Abstract
The current standard of care for the management of estrogen receptor (ER)-positive and human epidermal growth factor receptor 2 (HER2)-negative breast cancer has been redefined by the introduction of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors. Although adults aged 65 years and older account for the majority of patients with breast cancer, limited data are available about the age-specific dosing, tolerability, and benefit of CDK4/6 inhibitors in this growing population. Older adults are under-represented in clinical trials and as a result, clinicians are forced to extrapolate from findings in younger and healthier patients when making treatment decisions for older patients. In this article, we review the limited age-specific evidence on the efficacy, toxicity, and quality of life (QoL) outcomes associated with the use of CDK4/6 inhibitors in older adults. We also describe ongoing trials evaluating CDK4/6 inhibitors in the older population and highlight that only a minority of adjuvant and metastatic trials of CDK4/6 inhibitors in the general breast cancer population includes geriatric assessments. Finally, we propose potential strategies to help guide decision making for fit and unfit older patients based on disease endocrine sensitivity, the need for rapid response and geriatric assessment.
Keywords: breast cancer, CDK4/6 inhibitors, endocrine therapy, older adults
Introduction
Aging is a risk factor for cancer whose incidence will increase in the next decades due to the increasing longevity of the population.1,2 Breast cancer is the most common cancer diagnosed in women.3 According to the Surveillance, Epidemiology and End Results Program (SEER), there were 266,120 new breast cancer diagnoses in 2018 in the United States, which constitute 15.3% of new cancer diagnoses.3 Over 40% of breast cancers are diagnosed in older patients (65–74 years: 24.1%, 75–84 years: 13.6%, 84+ years: 5.5%) and over 50% of breast cancer deaths occur in the older adult population (65–74 years: 24.1%; 75–84 years: 19.7%; 84+ years: 16.9%).
Approximately 80% of breast cancers are estrogen receptor (ER) positive and 20% are human epidermal growth factor receptor 2 (HER2) positive.4 ER-positive breast cancer patients tend to be older, have well-differentiated disease, smaller tumor size and negative lymph node status, which, along with a more indolent biology, contributes to the better survival compared with other disease subtypes.4 Yet, older women account for the majority of breast cancer deaths despite significant advances having been achieved in its diagnosis and treatment. In fact, older adults are historically under-represented in clinical trials, and reports indicate that the accrual rates of older adults have not improved in the last decade.5,6 The age of older patients enrolled in registration trials supporting United States Food and Drug Administration (FDA) approval from 2005 to 2015 continues to be low (65–74 years: 17%; 75–79 years: 3%; 80+: 1%).7 Furthermore, older patients on clinical trials may be fitter and not necessarily representative of the older population as a whole. It is also well recognized that older patients with breast cancer may have impairments that correlate with chemotherapy toxicity8 and are frequently underdetected in routine practice.9 These discrepancies between patients seen in the real world and trial participants affect the applicability of therapeutic recommendations for older patients.10
Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors have been a significant breakthrough in the management of ER-positive, HER2-negative breast cancer. In this review, we discuss the rationale for targeting CDK4/6 and clinical trials evaluating CDK4/6 inhibitors, highlighting efficacy, toxicity, and quality of life (QoL) outcomes relevant to older adults based on age-specific data published in literature or provided by the manufacturing companies where available.
Targeting CDK4/6
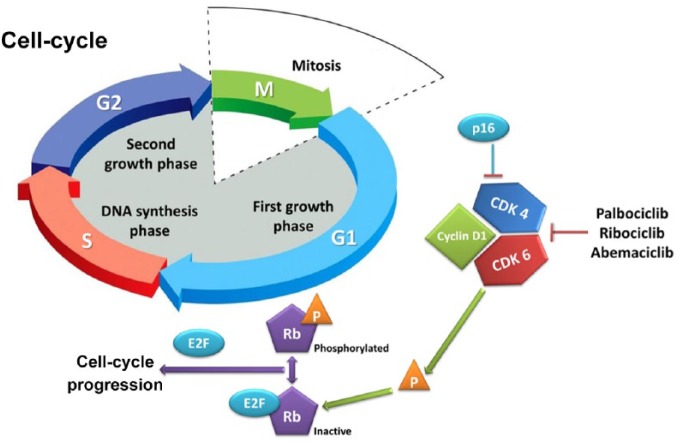
One of the hallmarks of cancer is the ability to circumvent programs regulating cell proliferation, senescence, and apoptosis.11 Each step of the cell cycle has checkpoints strictly governed by CDK, which include a cyclin (protein) and a kinase (enzyme).12 A critical step is the transition from the G1 phase, in which cells grow, to the S phase, in which deoxyribonucleic acid (DNA) replication occurs. This step requires binding of cyclin D1 to CDK4 and/or CDK6, phosphorylation of the retinoblastoma protein (Rb), and activation of the E2F transcription factor, all of which promote cellular transition through the G1 phase (Figure 1).13 In normal tissues, cell-cycle checkpoints provide mechanisms to halt cellular growth and to remove damaged cells by apoptosis. In malignant cells, however, checkpoint defects lead to uncontrolled growth and tumorigenesis.14
Figure 1.
The retinoblastoma-E2F pathway.
Phosphorylation by the CDK4/6 complex causes conformational changes to the structure of Rb structure and releases E2F, which is necessary for the expression of S-phase genes. Both p16 and the CDK4/6 inhibitors exert their mechanism of action by blocking the phosphorylation of Rb.
CDK4/6, cyclin-D-dependent kinase 4/6; Rb, retinoblastoma.
Cyclin D1 is overexpressed or amplified in a significant proportion of breast cancer, particularly in the ER-positive subtype.15,16 Additionally, antiestrogen medications rapidly downregulate cyclin D1, and deregulated cyclin D1 expression may cause resistance to these agents. Since CDK4 and 6 are important for cyclin-mediated G1–S transition, their inhibition represents an attractive therapeutic option, particularly in ER-positive breast cancer.17 The first CDK4/6 inhibitor, palbociclib, inhibited the growth of ER-positive breast cancer cell lines, and subsequently demonstrated its efficacy in clinical trials. Since the advent of palbociclib, two additional CDK4/6 inhibitors (ribociclib and abemaciclib) have been approved for use in ER-positive breast cancer.18
The cell cycle represents a convergent point for both cancer and aging,19 suggesting that normal aging may interact with cell-cycle targeting. CDK4/6 inhibition leads to a senescent-like cellular phenotype, which, in contrast with that induced by aging, may be reversible upon drug discontinuation.18 Aging has various effects on cell-cycle components which may also influence the efficacy of CDK4/6 inhibitors. The expression of the cell-cycle regulator (and CDK4/6 inhibitor) p16Ink4a in lymphocytes, for example, exponentially increases with chronological age.20
Although CDK4/6 inhibitors act on one of the interfaces between cancer and aging, the effect of cellular senescence on their efficacy has not been adequately studied. Additionally, little is known about the influence of chronological and biological age on CDK4/6 inhibitors’ toxicity.
Potential challenges of CDK4/6 inhibition in older patients
The most common toxicity of CDK4/6 inhibitors palbociclib and ribociclib is myelosuppression, since CDK6 is responsible for the promotion of hematologic precursor proliferation.21 Neutropenia is mediated by cell-cycle arrest without apoptosis, a mechanism distinct to that seen in chemotherapy-related agranulocytosis.22 It is dose related, not cumulative, and its severity often decreases in consecutive cycles. Generally, for grade 1/2 toxicities, no treatment interruptions or dose adjustments are needed. In the case of grade 3 neutropenia on palbociclib, dose adjustments are not required except on day 1 or with concomitant fever. In the case of ribociclib, dose should be held until recovery to grade ⩽ 2 and ribociclib should be resumed on a reduced dose. For abemaciclib, dose should be held until recovery to grade ⩽ 2 and resumed on a reduced dose if grade 3 neutropenia is recurrent. In the case of grade 4 neutropenia, all three drugs require dose interruption until recovery to grade ⩽ 2 followed by dose reduction.23–25 Despite the high rates of neutropenia, febrile neutropenia remains rare, reflecting the mechanistic differences compared with chemotherapy. Other toxicities associated with CDK4/6 inhibitors include: fatigue, mucositis, gastrointestinal toxicities, liver function test abnormalities, and QT prolongation. The rates and significance of these in older patients with the individual agents is discussed in the following sections.
Palbociclib undergoes hepatic metabolism involving oxidation and sulfonation through the cytochrome P450 3A4 (CYP3A) and sulfotransferase enzyme SULT2A1.23 Ribociclib and abemaciclib are also metabolized in the liver, mainly through oxidation via CYP3A4.24,25 Feces is the major route of excretion of CDK4/6 inhibitors. No dose adjustments are required in cases of mild hepatic impairment [total bilirubin ⩽ upper limit of normal (ULN) and AST > ULN, or total bilirubin > 1.0–1.5 × ULN and any AST], which does not impact on their exposure; however, the pharmacokinetics of palbociclib has not been studied in the presence of moderate to severe hepatic impairment. In patients with Child–Pugh B or C, it is recommended to start ribociclib at a reduced dose (400 mg). In patients with Child–Pugh C, it is recommended that abemaciclib be administered once daily.23–25 Similarly, no dose adjustments are required in cases of mild to moderate renal impairment [30 ml/min ⩽ creatinine clearance (CrCl) < 90 ml/min]. The impact of severe renal impairment (CrCl < 30 ml/min) on the pharmacokinetics and exposure of the three drugs is unknown.23–25 Of interest, abemaciclib was shown to inhibit renal tubular secretion transporters and can increase serum creatinine without affecting the glomerular filtration.26 This typically occurs within the first 28 days of treatment and is reversible upon discontinuation. In older adults, this may be erroneously interpreted as renal impairment. Therefore, the measurement of alternative markers including urea, cystatin C and calculated glomerular filtration rate may be considered.
The aging process is associated with decreased physiologic reserve of multiple systems, which may also be affected by comorbidities, drug interactions and cancer itself. Data evaluating the pharmacokinetics and safety of CDK4/6 inhibitors in older adults are lacking. Of particular importance is the fact that the decreased bone marrow reserve seen in older patients may enhance the risk of myelosuppression, which is a common side effect of this class of drugs. Liver metabolism also decreases with aging and this can lead to increased drug exposure and adverse events. The progressive decreases in glomerular filtration rate and renal blood flow may potentially enhance the severity of dehydration in older patients experiencing diarrhea or nausea as a result of treatment. The prolongation of the corrected QT (QTc) interval intrinsic with aging needs to be considered, as it may possibly increase the risk of cardiac adverse events in older patients receiving ribociclib.
A key consideration prior to initiating CDK4/6 inhibitors and when managing toxicities is the potential for drug interactions. This is of particular importance in older patients who are more likely to be taking concurrent medications. Particular care must be taken when drugs affecting the function of CYP3A4 are used concomitantly with CDK4/6 inhibitors. Their hepatic metabolism may be affected by various pharmacological agents which are frequently used in older adults (Table 1).27 Concomitant use of strong CYP3A4 and CDK4/6 inhibitors should be avoided, and alternative therapeutic approaches should be considered. However, if CYP3A4 inhibitors must be administered then dose reductions of CDK4/6 inhibitors are recommended. A list of potentially relevant drugs is shown in Table 1. One particularly common scenario would be the use of direct oral anticoagulants concurrently with CDK4/6 inhibitors. CYP3A4 is involved in the metabolism of apixaban and rivaroxaban, with a minimal role in the metabolism of edoxaban, which is eliminated instead mainly via the efflux transporter P-glycoprotein (P-gp)/ABCB1 system. Palbociclib, ribociclib and abemaciclib inhibit P-gp but are only weak, time-dependent inhibitors of CYP3A4. No data about interactions with edoxaban are available, although the inhibition of P-gp mediated by CDK4/6 inhibitors can theoretically lead to greater exposure to edoxaban. Therefore, either apixaban or rivaroxaban would be a better choice if patients require concomitant oral anticoagulation. While the consideration of concurrent medications is relevant to all patients, the higher rate of comorbid health conditions and therefore polypharmacy in older patients makes this issue particularly pertinent in this population.
Table 1.
Potential drug interactions with CDK4/6 inhibitors and their management.27
| Cytochrome P450 3A | Drug class/agents | Effect | Management |
|---|---|---|---|
| Strong inducers | Anticonvulsants: carbamazepine, phenytoin, barbiturates Cytostatic: mitotane Antibiotics: rifampicin Herbs: St. John’s wort |
Decreased exposure of CDK4/6 inhibitors | Concomitant administration should be avoided |
| Moderate inducers | Endothelin antagonist: bosentan Antiviral: efavirenz, etravirine |
||
| Strong inhibitors | Antiretrovirals: boceprevir, cobicistat, danoprevir and ritonavir, elvitegravir, paritaprevir, ombitasvir, dasabuvir AVP receptor inhibitor: conivaptan Antifungals: itraconazole, ketoconazole, posaconazole, voriconazole Others: grapefruit and grapefruit juice |
Increased exposure of CDK4/6 inhibitors | Concomitant administration should be avoided or dose reduction of CDK4/6 inhibitor is recommended |
| Moderate inhibitors | NK-1 antagonist: aprepitant H2-receptor antagonist: cimetidine Antibiotics: ciprofloxacin, erythromycin Antifungals: clotrimazole, fluconazole Antiarrhythmics: dronedarone Benzodiazepines: tofisopam Protein kinase inhibitor: crizotinib, imatinib Immunosuppressant: cyclosporine SSRI: fluvoxamine |
Increased exposure of CDK4/6 inhibitors | Concomitant administration should be avoided or dose reduction of CDK4/6 inhibitor is recommended |
| Sensitive substrates | Opioids: alfentanil PDE inhibitors: avanafil, vardenafil, sildenafil Antidepressants/anxiolytics: buspirone AVP receptor inhibitor: conivaptan Selective muscarinic M3 antagonist: darifenacin Antiretrovirals: darunavir, saquinavir, tipranavir H1 antihistamine: ebastine mTOR inhibitors: everolimus, sirolimus, tacrolimus Tyrosine kinase inhibitor: ibrutinib MTP inhibitor: lomitapide Statins: lovastatin, simvastatin, atorvastatin Benzodiazepines: midazolam, triazolam, alprazolam Opioid-antagonist: naloxegol Calcium channel blocker: amlodipine, felodipine, diltiazem, nisoldipine, verapamil |
Resulted in increased exposure of the concomitant drugs | Close monitoring of symptoms/dose reduction of concomitant drugs is recommended |
| Moderately sensitive substrates | Benzodiazepines: alprazolam NK-1 antagonist: aprepitant Uricosuric agents: colchicine Glucosylceramide synthase inhibitor: eliglustat Antipsychotic: pimozide, Antiretrovirals: rilpivirine Anticoagulant: rivaroxaban PDE inhibitor: tadalafil |
Resulted in increased exposure of the concomitant drugs | Closely monitoring of symptoms/dose reduction of concomitant drugs is recommended |
| For ribociclib only | |||
| QT-interval-prolonging drugs | Analgesics: methadone Antiarrhythmics: amiodarone, adenosine, disopyramide, flecainide, ibutilide, procainamide, propafenone, quinidine, sotalol Anticonvulsants: fosphenytoin Antidepressants: amitriptyline, citalopram, desipramine, doxepin, fluoxetine, imipramine, maprotiline, nortriptyline, paroxetine, sertraline, venlafaxine Antihistamines: clemastine, diphenhydramine, loratadine Antibiotics: clarithromycin, erythromycin, gatifloxacin, levofloxacin, moxifloxacin, trimethoprim sulfamethoxazole Antifungals: fluconazole, ketoconazole Antivirals: foscarnet, ganciclovir Antimalarials: mefloquine, halofantrine, quinine Antiprotozoals: pentamidine Antipsychotics: chlorpromazine, clozapine, haloperidol, quetiapine, risperidone, ziprasidone Cancer chemotherapeutic agents: arsenic trioxide SERM: tamoxifen Cardiovascular nonantiarrhythmics: Indapamide, Probucol 5-HT-antagonists: ondansetron, dolasetron Gastrointestinal agents: octreotide, droperidol Migraine agents: naratriptan, sumatriptan, rizatriptan, zolmitriptan |
Prolongation of QT interval Torsade de pointes |
Coadministration with ribociclib should be avoided |
AVP, arginine vasopressine; CDK4/6, cyclin-dependent kinase 4/6; 5-HT, 5-hydroxytryptamine; mTOR, mammalian target of rapamycin; MTP, microsomal triglyceride transfer protein; SERM, selective estrogen receptor modulator; SSRI, selective serotonin reuptake inhibitor; PDE, phosphodiesterase.
Palbociclib
Palbociclib was the first CDK4/6 inhibitor to be investigated. However, specific evidence to guide its use in older adults is limited. The phase II PALOMA-1 study tested the safety and efficacy of palbociclib in combination with letrozole versus letrozole alone in the first-line setting for patients with ER-positive HER2-negative advanced breast cancer.28 A total of 76 out of 165 enrolled patients were aged 65+ and derived a median progression-free survival (PFS) of 26.2 months [95% confidence interval (CI) 12.6–not reached (NR)] with palbociclib plus letrozole versus 12.9 months (95% CI 5.7–22.2) with letrozole alone. Grade 3–4 adverse events, dose reduction and discontinuation rates were not influenced by age.
Based on the positive results from PALOMA-1, a large phase III randomized double-blind study (PALOMA-2) was initiated. This study recruited 666 postmenopausal treatment-naïve women with ER-positive, HER2-negative advanced breast cancer. Compared with letrozole and placebo, the combination of palbociclib and letrozole was associated with improved PFS [24.8 versus 14.5 months; hazard ratio (HR) 0.58; 95% CI 0.46–0.72] and objective response rate (ORR; 42% versus 35%).29 Neutropenia was common with the combination compared with letrozole alone (89.5% versus 6.3%), along with increased rates of fatigue, nausea, and alopecia. Overall survival (OS) data are still immature. The PALOMA-2 study enrolled 262 patients (39.3%) aged 65+, who represented 40.8% (n = 181) of those enrolled in the combination arm and 36.5% (n = 81) in the placebo arm. The median age in the experimental arm was 62 years (range: 30–89) and 61 years (range: 28–88) in the placebo arm. In the subgroup analysis, the benefit in PFS was maintained in patients 65+ with an HR of 0.57 (95% CI 0.39–0.84) versus 0.57 (95% CI 0.43–0.74) in patients younger than 65. No age-specific data about toxicities in first line are available.
The double-blind phase III PALOMA-3 randomized 521 women with ER-positive, HER2-negative advanced breast cancer to either palbociclib and fulvestrant or placebo and fulvestrant.30,31 These patients either relapsed during or within 1 year after completion of adjuvant endocrine therapy or progressed on prior palliative hormonal treatment. The combination improved median PFS (9.5 versus 4.6 months; HR 0.46; 95% CI 0.36–0.59), but with higher rates of neutropenia (65% versus 1%, respectively) and fatigue (39% versus 28%). OS data are still immature. One hundred and twenty-nine patients (24.8%) were aged 65+. In the experimental arm, the median age was 57 (range: 30–88) and in the placebo arm it was 56 (range: 29–80). Individuals aged 65+ represented 24.8% (86 patients) and 24.7% (43 patients) of the group, respectively. In the subgroup analysis, older patients derived PFS benefit with an HR of 0.35 (95% CI 0.19–0.62) compared with 0.44 (95% CI 0.32–0.61) in patients younger than 65.
In the PALOMA-3 study, age did not significantly increase the risk of grade 3–4 neutropenia. In patients aged younger than 50, 50–69, and 70+, grade 3–4 neutropenia occurred in 28.7, 57.4, and 31.9%, respectively.32 In multivariate analysis, a trend for association of grade 3–4 neutropenia and infections was detected in patients aged 70+ versus 50–69 and in patients aged 70+ versus younger than 50, although it was not statistically significant.
In a pooled analysis of data from 872 patients enrolled in the randomized phase II and III studies (PALOMA-1, -2 and -3) investigating safety and efficacy of palbociclib plus letrozole or fulvestrant in ER-positive, HER2-negative breast cancer, 221 (25%) of the participants were aged 65–74 and 83 (10%) were aged 75+.33 In the first-line setting for all patients, median PFS was 24.4 months (95% CI 22.0–26.2) in the combined palbociclib and letrozole group versus 13.6 months (95% CI 11.1–16.4) in the placebo and letrozole group with an HR of 0.53 (95% CI 0.44–0.64). In the pretreated group, median PFS was 9.5 months (95% CI 9.2–11.0) in the combined palbociclib and fulvestrant group versus 4.6 months (95% CI 3.5–5.6) in the placebo and fulvestrant group with an HR of 0.46 (95% CI 0.36–0.59). The PFS benefit was observed in patients aged 65–74 and 75+. No new safety concerns were identified in the older population, and no more than 5% had grade 3–4 adverse events except for neutropenia and leukopenia. Neutropenia occurred in 81% of patients treated with palbociclib compared with 5% in the control group; these were consistent in both age groups (77% versus 1% in patients aged 65–74; 90% versus 3% in those aged 75+). Eleven patients (1%) experienced febrile neutropenia on palbociclib compared with none in the placebo group. A total of 166 patients (19%) treated with palbociclib developed serious adverse events of any grade (25% of those aged 65–74 and 30% of those 75+), and 77 patients (9%) had to discontinue it owing to adverse events compared with 22 (5%) in the control group. Neutropenia remained the most frequent reason for discontinuation in 14 patients (1.6%) [4 patients (1.8%) aged 65–74; 4 patients (4.8%) aged 75+]. The average clearance of palbociclib was lower for older patients, but the observed differences were unlikely to be clinically relevant in view of the observed safety and efficacy profile.
An FDA pooled analysis of two studies of CDK4/6 inhibitors also suggested similar efficacy and safety in older women (aged 65+ and 70+) compared with their younger counterparts, although greater serious adverse events and discontinuations occurred in patients aged 65+.34 A recent retrospective analysis of 160 patients aged 65+ (including 92 aged 70+) revealed similar findings.35
The QoL data for palbociclib trials have not been reported by age. In PALOMA-3, using the EORTC QLQ-C30 and BR23 measures, patients in the palbociclib group had less reduction in global QoL from baseline, and experienced a longer time to deterioration of QoL, compared with the placebo group.36 Notably, both groups experienced significant worsening of cognitive and role functioning from baseline. In the palbociclib group, there was a significant decrease in pain from baseline (which was also shown in the PALOMA-2 trial), and improvement in emotional functioning. In PALOMA-2, the two treatment groups did not differ in the QoL change from baseline using Functional Assessment of Cancer Therapy—Breast cancer (FACT-B), Functional Assessment of Cancer Therapy (FACT)—General, and EuroQOL—5 dimensions (EQ-5D) scales.37 However, the palbociclib group experienced significant improvement in pain scores. In both treatment groups, patients without progression of disease had delayed worsening of QoL measured by FACT-B. To what extent these QoL benefits are experienced by older patients has not been described. However, it seems reasonable to conclude that similar benefits are likely, in particular, as use of a CDK4/6 inhibitor may defer the time to cytotoxic chemotherapy in older patients.
Ribociclib
Ribociclib is a CDK4/6 inhibitor with similar preclinical properties to palbociclib. To date, ribociclib has been evaluated in three pivotal trials: MONALEESA-2 and MONALEESA-3, and MONALEESA-7.38–40 Based on the findings of the MONALEESA-2 trial, ribociclib was approved by the FDA in March 201741 and the European Medicines Agency in August 201742 for use in combination with an aromatase inhibitor (AI) as a first-line treatment for HR-positive/HER2-negative advanced or metastatic breast cancer in postmenopausal women. Recently, the FDA has also approved its use in combination with fulvestrant in first or second line, based on the MONALEESA-3 study findings.24 Despite pronounced changes in drug metabolism, absorption, and distribution with increasing age, limited evidence exists to guide therapy with ribociclib in the increasing number of older adults with breast cancer. Clinical trials evaluating ribociclib primarily included women less than age 70. Thus, there exists a significant knowledge gap in the safety and tolerability of delivering ribociclib to older adults with cancer.
In the phase III, randomized, double-blinded MONALEESA-2 study, 668 postmenopausal women ranging from ages 23–91 (median: 62 years) with advanced ER-positive/HER-2 negative breast were assigned to receive either letrozole and ribociclib or letrozole and placebo. Of 334 patients who received ribociclib and letrozole, 150 patients (45%) were 65+, and per the package insert, only 35 patients (11%) were 75+. Moreover, there has been no published cohort data for enrolled patients aged 70+. Similarly, in MONALEESA-3, among the 484 patients who received ribociclib and fulvestrant, the median age was 63 (range: 31–89), but unlike in MONALEESA-2, there are currently no published data on women 65+ who participated in this trial.
A subgroup analysis of the efficacy and safety of MONALEESA-2 patients was conducted based on prespecified age cut off of 65, which included 373 patients less than age 65 years and 295 patients aged 65+.43,44 Among patients receiving ribociclib and letrozole, the median PFS for patients aged 65+ was NR (95% CI 19.3–NR) compared with 18.4 months (95% CI 15.0–NR) in older patients who received letrozole alone. There was no significant difference in the effect of ribociclib treatment on PFS between older (65+; HR 0.608; 95% CI 0.394–0.937) and younger (younger than 65; HR 0.523; 95% CI 0.378–0.723) patients. ORR was greater in the ribociclib arm across both age groups [younger than 65: ribociclib arm, 44% (95% CI: 36–51) and letrozole-only arm 25% (95% CI: 19–31); 65+ years: ribociclib arm, 37% (95% CI: 30–45) and letrozole-only arm, 31% (95% CI: 24–39)].44 The maintained PFS benefit in patients 65+ was confirmed on a more updated analysis with an HR of 0.658 (95% CI 0.466–0.928).38
Among the three pivotal trials studying ribociclib, the most common adverse events reported are neutropenia, leukopenia, and fatigue.38–40 In particular, MONALEESA-2 patients receiving combination ribociclib and letrozole versus experienced grade 3–4 neutropenia (59.3% versus 0.9% in letrozole alone), leukopenia (21% versus 0.6% in letrozole alone), and fatigue (2.4% versus 1% with letrozole).38 Patients receiving ribociclib with letrozole experienced a pulmonary embolism at a rate of 0.6%. Among patients in the ribociclib and letrozole group, 7.5% discontinued due to adverse events, whereas only 2.1% of the letrozole group discontinued the study. In MONALEESA-2, The safety profile of the subgroup analysis by the prespecified age cut off of 65 did not show any apparent differences in systemic exposure related to age. Nausea, alopecia, diarrhea, and vomiting were adverse events reported in >10% of patients in the ribociclib arm over the placebo arm, regardless of the age subgroup. However, there was a >10% increase in the incidence of fatigue and grade 1–2 anemia reported in the ribociclib plus letrozole over the placebo plus letrozole arm in patients 65+.44 The incidence of anemia, hypertension, and asthenia were also higher in older patients, although this was irrespective of treatment arm.
Cardiac toxicity in the form of QT-interval prolongation has been reported with ribociclib, which may cause cardiac repolarization abnormalities by affecting subunits of voltage-gated channels.45 Concomitant use of QT-prolonging medications, such as those shown in Table 1, should be avoided. In the phase III MONALEESA-2 trial, one case of sudden cardiac death was reported46 and found to be related to concomitant use of methadone.46,47 An electrocardiogram (ECG) is required at baseline and treatment can be initiated in cases where the QTc interval is <450 msec on Fridericia’s formula (QTcF); repeat ECGs are recommended on cycle 1, day 14, and cycle 2, day 1, and subsequently as clinically indicated. In cases where the QTcF > 480 msec, a dose interruption is indicated until resolution. In cases where the QTcF > 500 msec, a dose interruption followed by a dose reduction is recommended.24 For the same reason, serum electrolytes need to be monitored and abnormalities should be corrected, especially during the first six cycles of treatment.24 Older adults have a higher baseline risk of QT prolongation due to increasing age itself, as well as pre-existing heart conditions.48–50 Therefore, in older patients with cardiac comorbidities and polypharmacy, ribociclib must be used with caution after a cardiology evaluation, or alternative options including palbociclib can be considered. In cases of long-QT syndrome, recent myocardial infarction, congestive heart failure, unstable angina, bradyarrhythmias or electrolyte abnormalities, the use of ribociclib should be avoided.
Health-related QoL (HRQoL) data in patients 65+ enrolled in MONALEESA-2 were presented at the 2018 American Society of Clinical Oncology Annual Meeting.38 Based on the EORTC QLQ-C30 and BR-23, HRQoL was similar in the two treatment groups. A clinically meaningful improvement was noted for pain in the ribociclib group through the first year of treatment. Both groups had an approximate 28-month median time to deterioration of HRQoL.
Although age-specific data show that patients 65+ benefit from ribociclib and have similar toxicity profile compared with younger patients, very few patients 70+ were included. Because of this, further efforts are needed to prospectively evaluate the safety and tolerability of ribociclib and endocrine therapy in older adults 70 years and above. In this respect, the phase IIIb COMPLEEMENT-1 trial has recruited over 3000 patients, of whom over 20% were aged 70+, and all of whom received letrozole and ribociclib. The results of this study are awaited.
Abemaciclib
One single-arm phase II single-agent study (MONARCH-1) and two randomized studies of abemaciclib plus endocrine therapy have been published (MONARCH 2 and 3). Despite this, the evidence to guide the use of abemaciclib in older patients with breast cancer is, again, limited.
The phase III, randomized, double-blinded MONARCH-2 study included 669 patients with ER-positive, HER2-negative advanced breast cancer who progressed on adjuvant or neoadjuvant endocrine therapy, or progressed following first-line palliative endocrine therapy.51 The study included 245 patients (36.6%) aged 65+. Results demonstrated a statistically significant improvement in PFS of 16.4 versus 9.3 months (HR 0.55; 95% CI 0.45–0.68). Subgroup analyses showed no statistically significant differences in benefit between younger and older patients (HR 0.52 and 95% CI 0.42–0.68 in patients younger than 65 versus HR 0.62 and 95% CI 0.41–0.94 in patients 65+).
The phase III, randomized, double-blinded MONARCH-3 study included 493 patients with ER-positive, HER2-negative advanced breast cancer. The median age of patients was 63 years (range: 32–88) and they had an Eastern Cooperative Oncology Group performance status of 0 or 1. There was a statistically significant improvement in median PFS with abemaciclib in combination with endocrine treatment versus placebo plus endocrine treatment (HR 0.54; 95% CI 0.41–0.72).52 Subgroup analyses showed no differences in efficacy between patients younger than 65 and 65+ (HR 0.53 and 95% CI 0.33–0.77 versus HR 0.57 and 95% CI 0.36–0.90, respectively).
There are no age-specific toxicity data which have been published. In the MONARCH-1 study, all patients had at least one treatment-related adverse event; most importantly, diarrhea (grade 3 in 19.7% of cases), fatigue, nausea, and grade 1–3 renal dysfunction. Similarly, 98.8% of patients treated with abemaciclib in the MONARCH-2 trial and 98.5% in the MONARCH-3 study had an adverse event (grade 3 in 48.5% and grade 4 in 6.4% of patients), especially diarrhea, neutropenia, fatigue, and infections.
It is worth noting that gastrointestinal toxicities, including nausea, vomiting and diarrhea, are more commonly associated with abemaciclib than with the other CDK4/6 inhibitors (although there are no direct comparative data). This different toxicity profile may be due to the fact that abemaciclib has higher selectivity for CDK4, which is the main regulator of the intestinal cell cycle.53 Therefore, while abemaciclib-associated myelosuppression is less prevalent, gastrointestinal toxicity and fatigue are more common.51,52 Gastrointestinal toxicities should be managed with standard nonpharmacological interventions and antidiarrhoeal agents, and clinically relevant drug interactions should be considered (Table 1).22 Prophylactic antidiarrhoeal treatment is usually not necessary, but clinicians should consider that among older patients, even low-grade toxicities such as grade 2 diarrhea may lead to functional decline, and more aggressive interventions may be necessary in this population.54 Data recently presented from MONARCH-2 using the EORTC QLQ-C30, BR-23, and Brief Pain Inventory short form showed no significant difference in HRQoL between the two treatment groups; however diarrhea, appetite loss, and nausea/vomiting were worse in the abemaciclib arm.55 These data were not specific to older patients. Further analysis indicated appetite loss and nausea/vomiting were worse with early cycles and returned to near baseline after cycle 7, whereas diarrhea returned to near baseline post-treatment.
Ongoing research
Major efforts are ongoing to further refine the role of CDK4/6 inhibitors in the management of breast cancer, as shown in Table 2.
Table 2.
Ongoing trials of CDK4/6 inhibitors.
| Trial title | ClinicalTrials.gov identifier | Setting | Primary endpoints |
Secondary endpoints |
Key eligibility criteria relevant to older adults | Geriatric assessments included |
|---|---|---|---|---|---|---|
| Multiple CDK4/6 inhibitors | ||||||
| Endocrine therapy plus CDK4/6 inhibition in first or second line for HR+ advanced breast cancer | NCT03425838 | Palliative | PFS after two lines of treatment | OS; HRQoL; safety; cost effectiveness; ORR | ⩾18 years CrCl ⩾ 30 ml/min No other malignancies |
No |
| Treatment of Canadian postmenopausal women with ER+ advanced breast cancer in the real-world setting with hormone therapy ± targeted therapy | NCT02753686 | Palliative | Duration of treatment | AEs; TTF; treatment adherence and sequencing; PFS; OS; healthcare resource utilization; HRQoL; work-related productivity | ⩾18 years | No |
| Palbociclib | ||||||
| Fulvestrant and palbociclib in treating older patients with hormone-responsive breast cancer that cannot be removed by surgery | NCT02760030 | Palliative | Treatment failure-free survival | Change in: cognition, comorbidities, depression, functional status, falls, nutritional status, social activity and support; adverse events; PFS | ⩾70 years Patients vulnerable or frail by Balducci criteria Serum creatinine 1.5 × ULN |
Blessed Orientation–Memory–Concentration Test; Charlson comorbidity index; Geriatric Depression Scale; falls, Timed Up and Go test; Instrumental Activities of Daily Living; Mini Nutritional Assessment®; Medical Outcome Study; Social Activity Limitations Survey |
| Polaris: palbociclib in HR+ advanced breast cancer: a prospective multicenter non-interventional study | NCT03280303 | Palliative | PFS; QoL; geriatric assessments; biomarkers | ⩾18 years | Geriatric 8; Activities of Daily Living | |
| PALbociclib CoLlaborative Adjuvant Study: a randomized phase III trial of palbociclib with standard adjuvant endocrine therapy versus standard adjuvant endocrine therapy alone for HR+/HER2− early breast cancer (PALLAS) | NCT02513394 | Adjuvant | Invasive DFS | Safety; distant RFS; locoregional RFS; OS | ⩾18 years ECOG PS 0–1 Hb ⩾ 10 g/dl Serum creatinine below ULN or CrCl ⩾ 60 ml/min/1.73 m2 No history of any malignancy |
No |
| Tucatinib, palbociclib and letrozole in metastatic HR+ and HER2− breast cancer | NCT03054363 | Palliative | Safety; PFS | PK assessment of tucatinib and palbociclib | ⩾18 years ECOG PS 0–1 Hb ⩾9 g/dl Serum creatinine ⩽ 1.5 mg/dl INR and aTTP ⩽1.5 × ULN LVEF ⩾ 50% No cardiovascular disease |
No |
| Fulvestrant, palbociclib and erdafitinib in ER+/HER2−/FGFR-amplified metastatic breast cancer | NCT03238196 | Palliative | Safety | PFS; ORR; CBR; PK assessment of erdafitinib; tolerability; measures of serum phosphate calcium, vitamin D, parathyroid hormone; FGFR1 amplification; next generation sequencing; cfDNA | ⩾18 years ECOG PS 0–1 Hb ⩾ 9 g/dl CrCl ⩾ 40 ml/min/1.73 m2 Albumin ⩾ 2.0 g/dl Normal potassium No corneal or retinal abnormalities No uncontrolled intercurrent illness including chronic liver or renal failure |
No |
| Palbociclib in combination with tamoxifen as first-line therapy for metastatic HR+ breast cancer | NCT02668666 | Palliative | Response rates (partial and complete) | Safety and tolerability; PFS; CBR; OS | ⩾18 years ECOG PS 0–2 No concurrent malignancy or malignancy within 3 years |
No |
| A study of palbociclib (PD-0332991) + letrozole versus placebo + letrozole for first-line treatment of Asian postmenopausal women with ER+/HER2− advanced breast cancer (PALOMA-4) | NCT02297438 | Palliative | PFS | OS; ORR; DoR; HRQoL; functional assessment; tumor tissue biomarkers; survival probability | 18–70 years ECOG PS 0–1 Adequate organ and marrow function |
No |
| Palbociclib After CDK and Endocrine therapy (PACE) | NCT03147287 | Palliative | PFS | ORR; safety and tolerability | ⩾18 years ECOG PS 0–1 0–1 prior lines of palliative chemotherapy 1–2 prior lines of palliative endocrine therapy |
|
| Ribociclib | ||||||
| Ribociclib and aromatase inhibitor in treating older participants with HR+ metastatic breast cancer | NCT03477396 | Palliative | Incidence of grade 2 and above toxicities related to ribociclib | AEs; dose reductions, dose delays, dose discontinuations; ORR; CBR; PFS; OS; PK | ⩾70 years Hb ⩾ 9.0 g/dl Normal electrolytes Serum creatinine < 1.5 mg/dl or CrCl ⩾ 50 ml/min No concurrent malignancy or malignancy within 3 years No clinically significant, uncontrolled heart disease or cardiac repolarization abnormalities Child–Pugh score A |
Yes |
| Study to assess the safety and efficacy of ribociclib (LEE011) in combination with letrozole for the treatment of men and pre/postmenopausal women with HR+/HER2− advanced breast cancer (COMPLEEMENT-1) | NCT02941926 | Palliative | AEs: grade 3/4 AEs and SAEs | TTP, ORR, CBR, patient reported outcome | ECOG PS 0–2 Adequate organ and bone marrow function QTcF interval at screening < 450 msec |
No |
| Study of ribociclib with everolimus + exemestane in HR+/HER2− locally advanced/metastatic breast cancer post progression on CDK4/6 inhibitor (TRINITI-1) | NCT02732119 | Palliative | Maximum tolerated dose/recommended dose; clinical benefit | PFS; OS; ORR; DoR; PS deterioration | ⩾18 years ECOG PS 0–1 Adequate organ and bone marrow function No clinically significant, uncontrolled heart disease or recent cardiac events |
No |
| Phase Ib trial of LEE011 with everolimus (RAD001) and exemestane in the treatment of HR+/HER2− advanced breast cancer | NCT01857193 | Palliative | Dose-limiting toxicities; safety and tolerability | Plasma concentration–time profiles; ORR; DoR; PFS; DCR | ⩾18 years ECOG PS 0–1 Fasting serum cholesterol ⩽ 300 mg/dl or 7.75 mmol/l and fasting triglycerides ⩽ 2.5 × ULN No clinically significant, uncontrolled heart disease LVEF ⩾ 50% |
No |
| Study of efficacy and safety of LEE011 in men and postmenopausal women with advanced breast cancer (MONALEESA-3) | NCT02422615 | Palliative | PFS | OS; ORR; PS deterioration; safety; HRQoL; CBR; time to response; DoR | ⩾18 years ECOG PS 0–1 Adequate organ and marrow function No clinically significant, uncontrolled heart disease |
No |
| Study of LEE011, BYL719 and letrozole in advanced ER+ breast cancer | NCT01872260 | Palliative | Dose-limiting toxicities; safety and tolerability; PK profiles | Plasma concentration–time profiles; ORR; DoR; PFS; PK assessment | Impaired cardiac function Clinically manifested diabetes mellitus |
No |
| CDK4/6 inhibitor, LEE011 (ribociclib), in combination with adjuvant endocrine therapy at varying duration for ER+ breast cancer (LEADER) | NCT03285412 | Adjuvant | Completion of treatment/safety and tolerability | AEs; switch in endocrine therapy; DFS | ⩾18 years ECOG PS 0–1 No prior history of other malignancies within past 5 years Hb ⩾ 9.0 g/dl Serum creatinine <1.5 mg/dl or CrCl ⩾ 50 ml/min Normal electrolytes Fasting plasma glucose < 140 mg/dl/ 7.7 mmol/l No uncontrolled intercurrent illness No clinically significant, uncontrolled heart disease LVEF ⩾ 50% |
No |
| Adjuvant ribociclib with endocrine therapy in HR+/HER2− high-risk early breast cancer (EarLEE-1) | NCT03078751 | Adjuvant | Safety and tolerability | – | ECOG PS 0–1 Adequate organ function Electrolytes within normal limits QTcF interval at screening < 450 msec No clinically significant, uncontrolled heart disease |
No |
| New Adjuvant TriAl with LEE (NATALEE)* | – | Adjuvant | Invasive DFS | – | – | – |
| Study of efficacy of ribociclib after progression on CDK4/6 inhibition in patient with HR+/HER2− advanced breast cancer (MAINTAIN) | NCT02632045 | Palliative | PFS | ORR | ⩾18 years ECOG PS 0–1 Normal electrolytes Serum creatinine level ⩽ 1.5 mg/dl or estimated GFR > 50 ml/min INR ⩽ 1.5 No concurrent malignancy or malignancy diagnosed within 5 years LVEF ⩾ 50% No clinically significant, uncontrolled heart disease |
No |
| Abemaciclib trials | ||||||
| Endocrine therapy with or without abemaciclib (LY2835219) following surgery in participants with breast cancer (monarchE) | NCT03155997 | Adjuvant | Invasive DFS | Invasive DFS for participants with Ki67 ⩾ 20%; distant RFS; OS; PK; change from baseline of functional assessment; change from baseline of QoL | ⩾18 years ECOG PS 0–1 Adequate organ function No serious pre-existing medical conditions No history of cardiovascular syncope, ventricular arrhythmia or sudden cardiac arrest No history of VTE |
No |
| A study of abemaciclib (LY2835219) in participants with breast cancer (MONARCH plus) | NCT02763566 | Palliative | PFS | OS; ORR; DoR; DCR; CBR; change from baseline in symptom burden; PK | ⩾18 years ECOG PS 0–1 Adequate organ function Hb ⩾ 8 g/dl Serum creatinine ⩽ 1.5 × ULN No serious pre-existing medical conditions No syncope of cardiovascular etiology, ventricular tachycardia, ventricular fibrillation, or sudden cardiac arrest No history of any other cancer |
No |
| A study of abemaciclib (LY2835219) plus tamoxifen or abemaciclib alone in women with metastatic breast cancer (nextMONARCH 1) | NCT02747004 | Palliative | PFS | ORR; DoR; OS; PK; change from baseline in symptom burden | ⩾18 years ECOG PS 0–1 Adequate organ function No syncope of cardiovascular etiology, ventricular tachycardia, ventricular fibrillation, or sudden cardiac arrest No history of any other cancer |
No |
Please note that this study has not been registered with ClinicalTrials.gov yet and available information has been derived from a press release.56
AEs, adverse events; aTPP, activated partial thromboplastin time; CBR, clinical benefit rate; cfDNA, plasma-cell-free deoxyribonucleic acid; CrCl, creatinine clearance; DCR, disease control rate; DFS, disease-free survival; DoR, duration of response; ECOG, Eastern Cooperative Oncology Group; FGFR1, fibroblast growth factor receptor; GFR, glomerular filtration rate; Hb, hemoglobin; HER2−, Human epidermal growth factor receptor 2 negative; HR+, hormone receptor-positive; HRQoL, health-related quality of life; INR, international normalized ratio; LVEF, left ventricular ejection fraction; ORR, objective response rate; OS, overall survival; PDE, ; PFS, progression-free survival; PK, pharmacokinetic; PS, performance status; QoL, quality of life; QTcF, corrected QT interval using Fridericia’s formula; RFS, recurrence-free survival; SAEs, serious adverse events; T-DM1, trastuzumab emtansine; TTF, time to treatment failure; TTP, time to progression; ULN, upper limit of the institutional normal range; VTE, venous thromboembolism.
The sequence of treatments is a key question being addressed by two ongoing studies, and this question is likely to be of great relevance to older patients. It is unclear at this point whether patients should be treated with a CDK4/6 inhibitor in the first-line setting, or after first progression on an AI. The implication for efficacy and QoL needs to be further investigated. To address this, the SONIA trial [ClinicalTrials.gov identifier: NCT03425838] is comparing the use of a CDK4/6 inhibitor plus a non-steroidal AI in first line followed by fulvestrant in second line with a nonsteroidal AI in first line followed by fulvestrant plus CDK4/6 inhibition in second line. The primary endpoint is PFS after two lines of treatment. The observational Treat ER+ight trial [ClinicalTrials.gov identifier: NCT02753686] is also focusing on the treatment patterns and sequence as well as the effectiveness and safety of various treatment options (including CDK4/6 inhibitors) for postmenopausal ER-positive, HER2-negative advanced breast cancer patients in a real-world setting.
Palbociclib trials
A phase II, single-arm trial is investigating the role of palbociclib specifically in an older population of patients [ClinicalTrials.gov identifier: NCT02760030], aimed at determining the treatment failure-free survival of its combination with fulvestrant in women aged 70+ with surgically or medically inoperable ER-positive, HER2-negative breast cancer.
Although not specific for the older population, an observational study [ClinicalTrials.gov identifier: NCT03280303] is evaluating the real-world use of palbociclib and including geriatric assessments for participants aged 70+. In the adjuvant setting, the multicenter, randomized, phase III PALLAS study [ClinicalTrials.gov identifier: NCT02513394] is evaluating the addition of 2 years of palbociclib to letrozole for patients, over letrozole alone. This study is highly relevant to patients with ER-positive, HER2-negative early breast cancer at higher risk of recurrence, which includes also older adults. In the palliative setting, palbociclib is being evaluated in combination with a number of options: tucatinib, an anti-HER2 tyrosine kinase inhibitor (TKI), in first and second line in combination with letrozole for ER-positive, HER2-positive disease in a phase Ib/II study [ClinicalTrials.gov identifier: NCT03054363]; fulvestrant and erdafitinib, a TKI-inhibiting FGFR, in a phase Ib study [ClinicalTrials.gov identifier: NCT03238196]; tamoxifen in a multicenter, nonrandomized, phase II trial [ClinicalTrials.gov identifier: NCT0266866]. Possible differences in pharmacokinetics and pharmacodynamics of palbociclib in postmenopausal Asian patients are being evaluated in the phase III PALOMA-4 trial [ClinicalTrials.gov identifier: NCT02297438] of palbociclib plus letrozole compared with placebo plus letrozole. However, the upper age limit here is 70 years and this may well limit generalizability of its findings. Finally, the phase II PACE (Palbociclib After CDK and Endocrine Therapy) study [ClinicalTrials.gov identifier: NCT03147287] is investigating the role of palbociclib in combination with fulvestrant with or without avelumab upon progression on CDK4/6 inhibitors.
Ribociclib trials
Ribociclib will be specifically evaluated in a population aged 70+ in a single-arm, phase IIa study [ClinicalTrials.gov identifier: NCT03477396] to assess its safety and tolerability along with AIs.
Other ongoing trials are not age specific. In the adjuvant setting, ribociclib is being assessed in the phase II, pilot LEADER study [ClinicalTrials.gov identifier: NCT03285412] along with standard endocrine therapy. In the metastatic setting, the phase I/II TRINITI-1 trial [ClinicalTrials.gov identifier: NCT02732119] is determining if the continued use of ribociclib beyond progression along with second-line everolimus and exemestane is still effective. The multicenter, randomized MAINTAIN trial [ClinicalTrials.gov identifier: NCT02632045] is also assessing ribociclib in combination with fulvestrant upon progression, on AI plus a CDK4/6 inhibitor. Another randomized phase Ib trial [ClinicalTrials.gov identifier: NCT01857193] is exploring the same triplet, as well as the combination of ribociclib with exemestane.
Abemaciclib trials
No trials specifically addressing the role of abemaciclib in older patients are currently ongoing. In the adjuvant setting, abemaciclib is being assessed within the monarchE study [ClinicalTrials.gov identifier: NCT03155997] in combination with standard adjuvant endocrine therapy in high-risk, node-positive patients. As in the PALLAS study, this study is highly relevant to older patients. In the metastatic setting, the MONARCH plus study [ClinicalTrials.gov identifier: NCT02763566] is randomizing postmenopausal women to abemaciclib plus nonsteroidal AI (NSAI) or fulvestrant versus placebo plus NSAI or fulvestrant to compare efficacy of these approaches in first line. Abemaciclib is also being evaluated along with tamoxifen in the phase II nextMONARCH-1 trial [ClinicalTrials.gov identifier: NCT02747004] in pretreated women with ER-positive, HER2-negative breast cancer. In the ER-positive, HER2-positive disease population, the results of the phase II monarcHER trial [ClinicalTrials.gov identifier: NCT02675231] of abemaciclib plus trastuzumab with or without fulvestrant or chemotherapy are awaited.
Conclusion
CDK4/6 inhibitors have revolutionized the management of advanced ER-positive, HER2-negative breast cancer. Nonetheless, specific data about their efficacy and safety profile in older patients are limited and mostly derived by subgroup analyses of the pivotal trials. Based on these analyses, CDK4/6 inhibitors appear to be equally effective in older as in younger patients, with very similar outcomes among the three drugs as shown in Table 3. There are limited data regarding adverse events and toxicity in older patients but available data suggest that older patients seem to derive similar efficacy with either similar to or slightly increased toxicity from these agents compared with their younger counterparts. QoL measures are also lacking; incorporating QoL in trials is important because some older patients may value QoL as important or more important than survival.57 No trials have specifically evaluated the role of CDK4/6 inhibitors in an older population of patients, who are typically subject to a shorter life expectancy, competing comorbidities, polypharmacy and an increased risk of treatment-related toxicities. Moreover, with only a few exceptions listed in Table 2, many ongoing trials are at risk of excluding older women due to narrow eligibility criteria or the lack of inclusion of geriatric parameters. These will hinder the applicability of their findings to a large population of patients with ER-positive, HER2-negative breast cancer. The lack of biomarkers able to define patients who are more likely to benefit from the addition of a CDK4/6 inhibitor58 is likely to add further uncertainty to the decision-making process in the older subgroup.
Table 3.
Key efficacy and toxicity outcomes of CDK4/6 inhibitors in older patients based on available data from the pivotal trials.
| Population | Outcome | Palbociclib | Ribociclib | Abemaciclib |
|---|---|---|---|---|
| Treatment naïve | PFS | PALOMA-2: 65+ years: HR 0.57 (95% CI 0.39–0.84) |
MONALEESA-2: +AI, 65+ years: HR 0.658 (95% CI 0.466–0.928) MONALEESA-3: +fulvestrant, over 65 years: HR 0.597 (95% CI 0.436–0.818)* |
MONARCH-3: 65+ years: HR 0.57 (95% CI 0.36–0.90) |
| Toxicity | 65+ years: any grade neutropenia 81%; febrile neutropenia 1% | 65+ years: nausea, alopecia, diarrhea and vomiting in >10% of patients; >10% increase in fatigue and grade 1–2 anemia | Age-specific data not available | |
| Pretreated | PFS | PALOMA-3: 65+ years: HR 0.35 (95% CI 0.19–0.62) | MONALEESA-3: +fulvestrant, 65+ years: HR 0.597 (95% CI 0.436– 0.818)* |
MONARCH-2: 65+ years: 0.620 (95% CI 0.447– 0.860) |
| Toxicity | 70+ years: grade 3–4 neutropenia 13.9% | Age-specific data not available | Age-specific data not available |
The MONALEESA-3 study included treatment-naïve and pretreated patients.
AI, aromatase inhibitor; CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
The management of ER-positive, HER2-negative advanced breast cancer should always take into account a number of factors, including patients’ performance status and preferences, life expectancy, comorbidities, previous lines of treatment and derived benefit, disease burden and extent, current symptoms or risk for developing symptoms, and organ function. Differentiating the apparently fit, older individual who is likely to benefit from and tolerate standard therapy, from the seemingly frail, older patient who is likely to experience unexpected side effects and requires a more personalized strategy, is important to reduce over- and undertreatment.59
Frailty is an increasingly recognized clinical state of vulnerability to stressors such as treatments or serious illness and involves an increased risk for adverse health outcomes, including functional decline and mortality.60 Despite frailty being increasingly prevalent in older age, chronological age alone does not define frailty. There is no gold standard for diagnosing frailty and multiple assessment tools have been developed,61 although commonly used measurements are physical function, gait speed, and cognition.62 The Balducci frailty criteria have traditionally defined vulnerable those individuals with some degree of dependence in routine activities and no more than two comorbidities; whereas frail adults are defined as dependent in activities of daily living (ADLs) and affected by either three or more comorbidities or a geriatric syndrome.63 On the other hand, the Fried frailty criteria identify frail individuals based on the presence of three or more of the following: unintentional weight loss (⩾10 lbs in the past year), self-reported exhaustion, weakness (grip strength), slow observed walking speed, or low physical activity.60
Aging is a highly individualized, multidimensional process and a comprehensive geriatric assessment (CGA) can fill the gap of knowledge to guide management of older cancer patients and the incorporation of novel treatment approaches, including CDK4/6 inhibitors, in the treatment of older adults, and offer the opportunity to look at all the factors potentially impacting on therapy outcomes, to address their needs, and ultimately to select patients suitable for a more aggressive approach. A CGA provides a detailed evaluation of medical, psychosocial, and functional problems as detailed in Table 4.64 Based on a CGA, frailty is defined as fulfilling one or more of the following criteria: dependency in ADLs; presence of severe comorbidities according to the Cumulative Illness Rating Scale; cognitive dysfunction (Mini Mental Status Examination score <24); depression (Geriatric Depression Scale score >13); malnutrition (Mini Nutritional Assessment score <17); polypharmacy (more than seven concomitant daily medications); or incontinence.65 An increasing amount of evidence supports the use of CGA to predict treatment adverse events,66,67 estimate survival,68 aid cancer treatment decisions,69 detect problems usually neglected by routine assessments,70 and improve mental health and well-being and pain control.71 CGA is recommended by the International Society of Geriatric Oncology (SIOG)72 and by the National Comprehensive Cancer Network guidelines.73 Nonetheless, its use is still limited as it is perceived to be time consuming and difficult to implement in a busy oncology practice. Hence, various screening tools have been used to select patients that will benefit from a formal CGA.74 For example, an abbreviated CGA, the Vulnerable Elders Survey-13, the Geriatric 8 tool, and the Flemish version of the Triage Risk Screening Tool have been developed.75 A CGA should also be a key resource for clinical trial design in order to improve the evidence base for management of older patients with cancer and the applicability of study findings.76
Table 4.
Comprehensive geriatric assessment domains and tools and abnormal scores useful for the diagnosis of frailty.64
| Domain | Tool | Abnormal score |
|---|---|---|
| Demographic and social status | Conditions of living, marital status, educational level, financial resources, social activities, family support Identification of the caregiver and burden (Zarit Burden Interview) |
>20 |
| Comorbidities | Charlson comorbidity index77
Cumulative Illness Rating Scale78 Cumulative Illness Rating Scale for Geriatrics79 Physical Health Section (subscale of OARS)80 Simplified comorbidity score81 |
|
| Polypharmacy | Beers criteria82
STOPP and START criteria83 |
|
| Functional status | ADL (Katz index)84
IADL (Lawton scale)85 Visual or hearing impairment, regardless of use of glasses or hearing aids Mobility problem (requiring help or use of walking aid) Timed Get Up and Go86 Handgrip strength Walking problems, gait assessment, and gait speed87,88 Self-reported number of falls (within different timeframes) |
<6 <8 ⩾14 s <1 m/s |
| Cognition | Mini Mental State Examination89,90
Montreal Cognitive Assessment91,92 Clock-drawing test93 Blessed Orientation–Memory–Concentration Test92 Mini-cog94 |
<24 <26 <5 >4 <4 |
| Mood | Geriatric Depression Scale (mini GDS, GDS-15, GDS-30)95,96
Hospital Anxiety and Depression Scale97,98 Distress thermometer |
Mini GDS: <1; GDS-15: >5; GDS-30: >10 >7 |
| Nutrition | Body mass index (weight and height index) Weight loss (unintentional loss in 3 or 6 months) Mini Nutritional Assessment (MNA®)99,100 Dentition |
<23 <24 |
| Fatigue | MOB-T69 | |
| Geriatric syndromes11 | Dementia Delirium Incontinence (fecal and/or urinary) Osteoporosis or spontaneous fractures Neglect or abuse Failure to thrive Pressure ulcer Sarcopenia |
ADL, Activities of Daily Living; IADL, Instrumental ADL.
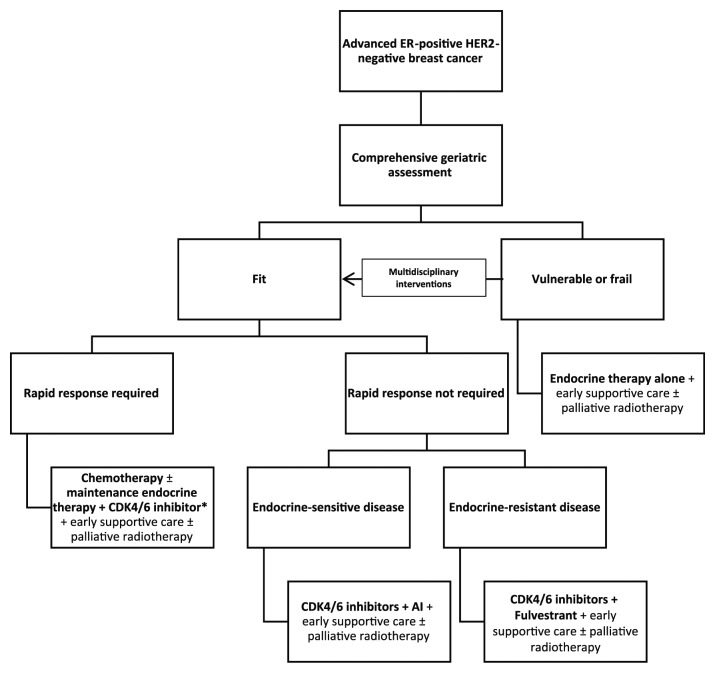
Therefore, we propose the algorithm shown in Figure 2 to guide the incorporation of CDK4/6 inhibitors in the management of ER-positive, HER2-negative advanced breast cancer in older patients based on a CGA. Fit patients with endocrine-sensitive disease should receive first-line AIs in combination with CDK/6 inhibitors; however, in the presence of endocrine-resistant breast cancer and if rapid response is not required, the association of fulvestrant and CDK4/6 inhibitors as per PALOMA-3 study findings may be used. For fit patients requiring rapid response due to severe symptoms, the use of first-line chemotherapy may be considered. It is debatable whether response rates are actually superior in a matched population of postmenopausal women with ER-positive, HER2-negative disease. Following induction chemotherapy, maintenance endocrine therapy plus CDK4/6 inhibition may be appropriate and this approach is currently under investigation in the CompLEEment-1 study, in which 22% of patients are aged 70+ and 19% received first-line chemotherapy.101 Less fit patients may be better suited to receive endocrine treatment alone, particularly if their life expectancy is thought to be short owing to competing causes of mortality. Vulnerabilities in CGA domains, such as functional status, cognition, polypharmacy, mood, nutrition and geriatric syndromes, may impact on the decision to use of CDK4/6 inhibitors in frail adults due to the risk of potential toxicities and increased burden of medications, required hospital visits, and blood tests. In certain cases, endocrine treatment alone without CDK4/6 inhibitors may be used to minimize the impact of potential toxicities (such as myelosuppression and diarrhea) and drug interactions on QoL. Nonetheless, frail patients should not be denied CDK4/6 inhibitors a priori as CGA may also help identifying those suitable for multidisciplinary interventions that have the potential to improve their vulnerabilities for consideration of treatment. Moreover, in patients who are frail as a consequence of cancer itself, another potential option is considering the introduction of CDK4/6 inhibitors at a later stage, once their disease responds to endocrine agents and their fitness subsequently improves, in order to maximize their chances of long-term remission. Endocrine therapy alone may also be a reasonable option in less fit older patients if they are asymptomatic, treatment naïve, and have predominantly bony metastatic involvement, but who are likely to remain fit enough for a CDK4/6 inhibitor in the second-line setting. Nevertheless, the merits of treatment sequencing are still under investigation in the SONIA trial. More studies investigating the tolerance of this class of drugs in real-world adults and their role in frail patients in the context of their preferences and values guided by shared decision making are warranted. Supportive care should also be incorporated early in the management of any older cancer patients, along with locoregional approaches if required and upon multidisciplinary discussion.
Figure 2.
Proposed initial approach to the management of ER-positive, HER2-negative advanced breast cancer in older patients.
*No definitive evidence supports the use of maintenance endocrine treatment.
CDK4/6, cyclin-dependent kinase 4/6; ER, estrogen receptor; HER2, Human epidermal growth factor receptor 2.
Based on the available data showing similar efficacy and toxicity profile in the older and young age groups, CDK4/6 inhibitors are an attractive option for older patients with advanced ER-positive, HER2-negative breast cancer. Ongoing adjuvant trials will further define their role in the management of early-stage disease. Nonetheless, better evidence is needed to guide their incorporation in the treatment strategy for this population, and trials specifically enrolling older adults that include geriatric parameters are warranted. Prospective observational studies and real-world experiences may also fill the current gap of knowledge.
Acknowledgments
The authors wish to acknowledge the support of Colm Doody and Jane Crimmin, pharmacists at The Royal Marsden NHS Foundation Trust, London, UK, for their help in outlining drug interactions relevant to CDK4/6 inhibitors. The authors wish to also acknowledge the support of Pfizer and Novartis in highlighting age-specific data when available. NMLB and AR wish to acknowledge the support of the National Institute for Health Research Biomedical Research Center for Cancer (The Royal Marsden NHS Foundation Trust and Institute of Cancer Research). All authors wrote the review and contributed to the final version. All authors reviewed the final version.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: AR has received speaker fees from Novartis and Pfizer and advisory board fees from Novartis, Pfizer and Lilly. The remaining authors declare no competing interests.
ORCID iDs: Nicolò Matteo Luca Battisti  https://orcid.org/0000-0002-1063-1717
https://orcid.org/0000-0002-1063-1717
Kah Poh Loh  https://orcid.org/0000-0002-6978-0418
https://orcid.org/0000-0002-6978-0418
Contributor Information
Nicolò Matteo Luca Battisti, Department of Medicine – Breast Unit, The Royal Marsden NHS Foundation Trust, Downs Road, Sutton, Surrey SM2 5PT, UK.
Nienke De Glas, Department of Medical Oncology, Leiden University Medical Center, Netherlands.
Mina S. Sedrak, Department of Medical Oncology & Therapeutics Research, City of Hope, USA
Kah Poh Loh, Division of Hematology/Oncology, University of Rochester Medical Center, USA.
Gabor Liposits, Department of Oncology, Regionhospitalet Herning, Denmark.
Enrique Soto-Perez-de-Celis, Department of Geriatrics, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Jessica L. Krok-Schoen, Division of Medical Dietetics and Health Sciences, The Ohio State University, Columbus, OH, USA
Ines B. Menjak, Department of Medicine, University of Toronto, Toronto, Ontario, USA
Alistair Ring, Department of Medicine, The Royal Marsden NHS Foundation Trust, UK.
References
- 1. Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009; 27: 2758–2765. [DOI] [PubMed] [Google Scholar]
- 2. Mistry M, Parkin DM, Ahmad AS, et al. Cancer incidence in the United Kingdom: projections to the year 2030. Br J Cancer 2011; 105: 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Cancer Institute. SEER cancer statistics factsheets: female breast cancer. Bethesda, MD: National Cancer Institute, https://seer.cancer.gov/statfacts/html/breast.html. (2018) [Google Scholar]
- 4. Onitilo AA, Engel JM, Greenlee RT, et al. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009; 7: 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol 2012; 30: 2036–2038. [DOI] [PubMed] [Google Scholar]
- 6. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999; 341: 2061–2067. [DOI] [PubMed] [Google Scholar]
- 7. Singh H, Kanapuru B, Smith C, et al. FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: a 10-year experience by the U.S. Food and Drug Administration. J Clin Oncol 2017; 35(Suppl. 15): 10009–10009. [Google Scholar]
- 8. Hamaker ME, Seynaeve C, Wymenga AN, et al. Baseline comprehensive geriatric assessment is associated with toxicity and survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: results from the OMEGA study of the Dutch breast cancer trialists’ group. Breast 2014; 23: 81–87. [DOI] [PubMed] [Google Scholar]
- 9. Girre V, Falcou MC, Gisselbrecht M, et al. Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci 2008; 63: 724–730. [DOI] [PubMed] [Google Scholar]
- 10. Battisti NML, Sehovic M, Extermann M. Assessment of the external validity of the national comprehensive cancer network and European Society For Medical Oncology guidelines for non-small-cell lung cancer in a population of patients aged 80 years and older. Clin Lung Cancer 2017; 18: 460–471. [DOI] [PubMed] [Google Scholar]
- 11. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 12. Hartwell LH, Kastan MB. Cell cycle control and cancer. Science 1994; 266: 1821–1828. [DOI] [PubMed] [Google Scholar]
- 13. Cooper GM. The eukaryotic cell cycle. In: The cell: a molecular approach. 2nd ed. Sunderland, MA: Sinauer Associates, 2000. [Google Scholar]
- 14. Foster I. Cancer: a cell cycle defect. Radiography 2008; 14: 144–149. [Google Scholar]
- 15. Caldon CE, Daly RJ, Sutherland RL, et al. Cell cycle control in breast cancer cells. J Cell Biochem 2006; 97: 261–274. [DOI] [PubMed] [Google Scholar]
- 16. Dean JL, Thangavel C, McClendon AK, et al. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 2010; 29: 4018–4032. [DOI] [PubMed] [Google Scholar]
- 17. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009; 11: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knudsen ES, Witkiewicz AK. The strange case of CDK4/6 inhibitors: mechanisms, resistance, and combination strategies. Trends Cancer 2017; 3: 39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Navarrete-Reyes AP, Soto-Perez-de-Celis E, Hurria A. Cancer and aging: a complex biological association. Rev Invest Clin 2016; 68: 17–24. [PubMed] [Google Scholar]
- 20. Pallis AG, Hatse S, Brouwers B, et al. Evaluating the physiological reserves of older patients with cancer: the value of potential biomarkers of aging? J Geriatr Oncol 2014; 5: 204–218. [DOI] [PubMed] [Google Scholar]
- 21. Zangardi ML, Spring LM, Blouin GC, et al. Ribociclib for post-menopausal women with HR+/HER2- advanced or metastatic breast cancer. Expert Rev Clin Pharmacol 2017; 10: 1169–1176. [DOI] [PubMed] [Google Scholar]
- 22. Spring LM, Zangardi ML, Moy B, et al. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendations. Oncologist 2017; 22: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palbociclib. Palbociclib: full prescribing information. New York, NY: Pfizer Inc, 2015. [Google Scholar]
- 24. Ribociclib. Ribociclib: full prescribing information, 2017. U. S. Food and Drug Administration. [Google Scholar]
- 25. Abemaciclib. Abemaciclib: full prescribing information, 2017. U. S. Food and Drug Administration. [Google Scholar]
- 26. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res 2017; 23: 5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. FDA Drug Interactions. Drug development and drug interactions: table of substrates, inhibitors and inducers, https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#transporter (2017, accessed 2 August 2018).
- 28. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 29. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 30. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 31. Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 32. Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist 2016; 21: 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rugo HS, Turner NC, Finn RS, et al. Palbociclib in combination with endocrine therapy in treatment-naive and previously treated elderly women with HR+ HER2- advanced breast cancer: a pooled analysis from randomized phase 2 and 3 studies. In: 39th Annual San Antonio breast cancer symposium (SABCS), San Antonio, TX, 2016. p. P4–22–03. [Google Scholar]
- 34. Singh H, Howie LJ, Boloomquist E, et al. A US Food and Drug Administration pooled analysis of outcomes of older women with hormone-receptor positive metastatic breast cancer treated with a CDK4/6 inhibitor as initial endocrine based therapy. In: 40th San Antonio Breast Cancer Symposium, San Antonio, TX, 2017. pp. GS5–06. [Google Scholar]
- 35. Clifton K, Kimmel J, Keating Litton J, et al. Examining progression free survival (PFS), overall survival (OS), and toxicities of palbociclib in a geriatric population. J Clin Oncol 2018; 36: abstract 10039. [Google Scholar]
- 36. Harbeck N, Iyer S, Turner N, et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: patient-reported outcomes from the PALOMA-3 trial. Ann Oncol 2016; 27: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rugo HS, Dieras V, Gelmon KA, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol 2018; 29: 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018; 29: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 39. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018; 36: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 40. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–915. [DOI] [PubMed] [Google Scholar]
- 41. US Food and Drug Administration. Approved drugs: ribociclib (Kisqali), https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm546438.htm (2017, accessed 1 June 2018).
- 42. European Medicines Agency. Kisqali, http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004213/human_med_002149.jsp&mid=WC0b01ac058001d124 (2017, accessed accessed 1 June 2018).
- 43. Sonke GS, Hart LL, Campone M, et al. Efficacy and safety of ribociclib (LEE011) + letrozole in elderly patients with hormone receptor-positive (HR+), HER2-negative (HER2-) advanced breast cancer (ABC) in MONALEESA-2. Eur J Canc 2017; 72: S1–S2. [Google Scholar]
- 44. Sonke GS, Hart LL, Campone M, et al. Ribociclib with letrozole vs letrozole alone in elderly patients with hormone receptor-positive, HER2-negative breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat 2018; 167: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coppola C, Rienzo A, Piscopo G, et al. Management of QT prolongation induced by anti-cancer drugs: target therapy and old agents. Different algorithms for different drugs. Cancer Treat Rev 2018; 63: 135–143. [DOI] [PubMed] [Google Scholar]
- 46. O’Shaughnessy J, Petrakova K, Sonke GS, et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2- advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat 2018; 168: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 48. Mangoni AA, Kinirons MT, Swift CG, et al. Impact of age on QT interval and QT dispersion in healthy subjects: a regression analysis. Age Ageing 2003; 32: 326–331. [DOI] [PubMed] [Google Scholar]
- 49. Rabkin SW. Impact of age and sex on QT prolongation in patients receiving psychotropics. Can J Psychiatry 2015; 60: 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Isbister GK. Risk assessment of drug-induced QT prolongation. Aust Prescr 2015; 38: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 52. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–3646. [DOI] [PubMed] [Google Scholar]
- 53. Beauchamp RD, Sheng HM, Shao JY, et al. Intestinal cell cycle regulations. Interactions of cyclin D1, CDK4, and p21Cip1. Ann Surg 1996; 223: 620–627; discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kalsi T, Babic-Illman G, Fields P, et al. The impact of low-grade toxicity in older people with cancer undergoing chemotherapy. Br J Cancer 2014; 111: 2224–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kaufman PA, Toi M, Neven P, et al. Health-related quality of life (HRQoL) in MONARCH 2: Abemaciclib plus fulvestrant in women with HR+, HER2- advanced breast cancer (ABC) who progressed on endocrine therapy. J Clin Oncol 2018; 36(Suppl. 15): 1049.29406801 [Google Scholar]
- 56. (TRIO) TRiO. TRIO awarded NATALEE study, largest single phase III breast cancer clinical trial in its history, https://globenewswire.com/news-release/2018/07/09/1534927/0/en/TRIO-Awarded-NATALEE-Study-Largest-Single-Phase-III-Breast-Cancer-Clinical-Trial-in-its-History.html (2018, accessed 8 September 2018).
- 57. Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. J Natl Cancer Inst 1994; 86: 1766–1770. [DOI] [PubMed] [Google Scholar]
- 58. Polk A, Kolmos IL, Kumler I, et al. Specific CDK4/6 inhibition in breast cancer: a systematic review of current clinical evidence. ESMO Open 2016; 1: e000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hamerman D. Toward an understanding of frailty. Ann Intern Med 1999; 130: 945–950. [DOI] [PubMed] [Google Scholar]
- 60. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 61. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016; 26: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc 2011; 59: 2129–3218. [DOI] [PubMed] [Google Scholar]
- 63. Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist 2000; 5: 224–237. [DOI] [PubMed] [Google Scholar]
- 64. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: young international society of geriatric oncology position paper. J Oncol Pract 2018; 14: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kristjansson SR, Nesbakken A, Jordhoy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol 2010; 76: 208–217. [DOI] [PubMed] [Google Scholar]
- 66. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012; 118: 3377–3386. [DOI] [PubMed] [Google Scholar]
- 67. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011; 29: 3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ramjaun A, Nassif MO, Krotneva S, et al. Improved targeting of cancer care for older patients: a systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol 2013; 4: 271–281. [DOI] [PubMed] [Google Scholar]
- 69. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol 2013; 24: 1306–1312. [DOI] [PubMed] [Google Scholar]
- 70. Extermann M, Meyer J, McGinnis M, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol 2004; 49: 69–75. [DOI] [PubMed] [Google Scholar]
- 71. Rao AV, Hsieh F, Feussner JR, et al. Geriatric evaluation and management units in the care of the frail elderly cancer patient. J Gerontol A Biol Sci Med Sci 2005; 60: 798–803. [DOI] [PubMed] [Google Scholar]
- 72. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014; 32: 2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. The National Comprehensive Cancer Network. Older adult oncology. Version 1, https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf (2018, accessed 7 July 2018). [DOI] [PubMed]
- 74. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendationsdagger. Ann Oncol 2015; 26: 288–300. [DOI] [PubMed] [Google Scholar]
- 75. Hamaker ME, Jonker JM, De Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 2012; 13: e437–e444. [DOI] [PubMed] [Google Scholar]
- 76. Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 2014; 32: 2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 78. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968; 16: 622–626. [DOI] [PubMed] [Google Scholar]
- 79. Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatr Res 1992; 41: 237–248. [DOI] [PubMed] [Google Scholar]
- 80. Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer 2005; 104: 1998–2005. [DOI] [PubMed] [Google Scholar]
- 81. Gironés R, Torregrosa D, Maestu I, et al. Comprehensive Geriatric Assessment (CGA) of elderly lung cancer patients: a single-center experience. J Geriatr Oncol 2012; 3: 98–103. [Google Scholar]
- 82. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 83. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983; 31: 721–777. [DOI] [PubMed] [Google Scholar]
- 85. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–186. [PubMed] [Google Scholar]
- 86. Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 87. Pamoukdjian F, Canoui-Poitrine F, Longelin-Lombard C, et al. Diagnostic performance of gait speed, G8 and G8 modified indices to screen for vulnerability in older cancer patients: the prospective PF-EC cohort study. Oncotarget 2017; 8: 50393–50402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51: 314–322. [DOI] [PubMed] [Google Scholar]
- 89. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 90. Kahle-Wrobleski K, Corrada MM, Li B, et al. Sensitivity and specificity of the mini-mental state examination for identifying dementia in the oldest-old: the 90+ study. J Am Geriatr Soc 2007; 55: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009; 73: 1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Loh KP, Pandya C, Zittel J, et al. Associations of sleep disturbance with physical function and cognition in older adults with cancer. Support Care Cancer 2017; 25: 3161–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 2000; 15: 548–561. [DOI] [PubMed] [Google Scholar]
- 94. Costa D, Severo M, Fraga S, et al. Mini-Cog and Mini-Mental State Examination: agreement in a cross-sectional study with an elderly sample. Dement Geriatr Cogn Disord 2012; 33: 118–124. [DOI] [PubMed] [Google Scholar]
- 95. Marc LG, Raue PJ, Bruce ML. Screening performance of the 15-item geriatric depression scale in a diverse elderly home care population. Am J Geriatr Psychiatry 2008; 16: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17: 37–49. [DOI] [PubMed] [Google Scholar]
- 97. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. J Affect Disord 2010; 126: 335–348. [DOI] [PubMed] [Google Scholar]
- 98. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 99. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009; 13: 782–788. [DOI] [PubMed] [Google Scholar]
- 100. Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999; 15: 116–122. [DOI] [PubMed] [Google Scholar]
- 101. De Laurentiis M, Neven P, Jerusalem G, et al. Ribociclib + letrozole in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer (ABC) with no prior endocrine therapy for abc: preliminary results from the phase 3b trial (abstract 1056). Chicago, IL: American Society of Clinical Oncology, 2018. [Google Scholar]