metabolites, even though oxycodone is technically an opiate, being a semisynthetic derivative of opium constituents such as morphine or codeine. A false positive occurs when a positive result is caused by a compound outside of the targeted drug or drug class.14-16 There are numerous documented examples of false positives, including a metabolite of labetalol (antihypertensive medication) cross-reacting with amphetamines immunoassays17-19 and quinolone antibiotics cross-reacting with some opiate immunoassays.20
A key attribute of UDT immunoassays is cross-reactivity, namely the degree to which any given compound (eg, drug, drug metabolite, or endogenous compound) can produce a signal on the assay.9,11,14 For UDT immunoassays, this may be expressed as the equivalent concentration of a compound that equals the cutoff concentration of the assay target (eg, the concentration of codeine or hydrocodone that produces the same signal as 300 ng/mL of morphine in an opiates screening assay) or as a percent cross-reactivity compared to a standard such as morphine. During the development of commercially marketed UDT immunoassays, assay manufacturers test drugs, drug metabolites, and endogenous compounds for cross-reactivity and report this data in the assay package insert.14 In addition to manufacturer information, the published literature contains reports of UDT cross-reactivity in case reports, case series, and sometimes more systematic investigations of multiple compounds.9,14-16
The proliferation of clinical laboratory and POC UDT immunoassays available on the market adds an additional element of complexity.9 The UDT immunoassays from different manufacturers vary in assay antibody specificity, calibrators, signal detection, and other factors, all of which can impact cross-reactivity. This is evident when analyzing results of UDT immunoassay proficiency testing.21 For pathologists and other health-care professionals who may be involved in interpreting UDT results, it is important to be able to access and interpret information related to assay cross-reactivity.22 In this report, we analyze the variability in how manufacturer cross-reactivity testing is summarized in package inserts for commercially available amphetamines, benzodiazepines, and opiates immunoassays, 3 broad drug classes commonly included in routinely used UDT panels. Specifically, we determined the number of compounds tested for cross-reactivity, manner in which cross-reactivity is measured, concentration units used, and how often compounds known to be cross-reactive with UDT immunoassays prior to 2010 had information in the various package inserts. We further assessed how often cross-reactivity data for some of the more widely known “designer drugs” (eg, “bath salts” such as mephedrone) were available in the package inserts.23-25 Lastly, we use 3 example clinical cases below to illustrate some of the practical challenges accessing and interpreting cross-reactivity data for UDTs.
Example Case #1
A 26-year-old pregnant woman is referred to a tertiary care medical center for management of preeclampsia. She previously tested presumptive positive for amphetamines on a POC UDT panel ordered by her primary obstetrician. No confirmatory testing was performed. At the medical center, a UDT panel performed in the central clinical laboratory also yielded a presumptive positive for amphetamines. The woman has no known history of nonmedical drug use. Her only known prescription medication is labetalol for hypertension during pregnancy. The obstetrician inquires whether the UDT results indicate use of amphetamine or methamphetamine.
Example Case #2
A 33-year-old man with chronic back pain is being monitored by UDT for adherence to therapy with oxycodone. As part of his controlled substance medication contract, he undergoes regular UDT to verify adherence to oxycodone therapy and to also monitor nonmedical drug use. He has had UDT performed at 2 different locations—a commercial laboratory site near his home and the medical center clinical laboratory affiliated with his pain specialist (more distant from the patient’s home). Urine drug testing for this patient has yielded inconsistent results with regard to opiate screens—those at the commercial laboratory are consistently negative for opiates, while those at the medical center laboratory have been consistently presumptive positive for opiates. The pain specialist consults the medical center pathologist-on-call to discuss the discordant results.
Example Case #3
An 18-year-old man is found unconscious during a rave party. He is brought by ambulance to the emergency department, and a friend accompanying him says the patient purchases “designer drugs” via the “dark web.” The emergency physician calls the clinical laboratory for information on how well the routine UDT panel used at the medical center (consisting of amphetamines, benzodiazepines, cocaine metabolite, opiates, and tetrahydrocannabinol) will detect the types of drugs this patient may have used.
Material and Methods
We compiled information from package inserts from marketed versions of 30 amphetamines, 23 benzodiazepines, and 28 opiates UDT immunoassays. These were chosen as they represent broad specificity immunoassays that have been commonly included on routine drug screening panels for decades, thereby having extensive literature on cross-reactivity.14 These included assays available as discrete applications for random access chemistry analyzers and also POC UDT panels (which typically require the user to run all assays simultaneously). Some of the assays are used by multiple vendors on different instrument platforms. The difference in numbers of inserts analyzed in this study is mainly due to multiple forms of amphetamines assays being marketed (including by the same manufacturer in varying assay specificities such as “amphetamines,” “amphetamine/methamphetamine,” or “amphetamine/ecstasy”) and that some POC UDT applications do not include a benzodiazepines screen. The package insert versions were obtained by direct access (assay being used within the authors’ health-care system), from publicly accessible web sites, or from contacting the manufacturer. A complete list of the assays is in Supplemental Table 1. Note that some assays have had recent updates (eg, additions to cross-reactivity data of an existing product or a more substantial change to a new assay version involving changes in assay antibodies, calibrators, etc), while others have been unchanged for years. An example of an assay that underwent an update with respect to cross-reactivity testing during the last 5 years is the Roche Diagnostics COBAS Benzodiazepines Plus assay, which added data to the package insert for some of the designer benzodiazepines discussed below.
The following information was extracted from each of the package inserts: concentration units for drugs and metabolites (ng/mL, μg/mL, or both units), whether cross-reactivity data were expressed in concentration equivalents (eg, 450 ng/mL of codeine equaled the reactivity of 300 ng/mL morphine in an opiates assay), whether cross-reactivity data were expressed in percent cross-reactivity (in some cases in addition to the data also being expressed in concentration equivalents), whether information was provided on therapeutically or toxicologically relevant urine concentrations, and whether the assay package insert was directly accessible online without restrictions. The package insert was considered not directly accessible if it was either not available at all online or was only available with special access (eg, online account only to those from institutions who were customers) or by request to vendor.
The package inserts were also analyzed for whether they contained information on cross-reactivity of “designer drugs” related to amphetamines, benzodiazepines, and opiates. Compounds examined included amphetamine-like drugs (cathinone, methcathinone, mephedrone, methylenedioxypyrovalerone [MDPV]), designer benzodiazepines (adinazolam, clonazolam, cloniprazepam, diclazepam, etizolam, flubromazepam, flubromazolam, flutazolam, ketazolam, phenazepam), and designer opioids (acetylfentanyl and other fentanyl analogs, AH-7921, MT-45, U-47700). The inserts were also examined for whether they contained cross-reactivity data for out-of-class compounds known to be cross-reactive with at least some marketed assays prior to 2010 based on a previously published detailed analysis of cross-reactivity data available at that time14: amphetamines assays (bupropion, ephedrine, labetalol, mexiletine, phenethylamine, phentermine, propylhexedrine), benzodiazepines assays (diazoxide, ketoprofen, lovastatin, modafinil, oxaprozin), and opiates assays (imipramine, meperidine, quinolone antibiotics [ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin, ofloxacin], ranitidine, rifampin).
The example patient scenarios in this study are hypothetical and do not identify any particular patient. However, these combine common cross-reactivity issues encountered by the corresponding author throughout years of experience.
Results
Variability of Information in Urine Drug Testing Package Inserts
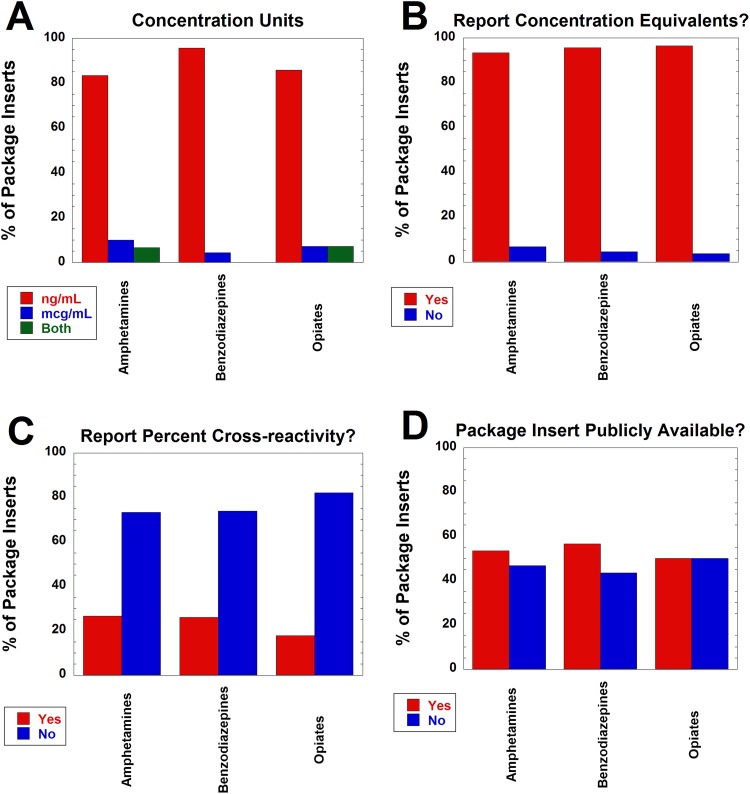
We analyzed information from package inserts from marketed versions of 30 amphetamines, 23 benzodiazepines, and 28 opiates UDT immunoassays with respect to concentration units used, how cross-reactivity was reported, and whether assay package insert was directly available online. Over 80% of package inserts for all 3 types of assays used ng/mL as the only concentration unit for drugs, drug metabolites, and other compounds tested for cross-reactivity. The remainder of the package inserts used either μg/mL or both ng/mL and μg/mL (Figure 1A). Those that contained both units typically used μg/mL for compounds with weak cross-reactivity (eg, expressing as 200 μg/mL instead of 200 000 ng/mL). Over 90% of the package inserts reported cross-reactivity in concentration equivalents (Figure 1B). Less than 30% reported cross-reactivity as a percentage compared to a standard (Figure 1C), although this calculation can be performed if concentrations equivalents are supplied (100 × [concentration of assay target that produces positive signal at intended cutoff]/[concentration of other compound producing equivalent signal]). Only approximately 50% of the package inserts were directly available online (Figure 1D). None of the package inserts analyzed provided information on clinically relevant concentrations of drug or drug metabolites in urine (eg, what concentrations might be expected during therapeutic treatment).
Figure 1.
Variability of information in urine drug testing package inserts for amphetamines (n = 30), benzodiazepines (n = 23), and opiates (n = 28) immunoassays. A, Breakdown on concentration units (ng/mL, μg/mL, or both units) used to describe cross-reactivity data. B, Breakdown of whether package insert reported concentration equivalents for cross-reactivity data. C, Breakdown of whether package insert reported percent cross-reactivity (relative to standard such as morphine for opiates). Note that some package inserts reported in both concentration equivalents and percent cross-reactivity. D, Breakdown of whether package inserts was directly available online. See Methods for more details on definitions.
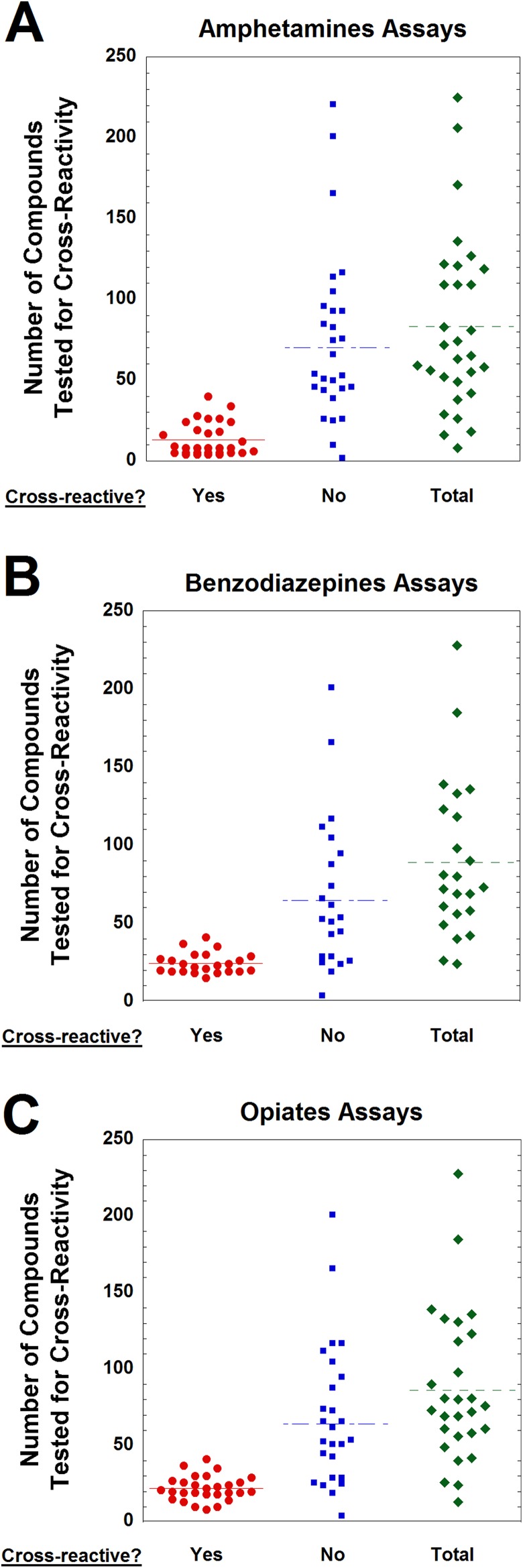
We next analyzed how many compounds were tested for cross-reactivity in the UDT package inserts (Figure 2). There was wide variability in both number of cross-reactive compounds reported and those with no cross-reactivity. For example, for the amphetamines assays, the range of cross-reactive compounds varied from 4 to 40, while the number of compounds with no reported cross-reactivity ranged from 0 to 221 (Figure 2A). A similar range was seen with both benzodiazepines (Figure 2B) and opiates (Figure 2C) immunoassays. Many of the package inserts with a high number of tested compounds with no cross-reactivity were from POC products that tested the same wide array of compounds on a device that performs multiple UDTs simultaneously.
Figure 2.
Number of compounds tested for cross-reactivity as reported in urine drug testing package inserts for (A) amphetamines (n = 30), (B) benzodiazepines (n = 23), and (C) opiates (n = 28) immunoassays. Data are broken down into compounds showing measurable cross-reactivity and those reported as having no cross-reactivity (at least within limits of concentrations tested by the manufacturer).
We next determined whether package inserts contained cross-reactivity data for “designer drugs” and for compounds with cross-reactivity to marketed UDTs known prior to 2010 (Table 1). The latter category includes compounds such as phentermine and the labetalol metabolite 3-amino-1-phenylbutane (APB) that are structurally close to amphetamine and methamphetamine, but not the intended targets of the assay.14,19 For the amphetamine UDT package inserts, ephedrine and pseudoephedrine were the compounds whose cross-reactivity data were most frequently found in package inserts (both 83.3%), followed by phentermine (66.7%), phenethylamine (53.3%), and bupropion (40.0%). Cross-reactivity data for labetalol were found in 20.0% of package inserts; however, only 6.7% had data for the metabolite APB that actually produces most of the cross-reactivity.14,19 Cross-reactivity data for designer amphetamine-like drugs were either absent in all package inserts (MDPV) or found in only 3.3% (cathinone, methcathinone) or 6.7% (mephedrone) of the inserts.
Table 1.
Data on Compound Cross-Reactivity for Amphetamines Urine Drug Testing (UDT Immunoassays).
| Compound | Category | Cross-Reactivity Data to Amphetamines UDTs Documented Before 2010* | Number and Percent of Package Inserts With Cross-Reactivity Data (n = 30) |
|---|---|---|---|
| Bupropion | Psychiatric medication | Yes | 40.0% |
| Cathinone | Cathinone (found in khat) | No | 3.3% |
| Ephedrine | Stimulant (substituted amphetamine) | Yes | 83.3% |
| Labetalol | Antihypertensive | No | 20.0% |
| Labetalol metabolite† | Metabolite of labetalol | Yes | 6.7% |
| MDPV | Designer drug (cathinone) | No | 0.0% |
| Mephedrone | Designer drug (cathinone) | No | 6.7% |
| Methcathinone | Designer drug (cathinone) | Yes | 3.3% |
| Mexiletine | Antiarrhythmic medication | Yes | 3.3% |
| Phenethylamine | Backbone of amphetamine structure | Yes | 53.3% |
| Phentermine | Stimulant (substituted amphetamine) | Yes | 66.7% |
| Propylhexedrine | Stimulant (substituted amphetamine) | Yes | 10.0% |
| Pseudoephedrine | Stimulant (substituted amphetamine) | Yes | 83.3% |
Abbreviation: MDPV, methylenedioxypyrovalerone.
*Whether cross-reactivity data for amphetamines UDTs were available in package inserts and/or published literature prior to 2010. For those who were available prior to 2010, all except labetalol (parent drug) have shown cross-reactivity with at least one marketed amphetamines UDT immunoassay.
†3-amino-1-phenylbutane (APB).
For the benzodiazepines UDT package inserts (Table 2), cross-reactivity data were mostly absent for 5 nonbenzodiazepine compounds known to be cross-reactive with some benzodiazepine UDTs prior to 2010 (diazoxide, ketoprofen, lovastatin, modafinil, oxaprozin). Of these compounds, cross-reactivity data were only present in package inserts for ketoprofen (26.1%), oxaprozin (17.4%), and diazoxide (8.7%). Cross-reactivity data were absent in all 23 package inserts for 4 of 10 designer benzodiazepines examined and only present in 4.3% (1 insert) or 8.7% (2 inserts) for the other 6 designer benzodiazepines: clonazolam (4.3%), diclazepam (4.3%), etizolam (4.3%), flubromazepam (4.3%), flubromazolam (4.3%), and ketazolam (8.7%).
Table 2.
Data on Compound Cross-Reactivity for Benzodiazepines Urine Drug Testing (UDT Immunoassays).
| Compound | Category | Cross-Reactivity Data to Benzodiazepines UDTs Documented Before 2010* | Number and Percent of Package Inserts With Cross-Reactivity Data (n = 23) |
|---|---|---|---|
| Adinazolam | Designer benzodiazepine | No | 0.0% |
| Clonazolam | Designer benzodiazepine | No | 4.3% |
| Cloniprazepam | Designer benzodiazepine | No | 0.0% |
| Diazoxide | Antihypertensive | Yes | 8.7% |
| Diclazepam | Designer benzodiazepine | No | 4.3% |
| Etizolam | Designer benzodiazepine | No | 4.3% |
| Flubromazepam | Designer benzodiazepine | No | 4.3% |
| Flubromazolam | Designer benzodiazepine | No | 4.3% |
| Flutazolam | Designer benzodiazepine | No | 0.0% |
| Ketazolam | Designer benzodiazepine | No | 8.7% |
| Ketoprofen | Nonsteroidal anti-inflammatory drug | Yes | 26.1% |
| Lovastatin | Lipid-lowering agent | Yes | 0.0% |
| Modafinil | Medication for excessive sleepiness | Yes | 0.0% |
| Oxaprozin | Nonsteroidal anti-inflammatory drug | Yes | 17.4% |
| Phenazepam | Designer benzodiazepine | No | 0.0% |
Abbreviation: UDT, urine drug testing.
*Whether cross-reactivity data for benzodiazepines UDTs were available in package inserts and/or published literature prior to 2010. For those who were available prior to 2010, all have shown cross-reactivity with at least one marketed benzodiazepines UDT immunoassay.
For the opiate UDT package inserts (Table 3), the compounds most frequently included for cross-reactivity data were meperidine (82.1%), imipramine (53.6%), and fentanyl (35.7%). Cross-reactivity data for 5 quinolone antibiotics (ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin, and ofloxacin) were low throughout, with data for only ciprofloxacin and norfloxacin included in 7.1% of inserts. Of the 4 designer opioids examined (fentanyl analogs, AH-7921, MT-45, U-47700), data were universally absent except for a single package insert (3.6%) with data for a fentanyl analog (acetylfentanyl).
Table 3.
Data on Compound Cross-Reactivity for Opiates Urine Drug Testing (UDT Immunoassays).
| Compound | Category | Cross-Reactivity to Amphetamines UDTs Documented Before 2010* | Number and Percent of Package Inserts With Cross-Reactivity Data (n = 28) |
|---|---|---|---|
| Ciprofloxacin | Quinolone antibiotic | Yes | 7.1% |
| AH-7921 | Designer opioid | No | 0.0% |
| Fentanyl | Synthetic opioid (nonopiate) | Yes | 35.7% |
| Fentanyl analog | Designer opioid | No | 3.6% |
| Imipramine | Tricyclic antidepressant | Yes | 53.6% |
| Levofloxacin | Quinolone antibiotic | Yes | 0.0% |
| Meperidine | Synthetic opioid (non-opiate) | Yes | 82.1% |
| Moxifloxacin | Quinolone antibiotic | Yes | 0.0% |
| MT-45 | Designer opioid | No | 0.0% |
| Norfloxacin | Quinolone antibiotic | Yes | 7.1% |
| Ofloxacin | Quinolone antibiotic | Yes | 0.0% |
| U-47700 | Designer opioid | No | 0.0% |
Abbreviation: UDT, urine drug testing.
*Whether cross-reactivity data for opiates UDTs were available in package inserts and/or published literature prior to 2010. For those who were available prior to 2010, all have shown cross-reactivity with at least one marketed opiates UDT immunoassay.
Resolution of Case #1
Labetalol is now well established to produce cross-reactivity on multiple marketed amphetamine UDTs mainly due to its metabolite APB, which has a chemical structure close to that of amphetamine.17-19 The shift toward labetalol as a preferred medication for managing hypertension in pregnancy means that cross-reactivity to amphetamines UDTs presents a special challenge within obstetrics. As indicated above, very few of the amphetamines UDT package inserts provide cross-reactivity data on the metabolite, although some do provide data on the parent drug (usually reporting as having no cross-reactivity). Thus, an examination of package inserts alone could easily lead to the conclusion that labetalol cross-reactivity would not be an explanation for the presumptive positive screens for this patient, which may lead to erroneous assignation of nonmedical drug use. This is a situation where literature search would be more insightful than package insert information. Confirmatory testing may also be indicated. If labetalol is the cause of the presumptive positive amphetamine UDT screens, standard amphetamines confirmatory testing would be expected to be negative.
Resolution of Case #2
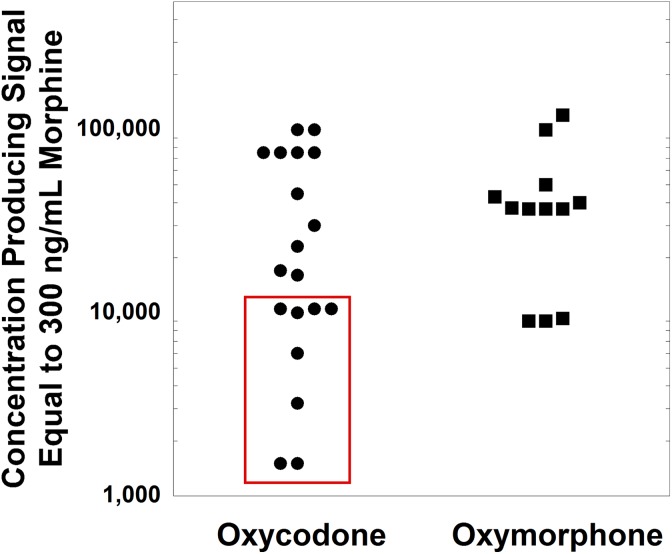
Variable cross-reactivity of traditional opiate UDTs to oxycodone has been well established, with most marketed assays having low cross-reactivity.14,16,21 This can be confusing, since oxycodone is a semisynthetic opiate sharing the core chemical structure of codeine and morphine. Using a 300 ng/mL positive cutoff (morphine as calibrator), the equivalent cross-reactive concentration of oxycodone varies from approximately 2500 to >75 000 ng/mL according to the package insert data analyzed in the present study. Figure 3 plots the concentrations of oxycodone and oxymorphone (main metabolite) that are reported in opiates package inserts to produce cross-reactivity equivalent to that of 300 ng/mL morphine (using package inserts analyzed in the present study). The oxycodone concentrations outlined by the red box in Figure 3 are within the range of concentrations seen in a detailed pharmacokinetic study of 20 mg oxycodone administered to healthy adult volunteers.26 As can be seen, 11 of the oxycodone assays plotted in Figure 3 have cutoffs for oxycodone higher than the upper limit of the concentrations observed in the pharmacokinetic study. The generally poor cross-reactivity of opiates UDT immunoassays to oxycodone has led to the development of oxycodone-specific immunoassays that provide sensitive and targeted detection of oxycodone and its metabolites.9,21,27 In the case scenario, use of an oxycodone immunoassay and/or confirmatory testing would provide more reliable assessment of adherence to therapy.
Figure 3.
Concentrations of oxycodone and oxymorphone (oxycodone metabolite) producing equivalent signal to 300 ng/mL for morphine urine immunoassays, as reported in package inserts analyzed in this study. The oxycodone concentrations outlined by the red box are within the range of concentrations seen in a detailed pharmacokinetic study of 20 mg oxycodone administered to healthy adult volunteers.26 The 4 data points plotted at 75 000 ng/mL for the oxycodone concentration were reported in the package inserts as >75 000 ng/mL.
Resolution of Case #3
This is a challenging and increasingly common question with the proliferation of designer drugs. Compounds misleadingly labeled “bath salts” are often amphetamine-like compounds such as mephedrone or MDPV.24,28,29 There are a wide array of other amphetamine-like drugs such as the cathinones and the 2C series. The abuse of designer benzodiazepines is a somewhat more recent development in the United States, although the drugs themselves often have long histories, developed by pharmaceutical companies in the 1960s and 1970s and then either abandoned for the clinical market or marketed in a limited number of countries.30-32 Synthetic opioids (including fentanyl analogs and other drugs such as U-47700) have also recently emerged as designer drugs of abuse.3,31,33,34 For the case scenario, the data in the present study should emphasize that package inserts are unlikely to contain data on these designer drugs. A literature search is more likely to reveal any information, if available. For example, a recent report contains data on how well multiple marketed benzodiazepines UDT immunoassay detect designer benzodiazepines, of which some do cross-react well with UDTs.35
Discussion
Interpretation of drug testing can be challenging yet have significant consequences for the patient.7,9,11,12,22 Some of the complications arise with the different purposes for which drug testing is used. In the context of emergency medicine, identification of drug overdoses may often be the primary consideration. In substance abuse programs and pain management, verification that a patient is actually adhering to therapy with a controlled substance may be as important as detecting nonmedical use of other substances.10,13,36 An additional challenge is that the downstream consequences of drug testing can involve a variety of personnel including nurses, pharmacists, social workers, probation officers, and medical review officers. The clinical laboratory may thus interact with personnel of diverse backgrounds seeking to understand UDT results. The multitude of vendors offering drug testing, including proliferation of many POC immunoassays, further complicates interpretation, as can be highlighted when discrepant results are seen for the same patient.22
Immunoassays are currently the most widely used method for UDT.22 In addition to immunoassays, mass spectrometry (MS)-based analytical methods such as gas chromatography/MS or liquid chromatography/tandem mass spectrometry (LC/MS/MS) provide specific identification of drugs and drug metabolites.7,11 Mass spectrometry–based methods are often used for confirmation of positive immunoassay screening results or for detection of drugs or drug metabolites undetectable or poorly detectable by immunoassays. An emerging but currently less common application of MS-based methods is as the direct method for UDT, bypassing initial screening by immunoassays.37 Although an increasing number of clinical laboratories are utilizing MS-based assays for UDT testing, relatively few hospital- or physician office–based laboratories have the capability to perform this testing and even fewer with rapid turnaround time.
The main barriers for adoption of MS-based technology by clinical laboratories include high cost of instrumentation (eg, LC/MS/MS analyzers often have capital purchase prices exceeding US$200 000), technical complexity of operation, and labor-intensive sample preparation and results analysis.38-40 There are relatively few FDA-cleared MS-based assays available in the United States for urine drug of abuse testing, requiring laboratories to validate their own “laboratory-developed tests” that would be high complexity under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) regulations.41 In contrast, most marketed immunoassays for drug analysis are in the CLIA moderate or waived complexity categories that have less stringent constraints than high-complexity tests.11
Although urine as a specimen offers a number of advantages for drug testing, there are a number of challenges with UDT immunoassays.9,14,15 These include variation in antibodies, assay format, vulnerability to interference, calibrators, and chosen cutoff concentration. Variability in drug metabolism and UDT assay cross-reactivity for metabolites add another complicated variable.
Shifting trends in drug use can influence how manufacturers design UDT immunoassays.16 For example, diazepam was the most commonly prescribed benzodiazepine of the 1970s and 1980s in the United States and was the overall most prescribed drug for some of those years. Not surprisingly, benzodiazepine UDTs developed in that era tended to target diazepam and/or diazepam metabolites such as nordiazepam or oxazepam. However, there has been a shift in benzodiazepine prescription patterns and 3 other benzodiazepines (alprazolam, clonazepam, and lorazepam) steadily increased in prescription volumes until all 3 overtook diazepam in frequency of prescriptions in the United States by 2002 (drug prescription trends compiled and analyzed in detail by Krasowski et al16; see especially supplemental data in that publication). This trend has continued to the present, as evidenced by data on outpatient prescription volumes.42 As a consequence, some manufacturers have redesigned benzodiazepine UDT immunoassays to better detect these historically “newer” benzodiazepines.
Cross-reactivity is an important aspect of UDT immunoassays.9,14,15 Even though interpretation can be complicated (eg, drugs with cross-reactive metabolites), cross-reactivity data can help explain results of testing. As the results in the present study show, there is wide variability in how manufacturers present cross-reactivity data (see Table 4 for summary), including units of concentration, manner in which cross-reactivity is expressed (percent cross-reactivity vs equivalent concentrations), number of compounds tested for cross-reactivity, and whether the package insert is directly available on the Internet. In our analysis of the number of compounds tested for cross-reactivity, there were as few as 4 positively cross-reactive compounds reported for one assay. Four UDT package inserts reported no compounds negative for cross-reactivity. On the other extreme, some package inserts reported cross-reactivity data for over 200 compounds.
Table 4.
Limitations of Cross-Reactivity Data in Package Inserts.
| Limitation | Comments |
|---|---|
| Variable number of compounds tested |
|
| Limited data on drug metabolites |
|
| Sparse data on designer drugs |
|
| Use of different concentration units |
|
| Variable availability of package inserts online |
|
High variability in data was also noted with cross-reactivity of compounds previously identified over 8 years ago to be cross-reactive on specific UDT immunoassays. Perhaps the best example of this is cross-reactivity of quinolone antibiotics to opiates immunoassays, a finding published in a high-impact general medical journal (JAMA) 17 years ago.20 Only 2 of 28 opiate immunoassay package inserts analyzed in the present study contained any information on quinolone antibiotic cross-reactivity. Similar low rates were seen for other compounds previously shown to be cross-reactive. The paucity of data makes it challenging to interpret UDT results. For the quinolone antibiotics, a literature search would reveal the 2001 publication that had analyzed 11 different quinolone antibiotics for cross-reactivity. However, without data on how more recent versions of UDT assays cross-react, it is not possible to predict cross-reactivity.
A general trend throughout package inserts is that they often do not contain cross-reactivity data on drug metabolites (unless the metabolites of one drug are themselves also a parent drug) and designer drugs.14,43 There are practical challenges in that metabolism of designer drugs may be poorly understood. Further, reference standards for drug metabolites and designer drugs may be difficult and costly to obtain. Labetalol is a good example of a drug whose metabolite has higher cross-reactivity to UDT immunoassays than the parent compound. The labetalol metabolite APB cross-reacts well with amphetamine UDT immunoassays (first reported in 1995), while the parent drug has much weaker or no cross-reactivity, as demonstrated in multiple publications.17-19 Yet in our analysis, data for the metabolite were found in only 2 of 30 current amphetamines UDT package inserts (both from the same manufacturer). The low amount of cross-reactivity data for designer drugs is not surprising, since some have only recently emerged as drugs of abuse.17-19 For designer drugs, published literature often provides more information, as illustrated with a recent publication on designer benzodiazepine cross-reactivity with UDT immunoassays.35
One regulatory challenge is that there are no established guidelines for which and how many compounds to test for cross-reactivity,14 although there are publications such as Guidance Document C-52 from the Clinical and Laboratory Standards Institute that can guide how to perform and report such testing once compounds are selected.44 Many package inserts utilize language such as “structurally related” compounds, although it is not clear how this is defined, either by manufacturers or the FDA. The FDA guidance does state that labeling of drug of abuse assays should include information on cross-reactivity/interferences, with cross-reactivity defined as how much the assay antibodies cross-react with “similar” drugs/metabolites/compounds.45 For the amphetamines, for example, ephedrine, phentermine, and pseudoephedrine show obvious similarity in chemical structure. Structural resemblance is less obvious with other compounds known to be cross-reactive (eg, quinolone antibiotics compared to morphine).
One systematic computational approach to this problem is molecular similarity, a computational technique that quantifies chemical similarity of one compound to the other. This approach has been used to rationalize and predict cross-reactivity of immunoassays used in UDT,14,16,43,46 therapeutic drug monitoring,47 and endocrinology.48 Regardless of approach, it would help to have more consistency in cross-reactivity testing, placing priority on compounds known to be cross-reactive in at least some assays and those with higher structural similarity to the target of the assay. This is an area of potential improvement for both regulators and manufacturers.
Overall, pathologists and other health-care professionals should be aware of the limitations of manufacturer information for UDT immunoassays. This can be especially challenging when managing patients who have had testing performed at other institutions or laboratories or in health-care systems utilizing a variety of different UDTs. Ideally, the number of different UDT systems should be minimized where feasible. In working up discrepant or confusing results, it can be time-consuming to determine which exact assays were used and attempt to obtain package inserts. For package inserts that are not readily available online, faxing or scanning the primary document can be challenging due to irregular sizes and small font. Literature searches for publications on cross-reactivity play an important role, particularly for designer drugs or other compounds that are unlikely to have been tested by the manufacturer for cross-reactivity. Confirmatory testing using MS should be considered when the clinical question cannot be answered by immunoassay screen alone. Lastly, pathologists and the clinical laboratory can play a helpful consultative role in drug test interpretation and assay selection.
Supplemental Material
APC-18-0033_Final_Supplemental_Table_1 for A Difficult Challenge for the Clinical Laboratory: Accessing and Interpreting Manufacturer Cross-Reactivity Data for Immunoassays Used in Urine Drug Testing by Justine M. Reschly-Krasowski, and Matthew D. Krasowski in Academic Pathology
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Matthew D. Krasowski, MD, PhD  https://orcid.org/0000-0003-0856-8402
https://orcid.org/0000-0003-0856-8402
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Akerele E, Olupona T. Drugs of abuse. Psychiatr Clin North Am. 2017;40:501–517. [DOI] [PubMed] [Google Scholar]
- 2. Lipari RN, Van Horn SL. Trends in Substance Use Disorders Among Adults Aged 18 or Older. CBHSQ Report. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2013:1–10. [PubMed] [Google Scholar]
- 3. Pergolizzi JV, Jr, LeQuang JA, Taylor R, Jr, Raffa RB; NEMA Research Group. Going beyond prescription pain relievers to understand the opioid epidemic: the role of illicit fentanyl, new psychoactive substances, and street heroin. Postgrad Med. 2018;130:1–8. [DOI] [PubMed] [Google Scholar]
- 4. Brady KT, McCauley JL, Back SE. Prescription opioid misuse, abuse, and treatment in the United States: an update. Am J Psychiatry. 2016;173:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weaver MF. Prescription sedative misuse and abuse. Yale J Biol Med. 2015;88:247–256. [PMC free article] [PubMed] [Google Scholar]
- 6. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. [DOI] [PubMed] [Google Scholar]
- 7. Mahajan G. Role of urine drug testing in the current opioid epidemic. Anesth Analg. 2017;125:2094–2104. [DOI] [PubMed] [Google Scholar]
- 8. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melanson SE. The utility of immunoassays for urine drug testing. Clin Lab Med. 2012;32:429–447. [DOI] [PubMed] [Google Scholar]
- 10. Magnani B, Kwong T. Urine drug testing for pain management. Clin Lab Med. 2012;32:379–390. [DOI] [PubMed] [Google Scholar]
- 11. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29:436–448. [DOI] [PubMed] [Google Scholar]
- 12. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clinic Proc. 2008;83:66–76. [DOI] [PubMed] [Google Scholar]
- 13. Cone EJ, Caplan YH, Black DL, Robert T, Moser F. Urine drug testing of chronic pain patients: licit and illicit drug patterns. J Anal Toxicol. 2008;32:530–543. [DOI] [PubMed] [Google Scholar]
- 14. Krasowski MD, Siam MG, Iyer M, Pizon AF, Giannoutsos S, Ekins S. Chemoinformatic methods for predicting interference in drug of abuse/toxicology immunoassays. Clin Chem. 2009;55:1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387–396. [DOI] [PubMed] [Google Scholar]
- 16. Krasowski MD, Pizon AF, Siam MG, Giannoutsos S, Iyer M, Ekins S. Using molecular similarity to highlight the challenges of routine immunoassay-based drug of abuse/toxicology screening in emergency medicine. BMC Emerg Med. 2009;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yee LM, Wu D. False-positive amphetamine toxicology screen results in three pregnant women using labetalol. Obstet Gynecol. 2011;117:503–506. [DOI] [PubMed] [Google Scholar]
- 18. Duenas-Garcia OF. False-positive amphetamine toxicology screen results in three pregnant women using labetalol. Obstet Gynecol. 2011;118:360–361. [DOI] [PubMed] [Google Scholar]
- 19. Gilbert RB, Peng PI, Wong D. A labetalol metabolite with analytical characteristics resembling amphetamines. J Anal Toxicol. 1995;19:84–86. [DOI] [PubMed] [Google Scholar]
- 20. Baden LR, Horowitz G, Jacoby H, Eliopoulos GM. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA. 2001;286:3115–3119. [DOI] [PubMed] [Google Scholar]
- 21. Melanson SE, Baskin L, Magnani B, Kwong TC, Dizon A, Wu AH. Interpretation and utility of drug of abuse immunoassays: lessons from laboratory drug testing surveys. Arch Pathol Lab Med. 2010;134:735–739. [DOI] [PubMed] [Google Scholar]
- 22. Ward MB, Hackenmueller SA, Strathmann FG; Education Committee of the Academy of Clinical Laboratory Physicians and Scientists. Pathology consultation on urine compliance testing and drug abuse screening. Am J Clin Pathol. 2014;142:586–593. [DOI] [PubMed] [Google Scholar]
- 23. Cottencin O, Rolland B, Karila L. New designer drugs (synthetic cannabinoids and synthetic cathinones): review of literature. Curr Pharm Des. 2014;20:4106–4111. [DOI] [PubMed] [Google Scholar]
- 24. German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2013;97:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Musselman ME, Hampton JP. “Not for human consumption”: a review of emerging designer drugs. Pharmacotherapy. 2014;34:745–757. [DOI] [PubMed] [Google Scholar]
- 26. Cone EJ, Heltsley R, Black DL, Mitchell JM, Lodico CP, Flegel RR. Prescription opioids. I. Metabolism and excretion patterns of oxycodone in urine following controlled single dose administration. J Anal Toxicol. 2013;37:255–264. [DOI] [PubMed] [Google Scholar]
- 27. Gingras M, Laberge MH, Lefebvre M. Evaluation of the usefulness of an oxycodone immunoassay in combination with a traditional opiate immunoassay for the screening of opiates in urine. J Anal Toxicol. 2010;34:78–83. [DOI] [PubMed] [Google Scholar]
- 28. Gunderson EW, Kirkpatrick MG, Willing LM, Holstege CP. Substituted cathinone products: a new trend in “bath salts” and other designer stimulant drug use. J Addict Med. 2013;7:153–162. [DOI] [PubMed] [Google Scholar]
- 29. Hall C, Heyd C, Butler C, Yarema M. “Bath salts” intoxication: a new recreational drug that presents with a familiar toxidrome. CJEM. 2013;15:1–6. [DOI] [PubMed] [Google Scholar]
- 30. Marin MJ, van Wijk XMR. Designer benzodiazepines: new drugs challenge laboratories. Clin Foren Toxicol News. 2018;2018:1–6. [Google Scholar]
- 31. Graddy R, Buresh ME, Rastegar DA. New and emerging illicit psychoactive substances. Med Clin North Am. 2018;102:697–714. [DOI] [PubMed] [Google Scholar]
- 32. Moosmann B, King LA, Auwarter V. Designer benzodiazepines: a new challenge. World Psychiatry. 2015;14:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu L, Wheeler SE, Venkataramanan R, et al. Newly emerging drugs of abuse and their detection methods: an ACLPS critical review. Am J Clin Pathol. 2018;149:105–116. [DOI] [PubMed] [Google Scholar]
- 34. Nelson ME, Bryant SM, Aks SE. Emerging drugs of abuse. Emerg Med Clin North Am. 2014;32:1–28. [DOI] [PubMed] [Google Scholar]
- 35. Pettersson Bergstrand M, Helander A, Hansson T, Beck O. Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays. Drug Test Anal. 2017;9:640–645. [DOI] [PubMed] [Google Scholar]
- 36. McMillin GA, Slawson MH, Marin SJ, Johnson-Davis KL. Demystifying analytical approaches for urine drug testing to evaluate medication adherence in chronic pain management. J Pain Palliat Care Pharmacother. 2013;27:322–339. [DOI] [PubMed] [Google Scholar]
- 37. McMillin GA, Marin SJ, Johnson-Davis KL, Lawlor BG, Strathmann FG. A hybrid approach to urine drug testing using high-resolution mass spectrometry and select immunoassays. Am J Clin Pathol. 2015;143:234–240. [DOI] [PubMed] [Google Scholar]
- 38. Garg U, Zhang YV. Mass spectrometry in clinical laboratory: applications in therapeutic drug monitoring and toxicology. Methods Mol Biol. 2016;1383:1–10. [DOI] [PubMed] [Google Scholar]
- 39. Wu AH, French D. Implementation of liquid chromatography/mass spectrometry into the clinical laboratory. Clin Chim Acta. 2013;420:4–10. [DOI] [PubMed] [Google Scholar]
- 40. Zhang YV, Wei B, Zhu Y, Zhang Y, Bluth MH. Liquid chromatography-tandem mass spectrometry: an emerging technology in the toxicology laboratory. Clin Lab Med. 2016;36:635–661. [DOI] [PubMed] [Google Scholar]
- 41. Weiss RL. The long and winding regulatory road for laboratory-developed tests. Am J Clin Pathol. 2012;138:20–26. [DOI] [PubMed] [Google Scholar]
- 42. Kane SP. ClinCalc DrugStats Database, version 17.0. 2017. http://clincalc.com/DrugStats. Accessed October 5, 2018.
- 43. Krasowski MD, Ekins S. Using cheminformatics to predict cross reactivity of “designer drugs” to their currently available immunoassays. J Cheminform. 2014;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clinical and Laboratory Standards Institute. Toxicology and Drug Testing in the Medical Laboratory. CLSI Guideline C52, 3rd ed Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 45. Lias CH. FDA Regulation of Drugs of Abuse Tests. SAMHSA DTAB Meeting. 2016. https://www.samhsa.gov/sites/default/files/meeting/documents/dtab-fda-regulation-drugs-abuse-tests-clearance.pdf. Accessed October 5, 2018.
- 46. Petrie M, Lynch KL, Ekins S, et al. Cross-reactivity studies and predictive modeling of “Bath Salts” and other amphetamine-type stimulants with amphetamine screening immunoassays. Clin Toxicol (Phila). 2013;51:83–91. [DOI] [PubMed] [Google Scholar]
- 47. Krasowski MD, Siam MG, Iyer M, Ekins S. Molecular similarity methods for predicting cross-reactivity with therapeutic drug monitoring immunoassays. Ther Drug Monit. 2009;31:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol. 2014;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APC-18-0033_Final_Supplemental_Table_1 for A Difficult Challenge for the Clinical Laboratory: Accessing and Interpreting Manufacturer Cross-Reactivity Data for Immunoassays Used in Urine Drug Testing by Justine M. Reschly-Krasowski, and Matthew D. Krasowski in Academic Pathology