Abstract
BACKGROUND:
While downregulation of several growth factors in major depressive disorder is well established, less attention has been paid to the upregulation of other growth factors. Yet, upregulated growth factors may offer better therapeutic targets. We show that connective tissue growth factor (CTGF) represents a target based on its upregulation in major depressive disorder and studies in animal models implicating it in negative affect.
METHODS:
CTGF gene expression was first evaluated in the postmortem human amygdala. The findings were followed up in outbred rats and in two rat lines that were selectively bred for differences in novelty-seeking and anxiety behavior (bred low responders and bred high responders). We studied the impact of social defeat and early-life treatment with fibroblast growth factor 2 on CTGF expression. Finally, we assessed the ability of an anti-CTGF antibody (FG-3019) to alter CTGF expression and emotionality.
RESULTS:
In the human amygdala, CTGF expression was significantly increased in major depressive disorder compared with control subjects. CTGF expression was also significantly increased in the dentate gyrus of adult bred low responders compared with bred high responders. Social defeat stress in bred low responders significantly increased CTGF expression in the dentate gyrus. Early-life fibroblast growth factor 2, a treatment that reduces anxiety-like behavior throughout life, decreased CTGF expression in the adult dentate gyrus. In outbred rats, CTGF administration increased depression-like behavior. Chronic treatment with FG-3019 decreased CTGF expression, and acute and chronic treatment was antidepressant.
CONCLUSIONS:
This study is the first to implicate CTGF as a prodepressant molecule that could serve as a target for the development of novel therapeutics.
Keywords: Amygdala, Dentate gyrus, Depression, FGF2, Hippocampus, Stress
The hypothesis that growth factors play a key role in major depressive disorder (MDD) has gained considerable support from animal and human studies (1–3). However, translating these findings into therapeutic strategies is challenging. In part, this is because most studies have focused on a decrease in growth factor function in mood disorders, and activating growth factors can have deleterious side effects including increased vulnerability to neoplasia. However, growth factors exist in highly interactive molecular matrices, often with opposing regulatory functions. We have recently shifted our focus to identifying growth factors whose expression increases in MDD and may act as prodepressants, as they would represent novel targets for blockade as a therapeutic strategy (4). Through a convergence of evidence, we have identified connective tissue growth factor (CTGF) as an important player in the control of affect, and this report provides the first evidence of its role as a prodepressant and novel therapeutic target.
CTGF is involved in adhesion, proliferation, migration, angiogenesis, and matrix production (5). CTGF (also known as CCN2) is an extracellular matrix protein that binds and modulates various growth factors, such as insulin-like growth factor, transforming growth factor-β, and fibroblast growth factor 2 (FGF2) (6). CTGF can bind the FGF receptors FGFR2 and FGFR3 and dose-dependently enhance signaling through their canonical ligands (7). This is especially notable because FGFR3 is the primary receptor for FGF9, a prodepressant whose expression is increased in postmortem brains of MDD subjects (4).
Additionally, CTGF can interact with various extracellular matrix proteins and cell surface receptors, such as the integrins, heparan sulfate proteoglycans, tropomysin-related kinase A, lipoprotein receptor–related protein, and transforming growth factor-β receptors (8). Thus, the net effect of CTGF depends on other molecules in the region. Most of the current functions of CTGF have been attributed to pathological conditions, such as fibrosis and cancer, where it is typically increased; however, very little is known about this growth factor under physiological conditions (9,10). Even less is known about its role in the brain.
Recently, CTGF was shown to play a role in information processing in excitatory glutamate neurons of the olfactory bulb (11). Other studies have found increased CTGF in astrocytes of the cortex following brain injury (12). Together, the studies suggest that CTGF may function as a proapoptotic molecule, and indeed, knocking down CTGF rescued apoptosis in the olfactory bulb (11).
It is possible that CTGF performs similar functions in other regions, such as the hippocampus or amygdala (13,14). Because neuronal survival in the hippocampus is involved in anxiolytic and antidepressant effects (15), CTGF may also be involved in the modulation of affect. Furthermore, many of the conditions in which CTGF is upregulated (e.g., cancer, diabetes, Alzheimer’s disease) are comorbid with MDD (6).
Here, we utilize a multipronged approach to evaluate the role of CTGF in affect. We assessed its expression in the postmortem brain of individuals with MDD. We also assessed its expression in our rodent models of differential locomotor response to a novel environment. The bred low responders (bLRs) exhibit less locomotor behavior to a novel environment compared with the bred high responders (bHRs). bLRs exhibit greater anxiety-like and depression-like behavior compared with the bHRs and greater reactivity to social stress, and differ on measures of neurogenesis (16–18). We hypothesized that CTGF expression would be increased in postmortem brains of individuals with MDD, and in rats that display higher levels of negative affect. We also hypothesized that these differences in gene expression would be modulated by stress and functionally relevant. We tested functionality mechanistically by either enhancing or blocking the activity of CTGF and ascertaining the behavioral outcomes.
METHODS AND MATERIALS
RNA Extraction in MDD
The tissue samples were obtained from the Brain Donor Program at the University of California, Irvine, with the consent of the next of kin (4,19). All of the individuals were men, and all of the MDD subjects committed suicide. The average age for MDDs and control subjects was 48.5 ± 6.28 years and 50.8 ± 7.51 years, respectively. The average pH was 6.84 ± 0.14 and 6.84 ± 0.16, respectively. All brains had a pH above 6.5 and an agonal factor score of zero (20). The average postmortem interval was 20.3 ± 2.42 hours and 21.8 ± 2.38 hours, respectively (t18 = 0.46, p = .65).
The amygdala was identified by an experienced neuroanatomist and dissected using previously published methods (4,21). Following dissection of amygdala-enriched blocks, the blocks were sectioned at 10 μm onto Superfrost Plus slides (Thermo Fisher, Waltham, MA). The amygdala nuclei were identified by performing an acetylcholinesterase stain on every 50th section, as previously described (22–24). Anatomical alignment was performed and three randomly chosen sections were selected from the amygdala (V. Sharma et al., Ph.D., unpublished data, February 14, 2015). The accessory basal, amygdalohippocampal, lateral, anterior amygdaloid area (AAA); basal, medial, central, cortical, and periamygdaloid cortices; and paralaminar nuclei were captured by laser capture microdissection on the ArcturusXT system (Thermo Fisher). The tissue was captured onto Arcturus CapSure Macro LCM caps (Thermo Fisher). The RNA from each nucleus was extracted from three sections onto three caps and pooled for processing. Total RNA was extracted and amplified, as previously described (25). RNA was quantified using the Quant-iT RiboGreen RNA Reagent Kit (Thermo Fisher) and quality was assessed using the Agilent RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA). Preliminary results from gene expression profiling of the amygdala nuclei showed that CTGF was significantly increased in several nuclei in MDD subjects, suggesting it as a target for validation (Table S1 in Supplement 1).
Quantitative Real-Time Polymerase Chain Reaction Analysis of CTGF in the Human Amygdala
Amplified RNA (1 μg) from the above-mentioned subjects was converted to complementary DNA using the iScript complementary DNA synthesis kit (Bio-Rad, Hercules, CA) for the accessory basal, amygdalohippocampal, lateral, and AAA nuclei. Then, 1 μL of complementary DNA was used in the polymerase chain reaction (PCR) using SsoAdvanced SYBR Green Supermix (Bio-Rad, Hercules, CA). The CTGF primer sequences were as follows: forward (TGGAGTTCAAGT GCCCTGAC) and reverse (ACTGCTCCTAAAGCCACACC). GAPDH was used as the housekeeping gene, and the primer sequences were as follows: forward (GGCCTCCAAGGAG TAAGACC) and reverse (AGGGGTCTACATGGCAACTG). PCR was performed on the Bio-Rad CFX Connect machine. Cycle threshold values and fold changes were calculated using the Livak method, as previously described (18).
Microarray Analysis in the Dentate Gyrus of Selectively Bred High and Low Responders
A subset of the methods and results pertaining to the effects of early-life FGF2 treatment on gene expression in the dentate gyrus of bLRs was previously published (18). In brief, rats were from generation F21 and selectively bred as previously described (26). All rats were maintained on a 12-hour light/dark cycle with food and water available ad libitum. Male and female bHR and bLR rats were injected with either FGF2 (20 ng/g in0.1 M phosphate-buffered saline with 0.1% bovine serum albumin, subcutaneous) or vehicle (0.1 M phosphate-buffered saline with 0.1% bovine serum albumin) the day after birth. However, only adult male rats were used in the subsequent studies. There were 6 bHR-vehicle, 5 bLR-vehicle, 5 bHRFGF2, and 4 bLR-FGF2 rats. The rats remained untouched until adulthood. All of the procedures were performed in accordance with the National Institutes of Health Guidelines on Laboratory Animal Use and Care and in accordance with the guidelines set by the university committee on use and care of rats at the University of Michigan. Following euthanasia by rapid decapitation and brain removal, the dentate gyrus was dissected using laser capture microscopy and RNA from the tissue samples was profiled with microarray using Illumina RatRef-12 Expression BeadChips (Illumina, Inc., San Diego, CA) (18). The data and results were analyzed in BeadStudio (Illumina, Inc.), and Ingenuity Pathway Analysis (Qiagen, Inc., Germantown, MD) was conducted, as previously described (18). Only transcripts from the dataset that were significantly detected in both groups and had a p value <.05 were considered for unbiased pathway analysis.
Quantitative Real-Time PCR Analysis of CTGF in the Amygdala of Selectively Bred High and Low Responders
The left amygdala was punched out (2 mm) of adult male bHRs (n = 6) and bLRs (n = 7) at approximately postnatal day 80 from generations F32 to F45 using 300-μm sections. This resulted in three punches/subject from bregma −2.56 to −3.8 (see Supplemental Methods in Supplement 1). The rat CTGF primer sequences were as follows: 5’-AGAGTGGAGATGCCAGGAGA-3’ and 5’-CACACACCCAGCTCTTGCTA-3’. Actin was used as the housekeeping gene. PCR was performed on the Bio-Rad CFX Connect machine. Cycle threshold values were generated and fold changes were calculated using the Livak method, as previously described (18).
Social Defeat Stress in bLRs
For social defeat stress, bLRs male rats were from generation F49 (see Supplemental Methods in Supplement 1). bLRs underwent social defeat starting on postnatal day 61 for 15 minutes a day for 4 days. The rats were separated by a wire mesh cage after the bLR was pinned by the resident aggressor and remained there until the end of the 15 minutes. The bLRs were sacrificed 4 days after social defeat testing ended. The rats were sacrificed on postnatal day 68, and the tissue was processed as described below for CTGF messenger RNA (mRNA) in situ hybridization.
Stereotaxic Surgery
Under isoflurane, a cannula was implanted in the left lateral ventricle of outbred rats, as previously described, with the following differences (27). Adult male rats were administered three total injections of flunixamine hydrochloride (2.5 mg/kg, intraperitoneal), one immediately before the start of surgery and two subsequent injections over the next 48 hours. The rats remained undisturbed for a minimum of 5 days before testing. For CTGF and FG-3019, the anti-CTGF antibody, 1 μL was injected over 1 minute, and the injector was removed after an additional 3 minutes to allow for diffusion.
Drug Administration and Behavioral Testing
For the acute microinjection studies, the elevated plus maze (EPM) was used to evaluate anxiety-like behavior, and the forced swim test (FST) was used to assess depression-like behavior. For CTGF, the rats were microinjected with CTGF (400 ng in vehicle, intracerebroventricular [ICV]; Sigma-Aldrich, St. Louis, MO) or vehicle (artificial extracellular fluid with 0.1% bovine serum albumin, ICV) 10 minutes before the EPM. Five days later, the groups were counterbalanced and the rats were tested on the FST. The rats were microinjected 1 hour after day 1 of the FST and tested on the FST 24 hours later (day 2). For FG-3019, the microinjection and testing protocols were identical to those used for CTGF, except that the rats were microinjected with FG-3019 (20 mg, ICV; gift from FibroGen, San Francisco, CA) or vehicle (human IgG, 20 mg, ICV; gift from FibroGen). For the EPM, the time spent (seconds) in the open arms, closed arms, and center was analyzed for the 5-minute test. For the FST, the percent total duration of climbing, swimming, and immobility was assessed as previously described (27) (see Supplemental Methods in Supplement 1 for descriptions of the EPM and FST).
For the chronic microinjection studies, FG-3019 (20 μg, ICV) or vehicle (human IgG, 20 mg, ICV) was administered every other day for 14 days. Locomotor behavior was assessed on day 11 as previously described (18), anxiety-like behavior was assessed by the EPM on day 12, and depression-like behavior was assessed by the FST on days 13 and 14. Rats were sacrificed if there was any evidence of piloerection or hunched posturing else immediately after day 2 of the FST. The brains were collected and frozen in isopentane (−80°C).
mRNA In Situ Hybridization for CTGF and Platelet-Derived Growth Factor-β
For all studies, the brains were sectioned in a −20°C cryostat at 10 μm. Sections were mounted on Fisher Superfrost Plus slides and separated by 200 μm. The slides were processed by in situ hybridization, as previously described (28). The rat accession number for CTGF probe was NM_022266 and represented the nucleotide region 918–1282. The CTGF pathway member platelet-derived growth factor-β (PDGFβ) was also assessed. The rat accession number for PDGFβ probe was NM_031524 and represented the nucleotide region 567–1105. The exposure time for CTGF was 21 days, and the exposure time for PDGFβ was 17 days. Probe synthesis reactions were as previously described (28). The films (Kodak Biomax MR, Sigma-Aldrich) were then developed and scanned, as previously described (28). The dentate gyrus of the hippocampus was quantified using the selection brush tool in ImageJ, version 1.50b (National Institutes of Health, Bethesda, MD) to capture the signal from the pixel area defined by the brush tool. The optical density represents the mean signal minus the background. The background is defined by placing a box in the molecular layer of the hippocampus. The optical density values from eight to 10 sections were averaged for each animal.
Statistical Analyses
For all quantitative real-time (qRT)-PCR, behavioral, and mRNA in situ hybridization results, a two-tailed Student’s t test was used to analyze the results.
RESULTS
CTGF Was Altered in the Postmortem Amygdala of Individuals With MDD
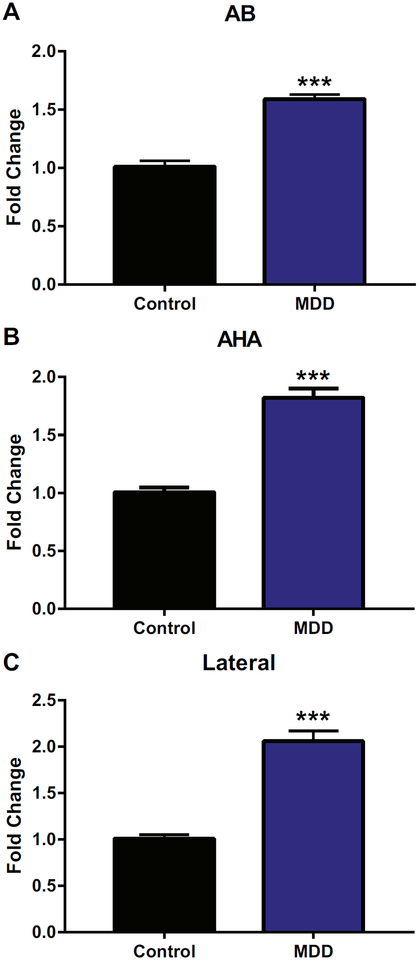
We initially used gene expression profiling in postmortem human amygdala nuclei as a first step to ascertain changes in growth factors and other related molecules associated with severe depression (V. Sharma et al., Ph.D., unpublished data, Februrary 14, 2015). Profiling results suggested that CTGF was consistently elevated in multiple amygdala nuclei in individuals with MDD (see Table S1 in Supplement 1). One of the CTGF probes ranked in the top 1.2% of all probes. The observed differences in CTGF gene expression led us to carry out targeted analysis using qRT-PCR. In Figure 1A, the accessory basal nucleus was the most significantly increased in MDD (t18 = −9.04, p < .001) compared with control subjects. In Figure 1B, the amygdalohippocampal nucleus was significantly increased in MDD (t18 = −8.72, p < .001) compared with control subjects. In Figure 1C, the lateral nucleus was significantly increased in MDD (t18 = −8.75, p < .001) compared with control subjects. There was also a nonsignificant trend for the AAA to also be increased in individuals with MDD compared with control subjects (t18 = 1.79, p = .09). Thus, CTGF was increased in the amygdala in individuals with MDD, suggesting that it may play a role in the etiology or expression of depression.
Figure 1.
Connective tissue growth factor expression was significantly increased in three amygdala nuclei in individuals with major depressive disorder (MDD) compared with control subjects. Fold changes are shown for the (A) accessory basal (AB), (B) amygdalohippocampal (AHA), and(C) lateral nuclei, all of which were significantly increased in individuals with MDD compared with control subjects. n = 10 per group. All values are mean ± SEM. ***p < .001.
The CTGF Pathway Was Altered in the Dentate Gyrus in Rats That Differ in Emotionality
To determine whether the increased CTGF expression observed in a psychiatric disorder is also present basally in rats that naturally differ in emotionality, we assessed CTGF gene expression in bHRs and bLRs. Although not significant, bLRs had twice the amount of CTGF mRNA than bHRs in the amygdala, as assessed by qRT-PCR (t6.93 = −1.50, p = .18). Given the known role of the dentate gyrus in neurogenesis and antidepressant response (15), we assessed CTGF expression in the dentate gyrus of bHRs and bLRs by microarray analysis. CTGF gene expression in the dentate gyrus was significantly increased in the bLRs that exhibit higher anxiety-like and depression-like behavior compared with bHRs. Several other CTGF pathway members, as defined by Ingenuity Pathway Analysis, were also altered in bHRs compared with bLRs (see Table 1). A complete list of significant gene expression changes in bLRs compared with bHRs is available in Table S2 in Supplement 2. We have previously reported that early-life administration of FGF2, a treatment known to decrease anxiety-like behavior in bLRs, significantly decreased CTGF expression in the dentate gyrus of bLRs (18). Referencing those results, it is noteworthy that many of the differences in the CTGF pathway currently identified between bLRs and bHRs in the dentate gyrus were reversed in bLRs treated with FGF2 compared with bLRs treated with vehicle (Table 1).
Table 1.
The Expression of CTGF Pathway Members Was Altered in bLRs in the Dentate Gyrus
| Transcript | bLR-VEH vs. bHR-VEH | bLR-FGF2 vs. bLR-VEH |
|---|---|---|
| CTGF | Upa | Downa |
| ECM2 | Down | |
| EGR1 | Downa | |
| BCL2L2 | Upa | Upa |
| IGFBP7 | Up | Downa |
| P4HA1 | Downa | |
| PDGFβ | Down | |
| MAPKAPK5 | Upa | |
| MAPK8IP3 | Upa | |
| PKN1 | Upa | |
| PRKAG1 | Upa | |
| CREB1 | Down | |
| RHOG | Upa | |
| RHOA | Downa | Upa |
| SOX4 | Upa | |
| STK3 | Upa |
BCL2L2, Bcl-2-like protein 2; bHR, bred high responder; bLR, bred low responder; CREB1, cyclic adenosine monophosphate response element binding protein 1; CTGF, connective tissue growth factor; ECM2, extracellular matrix protein 2; EGR1, early growth response protein 1; IGFBP7, insulin-like growth factor–binding protein 7; MAPK8IP3, mitogen-activated protein kinase 8 interacting protein 3; MAPKAPK5, mitogen-activated protein kinase–activated protein kinase 5; P4HA1, prolyl 4-hydroxylase subunit alpha-1; PKN1, protein kinase novel 1; PRKAG1, protein kinase adenosine monophosphate–activated noncatalytic subunit gamma 1; PGDFβ, platelet-derived growth factor-β; RHOA, Ras homolog gene family member G; RHOG, homolog gene family member G; SOX4, (sex determining region Y)-box 4; STK3, serine/threonine kinase 3; VEH, vehicle.
Significant effects (p < .05).
When unbiased Ingenuity Pathway Analysis was performed on the dataset, the top four significant physiological functions were nervous system development and function, tissue development, cardiovascular system development and function, and connective tissue development and function. The top four significant networks for diseases and disorders were cardiovascular disease, neurological disease, organismal injury and abnormalities, and connective tissue disorders. The significant functional and network alterations in connective tissue disorders is especially interesting given that this function was also altered in bLRs treated with FGF2 compared with bLRs treated with vehicle (18), as well as in the choroid plexus of individuals with MDD (25).
Social Defeat Stress Increases CTGF Expression in bLRs
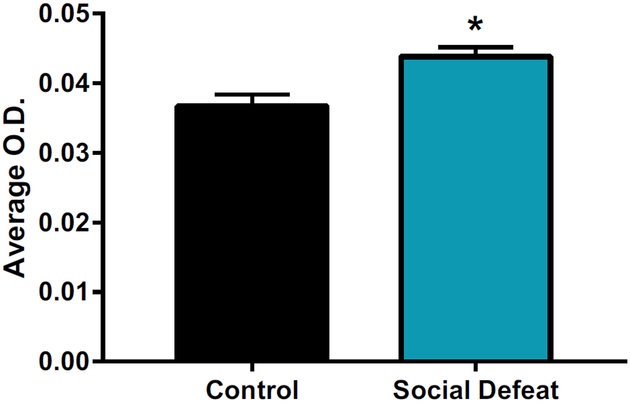
Because bHRs and bLRs differ basally in affect and their response to stress (16), we sought to determine whether stress might also alter CTGF expression in the hippocampus. Given that the bLRs are more susceptible to stress and changes in their environment, we assessed their response to 4 days of social defeat stress in adulthood. Four days of social defeat increased CTGF expression in bLRs in the dentate gyrus (t9 = 2.46, p < .05) (see Figure 2). These results suggest that social stress may increase CTGF expression levels akin to the increase observed in MDD.
Figure 2.
Stress in bred low responders increased connective tissue growth factor gene expression. Four days of social defeat stress increased connective tissue growth factor expression in the dentate gyrus compared with control animals. n = 5–6 per group. All values are mean ± SEM. *p < .05. O.D., optical density.
Acute Administration of CTGF to Outbred Rats Increased Depression-like Behavior
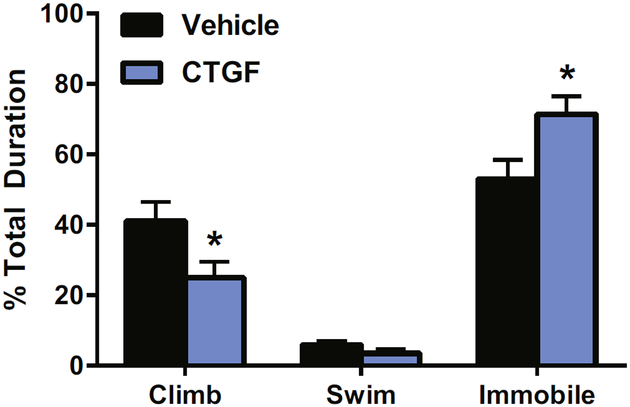
The role that CTGF plays in emotionality was previously unknown. To determine whether CTGF may modulate affective behavior, we assessed the ability of CTGF to alter anxiety-like and depression-like behavior in outbred rodents. Acutely, CTGF microinjections into the left lateral ventricle did not alter anxiety-like behavior on the EPM (open [t21 = −1.55, p = .14], closed [t21 = 0.84, p = .41]) (see Table S3 in Supplement 1). However, ICV administration of 400 ng of CTGF altered depression-like behavior. Specifically, CTGF increased the time spent immobile in the FST (t21 = −2.39, p < .05) (see Figure 3). It also decreased the time spent climbing (t21 = 2.16, p < .05). This suggests that CTGF may be a prodepressant molecule.
Figure 3.
Connective tissue growth factor (CTGF) increased depression-like behavior. Acute administration of CTGF to outbred rats increased time spent immobile and decreased the time spent climbing in the forced swim test. n = 10–13 per group. All values are mean ± SEM. *p > .05.
An Antibody to CTGF (FG-3019) Altered Gene Expression of the CTGF Pathway
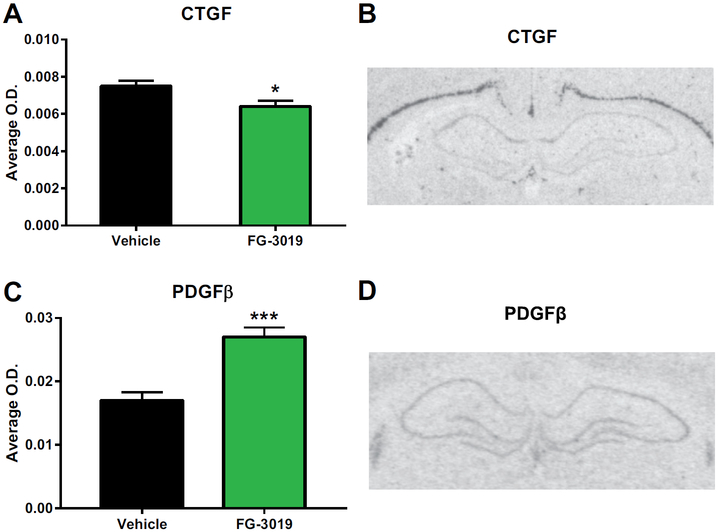
To verify that the antibody to CTGF, FG-3019, decreased its own gene expression, mRNA in situ hybridization was performed in the dentate gyrus, the same region we showed to be different basally between bLRs and bHRs in adulthood. Indeed, chronic treatment with FG-3019 decreased CTGF expression in the dentate gyrus (t13 = 2.46, p < .05) (see Figure 4A, B). To determine whether altering the antibody could alter other signaling partners (see Table 1), we also assessed the gene expression of another growth factor, PDGFβ. Because CTGF was increased and PDGFβ was decreased in bLRs, and FG-3019 decreased CTGF, we hypothesized that the PDGFβ would be increased after FG-3019. As expected, FG-3019 increased PDGFβ gene expression in the dentate gyrus (t12 = −4.69, p = .001) (see Figure 4C, D). These results suggest that FG-3019 is effective at decreasing CTGF expression in brain and can alter the CTGF pathway.
Figure 4.
FG-3019, an anti–connective tissue growth factor (CTGF) antibody, altered gene expression in the CTGF pathway in the dentate gyrus. (A) Chronic treatment with FG-3019 decreased CTGF gene expression in the dentate gyrus. n = 7–8 per group. All values are mean ± SEM. *p < .05. (B) Representative CTGF messenger RNA expression at the level of the hippocampus. (C) Chronic treatment with FG-3019 increased platelet-derived growth factor-β (PDGFβ) in the dentate gyrus. n = 7 per group. All values are mean ± SEM. ***p = .001. (D) Representative PDGFβ messenger RNA expression at the level of the hippocampus. O.D., optical density.
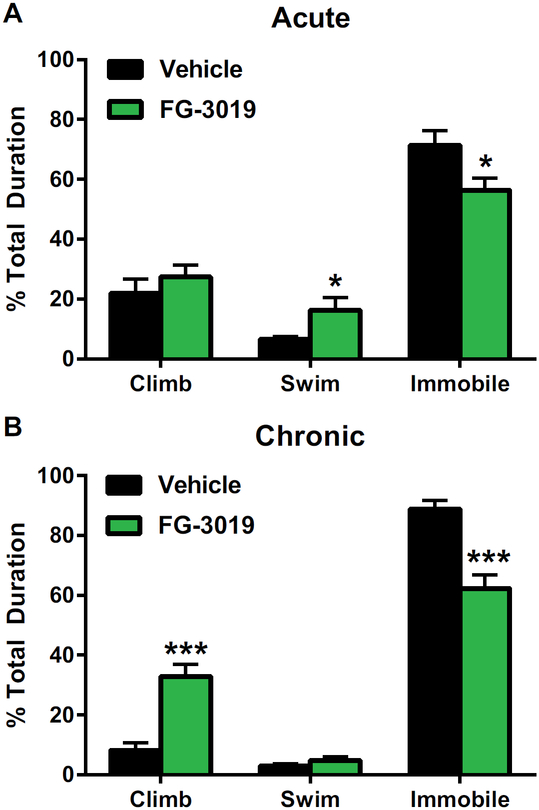
Acute and Chronic Administration of an Antibody to CTGF (FG-3019) Decreased Depression-like Behavior in Outbred Rats
Because CTGF was acutely prodepressant, decreasing its expression may be antidepressant. We studied the ability of FG-3019, a humanized monoclonal antibody to CTGF, to alter affective behavior in rodents. Acutely, FG-3019 was antidepressant and decreased immobility in the FST (t21 = 2.36, p < .05) while increasing the time spent swimming (t21 = 22.30, p < .05) (see Figure 5A). There was no effect on anxiety-like behavior in the EPM (closed arms [t21 = −0.98, p = .34], open arms [t21 = 1.07, p = .30]) (see Table S3 in Supplement 1). Chronically, there was no effect on either locomotor behavior (t18 = 0.01, p = .99) or anxiety-like behavior (closed arms [t17 = −0.06, p = .95], open arms [t17 = −0.62, p = .54]) (see Table S3 in Supplement 1). However, FG-3019 was antidepressant and decreased the time spent immobile (t13 = 5.05, p < .001) while increasing the time spent climbing (t13 = −5.42, p < .001) in the FST (see Figure 5B). Thus, an antibody to CTGF may be an effective and novel antidepressant.
Figure 5.
FG-3019, an anti–connective tissue growth factor antibody, decreased depression-like behavior. (A) Acute administration of FG-3019 decreased time spent immobile and increased time spent swimming in the forced swim test. n = 11–12 per group. All values are mean ± SEM. *p < .05. (B) Chronic administration of FG-3019 decreased time spent immobile and increased time spent climbing in the forced swim test. n = 7–8 per group. All values are mean 6 SEM. ***p < .001.
DISCUSSION
This study is the first to implicate CTGF as a prodepressant molecule that could serve as a target for the development of novel therapeutics. This is based on several lines of convergent evidence from postmortem human brains and animal studies: 1) CTGF gene expression is significantly increased in several nuclei of the amygdala in MDD; 2) CTGF gene expression is increased in the dentate gyrus of rats that display increased anxiety-like and depression-like behavior; 3) an early-life manipulation that decreases anxiety behavior in vulnerable rats also decreases CTGF expression; 4) CTGF gene expression is increased following social defeat stress; 5) central administration of CTGF increases depression-like behavior in outbred rats; 6) conversely, an antibody to CTGF, FG-3019, decreases depression-like behavior; and 7) in response to FG-3019, CTGF expression is decreased while PDGFβ expression is increased in the dentate gyrus. Overall, these findings suggest that CTGF is a novel prodepressant molecule and decreasing its expression may have antidepressant effects.
We assessed gene expression in the postmortem human amygdala of individuals with MDD. Our results indicated that CTGF was strongly increased in several of the amygdala nuclei. However, it should be mentioned that all of the subjects with MDD committed suicide in the qRT-PCR validation. Therefore, the results may represent a specific phenotype of MDD that may contribute to our understanding of suicidal behavior. Only men were included, as there were not enough female suicide brains to perform a meaningful statistical comparison. The amygdala nuclei studied interact with a variety of brain regions, including the hypothalamus, cortex, striatum, thalamus, and insula, to coordinate the external stimuli and internal environment, thus producing an integrated response relevant to attention, motivation, and reward (29). This led us to the hypothesis that CTGF may be contributing to the negative affect in depressed individuals.
To determine whether the alterations observed in MDD could be recapitulated in our animal model, we assessed CTGF gene expression in the amygdala and dentate gyrus in male rats only in an attempt match the human findings. For the amygdala, we used brains from bHRs and bLRs across several generations to determine if this effect could be generalized in our animal model. Although not statistically significant, bLRs had twice the amount of CTGF expression in the amygdala than bHRs, paralleling the human findings. The amygdala is strongly implicated in human depression (30–32), and the profound effects we observed in the amygdala are likely due to the chronic condition of the disorder. In contrast, animal models tend to measure spontaneous, acute depression-like behavior in which the hippocampus appears to be the relevant brain area (33). We then performed a microarray study in the dentate gyrus and found increased CTGF expression in bLRs that exhibit increased anxiety-like and depression-like behavior compared with bHRs. These findings are congruent with previous indications that CTGF was upregulated in the hippocampus of another animal model of depression, the Wistar-Kyoto more immobile rat strain (34).
Moreover, the expression of CTGF could be altered by manipulations that also alter affective responses. Early-life FGF2 administration to bLRs is known to decrease anxiety-like behavior in adulthood (18), and this manipulation decreased the basal level of CTGF in the bLRs. Indeed, several molecules that interact with or signal through CTGF (35) were upregulated in bLRs and normalized by FGF2 treatment. Thus, the CTGF signaling pathway may represent an interesting target for further exploration.
Four days of social defeat stress in bLRs increased CTGF expression in the dentate gyrus. This is congruent with a previous study showing that 8 days of stress increased CTGF (36). Because CTGF is also known to interact with numerous molecules, and the signaling and feedback is extremely complex, more research on CTGF is needed to elucidate the dynamics of the stress response.
With mounting evidence that high levels of CTGF are associated with anxiety and depression, we asked whether we could implicate CTGF in the regulation of affect. Our studies demonstrate that CTGF can induce depression-like behavior, whereas blockade of CTGF levels using an antibody approach decreases depression-like behavior. Interestingly, decreasing CTGF expression also resulted in changes in another molecule in the CTGF pathway, PDGFβ. Typically, CTGF and PDGFβ are both increased in fibrosis models and are consider profibrotic agents (35,37). Thus, it is possible that the alteration in PDGFβ is a compensatory effect based on its weak association with CTGF and not a therapeutic effect (38).
CTGF is a critical mediator of fibrosis, and FG-3019 has beneficial results in open-label clinical trials of fibrotic conditions, such as idiopathic pulmonary fibrosis (10,39). It is possible that a depressed brain may have similar sequelae of gene changes associated with a fibrotic state. Because CTGF can interact with a variety of players and influence a multitude of downstream receptors and pathways, decreasing its expression may be a valuable therapeutic avenue in treating depression. Agents that are not as selective or specific, such as the small molecule inhibitors of CTGF, may also offer valuable therapeutic opportunities.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by National Institute of Mental Health Conte Center Grant No. P50 MH60398 (to WEB), National Institute of Mental Health Grant No. R01 MH104261 (to HA, SJW), National Institute on Drug Abuse Grant No. U01 DA043098 (to HA), the Hope for Depression Research Foundation, Office of Naval Research Grant Nos. N00014-09-1-0598 and N00014-12-1-0366 (to HA), and the Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC (to HA, SJW, RMM, WEB, AFS, FSL, JDB). The authors are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund LLC. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan; Stanford University; Weill Medical College of Cornell University; the University of California, Irvine; and the HudsonAlpha Institute for Biotechnology to encourage the development of appropriate findings for research and clinical applications.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2018.04.013.
REFERENCES
- 1.Duman RS, Monteggia LM (2006): A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V, Nestler EJ (2010): Linking molecules to mood: New insight into the biology of depression. Am J Psychiatry 167:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner CA, Watson SJ, Akil H (2012): The fibroblast growth factor family: Neuromodulation of affective behavior. Neuron 76:160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aurbach EL, Inui EG, Turner CA, Hagenauer MH, Prater KE, Li JZ, et al. (2015): Fibroblast growth factor 9 is a novel modulator of negative affect. Proc Natl Acad Sci U S A 112:11953–11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik AR, Liszewska E, Jaworski J (2015): Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front Cell Neurosci 9:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jun JI, Lau LF (2011): Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 10:945–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoyama E, Kubota S, Takigawa M (2012): CCN2/CTGF binds to fibroblast growth factor receptor 2 and modulates its signaling. FEBS Lett 586:4270–4275. [DOI] [PubMed] [Google Scholar]
- 8.Mason RM (2013): Fell-Muir lecture: Connective tissue growth factor (CCN2) – A pernicious and pleiotropic player in the development of kidney fibrosis. Int J Exp Pathol 94:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubota S, Takigawa M (2015): Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin Sci (Lond) 128:181–196. [DOI] [PubMed] [Google Scholar]
- 10.Lipson KE, Wong C, Teng Y, Spong S (2012): CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 5(suppl 1): S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khodosevich K, Lazarini F, von Engelhardt J, Kaneko H, Lledo PM, Monyer H (2013): Connective tissue growth factor regulates inter-neuron survival and information processing in the olfactory bulb. Neuron 79:1136–1151. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Liu Z, Li X, Luo B, Xiong J, Gan W, et al. (2014): Accumulation of connective tissue growth factor1 cells during the early phase of rat traumatic brain injury. Diagn Pathol 9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuer H, Christ S, Friedrichsen S, Brauer D, Winckler M, Bauer K, et al. (2003): Connective tissue growth factor: a novel marker of layer VII neurons in the rat cerebral cortex. Neuroscience 119:43–52. [DOI] [PubMed] [Google Scholar]
- 14.Kondo Y, Nakanishi T, Takigawa M, Ogawa N (1999): Immunohisto-chemical localization of connective tissue growth factor in the rat central nervous system. Brain Res 834:146–151. [DOI] [PubMed] [Google Scholar]
- 15.Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000): Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinton S, Miller S, Watson SJ, Akil H (2008): Prenatal stress does not alter innate novelty-seeking behavioral traits, but differentially affects individual differences in neuroendocrine stress responsivity. Psycho-neuroendocrinology 33:162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H (2009): A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci 29:6379–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner CA, Clinton SM, Thompson RC, Watson SJ Jr, Akil H (2011): Fibroblast growth factor-β (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proc Natl Acad Sci U S A 108:8021–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. (2004): Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A 101:15506–15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomita H, Vawter MP, Walsh DM, Evans SJ, Choudary PV, Li J, et al. (2004): Effect of agonal and postmortem factors on gene expression profile: Quality control in microarray analyses of postmortem human brain. Biol Psychiatry 55:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones EG, Hendry SH, Liu XB, Hodgins S, Potkin SG, Tourtellotte WW (1992): A method for fixation of previously fresh-frozen human adult and fetal brains that preserves histological quality and immunoreactivity. J Neurosci Methods 44:133–144. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Amado M, Prensa L (2012): Stereological analysis of neuron, glial and endothelial cell numbers in the human amygdaloid complex. PLoS One 7:e38692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Amado M, Prensa L (2013): Distribution of dopamine transporter immunoreactive fibers in the human amygdaloid complex. Eur J Neurosci 38:3589–3601. [DOI] [PubMed] [Google Scholar]
- 24.Schumann CM, Amaral DG (2005): Stereological estimation of the number of neurons in the human amygdaloid complex. J Comp Neurol 491:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner CA, Thompson RC, Bunney WE, Schatzberg AF, Barchas JD, Myers RM, et al. (2014): Altered choroid plexus gene expression in major depressive disorder. Front Hum Neurosci 8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, et al. (2006): Selective breeding for divergence in novelty-seeking traits: Heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet 36:697–712. [DOI] [PubMed] [Google Scholar]
- 27.Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H (2008): Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res 1224:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Litvin Y, Turner CA, Rios MB, Maras PM, Chaudhury S, Baker MR, et al. (2016): Fibroblast growth factor 2 alters the oxytocin receptor in a developmental model of anxiety-like behavior in male rat pups. Horm Behav 86:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson LW, Petrovich GD (1998): What is the amygdala? Trends Neurosci 21:323–331. [DOI] [PubMed] [Google Scholar]
- 30.Bellani M, Baiano M, Brambilla P (2011): Brain anatomy of major depression II. Focus on amygdala. Epidemiol Psychiatr Sci 20: 33–36. [DOI] [PubMed] [Google Scholar]
- 31.Kim YK, Won E (2017): The influence of stress on neuroinflammation and alterations in brain structure and function in major depressive disorder. Behav Brain Res 329:6–11. [DOI] [PubMed] [Google Scholar]
- 32.Mears D, Pollard HB (2016): Network science and the human brain: Using graph theory to understand the brain and one of its hubs, the amygdala, in health and disease. J Neurosci Res 94:590–605. [DOI] [PubMed] [Google Scholar]
- 33.Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, et al. (2018): Treatment resistant depression: A multi-scale, systems biology approach. Neurosci Biobehav Rev 84:272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pajer K, Andrus BM, Gardner W, Lourie A, Strange B, Campo J, et al. (2012): Discovery of blood transcriptomic markers for depression in animal models and pilot validation in subjects with early-onset major depression. Transl Psychiatry 2:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kok HM, Falke LL, Goldschmeding R, Nguyen TQ (2014): Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat Rev Nephrol 10:700–711. [DOI] [PubMed] [Google Scholar]
- 36.Stankiewicz AM, Goscik J, Majewska A, Swiergiel AH, Juszczak GR (2015): The effect of acute and Cchronic social stress on the hippo-campal transcriptome in mice. PLoS One 10:e0142195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park HJ, Kim HG, Wang JH, Choi MK, Han JM, Lee JS, et al. (2016): Comparison of TGF-beta, PDGF, and CTGF in hepatic fibrosis models using DMN, CCl4, and TAA. Drug Chem Toxicol 39:111–118. [DOI] [PubMed] [Google Scholar]
- 38.Pi L, Chung PY, Sriram S, Rahman MM, Song WY, Scott EW, et al. (2015): Connective tissue growth factor differentially binds to members of the cystine knot superfamily and potentiates platelet-derived growth factor-B signaling in rabbit corneal fibroblast cells. World J Biol Chem 6:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raghu G, Scholand MB, de Andrade J, Lancaster L, Mageto Y, Goldin J, et al. (2016): FG-3019 anti-connective tissue growth factor monoclonal antibody: Results of an open-label clinical trial in idiopathic pulmonary fibrosis. Eur Respir J 47:1481–1491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.