Abstract
Background and Aims:
A simple, safe, targeted and efficient in vivo DNA delivery system is necessary for clinical-grade liver targeted gene therapy in humans. Intravascular hydrodynamic gene delivery has been investigated in large animal models but translation to humans has been hampered by its technical challenges, invasiveness and potential for significant cardiovascular adverse events. We posited that intrabiliary delivery of DNA plasmids via ERCP-guided hydrodynamic injection overcomes these obstacles.
Methods:
Twelve pigs (40–50 kg) were divided into 3 groups (n=4 per group) and survived 21, 30, or 60 days. ERCP was performed by inflating a balloon catheter in the common hepatic duct and creating a closed space between it and the liver parenchyma. Last, a solution composed of plasmid/sleeping beauty (SB) mix was injected under pressure through the catheter into the closed space. Swine were killed at the 3 different time points and liver tissue harvested. Plasmid DNA expression and functional translated protein expression were assessed.
Results:
ERCP-guided hydrodynamic delivery of naked plasmid DNA facilitated by pCMV-Sleeping Beauty transposons was technically feasible and devoid of cardiovascular and local adverse events in all 12 pigs. Furthermore, plasmid DNA (both single and combination) was successfully transferred into swine hepatocytes in all 12 pigs. Additionally, stable integration of the DNA constructs in hepatocyte genomic DNA were reliably noted at all 3 time points. In the 4 swine that were kept alive to 60 days, successful genomic integration and subsequent protein expression was observed in the targeted liver tissue.
Conclusions:
ERCP-guided hydrodynamic delivery of gene therapy may usher in the next chapter in gene therapy with the potential to impact a variety of single-gene, complex genetic and epigenetic liver diseases. It also raises the possibility that other nucleic acid therapeutics (microRNA, lncRNA, siRNA, shRNA) could similarly be delivered.
Keywords: Hydrodynamic injection, hydrodynamic gene delivery, liver targeted gene delivery, biliary occlusion, plasmid, non-viral gene delivery, Sleeping Beauty
Introduction
The liver is affected in many acquired and inherited gene disorders. Devastating single gene disorders such as alpha-1 antitrypsin deficiency, cystic fibrosis, and many others could theoretically be treated by inserting a corrected copy of the defective gene into affected liver cells. This presents an opportunity for the application of liver targeted gene therapy, where the replacement of single gene has been shown to have a significant clinical impact.1 However, a technically simple, free of fatal adverse events, liver-specific method to deliver gene therapy does not currently exist. The lack of such a method is a major drawback to the effective treatment of millions of patients, many of whom are children.2 Previous attempts at treatment of some of these disorders highlighted the potentially catastrophic side effects associated with the delivery vehicle, as well as with the method of delivery.3, 4
Therefore, a critical, but elusive step is the development of a clinical-grade, simple, safe, and efficient in vivo nucleic acid delivery system. The ideal system would include a non-viral carrier as well as a methodologic approach that would be specific for the liver, minimally-invasive, and with the potential to be performed in an outpatient setting.
The sleeping beauty (SB) transposon system has been used to promote the integration of transgenes in mammalian cells via a cut-and-paste mechanism.5 The system has found its applications mainly in small animals with few in vivo large animal 6 or human studies.7 The system consists of plasmids containing 2 transcription units, one expressing the enzyme SB transposase and the other expressing the transgene DNA to be inserted into the host genome. In rodents, this technique has resulted in successful expression of coagulation factor IX,8 factor VIII,9 alpha-1 antitrypsin 10 and many other proteins such that short-term correction of the diseased phenotype was observed. A critical requirement of non-viral gene delivery vehicles, such as SB, is a method to introduce the plasmids in the nucleus of target cells. Several methods have been investigated, mostly in small animals, to deliver non-viral vectors to the liver.11 The most promising, to date, appears to be hydrodynamic injection via a vein12–21. This vascular route, as documented22, 23, is technically challenging, time-consuming and by extension expensive, and has, expectedly, cardiovascular side effects. These studies relied on creating relatively high hydrostatic pressure in the vascular bed that promoted plasmid uptake into the target cells.
Although the intravascular hydrodynamic injection has been the most commonly used route12–16, the delivery of plasmids via the bile duct represents an alternative pathway, but has only been evaluated in rodents, through invasive surgical approaches 24–26. If intrabiliary delivery of plasmids via ERCP-guided hydrodynamic injection in a large animal model could overcome the current challenges faced by intravascular injection, this may promote the commencement of liver targeted gene therapy in humans.
Herein, we describe, for the first time, an ERCP-directed hydrodynamic delivery of SB and associated plasmids to swine liver. The bile duct route has theoretical advantages over intravascular delivery including significantly smaller volume of injection, absence of adverse cardiorespiratory events and reduced risk of systemic toxicity and of systemic dispersal of plasmids, and by extension, increased specificity. Additionally, there is some evidence that bile may contain fewer nucleases versus blood and therefore the intrabiliary route offers the theoretical advantage of improved DNA stability.27 In the current study, we selected pT3-EF1a-NICD, pT3-EF1a-AKT, and pT3-N90-beta-catenin to be delivered as target constructs as they have demonstrated the capability to be integrated into somatic cells in vivo with the guidance of pCMV-SB transposon.28–30. The overarching goal of this study was to establish that ERCP-directed, hydrodynamically-mediated nonviral liver gene therapy is simple, effective and safe and to provide the preclinical backdrop for further human clinical trials.
The specific objectives of this study were to (1) ascertain the parameters necessary for intrabiliary-delivered hydrodynamic gene delivery; (2) demonstrate feasibility of liver cell transduction using ERCP; and (3) assess whether successful transduction results in stable expression of the delivered plasmid proteins. The long-term goal of our research is to establish a minimally invasive method of non-viral gene delivery to the liver.
Methods
Animal and study conditions.
A total of 15 Yorkshire pigs (Sus scrofa domestica) weighing 40 to 50 kg and aged 4 to 6 months at study initiation were obtained from a commercial, closed herd swine vendor (Archer Farms, Darlington, Md). Environmental acclimation was at 72°F ±2°F, 30 % to 70% relative humidity, 14 hours:10 hours (light: dark cycle), and approximately 15 air changes/hour occurred for 1 week before study initiation. Swine were housed individually in 24 ft2 indoor runs and fed Teklad Mini-swine diet (No. 8753, Harlan Tekland, Madison, Wisc). Water was provided ad libitum before study initiation. All experimental animal procedures were approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University and conducted in compliance with the Animal Welfare Act, applicable Animal Welfare Regulations, and the Guide for the Care and Use of Laboratory Animals at an AAALAC-accredited facility. 31–33
Determination of maximal tolerable injection parameters of the swine bile duct.
Initially, we performed ex vivo experiments using standard endoscopic accessories on 3 swine livers to determine the maximal tolerated injection parameters necessary to rupture the biliary tree (see Appendix 1 for further details). These ex vivo studies were used to inform parameters for initial in vivo nonsurvival studies on 3 swine. After the swine were anesthetized, the duodenoscope (ED-3490 TK, Pentax, Montvale, NJ) was inserted through the mouth and positioned in the proximal duodenum in front of the biliary orifice. The common bile duct (CBD) was cannulated with an extraction balloon preloadass). Selective biliary cannulation was technically simple and safe (no risk of pancreatitis) as the opening of the pancreatic duct is separate in swine. Under fluoroscopic guidance (Allura Xper FD20, Philips Medical Systems N.A., Bothell, Wash), 3 mL boluses of one-third strength iohexol contrast medium (Omnipaque, 350 mg/mL, GE Health Co, Princeton, NJ) were injected into the biliary tree to opacify important landmarks (cystic duct, hepatic hilum and main intrahepatic ducts). The guidewire was then inserted into the intrahepatic ducts, and an extraction balloon was advanced to the common hepatic duct (CHD). The extraction balloon was subsequently advanced to the CHD as the injection from this location would allow the entire liver to be exposed to the injected solution. At this point, the balloon was inflated with air to 12 mm such that it was wedged against the duct wall. The guidewire was removed and the angiographic injector apparatus was connected to the guidewire port of the extraction balloon. Under fluoroscopy, X mL injection volumes (X= 10, 20, 30, 40) of one-third strength iohexol contrast medium were injected at Y mL/s injection rate (Y= 1, 2, 3) with the maximal pressure set to 999 psi. The balloon remained inflated for 30 seconds after completion of each injection. Injection parameters were sequentially tested at 10-minute intervals in ascending order until the rupture of the bile duct as evidenced by extravasation of contrast medium. The duodenoscope was then withdrawn, and the swine euthanized.
Hydrodynamic gene delivery to effectuate plasmid transduction.
The preparation of the pigs (n=12) and the instruments used were similar to the aforementioned experiments. The bile duct was cannulated, 3 mL of contrast medium injected and the balloon advanced over the guidewire into the CHD. The extraction balloon was inflated to 12 mm and the guidewire removed. The guidewire port was primed with 1.5 mL of plasmids. Then 30 mL of plasmids was injected at 2 mL/s (based on the experiment above) with the maximal pressure set to 999 psi. The balloon remained inflated for 2 minutes. After balloon deflation, to confirm the integrity of the biliary tree, a balloon occlusion cholangiogram was performed by withdrawing the extraction balloon to the distal CBD, inflating the balloon to 12 mm, and injecting 5 mL of contrast medium. The duodenoscope was then withdrawn and swine recovered under veterinary supervision until supine. The clinical health of the animals was assessed at least once daily over the duration of the study.
Plasmids and their allocation.
For the purposes of stable gene expression in target liver of swine, we chose target plasmids combined with Sleeping Beauty-mediated somatic integration. The constructs pT3-EF1a-NICD, pT3-EF1a-AKT, pT3-N90-beta-catenin, and transposon plasmid pCMV-SB were used. The allocation of the constructs into each of the three groups are shown in Table 1. Note, 8 swine received a single plasmid (AKT or NICD), and 4 swine received a combination of 2 plasmids (AKT and beta-catenin) (Table 1). Transduction of plasmids to each of the 12 swine livers were assessed via PCR. Protein expression was assessed in the 4 swine that received the combination of 2 plasmids and were kept alive to 60 days. Protein expression was illustrated by Western blot and the location of integrated gene protein expression was determined by immunofluorence staining.
Table1: Swine liver ERCP hydrodynamic injection with different plasmids combination.
Twelve swine were randomly divided into 3 groups. Hydrodynamic injection with different groups of plasmids were performed and swine were killed at different time points. Tissue was subsequently harvested for the experiments undertaken.
| Swine | Plasmids combination | Survival time |
|---|---|---|
| 4 | PT3-EF1a-AKT+PCMV-Sleeping beauty | 21 days |
| 4 | PT3-EF1a-NICD+PCMV-Sleeping beauty | 30 days |
| 4 | PT3-EF1a-AKT and PT3-N90-beta-catenin+PCMV- Sleeping Beauty |
60 days |
Additional methods are presented in Appendix 1.
Results
Hydrodynamic injection parameters.
Initial experiments were performed ex vivo with the specific purpose of clarifying the upper limits of safe injection speed and volumes. We injected normal saline through the catheter and noted transient swelling of the liver during and immediately after the hydrodynamic injection. Experiments revealed that the bile duct ruptured when 50 mL of solution was injected at a speed of 3 mL/s. In each of the livers, rupture occurred immediately distal to the tip of the balloon just below the hepatic hilum (Supplementary Figure 1A).
The segmental anatomy of the swine liver is similar to that of a human with regard to vascularity and the biliary tree distribution.34 The liver contains 6 lobes including the quadrate, caudate, right medial and lateral, left medial, and lateral. The common hepatic duct (CHD) enters the liver caudal and dorsal to the gallbladder. The CHD is found in the porta hepaticus, ventral to the portal vein and the hepatic artery.34 As reported, we found the biliary orifice located approximately 2 cm distal to the pylorus on the posterior inferior wall.34 For all ERCP procedures, the duodenoscope was placed in the short position en face to the biliary orifice. In vivo experiments revealed that cannulation of the common bile duct (CBD) and intrahepatic ducts was technically simple. Contrast injection demonstrated that the diameters of CBD was 6 to 8 mm, CHD 3 to 5 mm, and the main intrahepatic ducts 2 mm, respectively. Despite injecting volumes as high as 20 mL at rates of 4 mL/s, no contrast medium escaped around the balloon into the CBD during hydrodynamic injection. This indicated that an adequate seal was created to facilitate the generation of hydrostatic pressure. Injecting volumes greater or equal to 20 mL, regardless of rate, demonstrated acinarization of the liver parenchyma indicating that contrast had exited the biliary tree. Injecting 40 mL at 2 mL/s resulted in rupture of the proximal CHD during the process of injection (Supplementary Figure 2, Supplementary Video 1). Injecting 30 mL at 2 mL/s resulted in acinarization of all liver segments without rupture of the bile duct wall (Figure 1, Supplementary Video 2). For the following experiments we have used these parameters and encountered no rupture or other local adverse events.
Figure 1: A-F: Fluoroscopic snap shot of swine bile duct distribution after contrast injection via ERCP.
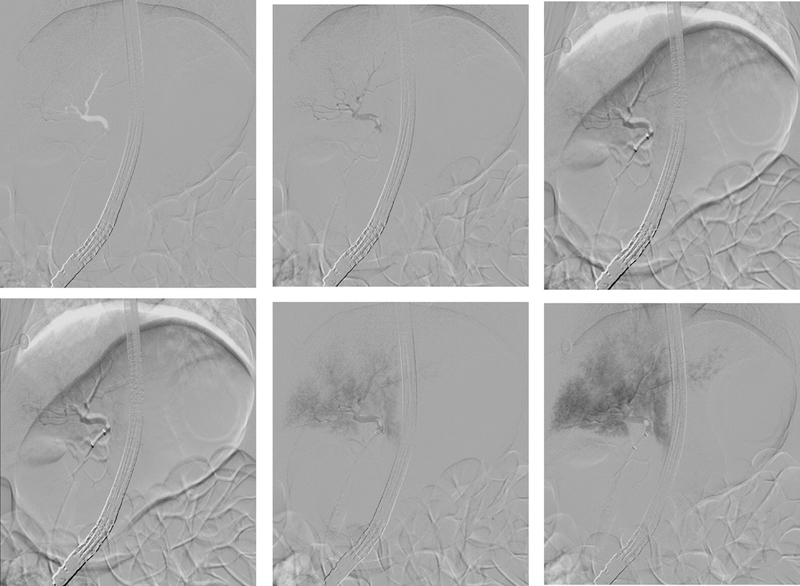
Rapid sequence fluoroscopic images (every 3 seconds) during injection of 30 mL of contrast medium at 2 mL/s. This resulted in acinarization of right and subsequently left liver segments without rupture of the bile duct wall (A-F). Therefore, these parameters were deemed optimal for hydrodynamic injection.
Tolerability of hydrodynamic injection.
Intravascular delivery of hydrodynamic therapy in animals is associated with a high rate of cardiovascular stress.11 Establishing a novel method to deliver hydrodynamic therapy that would be void of any cardiovascular adverse events was one of the main motivations for the current study. Therefore, we carefully monitored for any systemic, cardiovascular or respiratory adverse events. Through these experiments, we demonstrated that there were no changes in physiological parameters observed during or after the injection, including electrocardiogram, heart rate, respiration rate, temperature, blood pressure, and oxygen saturation. There were no intra- or postprocedural adverse events observed. The animals were alert, responsive, defecating, urinating, drinking, and eating well in after the procedure and for the following days. Animals did not exhibit signs of sepsis, peritonism or jaundice. Laboratory test results, including liver function tests and complete blood count, demonstrated values within normal limits at 7 days and at the time of death (day 21, 30, or 60) (data not shown). These results appear to suggest that the process of ERCP and intrabiliary hydrodynamic gene delivery has no significant negative physiological or functional impact.
Impact of hydrodynamic injection on hepatic tissue.
The balloon occlusion cholangiogram on the day of animal death revealed mild diffuse dilation of the extra- and intrahepatic biliary tree by approximately 25%. There was no focal dilation or stricture of the bile ducts. At necropsy, gross examination of the abdomens were within normal limits. There was no ascites and the liver surface had a normal appearance. The liver was sectioned in 1 cm thick slices with normal hepatic parenchyma observed. Microscopic examination revealed no evidence of chronic liver injury or accumulation of lymphocytes. There was no enlargement of hepatic sinusoids (Supplementary Figure 1B). These results suggest that hydrodynamic gene delivery via the biliary tree is safe. It is unclear if the observed mild dilation in the biliary tree is specific to swine or likely to occur in humans. Furthermore, even if it were to occur, it is questionable that there would be any consequence as a result of the mild dilation. Of note, dilation in the common bile duct is sometimes observed after cholecystectomy with no known negative consequences.35, 36
Plasmid DNA can successfully be transferred into swine liver hepatocytes.
Swine livers specimens (6 from each swine) from day 21 and day 30, respectively, post hydrodynamic injections were harvested and DNA was extracted (Figures 2A and 2B). The 249 base pair (bp) PCR product of AKT and 213 bp product of NICD, respectively, were detected in the left (proximal and distal), right (proximal and distal), caudate liver lobe and CHD in each of the 8 swine at day 21 and 30, respectively. Therefore, based on PCR data, these 2 groups of experiments demonstrated that single genes (AKT or NICD) can be successfully transduced into liver tissue. Considering these initial positive results, we then wanted to assess whether a combination of 2 plasmids can also be delivered. To this end, AKT + beta-catenin in 1:1 ratio were delivered together with pCMV-SB at the same ratio of 25:1 gene DNA:SB DNA. Thus, another 4 swine underwent ERCP and hydrodynamic injection and were survived for 60 days. Both AKT and beta-catenin plasmid DNA were detected after 60 days in each of the swine throughout all liver lobes as well as in CHD (data not shown). These results demonstrate hydrodynamic injection of PT3-EF1a-AKT and PT3-N90-beta-catenin can successfully transduce hepatocytes and the presence after 2 months indicates stable integration of the constructs in the hepatocyte genomic DNA.
Figure 2: PCR analysis performed on genomic DNA collected from swine by amplification with primers for the human AKT and NCID gene in swine liver and bile duct.
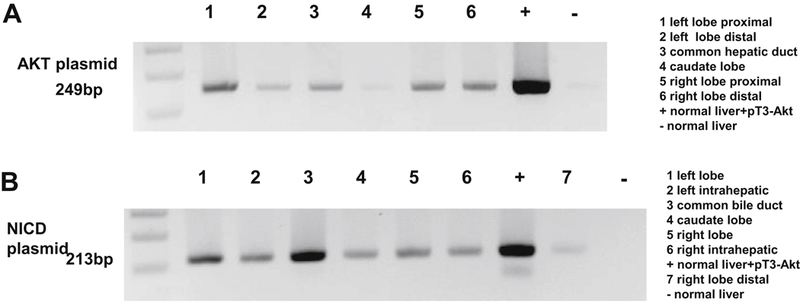
A: Plasmid DNA AKT presence in liver tissue 21 days after ERCP and hydrodynamic injection. At day 21, swine liver were harvested, and DNA was extracted. PCR analysis of 6 samples from each swine liver was performed on genomic DNA by 35 cycles of amplification, denature at 94°C denature, anneal at 59°C anneal with primer for human AKT gene. B: Plasmid DNA NICD presence in liver tissue 30 days after ERCP and hydrodynamic injection. At day 30, swine liver were harvested, and DNA was extracted. PCR analysis of 6 samples from each swine liver was performed on genomic DNA by 35 cycles of amplification, denature at 94°C denature, anneal at 63°C anneal with primer for human NICD gene. Results show that each location has the plasmid AKT and NICD sequence expression.
Protein expression of the delivered genes in swine liver tissue.
Successful and long lasting in vivo gene therapy requires DNA integration versus episomal expression of the delivered gene. Although we demonstrated successful transduction of swine liver via PCR, we sought to determine whether the delivered plasmid genes were integrated and successfully replicated, transcribed and translated into functional proteins. The same 4 swine that underwent ERCP and hydrodynamic injection with the combination of 2 plasmids (AKT + beta-catenin) were used to ascertain whether protein expression occurs at 60 days after the procedure. The livers from these 4 swine were harvested and lysed, swine liver lysates were randomly collected (left, caudate, right lobes, and CHD samples) and analyzed for the expression of the tagged AKT or beta-catenin by Western blot. The housekeeping gene beta-actin was used as internal control by Western blot. We found that each analyzed liver tissue expressed the delivered AKT and beta-Catenin proteins (Figure 3), indicating successful genomic (as opposed to episomal) integration, transcription and protein synthesis. The 8 swine that had single genes transduced were not assessed for protein expression as logically, it would be expected to occur with a similar or greater degree of likelihood in animals which were survived for fewer days.37
Figure 3: Western blot shows AKT and Beta-catenin protein expressed in two swine livers.
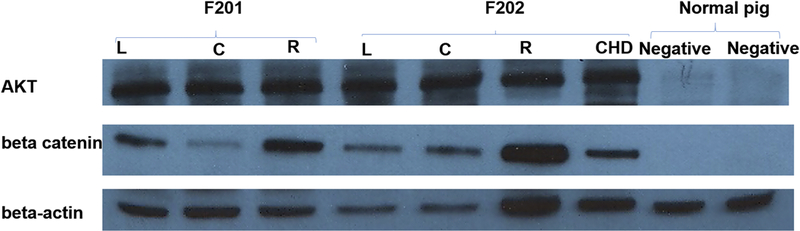
Sixty days after ERCP plasmid hydrodynamic injection, swine (F201 and F202) liver tissue were harvested, lysed and analyzed via western blot. A–B demonstrate successful plasmid DNA integration and transcription into protein. C: Beta-actin functioned as an internal control (C). L (left lobe). C (caudate lobe), R (right lobe), CHD (common hepatic duct), normal pig liver tissue was used as negative control.
To determine the extent and exact location of the integrated gene protein expression and whether both constructs can be integrated into the same cells, liver specimen of four swine in the third group were harvested, embedded in paraffin and sectioned. These slides were stained with anti-HA-tag-AKT (green fluorescence) and MYC-tag-beta-catenin (red fluorescence). Pockets of liver tissue express both AKT and beta-catenin in the same cells (Figure 4). In addition, beta-catenin is highly expressed in the hepatic parenchyma and nearby bile duct, whereas AKT only expressed in hepatic parenchyma (Figure 5). Thus, immunostaining further confirmed that we can successfully deliver target genes to the liver via ERCP hydrodynamic injection, and that the genes can integrate, replicate together and remain expressed in the liver cells long term.
Figure 4: HA-Tag AKT and MYC-tag beta catenin protein were expressed in swine liver tissue.
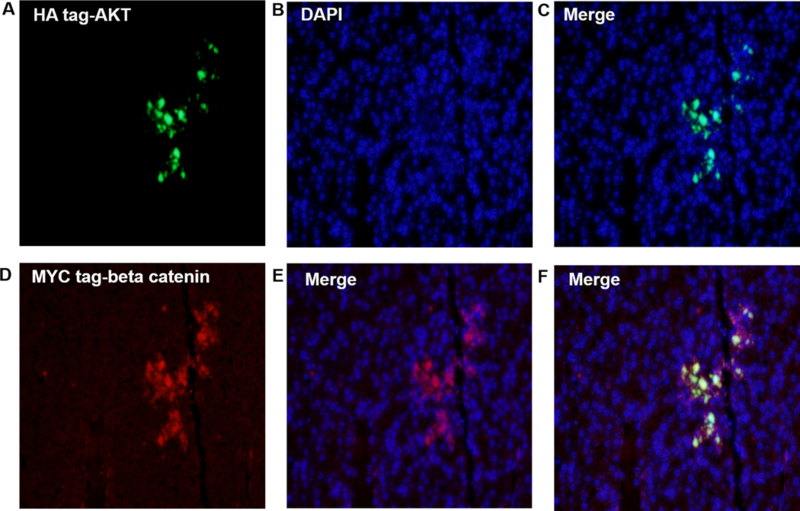
Swine liver tissue hydrodynamic injected with AKT and beta-catenin plasmids were harvested at day 60, and tissues were analyzed for the expression of plasmid AKT and beta catenin protein via fluorescence microscopy. A–C shows the AKT protein integrated and stably expressed in hepatocytes. D–F shows beta-catenin protein integrated and stably expressed in hepatocytes. This figure demonstrates that hydrodynamically injected plasmids can be stably integrated and expressed in swine hepatocytes (amplification: 20X).
Figure 5: HA-Tag AKT and MYC-tag beta catenin protein were expressed in swine hepatocytes and bile duct.
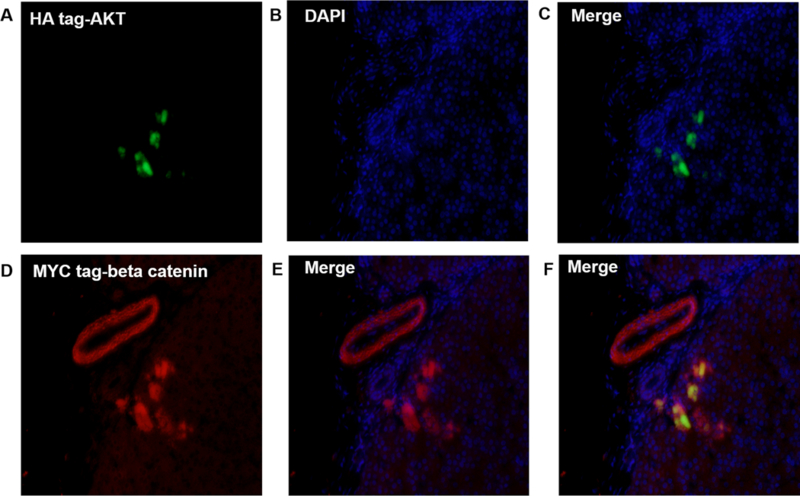
Swine liver tissue that underwent hydrodynamic injection with AKT and beta-catenin plasmids were harvested at day 60, and tissues were analyzed for the expression of plasmid AKT and beta catenin protein via fluorescence microscopy. A–C show the anti HA-tag AKT protein inside hepatocytes. D–E demonstrates beta-catenin expressed in bile duct and hepatocytes. F illustrates that some hepatocytes express both AKT and beta-catenin. The figure demonstrate that hydrodynamic injection of plasmids can be stably integrated and expressed in swine hepatocytes and bile duct (amplification: 20X).
Discussion
To date, it has been impossible to perform liver specific hydrodynamic gene delivery in a large animal model with direct translatability to human trials. Non-rodent studies thus far have exclusively reported hydrodynamic gene delivery through the vascular system (portal, hepatic veins, or IVC). Studies using intravascular hydrodynamic gene delivery have demonstrated the technique to be invasive, technically challenging, cumbersome, and associated with severe cardiorespiratory compromise.16 To avoid the adverse cardiorespiratory events, “lobe specific” gene delivery has been reported with the notion that sequential targeting of several liver lobes will be necessary.12–15 To circumvent these problems, here we investigated the feasibility and safety of intrabiliary hydrodynamic gene delivery.
Using an ERCP technique, we could identify safe injection parameters and successfully transduce hepatocytes with genes and express the protein in liver cells. Furthermore, only relatively small volumes of plasmids were necessary to target all liver segments and no biliary or liver parenchymal injury was observed. Our results indicate that intrabiliary hydrodynamic gene delivery is minimally invasive, technically simple, and safe. Cumulatively, the data presented establishes the utility of ERCP-mediated hydrodynamically delivered liver-targeted gene therapy and suggest that this technique deserves further development for potential utilization in human patients.
There were several anatomic and physiologic reasons for which we hypothesized that intrabiliary hydrodynamic injection will be successful for our gene therapy application for liver cells in vivo and for which we used swine models. First, swine (40–50 kg) have similar hepatobiliary anatomy and duct caliber to humans. In addition, similar to the anatomy of humans, the swine liver allows for two potential mechanisms to promote gene uptake by hepatocytes through intrabiliary injection. Hepatocytes can be exposed to bile duct retrograde flow through biliary canaliculi, though canalicular tight junctions might be expected to restrict DNA uptake by hepatocytes to the canalicular membrane of the hepatocyte. However, canaliculi are of sufficient size (1 µm in diameter) to permit access of plasmids to the space of Disse and subsequently be taken by the apical membrane of hepatocytes. Finally, rodent studies describing intrabiliary injection of plasmids have yielded promising results.24–26 Zhang et al25 demonstrated that plasmids had similar levels of access to the liver by the intrabiliary or portal vein route; however, plasmid complexes persisted in the liver much longer after intrabiliary delivery. Otsuka et al38 compared intrabiliary versus portal vein liver directed gene transfer in rats and pigs using liposomes and found that transgene expression in the intrabiliary group lasted longer and lower amounts of DNA were required to achieve the same outcome. These studies suggested that intrabiliary delivery of DNA might be successful, but certain elements - such as utilization in small animals and slow delivery of plasmid DNA - precluded any potential generalization to a clinical setting.
Intrabiliary hydrodynamic gene delivery was minimally invasive, technically simple, and well tolerated. Only one proceduralist and one assistant were used to perform ERCP and hydrodynamic injection. Postprocedure pain was not observed without any analgesics used throughout the study. Additionally, no evidence of cholangitis or liver abscess was noted despite the absence of periprocedural antibiotics. Liver biochemistry did not reveal evidence of hepatocellular or biliary epithelial injury, which is in contrast to intravascular hydrodynamic injection.12–15 Although at this point we can only hypothesize that this procedure will be equally benign in human patients, it appears that its safety profile may not be a barrier to clinical trials. It must be acknowledged that in swine models, scenarios such as failed biliary cannulation and post-ERCP pancreatitis are extremely unlikely as the pancreatic orifice is several centimeters downstream from the biliary orifice. Although there are potential adverse events associated with ERCP in the clinical setting (including the pediatric population), it remains a widely used procedure.39–41 Furthermore, if there is suboptimal efficiency of transduction, one can consider performing repeated hydrodynamic injections over the course of treatment. The procedure time and the risk of ERCP related adverse events are significantly reduced in the subsequent procedure. Endoscopic ultrasound guided biliary access is disseminating and likely reduces the risk of post ERCP pancreatitis. However, it would be challenging to create a closed system and generate the hydrodynamic pressures necessary with current needle systems.
Despite successful and safe transduction of plasmids throughout the liver, there is room for further investigation into our technique. In the current study, we did not measure the pressure generated within the biliary tree and our methods were geared to identifying the maximal tolerable pressure. Identifying the minimal pressure parameters for transduction will increase the safety. Second, although we demonstrated 100% plasmid DNA transduction, we did not assess the efficiency of transduction as a function of procedural parameters, such as pressure, volume, time of delivery or plasmid concentration. Third, we did not assess for non-target (kidney, lung, heart etc) delivery of plasmids, although conceivably less of a problem compared to the vascular route. However, even if plasmid DNA escaped the liver and circulated to a different organ, there would be no pressure applied in the distant organ that would force the plasmids past the cell membrane. Therefore, it is tempting to hypothesize that this technique would have no clinically relevant distant gene transduction. Fourth, this technique may need to be adapted to patients who already are significantly diseased (i.e liver cirrhosis). Fifth, the inherent limitations of studies performed in animals mandate careful consideration before validation in human patients.
Hydrodynamic gene delivery via the bile duct represents an important step towards the clinical use of non-viral gene delivery. Herein we demonstrate that a minimally invasive, low cost and safe technique was able to transduce hepatocytes throughout the liver with a single injection. Furthermore, we demonstrate that effective DNA integration and protein production in the liver of large animals of hydrodynamically delivered plasmids is feasible without systemic side effects. The clinical applications made possible by this approach will depend on the precise localization of gene expression (hepatocytes, cholangiocytes, sinusoidal epithelial cells), the efficiency of gene transfer (proportion of cells expressing the gene) and the longevity of gene expression. Clarification of the aforementioned outcomes will be necessary before clinical utilization of intrabiliary hydrodynamic gene delivery.
Supplementary Material
Acknowledgements:
This work was supported by National Institutes of Health Grant, DK090154, R01CA190040, and R01EB017742 to Florin M Selaru
Funding Sources: This work was supported by National Institutes of Health Grant, K Award, DK090154 to Florin M Selaru
Abbreviations:
- ERCP
endoscopic retrograde cholangio pancreatography
- SB
Sleeping Beauty
- TKX
tiletamine, zolazepam, ketamine, and xylazine
- CHD
common hepatic duct
- CBD
common bile duct
- bp
base pair
- AKT
Protein kinase B
- NICD
notch intracellular domain
Acronyms:
- (IVC)
inferior vena cava
- (ERCP)
endoscopic retrograde cholangiopancreatography
- (CHD)
common hepatic duct
- (CBD)
common bile duct
- (TKX)
tiletamine, zolazepam, ketamine, and xylazine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Vivek Kumbhari is a consultant for Boston Scientific and Apollo Endosurgery Anthony Kalloo is a founding Member, equity Holder and consultant for Apollo Endosurgery. All other authors have no relevant disclosures
REFERENCES
- 1.Flotte TR, Trapnell BC, Humphries M, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther 2011;22:1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogliolo M, Surralles J. Fanconi anemia: a model disease for studies on human genetics and advanced therapeutics. Curr Opin Genet Dev 2015;33:32–40. [DOI] [PubMed] [Google Scholar]
- 3.Lehrman S Virus treatment questioned after gene therapy death. Nature 1999;401:517–8. [DOI] [PubMed] [Google Scholar]
- 4.Raper SE, Yudkoff M, Chirmule N, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther 2002;13:163–75. [DOI] [PubMed] [Google Scholar]
- 5.Aronovich EL, McIvor RS, Hackett PB. The Sleeping Beauty transposon system: a non-viral vector for gene therapy. Hum Mol Genet 2011;20:R14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrels W, Mates L, Holler S, et al. Germline transgenic pigs by Sleeping Beauty transposition in porcine zygotes and targeted integration in the pig genome. PLoS One 2011;6:e23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiti SN, Huls H, Singh H, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother 2013;36:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yant SR, Meuse L, Chiu W, et al. Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat Genet 2000;25:35–41. [DOI] [PubMed] [Google Scholar]
- 9.Ohlfest JR, Frandsen JL, Fritz S, et al. Phenotypic correction and long-term expression of factor VIII in hemophilic mice by immunotolerization and nonviral gene transfer using the Sleeping Beauty transposon system. Blood 2005;105:2691–8. [DOI] [PubMed] [Google Scholar]
- 10.Mikkelsen JG, Yant SR, Meuse L, et al. Helper-Independent Sleeping Beauty transposon-transposase vectors for efficient nonviral gene delivery and persistent gene expression in vivo. Mol Ther 2003;8:654–65. [DOI] [PubMed] [Google Scholar]
- 11.Aravalli RN, Belcher JD, Steer CJ. Liver-targeted gene therapy: Approaches and challenges. Liver Transpl 2015;21:718–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamimura K, Kanefuji T, Yokoo T, et al. Safety assessment of liver-targeted hydrodynamic gene delivery in dogs. PLoS One 2014;9:e107203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamimura K, Suda T, Xu W, et al. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther 2009;17:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamimura K, Suda T, Zhang G, et al. Parameters Affecting Image-guided, Hydrodynamic Gene Delivery to Swine Liver. Mol Ther Nucleic Acids 2013;2:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khorsandi SE, Bachellier P, Weber JC, et al. Minimally invasive and selective hydrodynamic gene therapy of liver segments in the pig and human. Cancer Gene Ther 2008;15:225–30. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Gao X, Song YK, et al. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther 2004;11:675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue W, Chen S, Yin H, et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 2014;514:380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakili S, Ebrahimi SS, Sadeghi A, et al. Hydrodynamic-based delivery of PTP1B shRNA reduces plasma glucose levels in diabetic mice. Mol Med Rep 2013;7:211–6. [DOI] [PubMed] [Google Scholar]
- 19.Hibbitt OC, Harbottle RP, Waddington SN, et al. Delivery and long-term expression of a 135 kb LDLR genomic DNA locus in vivo by hydrodynamic tail vein injection. J Gene Med 2007;9:488–97. [DOI] [PubMed] [Google Scholar]
- 20.Feng DM, He CX, Miao CY, et al. Conditions affecting hydrodynamics-based gene delivery into mouse liver in vivo. Clin Exp Pharmacol Physiol 2004;31:850–5. [DOI] [PubMed] [Google Scholar]
- 21.Hagstrom JE. Plasmid-based gene delivery to target tissues in vivo: the intravascular approach. Curr Opin Mol Ther 2003;5:338–44. [PubMed] [Google Scholar]
- 22.Zhang G, Vargo D, Budker V, et al. Expression of naked plasmid DNA injected into the afferent and efferent vessels of rodent and dog livers. Hum Gene Ther 1997;8:1763–72. [DOI] [PubMed] [Google Scholar]
- 23.Racz Z, Godo M, Revesz C, et al. Immune activation and target organ damage are consequences of hydrodynamic treatment but not delivery of naked siRNAs in mice. Nucleic Acid Ther 2011;21:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, Zhang X, Dong X, et al. A remarkable permeability of canalicular tight junctions might facilitate retrograde, non-viral gene delivery to the liver via the bile duct. Gut 2005;54:1473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Collins L, Sawyer GJ, et al. In vivo gene delivery via portal vein and bile duct to individual lobes of the rat liver using a polylysine-based nonviral DNA vector in combination with chloroquine. Hum Gene Ther 2001;12:2179–90. [DOI] [PubMed] [Google Scholar]
- 26.Chen CY, Liu HS, Lin XZ. Hydrodynamics-based gene delivery to the liver by bile duct injection of plasmid DNA--the impact of lasting biliary obstruction and injection volume. Hepatogastroenterology 2005;52:25–8. [PubMed] [Google Scholar]
- 27.Ajiki T, Onoyama H, Yamamoto M, et al. Detection of point mutations in K-ras gene at codon 12 in bile from percutaneous transhepatic choledochal drainage tubes for diagnosis of biliary strictures. Int J Pancreatol 1995;18:215–20. [DOI] [PubMed] [Google Scholar]
- 28.Stauffer JK, Scarzello AJ, Andersen JB, et al. Coactivation of AKT and beta-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res 2011;71:2718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest 2012;122:2911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calvisi DF, Wang C, Ho C, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 2011;140:1071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Animal Welfare Act as Amended Volume 7 USC §2131–2159, 2008. [Google Scholar]
- 32.Animal Welfare Regulations Volume 9 CFR §1–4, 2013. [Google Scholar]
- 33.Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals 8th Edition: National Academies Press, 2011. [Google Scholar]
- 34.Swindle MM. Swine in the Laboratory: Surgery, Anesthesia, Imaging and Experimental Techniques, 2nd Ed. Boca Raton (FL):: Taylor and Francis Group, 2007. [Google Scholar]
- 35.Atkinson CJ, Lisanti CJ, Schwope RB, et al. Mild asymptomatic intrahepatic biliary dilation after cholecystectomy, a common incidental variant. Abdom Radiol (NY) 2017;42:1408–1414. [DOI] [PubMed] [Google Scholar]
- 36.McArthur TA, Planz V, Fineberg NS, et al. CT evaluation of common duct dilation after cholecystectomy and with advancing age. Abdom Imaging 2015;40:1581–6. [DOI] [PubMed] [Google Scholar]
- 37.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 1999;6:1258–66. [DOI] [PubMed] [Google Scholar]
- 38.Otsuka M, Baru M, Delriviere L, et al. In vivo liver-directed gene transfer in rats and pigs with large anionic multilamellar liposomes: routes of administration and effects of surgical manipulations on transfection efficiency. J Drug Target 2000;8:267–79. [DOI] [PubMed] [Google Scholar]
- 39.Yachimski PS, Ross A. The Future of Endoscopic Retrograde Cholangiopancreatography. Gastroenterology 2017;153:338–344. [DOI] [PubMed] [Google Scholar]
- 40.Luo H, Zhao L, Leung J, et al. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet 2016;387:2293–2301. [DOI] [PubMed] [Google Scholar]
- 41.Rosen JD, Lane RS, Martinez JM, et al. Success and safety of endoscopic retrograde cholangiopancreatography in children. J Pediatr Surg 2017;52:1148–1151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


