Abstract
Intimate partner violence (IPV) victimization is associated with a wide range of mental and physical health problems, but little is known about the effect of IPV on cognitive decline. Previous research suggests an association between IPV victimization and cognitive dysfunction, but the few studies that have examined this phenomenon were cross-sectional in design and focused only on female victims of IPV. The current study examined cognitive function over time among a diverse population of both male and female victims of IPV. Regression analyses indicated increased completion time on Trail Making Test (TMT) A for both male and female victims of IPV living below poverty, as well as for female victims of IPV without previously depressive symptomatology. Results also indicated increased completion time on TMT B for male victims of IPV. Our findings support an association between IPV victimization and increased cognitive decline that is moderated by poverty status and previous depressive symptomatology.
Keywords: cognition, intimate partner violence, domestic violence, executive function, attention
Introduction
Cognitive performance, the “processes of knowing, including attending, remembering, and reasoning” (American Psychological Association, 2014), shifts and changes over the adult lifespan, and is influenced by numerous demographic and health-related factors such as age, sex, socioeconomic status, and mental and physical health. Although there is considerable debate about the mechanisms underlying cognitive change over time (Glisky, 2007), some cognitive decline is considered a “normal” component of aging (Deary et al., 2009). Cognitive deficits are common among individuals with traumatic brain injuries and neurodegenerative disorders, but less is known about the role of psychiatric and psychological conditions in cognitive performance.
Psychological symptoms such as those associated with depression (McDermott & Ebmeier, 2009) and emotional trauma (Brandes et al., 2002) reduce cognitive performance. Depressive symptoms are associated with cognitive decline, particularly executive function (Elliott et al., 1996; McDermott & Ebmeier, 2009) processing speed (Beats, Sahakian, & Levy, 1996; Elliott et al., 1996; McDermott & Ebmeier, 2009), spatial recognition and attentional shifting (Beats et al., 1996). Similarly, psychological symptoms associated with exposure to trauma can lead to deficits in attention, memory, learning, and executive function (Brandes et al., 2002; Flaks et al., 2014; Yehuda et al., 2006). It is unclear whether cognitive dysfunction following exposure to a traumatic event is mediated by the emergence of post-traumatic stress disorder (PTSD). Some studies suggest that a decline in cognitive performance following trauma exposure is associated with the development of PTSD (e.g., Flaks et al., 2014); however, other findings suggest that individuals exposed to trauma experience significantly greater cognitive decline over time than unexposed comparisons, regardless of PTSD symptom severity (e.g., Yehuda et al., 2006).
Psychological and cognitive responses to trauma may differ based on sex (Polak, Witteveen, Reitsma, & Olff, 2012; Vrana & Lauterbach, 1994). Although men have higher rates of trauma exposure, women tend to have higher rates of psychological consequences, such as PTSD, following traumatic events (Breslau, 2009). Further, Vrana and Lauterbach (1994) found that men exposed to traumatic events were affected more negatively by events they felt they could not discuss, whereas women were affected more negatively by exposure to violent deaths or injury.
Few studies have examined sex differences in cognitive function following exposure to traumatic events; however, one study (Polak et al., 2012) found that male PTSD patients performed worse on measures of executive function, whereas female PTSD patients did not exhibit any signs of executive dysfunction. Although the mechanisms of change are unclear, research suggests that traumatic experiences have a negative impact on cognitive performance. Despite these findings, few studies have examined the association between IPV victimization and cognitive function.
Intimate partner violence (IPV) is a noteworthy source of psychological trauma and a serious public health concern that affects both men and women in all racial and socioeconomic groups (Bonomi et al., 2009). IPV prevalence estimates vary by sample and detection method, particularly with respect to age, income, race, and sex. IPV victimization is more common among younger adults (Bonomi, Anderson, Rivara, & Thompson, 2007; Jones et al., 1999; Thompson et al., 2006) and those with lower household income (Bonomi et al., 2009; Campbell et al., 2002; Coker et al., 2002; Jones et al., 1999; Thompson et al., 2006). Although some studies found no significant differences in rates of IPV victimization with respect to race (Coker, Smith, Bethea, King, & McKeown, 2000; Thompson et al., 2006), other studies have found higher rates of reported IPV among African Americans than European Americans (Campbell et al., 2002; Jones et al., 1999). Racial disparities appear to be largely accounted for by socioeconomic factors (Cazenave & Straus, 1990; Grossman & Lundy, 2007).
There is a considerable degree of controversy regarding prevalence of IPV with respect to sex. Some theorists argue that IPV is often expressed through mutual physical contact, but women bear the brunt of injury, leading to higher reporting rates in medical and legal contexts (Archer, 2000). Further, a significant amount of research has focused primarily on IPV victimization among women (e.g., Adams, Bybee, Tolman, Sullivan, & Kennedy, 2013; Bonomi et al., 2007; Thompson et al., 2006). The tendency for researchers to exclude male victims in their analyses may lead to inaccurate perceptions of the prevalence of IPV with respect to sex. Despite these discrepancies, it is important to accurately understand the prevalence of IPV among these specific populations in order to appropriately address the consequences stemming from this type of abuse.
IPV is associated with a range of psychological and physical health problems. Psychological consequences of IPV include depression, PTSD, suicidality (Golding, 1999), anxiety (e.g., Adams et al., 2013; Bonomi et al., 2009), and substance abuse (e.g., Coker et al., 2002; Coker et al., 2000). These outcomes may be moderated by sex, with women reporting more symptoms related to PTSD (Golding, 1999) and men reporting more depressive symptoms following their abuse (e.g., Reid et al., 2008). Although findings from research examining mental health outcomes of female victims of IPV have linked reported abuse to depression, suicidality, PTSD, substance abuse (e.g., Golding, 1999) and anxiety (Adams et al., 2013), only depression and substance abuse were reported in studies that included male victims of IPV in their sample.
In addition to the psychological consequences associated with IPV, many victims of IPV also suffer from physical health problems associated with their abuse. Direct effects include contusions, soft-tissue injuries, sprains, strains, fractures, maxillofacial injuries, and traumatic brain injuries (Mitchell & Anglin, 2009). Men and women with a reported history of IPV victimization tend to report poorer overall health than their nonabused counterparts (Carbone-Lopez, Kruttschnitt, & Macmillan, 2006; Coker et al., 2002). Specifically, findings from research examining physical health outcomes of both male and female victims of IPV have linked reported abuse to chronic disease (e.g., Coker et al., 2002), joint disease, asthma, smoking, activity limitations, heaving drinking, (e.g., Breiding, Black, & Ryan, 2008) and physical disability (e.g., Carbone-Lopez et al., 2006). Physical health outcomes associated with IPV may be moderated by sex. One study (Breiding et al., 2008) reported greater risk for heart attacks, strokes, and high cholesterol for women, but not men with a reported history of IPV. Although a considerable amount of research has focused on the mental and physical health outcomes associated with IPV, less literature is available on the cognitive consequences of abuse.
Previous research on the association between IPV victimization and cognitive dysfunction, although limited, suggests that IPV victimization may negatively affect cognitive function, particularly with respect to executive function (Seedat, Videen, Kennedy, & Stein, 2005; Stein, Kennedy, & Twamley, 2002), working memory (Stein et al., 2002), and processing speed (Twamley et al., 2009). For example, one study (Stein et al., 2002) found that female victims of IPV performed worse than nonabused controls on tasks of visuoconstruction, working memory, and executive function. Similarly, Seedat et al. (2005) found that female victims of IPV performed worse than controls on tests of cognitive inhibition and set shifting. Further, Twamley et al. (2009), found that women with PTSD related to IPV victimization performed significantly worse than healthy controls on a measure of processing speed.
The few studies that have examined the association between IPV victimization and cognitive function focused only on female victims of IPV (e.g., Seedat et al., 2005; Stein et al., 2002; Twamley et al., 2009). Further, each of the studies investigating the effects of IPV on cognitive function was cross-sectional. Due to the temporal association between age and neurocognitive decline, it is important to investigate the effects of IPV victimization on cognitive function over time. Although some level of cognitive decline is inevitable with age, it is important to identify risk factors that can lead to an accelerated rate of decline in cognitive function over time in order to better understand the mechanisms underlying this type of deterioration.
The present study examined the effect of IPV victimization on cognitive function among a mixed-sex sample of economically and racially diverse working-aged adults. Based on high rates of depression among both male and female victims of IPV, our study examined whether this effect is moderated by previous depressive symptomatology. Based on findings from research examining cognitive function following exposure to traumatic events, we expected to find an association between IPV victimization and cognitive decline over time. Although our literature review uncovered no studies that examined the association between IPV victimization and cognitive function among men, research suggests differential health and psychological outcomes for men and women following IPV victimization. Based on these findings, we expected to observe some sex differentials in the association between IPV victimization and cognitive performance. Further, based on findings from research examining the association between depression and cognitive function, we expected worse cognitive performance among individuals with previously depressive symptomatology.
Method
Participants
Participants were 63 men and women initially between the ages of 30–63 who were participants in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. HANDLS is an ongoing epidemiological study focusing on health disparities in Baltimore, Maryland. Participants are a fixed cohort of women and men recruited from 12 neighborhoods (contiguous census tracts) in Baltimore City that were predetermined based on the likelihood of yielding representative distributions of Baltimore City residents. The area probability sample was designed to sample a diverse group of participants with respect to a 4-way factorial design of age, sex, race, and SES. Poverty status was coded as a binary variable. Individuals were categorized as living in households with incomes at or below 125% of the federal poverty line or 125% above the federal poverty line based on their annual household income and household size. For example, in 2003, the federal poverty level was $18,400 for a family of four. Therefore, an individual in our sample in a household earning less than or equal to $18,400 per year with a household size of four was classified as living below the federal poverty line. Race was defined as African American or white and participants ranged from 30 to 64 years of age (Evans et al., 2010). Exclusionary criteria for the HANDLS study included a current pregnancy, a history of Auto-Immune Deficiency Syndrome (AIDS) or cancer.
Longitudinal data were collected at two time points (Wave 1 and Wave 3) spanning approximately 5 years between each visit for each participant. In order to investigated the rate of cognitive decline following a new incident of IPV victimization, the present study examined HANDLS participants who reported IPV victimization at Wave 3, but reported no history of IPV victimization before their participation in Wave 1. Overall, 38 participants (2.27%) reported IPV victimization since their last HANDLS visit; however, six participants were excluded due to their reporting victimization prior to Wave 1 and 8 were excluded because of missing data regarding IPV victimization prior to Wave 1. Of the 24 remaining participants reporting incident IPV victimization, 21 had complete cognitive data. We created a comparison sample by performing a 2:1 match on education and initial age for participants with no history of IPV. Matching is a way of controlling for certain criteria that removes the variation in that criteria which can then not be used as a comparisons classification. Therefore, we did not match on any variables that were included as covariates in our analyses. Although some studies use years of education as a proxy for SES, years of education and poverty status were not highly correlated in the sample for the current study (r = 0.17). Within the effective sample, 54% identified as female and 46% of participants identified as male. Further, 63% of participants identified as African American and 37% identified as white.
Measures
Cognitive function was evaluated using several measures, including the Trail Making Test (TMT) Parts A and B, and the Animal Naming (AN) Test. Although many cognitive measures were administered to HANDLS participants, only the scores from TMT A, TMT B, and the AN test were analyzed for the current study due to missing data for the participants in these analyses. These measures were administered to participants during their visits to the mobile research vehicles (MRVs) during Wave 1 and Wave 3.
Trail Making Test (TMT).
The Trail Making Test (TMT) consists of two parts. Part A of the TMT consists of a sheet of paper displaying circles labeled “1” through “25”. This task requires the participant to sequentially connect the 25 circles as quickly and as accurately as possible by drawing straight lines from one circle to the next (e.g., connect circle “1” to circle “2” and so on). Studies suggest that part A of the TMT is a valid measure of visuoperceptual abilities, attention, and information processing speed (1944; Lezak, 1995; Reitan, 1992).
Part B of the TMT consists of a sheet of paper displaying 25 circles with numbers (“1”-”12”) and letters (“A”-”L”). This portion of the TMT requires participants to connect the 25 circles, switching back and forth between numbers and letters (e.g., connect “1” to “A” and “A” to “2” and “2” to “B” and so on) as quickly and accurately as possible. Part B of the TMT serves as a measure of processing speed and cognitive flexibility. The scores for TMT parts A and B are derived from the number of seconds it takes the participant to complete each task, with higher scores indicating poorer neurocognitive performance (1944; Lezak, 1995; Reitan, 1992).
The Animal Naming (AN) Task.
The Animal Naming (AN) task is a measure of categorical verbal fluency that requires participants to recite the names of as many animals that they can in 60 seconds. Participants receive one point for each animal name that they recite. Duplicate and mythical animal names are not counted (Tombaugh, Kozak, & Rees, 1999).
The Center for Epidemiological Studies Depression Scale (CES-D).
Depression was assessed during Wave 1 using the Center for Epidemiological Studies Depression Scale (CES-D). The CES-D is a 20-item questionnaire used to assess the presence and frequency of depressive symptoms in the general population. Participants are asked to rate their depressive symptoms within the past week using a 4-point Likert scale ranging from “0” (“rarely or none of the time”) to “3” (“most or all of the time”). Scores for this measure range from 0 to 60. Scores greater than 16 are suggestive of significant depressive symptomatology (Radloff, 1977). The CESD variable was dichotomized based on the cut-off level for clinical significance.
Intimate Partner Violence.
Incident IPV victimization was assessed by participants’ reports of sexual or physical abuse by a partner in Wave 3, but no such abuse before Wave 1. IPV in Wave 1 was evaluated during each participant’s medical history interview. As part of a semi-structured interview, participants were asked whether or not they had ever been physically or sexually abused by an intimate partner in their lifetime. Participants responses were coded as “Yes” or “No.” IPV in Wave 3 was evaluated using audio computer-assisted self-interview (ACASI) software composed of various psychological measures. Participants’ reports of abuse were based on a response of “Yes” or “No” to either of the following questions: “Since your last HANDLS examination, have you been hit, slapped, kicked, or otherwise physically hurt by (a partner)?” or “Since your last HANDLS examination, has anyone (partner) forced you to have an unwanted sexual act?”
Procedure
Participants provided written informed consent before participating in the study. The study is approved by the Institutional Review Board of the National Institute of Environmental Health Sciences, NIH. Demographic information was obtained from each HANDLS participant during an interview conducted in the participant’s home. On a separate occasion, participants were administered various medical and psychological tests during an all-day appointment on mobile research vehicles parked in their neighborhoods including a detailed medical history at the initial visit and an interim medical history at the follow-up visit approximately 5 years later. Although a number of measures were administered, only the data from the measures related to IPV and cognitive function were analyzed. Participants received monetary compensation, as well as comprehensive physical examinations and clinical reports after they participated in the study. Detailed information regarding the procedure for the HANDLS study has been described previously (Evans et al., 2010).
Analyses
All statistical analyses were performed using the statistical software “R” (R Development Core Team, 2013).
Matching
Forty-two participants who did not meet the criteria for the IPV group were matched to the 21 participants who did meet the criteria for the IPV group. Participants were matched on years of education and age at baseline. The 2:1 matching procedure was performed using the “MatchIt” package (Ho, Imai, King, & Stuart, 2011) in R. Pearson’s Chi-squared test with Yates’ continuity correction was used to test differences in sex and race between the IPV group and matched controls. A Welch two-sample t-test was used to test differences in age at baseline and years of education between the IPV group and matched controls. No differences were found for sex (x2 = 0.008, p = 0.929), race (x2 = 0, p = 1), years of education (t(41.8) = 0.665, p = .510) or age (t(46.67) = − 0.486, p = 0.629) between the IPV group and matched controls.
Regression Models.
The longitudinal relationship between IPV victimization and cognitive function was examined with mixed-effects regression analyses (Singer & Willett, 2003) using unstructured correlation matrices. Mixed-effects regression analyses account for the correlation of repeated measurements over time among participants. Further, this technique is not affected by missing data and can adequately handle varying measurement intervals within and across participants. Backwards elimination was used in each regression model. Backwards elimination is a technique commonly used with mixed model regression analyses in which unnecessary and nonsignificant fixed-effects are removed from the regression analysis. The model begins with all candidate variables and nonsignificant variables are then removed in a step-wise fashion. However, if a fixed-effect corresponds to a significant interaction term or random effect then it is not removed from the analysis. The benefits of backward elimination with linear mixed-effect models are well-documented (e.g., Morrell, Pearson, & Brant, 1997).
Three separate regression analyses were performed for each outcome variable (i.e., Trail Making Test Part A, Trail Making Test Part B, Animal Naming Test) using the “lme4” (Bates, Maechler, Bolker, & Walker, 2014) and “lmerTest” (Kuznetsova, Brockhoff, & Christensen, 2013) packages in R. The lme4 package is used to fit linear and generalized linear mixed-effects regression models. The lmerTest package calls on functions within the lme4 package in order to perform F-tests for fixed effects, likelihood ratio tests for random effects, and backwards elimination of nonsignificant fixed effects. Models were adjusted for age at baseline and years of education using a matching procedure. Poverty status, depression, and sex were treated as dichotomous covariates. Age at Wave 1 and Wave 3 was modeled as a random effect to serve as an index of time. This technique is recommended for studies involving multiple waves of data collection (McArdle & Bell, 2000).
Model 1 for each of the three outcome variables examined whether a reported history of IPV, sex, poverty status, and their interactions were associated with a change in cognitive performance over time. In Model 2, separate, mixed-effects linear regression analyses added depression as covariate to test its influence of the relationship between IPV, sex, poverty status, and their interactions on each of the outcome variables (TMTA, TMTB, AN). Following a growth model formulation, age served a dual purpose as both the age of the participant and the measure of time. Random effects for both models included age and the subclass assigned to each participant in the matching procedure. Backwards elimination was performed for non-significant fixed effects (Morrell et al., 1997). P-values were calculated for t-tests based on Satterthwaite’s approximation for denominator degrees of freedom. Random effects were tested using log-likelihood ratio tests with one degree of freedom. Since the data for TMT A and TMT B were skewed, we performed a logarithmic transformation of the scores for these variables.
Results
Model 1
There was a significant IPV × sex interaction for Trail Making Test (TMT) Part B (b = 1.12, t (58.4) = 3.08, p = .003, g = 0.81). For men, a reported history of IPV was associated with a significant increase in completion time (worse performance) on TMT Part B. No significant main effects or interactions were found on TMT Part A or the Animal Naming (AN) test with respect to IPV. Coefficients and confidence intervals for regression model 1 are shown in Table 1.
Table 1.
Coefficients and confidence intervals for mixed model regression of IPV, age, sex, and poverty status on cognitive performance
| (log) Trail Making Test A |
(log) Trail Making Test B |
Animal Naming | ||||
|---|---|---|---|---|---|---|
| b | 95% CI | b | 95% CI | b | 95% CI | |
| IPV | - | - | − 0.41 | [−0.86, 0.03] | - | - |
| Age | 0.02*** | [0.01, 0.03] | 0.01 | [−0.02, 0.02] | −0.22** | [−0.38, −0.07] |
| Sex | - | - | − 0.15 | [−0.55, 0.26] | 2.45 | [−0.26, 5.17] |
| IPV*Sex | - | - | 1.12** | [0.41, 1.82] | - | - |
| Poverty | 0.22 | [−0.01, 0.31] | 0.55* | [0.06, 1.06] | −1.95 | [−4.92, 0.96] |
Note. N = 63. IPV = intimate partner violence.
p <. 05.
p <. 01.
p < .001.
Model 2
There was a significant age × IPV × poverty status interaction for TMT Part A (b = 0.11, t(73.9) = 2.94 p = .004, g = 0.78). Over time, a reported history of IPV, paired with living 125% below the poverty line, was associated with a significant increase in completion time (worse performance) on TMT Part A (Figure 1). There was also a significant age × IPV × sex × depression interaction for TMT Part A (b = 0.15, t(55.1) = 3.25, p = .002, g = 0.86). Over time, IPV was associated with a significant increase in completion time (worse performance) on TMT Part A for women without a history of depressive symptoms (Figure 2). Figure 1 depicts significant IPV x Poverty Status x Age interaction for TMT Part A. Figure 2 depicts significant IPV x Age x Depression x Sex interaction for TMT Part A. Both figures represent age-related change in cognitive performance as a function of IPV using all information in each model. After backwards elimination, the final regression models for TMT B and the Animal Naming (AN) test did not change. Coefficients and confidence intervals for regression model 2 are shown in Table 2.
Figure 1.
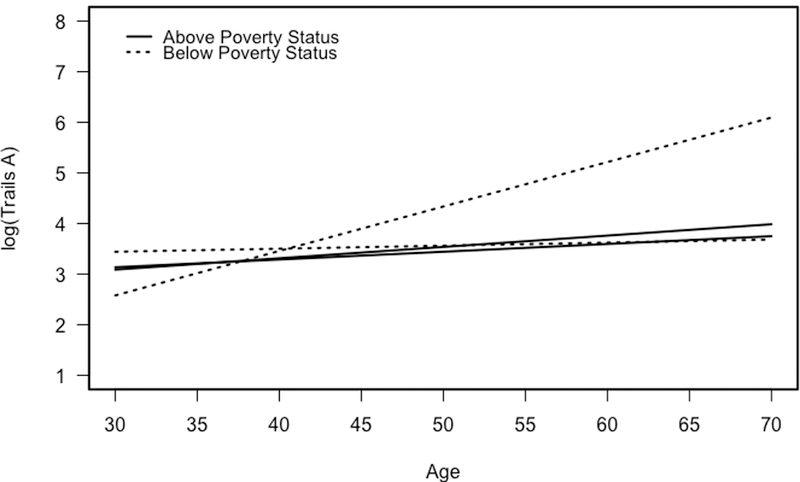
Longitudinal change in performance on the Trail Making Test Part A as a function of intimate partner violence (IPV) and poverty status.
Figure 2.
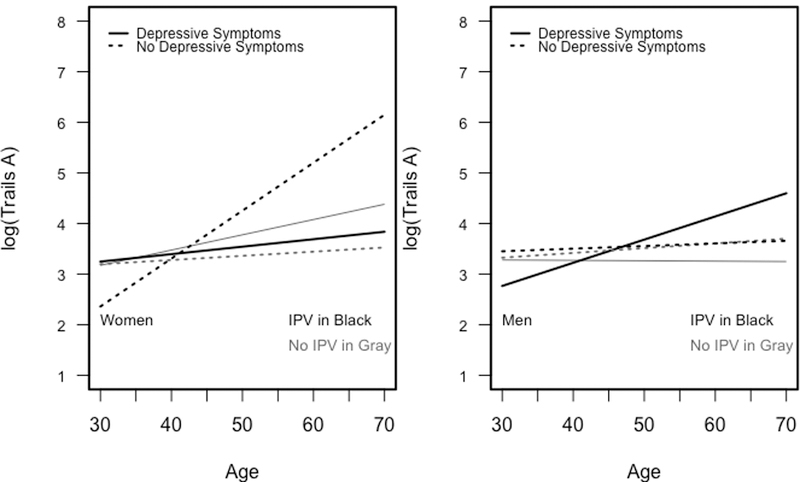
Longitudinal change in performance on the Trail Making Test Part A among men and women as a function of intimate partner violence (IPV) and depression.
Table 2.
Coefficients and confidence intervals for mixed model regression of IPV, age, sex, depression and poverty status on cognitive performance
| (log) Trail Making Test A |
(log) Trail Making Test B |
Animal Naming | ||||
|---|---|---|---|---|---|---|
| b | 95% CI | b | 95% CI | b | 95% CI | |
| IPV | 0.52 | [−0.00, 0.04] | −0.41 | [−0.86, 0.03] | - | - |
| Age | 0.02 | [0.02, 0.96] | 0.00 | [−0.02, 0.02] | −0.22** | [−0.38, −0.07] |
| IPV*Age | 0.05* | [0.00, 0.09] | - | - | - | - |
| Sex | 0.05 | [−0.32, 0.39] | −0.15 | [−0.55, 0.26] | 2.45 | [−0.26, 5.17] |
| IPV*Sex | −0.51 | [−1.47, 0.60] | 1.12** | [0.41, 1.82] | - | - |
| Poverty | 0.07 | [−0.31, 0.47] | 0.55* | [0.06, 1.06] | −1.95 | [−4.92, 0.96] |
| IPV*Poverty | 1.12* | [0.40, 1.92] | - | - | - | - |
| CES | 0.56* | [0.19, 0.92] | - | - | - | - |
| CES*IPV | −1.13** | [−1.74, −0.48] | - | - | - | - |
| IPV*Age*Sex | −0.06 | [−0.13, 0.02] | - | - | - | - |
| IPV*Age*Poverty | 0.11** | [0.05, 0.19] | - | - | - | - |
| IPV*Age*CES | −0.10** | [−0.15, −0.05] | - | - | - | - |
| IPV*Sex*Poverty | −1.05 | [−2.16, 0.00] | - | - | - | - |
| IPV*Sex*CES | 1.50* | [0.47, 2.50] | - | - | - | - |
| IPV*Age*Sex*Poverty | −0.09 | [−0.19, −0.00] | - | - | - | - |
| IPV*Age*Sex*CES | 0.15** | [0.06, 0.23] | - | - | - | - |
Note. N = 63. IPV = intimate partner violence. CES = depression.
p < .05.
p < .01.
Discussion
In this study, we examined the relationship between a reported history of IPV victimization and change in cognitive function in a mixed-sex sample of African-American and white participants between the ages of 30 and 63. We found that IPV victimization was significantly associated with decline in attention, visuoperceptual ability and executive function and that this relationship differed by poverty status and previous symptoms of depression. The current findings extend prior research demonstrating associations between IPV victimization and cognitive function by examining change in cognitive functioning over time in both men and women, and by examining interactive influences of poverty status, race, and previous depressive symptoms in an epidemiological sample.
IPV victimization was associated with a statistically significant increase in completion time (poorer performance) on Trail Making Test (TMT) Part A over time for men and women living below poverty. These results suggest that poverty status may moderate the effect of IPV victimization on attention and visuoperceptual ability. These results are consistent with others studies linking poverty to cognitive dysfunction (e.g., Mani, Mullainathan, Shafir, & Zhao, 2013; Turrell et al., 2002). A recent study (Mani et al., 2013) proposed a “cognitive constraint hypothesis” postulating that poverty itself contributes to cognitive decline because the stress of financial difficulty consumes a large majority of an individual’s cognitive resources.
IPV victimization was also associated with a significant decline in performance on TMT A for women without a history of significant depressive symptomatology. These findings suggest that IPV victimization was related to declines in attention and visuoperceptual ability over time for previously non-depressed women, but not for women with a history of significant depressive symptomatology. There are a couple of possible explanations for this phenomenon. First, despite numerous studies citing the relationship between cognitive dysfunction and depression, there is some evidence that the neurobiological changes associated with depressive symptoms may improve certain aspects of cognition (Andrews & Thomson, 2009). Andrews and Thomson (2009) proposed an analytical rumination hypothesis, suggesting that there are several adaptive features of depression, including attentional control. Specifically, the left ventrolateral prefrontal cortex (VLPFC) is activated during depressive episodes in order to facilitate focused attention on the target problem. Research suggests that activation of the left VLPFC can improve an individual’s ability to concentrate and withstand distractions (Andrews & Thomson, 2009).
Second, research suggests that women are more likely than men to seek psychological help for depression (e.g., Moller-Leimkuhler, 2002) and the skills and insight acquired in therapy can prevent a relapse of depressive episodes (e.g., Bockting, Spinhoven, Wouters, Koeter, & Schene, 2009). Further, women are also more likely to reach out for social support for psychological problems (Tamres, Janicki, & Helgeson, 2002). Women who have suffered previously from depressive symptomatology may be more familiar with available resources and may feel more comfortable reaching out for help following IPV victimization.
The results from TMT A for previously non-depressed women with a reported history of IPV are inconsistent with previous research on IPV victimization and time scores on TMT Part A (Stein et al., 2002; Twamley et al., 2009). However, both studies were cross-sectional in design and neither study included men in their sample.
IPV victimization was associated with a statistically significant increase in completion time (poorer performance) on TMT B for men, but not for women. These results suggest that IPV victimization may have a significantly greater effect on executive dysfunction in men over time. Previous research suggests differential frontal lobe activation patterns in response to stress between men and women, with greater involvement of the prefrontal regions for men compared to women (Wang et al., 2007). Considering that the frontal lobes are implicated in tasks involving cognitive flexibility and set-shifting (Dulay, Busch, Chapin, Jehi, & Najm, 2013) it is not surprising that male victims of IPV performed worse on a task of executive function.
Further, it is possible that this effect persists for men but not for women because of gender stereotypes regarding IPV victimization. Public perception of IPV victimization leans toward the idea that women are the victims and men are the perpetrators (Kimmel, 2002). Beyond men’s general reluctance to seek medical attention (Galdas, Cheater, & Marshall, 2005), gender stereotypes may further deter them from seeking help for their abuse, and may limit the help provided by police, social services, or health professionals. Research suggests that male victims of IPV are less likely than female victims of IPV to seek psychological help following their abuse (e.g., Tjaden & Thoennes, 2000). Female victims of IPV may be more likely to reach out for help, thus allowing them to better cope with their victimization. Lack of appropriate intervention may lead to more harmful and extended cognitive consequences for male victims of IPV.
Consistent with previous research (Stein et al., 2002), IPV victimization did not significantly influence performance on the Animal Naming test, suggesting that categorical fluency is not affected by a history of IPV victimization. Previous research suggests that the hippocampus is negatively impacted by traumatic stress (Bremner, 2006). Given the temporal lobe involvement in semantic fluency tasks (Baldo, Schwartz, Wilkins, & Dronkers, 2006), it is surprising that a measure of semantic fluency would not be impaired among victims of IPV victimization. However, our results were consistent with findings from Stein et al. (2002) in which victims of IPV did not perform worse on a measure of semantic fluency. These results suggest that cognitive function may be differentially impacted by specific types of trauma exposure, particularly in the domain of semantic fluency.
The current study is not without limitations. First, due to stringent inclusion criteria, only a small number of participants were included in the effective sample, thus lending to the possibility of over- or underestimating the results of our analyses. Future research would benefit from a larger sample size. A second limitation of the current study involves measuring IPV through participants’ self-reports of victimization on a 2-item screening instrument. Although self-report is arguably the best single method for detecting IPV victimization, underreporting may result from discrepancies in interpretation of item content or personal definitions of victimization (e.g., Perry & Fromuth, 2005). Screening instruments similar to items used in the current study tend to have good specificity, but lower sensitivity than more comprehensive measures (Nelson, Bougatsos, & Blazina, 2012). In addition, the phrasing of the physical IPV screening item included the term harm, which may have reduced endorsement by individuals who experienced physical partner aggression that was not perceived as harmful or injurious. Future research would benefit from implementing more comprehensive measures of IPV that do not rely solely on participants’ self-reports of abuse. Other limitations are a small number of cognitive tests over a relatively short interval. We used only a global measure of depressive symptomology and we did not have repeated measures for anxiety or symptoms of PTSD.
Finally, the incident rate of physical IPV victimization in the current study was 2% for women and 1.3% for men. These rates are considerably lower than incident rates of IPV reported among national samples. For example, 4% of women and 4.7% of men reported new cases of IPV victimization in the Center for Disease Control’s National Intimate Partner and Sexual Violence Survey (Breiding, Chen, & Black, 2014). However, the sample for the National Intimate Partner and Sexual Violence Survey included individuals 18 years of age and older, whereas the sample for the current study included individuals ages 30 to 64 years. Considering that rates of IPV are typically higher among younger individuals (Breiding et al., 2014), it is possible that the restricted age of the sample in the current study contributed to lower reported rates of incident IPV.
Nevertheless, our study has several strengths. We examined a mixed racial and economically diverse area probability sample of urban adults. HANDLS participants are generally forthcoming in discussing health problems, economic problems, and other vicissitudes of life with our staff physician and nurses. It is likely that IPV victimization would have been included had it occurred. Data on cognitive performance were longitudinal over about 5 years, and we examined only incident IPV. To date, no other study has examined changes in cognitive performance associated with incident IPV victimization.
The results of the current study highlight the need for further investigation into the association between IPV victimization and cognitive function and have important implications for future prevention education and treatment for victims of IPV. Identifying health risks associated with IPV victimization may facilitate more honest and comprehensive psychoeducation with victims of IPV. Further, although the issue of help-seeking for IPV victimization is complex, at-risk populations may be more likely to seek psychological intervention following experiences of abuse if they are well-informed of the health risks associated with partner violence victimization. Additionally, the results of our study contribute to a growing body of literature supporting prevention efforts aimed at both men and women. The U.S. Preventative Services Task Force (USPSTF) recommends that clinicians screen all women of child-bearing age for IPV (Moyer, 2013). Based on the results of the current study, it would likely be beneficial for clinicians to screen both male and female patients for IPV and to administer a brief cognitive assessment to patients who disclose abuse.
Acknowledgments
This research was supported by the National Institute on Aging Intramural Research Program of the National Institutes of Health (grant number Z01-AG000194).
Special thanks to the National Institute on Aging Intramural Research Program of the National Institutes of Health for supporting this research.
Footnotes
The authors declare they have no conflicts of interest.
Contributor Information
Megan R. Williams, National Institute on Aging Intramural Research Program, Baltimore, Maryland
Christopher M. Murphy, Department of Psychology, University of Maryland Baltimore County, Baltimore, Maryland
Gregory A. Dore, National Institute on Aging Intramural Research Program, Baltimore, Maryland
Michele K. Evans, National Institute on Aging Intramural Research Program, Baltimore, Maryland
Alan B. Zonderman, National Institute on Aging Intramural Research Program, Baltimore, Maryland.
References
- Adams AE, Bybee D, Tolman RM, Sullivan CM, & Kennedy AC (2013). Does job stability mediate the relationship between intimate partner violence and mental health among low-income women? American Journal of Orthopsychiatry, 83(4), 600–608. Retrieved from doi:10.1111/ajop.12053 [DOI] [PubMed] [Google Scholar]
- American Psychological Association. (2014). Glossary of Psychological Terms Retrieved from http://www.apa.org/research/action/glossary.aspx
- Andrews PW, & Thomson JA (2009). The Bright Side of Being Blue: Depression as an Adaptation for Analyzing Complex Problems. Psychological Review, 116, 620–654. doi:10.1037/A0016242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J (2000). Sex differences in aggression between heterosexual partners: A meta-analytic review. Psychological Bulletin, 126, 651–680. doi:10.1037MJ033-2909.I26.5.651 [DOI] [PubMed] [Google Scholar]
- Army Individiual Test Battery. (1944). Manual of directions and scoring Washington, DC: War Department, Adjutant General’s Office. [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, & Dronkers NF (2006). Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc, 12(6), 896–900. doi:10.1017/S1355617706061078 [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2014). lme4: Linear mixed-effects models using Eigen and S4 (Version R package version 1.14) Retrieved from http://lme4.r-forge.r-project.org/
- Beats BC, Sahakian BJ, & Levy R (1996). Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine, 26, 591–603. doi:10.1017/S0033291700035662 [DOI] [PubMed] [Google Scholar]
- Bockting CLH, Spinhoven P, Wouters LF, Koeter MWJ, & Schene AH (2009). Long-term effects of preventive cognitive therapy in recurrent depression: A 5.5-year follow-up study. Journal of Clinical Psychiatry, 70, 1621–1628. doi:10.4088/Jcp.08m04784blu [DOI] [PubMed] [Google Scholar]
- Bonomi AE, Anderson ML, Reid RJ, Rivara FP, Carrell D, & Thompson RS (2009). Medical and psychosocial diagnoses in women with a history of intimate partner violence. Archives of Internal Medicine, 169, 1692–1697. doi:10.1001/archinternmed.2009.292 [DOI] [PubMed] [Google Scholar]
- Bonomi AE, Anderson ML, Rivara FP, & Thompson RS (2007). Health outcomes in women with physical and sexual intimate partner violence exposure. Journal of Womens Health, 16, 987–997. doi:10.1089/Jwh.2006.0239 [DOI] [PubMed] [Google Scholar]
- Brandes D, Ben-Schachar G, Gilboa A, Bonne O, Freedman S, & Shalev AY (2002). PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Research, 110, 231–238. doi:10.1016/S0165-1781(02)00125-7 [DOI] [PubMed] [Google Scholar]
- Breiding MJ, Black MC, & Ryan GW (2008). Chronic disease and health risk behaviors associated with intimate partner violence - 18 US states/territories, 2005. Annals of Epidemiology, 18, 538–544. doi:10.1016/J.Annepidem.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Breiding MJ, Chen J, & Black MC (2014). Intimate Partner Violence in the United States — 2010 Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. [Google Scholar]
- Bremner JD (2006). Traumatic stress: effects on the brain. Dialogues Clin Neurosci, 8(4), 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N (2009). The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma, Violence, and Abuse, 10, 198– 210. doi:10.1177/1524838009334448 [DOI] [PubMed] [Google Scholar]
- Campbell JC, Jones AS, Dienemann J, Kub J, Schollenberger J, O’Campo P, … Wynne C (2002). Intimate partner violence and physical health consequences. Archives of Internal Medicine, 162, 1157–1163. doi:10.1001/Archinte.162.10.1157 [DOI] [PubMed] [Google Scholar]
- Carbone-Lopez K, Kruttschnitt C, & Macmillan R (2006). Patterns of intimate partner violence and their associations with physical health, psychological distress, and substance use. Public Health Reports, 121, 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave NA, & Straus MA (1990). Race, class, network embededness, and family violence: A search for potent support systems. . In Straus MA & Gelles RJ (Eds.), Physical Violence in American Families (pp. 321–340). New Brunswick, NJ: Transaction Publishers. [Google Scholar]
- Coker AL, Davis KE, Arias I, Desai S, Sanderson M, Brandt HM, & Smith PH (2002). Physical and mental health effects of intimate partner violence for men and women. American Journal of Preventive Medicine, 23, 260–268. doi:10.1016/S0749-3797(02)00514-7 [DOI] [PubMed] [Google Scholar]
- Coker AL, Smith PH, Bethea L, King MR, & McKeown RE (2000). Physical health consequences of physical and psychological intimate partner violence. Archives of Family Medicine, 9, 451–457. doi:10.1001/Archfami.9.5.451 [DOI] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, … Starr JM (2009). Age-associated cognitive decline. British Medical Bulletin, 92, 135–152. doi:10.1093/Bmb/Ldp033 [DOI] [PubMed] [Google Scholar]
- Dulay MF, Busch RM, Chapin JS, Jehi L, & Najm I (2013). Executive functioning and depressed mood before and after unilateral frontal lobe resection for intractable epilepsy. Neuropsychologia, 51(7), 1370–1376. doi:10.1016/j.neuropsychologia.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, & Paykel ES (1996). Neuropsychological impairments in unipolar depression: The influence of perceived failure on subsequent performance. Psychological Medicine, 26, 975–989. doi:10.1017/S0033291700035303 [DOI] [PubMed] [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, & Zonderman AB (2010). Healthy aging in neighborhoods of diversity across the life span (handls): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & Disease, 20, 267–275. [PMC free article] [PubMed] [Google Scholar]
- Flaks MK, Malta SM, Almeida PP, Bueno OFA, Pupo MC, Andreoli SB, … Bressan RA (2014). Attentional and executive functions are differentially affected by post-traumatic stress disorder and trauma. Journal of Psychiatric Research, 48, 32–39. doi:10.1016/J.Jpsychires.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Galdas PM, Cheater F, & Marshall P (2005). Men and health help-seeking behaviour: literature review. Journal of Advanced Nursing, 49, 616–623. doi:10.1111/J.1365-2648.2004.03331.X [DOI] [PubMed] [Google Scholar]
- Glisky EL (2007). Changes in Cognitive Function in Human Aging. In Riddle DR (Ed.), Brain aging: Models, methods, and mechanisms Boca Raton: CRC Press. [PubMed] [Google Scholar]
- Golding JM (1999). Intimate partner violence as a risk factor for mental disorders: A meta-analysis. Journal of Family Violence, 14, 99–132. doi:10.1023/A:1022079418229 [Google Scholar]
- Grossman SF, & Lundy M (2007). Domestic violence across race and ethnicity: implications for social work practice and policy. Violence against Women, 13(10), 1029–1052. doi:10.1177/1077801207306018 [DOI] [PubMed] [Google Scholar]
- Ho DE, Imai K, King G, & Stuart EA (2011). MatchIt: Nonparametric preprocessing for parametric causal inference. Journal of Statistical Software, 42(8). doi:10.1.1.332.3354 [Google Scholar]
- Jones AS, Gielen AC, Campbell JC, Schollenberger J, Dienemann JA, Kub J, … Wynne EC(1999). Annual and lifetime prevalence of partner abuse in a sample of female HMO enrollees. Womens Health Issues, 9, 295–305. doi:10.1016/S1049-3867(99)00022-5 [Google Scholar]
- Kimmel MS (2002). “Gender symmetry” in domestic violence: A substantive and methodological research review. Violence against Women, 8, 1332–1363. doi:10.1177/107780102237407 [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2013). lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package) (Version R package version 2.0–0). Retrieved from http://cran.r-project.org/package=lmerTest
- Lezak MD (1995). Executive functions and motor performance. In Lezak MD (Ed.), Neuropsychological Assessment (pp. 650–685). New York: Oxford University Press. [Google Scholar]
- Mani A, Mullainathan S, Shafir E, & Zhao J (2013). Poverty impedes cognitive function. Science, 341(6149), 976–980. doi:10.1126/science.1238041 [DOI] [PubMed] [Google Scholar]
- McArdle JJ, & Bell RQ (2000). An introduction to latent growth models for developmental data analysis . Mahwah, NJ: Earlbaum. [Google Scholar]
- McDermott LM, & Ebmeier KP (2009). A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders, 119(1–3), 1–8. doi:10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- Mitchell C, & Anglin D (Eds.). (2009). Intimate partner violence: A health-based perspective New York, NY: Oxford University Press. [Google Scholar]
- Moller-Leimkuhler AM (2002). Barriers to help-seeking by men: a review of sociocultural and clinical literature with particular reference to depression. Journal of Affective Disorders, 71(1–3), 1–9. [DOI] [PubMed] [Google Scholar]
- Morrell CH, Pearson JD, & Brant LJ (1997). Linear transformations of linear mixed-effects models. American Statistician, 51, 338–343. doi:10.2307/2685902 [Google Scholar]
- Moyer VA (2013). Screening for intimate partner violence and abuse of elderly and vulnerable adults: U.S. preventive services task force recommendation statement. Annals of Internal Medicine, 158, 478–486. doi:10.7326/0003-4819-158-6-201303190-00588 [DOI] [PubMed] [Google Scholar]
- Nelson HD, Bougatsos C, & Blazina I (2012). Screening women for intimate partner violence: a systematic review to update the U.S. Preventive Services Task Force recommendation. Annals of Internal Medicine, 156, 796–808. doi:10.7326/0003-4819-156-11-201206050-00447 [DOI] [PubMed] [Google Scholar]
- Perry AR, & Fromuth ME (2005). Courtship violence using couple data: Characteristics and perceptions. Journal of Interpersonal Violence, 20, 1078–1095. doi:10.1177/0886260505278106 [DOI] [PubMed] [Google Scholar]
- Polak AR, Witteveen AB, Reitsma JB, & Olff M (2012). The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders, 141, 11–21. doi:10.1016/J.Jad.2012.01.001 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2013). R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385 – 401. doi:10.1177/014662167700100306 [Google Scholar]
- Reid RJ, Bonomi AE, Rivara FP, Anderson ML, Fishman PA, Carrell DS, & Thompson RS (2008). Intimate partner violence among men: Prevalence, chronicity, and health effects. American Journal of Preventive Medicine, 34, 478–485. doi:10.1016/J.Amepre.2008.01.029 [DOI] [PubMed] [Google Scholar]
- Reitan RM (1992). Trail Making Test: Manual for administration and scoring Tuscon, AZ: Reitan Neuropsychology Laboratory. [Google Scholar]
- Seedat S, Videen JS, Kennedy CM, & Stein MB (2005). Single voxel proton magnetic resonance spectroscopy in women with and without intimate partner violence-related posttraumatic stress disorder. Psychiatry Research-Neuroimaging, 139, 249–258. doi:10.1016/J.Pscychresns.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Singer JD, & Willett JB (2003). Applied Longitudinal Data Analyses: Modeling Change and Event Occurence (1st ed.). New York, NY: Oxford University Press. [Google Scholar]
- Stein MB, Kennedy CA, & Twamley EW (2002). Neuropsychological function in female victims of intimate partner violence with and without posttraumatic stress disorder. Biological Psychiatry, 52, 1079–1088. doi:10.1016/S0006-3223(02)01414-2 [DOI] [PubMed] [Google Scholar]
- Tamres LK, Janicki D, & Helgeson VS (2002). Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Personality and Social Psychology Review, 6(1), 2–30. doi:10.1207/S15327957pspr0601_1 [Google Scholar]
- Thompson RS, Bonomi AE, Anderson M, Reid RJ, Dimer JA, Carrell D, & Rivara FP (2006). Intimate partner violence: Prevalence, types, and chronicity in adult women. American Journal of Preventive Medicine, 30(6), 447–457. doi:10.1016/J.Amepre.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Tjaden P, & Thoennes N (2000). Extent, nature, and consequences of intimate partner violence (NCJ 181867) Retrieved from Washington, DC: http://www.ojp.usdoj.gov/nij [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology, 14(2), 167–177. doi:10.1016/S0887-6177(97)00095-4 [PubMed] [Google Scholar]
- Turrell G, Lynch JW, Kaplan GA, Everson SA, Helkala EL, Kauhanen J, & Salonen JT (2002). Socioeconomic position across the lifecourse and cognitive function in late middle age. Journals of Gerontology Series B-Psychological Sciences and Social Sciences, 57(1), S43–S51. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Allard CB, Thorp SR, Norman SB, Cissell SH, Berardi KH, … Stein MB (2009). Cognitive impairment and functioning in PTSD related to intimate partner violence. Journal of the International Neuropsychological Society, 15(6), 879–887. doi:10.1017/S135561770999049x [DOI] [PubMed] [Google Scholar]
- Vrana S, & Lauterbach D (1994). Prevalence of Traumatic Events and Posttraumatic Psychological Symptoms in a Nonclinical Sample of College-Students. Journal of Traumatic Stress, 7(2), 289–302. doi:10.1007/Bf02102949 [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, … Detre JA (2007). Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci, 2(3), 227–239. doi:10.1093/scan/nsm018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Tischler L, Golier J, Grossman R, Brand S, Kaufman S, & Harvey P (2006). Longitudinal assessment of cognitive performance in Holocaust survivors with and without PTSD. Biological Psychiatry, 60, 714–721. doi:10.1016/j.biopsych.2006.03.069 [DOI] [PubMed] [Google Scholar]


