Abstract
Background:
The development of radiopharmaceuticals containing 225Ac for targeted alpha therapy is an active area of academic and commercial research worldwide.
Objectives:
Despite promising results from recent clinical trials, 225Ac-radiopharmaceutical development still faces significant challenges that must be overcome to realize the widespread clinical use of 225Ac. Some of these challenges include the limited availability of the isotope, the challenging chemistry required to isolate 225Ac from any co-produced isotopes, and the need for stable targeting systems with high radio-labeling yields.
Results:
Here we provide a review of available literature pertaining to these challenges in the 225Ac-radiopharmaceutical field and also provide insight into how performed and planned efforts at TRIUMF - Canada’s particle accelerator centre - aim to address these issues
Keywords: Actinium-225, targeted alpha therapy, isotope production, radiochemistry, radiolabeling, TRIUMF
1. INTRODUCTION
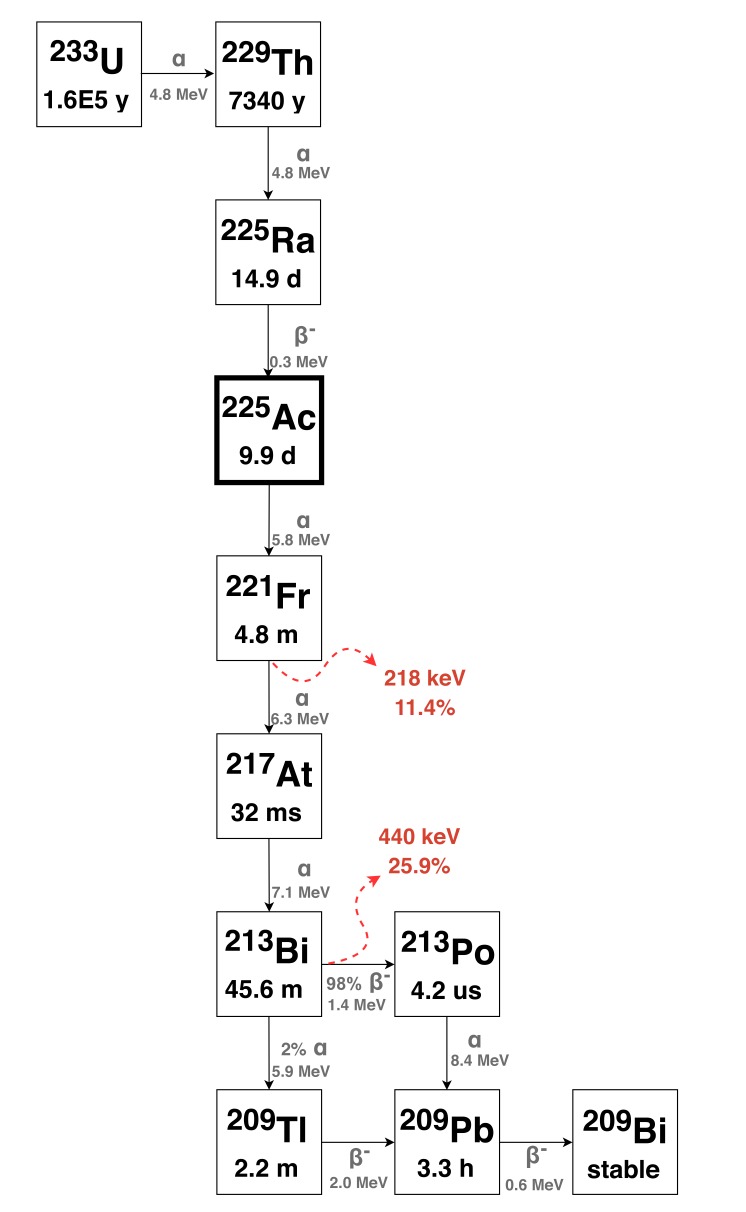
Targeted radionuclide therapy using alpha-emitting isotopes combined with disease-specific targeting vectors (antibodies or peptides) has the potential to treat metastatic disease by delivering a source of cytotoxic radiation directly to targeted cells [1-8]. Given specific targeting, the short range and high cytotoxicity of alpha particles result in the destruction of nearby diseased cells with limited harm to healthy tissues [9]. Due to this potential, the development of alpha-emitting radiopharmaceuticals for Targeted Alpha Therapy (TAT) is an active area of academic and commercial research worldwide. Though one alpha-emitting radiopharmaceutical, Xofigo (223RaCl2), is approved for clinical use, the clinical approval of an alpha-emitting isotope combined with a disease-targeting biomolecule has yet to occur and these radiopharmaceuticals remain in the development stages. Several candidate isotopes for TAT are currently under clinical and preclinical evaluation, including 149Tb, 211At, 212Bi, 212Pb, 213Bi, 223Ra, 224Ra, 227Th, and 225Ac. 225Ac is one promising candidate isotope for TAT due to its 9.9 day half-life - suitable for targeting with antibodies - and the emission of four alpha particles in its decay chain (it decays to stable 209Bi via four alpha- and two beta-decays - Fig. 1). 225Ac can also be used as a generator of 213Bi (t1/2 = 45.6 min), itself a promising TAT isotope.
Fig. (1).
Decay schematic showing the decay and production pathways for 225Ac. Gamma emissions useful for quantification of 225Ac are shown in red. (The color version of the figure is available in the electronic copy of the article).
While several clinical trials have demonstrated the potential of 225Ac or 213Bi radiopharmaceuticals to treat advanced cancers [10-13], the development of these drugs faces many challenges that have slowed progress, including: 1) the limited supply of 225Ac - currently only 63 GBq (1.7 Ci) globally annually - which could treat fewer than 1000 patients per year; 2) the need for adequate chemical purification processes required to separate 225Ac from co-produced stable or unstable isotopes during production; and 3) the need for stable targeting systems with high radiolabeling yields and appropriate pharmacodynamics, the development of which is hindered by a limited understanding of actinium chemistry and complicated by the need to also successfully retain 225Ac progeny isotopes at the target site despite recoiling daughter nuclei and changing chemistry as the decay chain progresses [14].
Each section of this review addresses one of these challenges. For each, we summarize the current literature including the current standard methods and any proposed alternatives, and also discuss how performed, planned, and potential activities at TRIUMF attempt to address these challenges facing the development of 225Ac-radiopharmaceu- ticals.
2. PRODUCTION OF 225Ac
2.1. Existing 225Ac Supplies
Current sources of 225Ac are primarily derived from the build-up of 229Th through the decay of 233U stockpiles (see Fig. (1) of the 233U decay chain). The majority of this 233U (t1/2 = 1.6 × 105 y) was produced between 1954 and 1970 via neutron irradiation of 232Th while being investigated for its use in nuclear weapons and reactors that were never fully deployed [15]. Between 1995 and 2005, 229Th (t1/2 = 7340 y) generated from 233U decay was extracted from stockpiles stored at Oak Ridge National Laboratory (ORNL, Oak Ridge, TN). This 229Th now exists in two sources: one at ORNL (∼5.55 GBq (150 mCi), or ∼704 mg) [16] and another (1.7 (46 mCi), or 215 mg) [17] transferred to the Institute for Transuranium Elements (ITU, Karlsruhe, Germany). A third 229Th source (5.55 GBq (150 mCi), 704 mg) obtained from Russia 233U stockpiles exists at the Leipunskii Institute for Physics and Power Engineering (IPPE, Obninsk, Russia) [18]. These three sources serve as generators of 225Ac and its parent 225Ra (t1/2 = 14.9 d) and act as the major 225Ac sources worldwide, producing approximately 26.6 GBq (720 mCi) (ORNL) [19] and 13.1 GBq (350 mCi) (ITU) [17] of 225Ac annually. While the IPPE source contains as much 229Th as the ORNL source, reported values indicate 225Ac production from this source is sporadic [18, 20]. Overall, the accepted global annual production from 229Th is 63 GBq (1.7 Ci) [21-25].
While a key advantage of this production method is an 225Ac product free of other actinium isotopes, 63 GBq (1.7 Ci) is insufficient to meet the current global demand for researchers and the development of new agents and will be even more inadequate should any 225Ac therapies become deployed clinically [19]. From research into fundamental 225Ac chemistry [9] to the most promising clinical trials [10], the development of 225Ac radiopharmaceuticals is slowed by the small supply and resulting high cost that makes 225Ac inaccessible to many researchers.
2.2. Leveraging Unique Facilities to Supply 225Ac Research
Due to the high cost of 225Ac, 225Ac-radiopharmaceutical development at TRIUMF currently relies on internal production using the Isotope Separator and Accelerator (ISAC) Facility [26]. Commissioned in 2000, ISAC produces beams of rare isotopes for experiments primarily studying nuclear structure and nuclear astrophysics. Irradiation of uranium or thorium targets with protons at 480 MeV results in the production of a number of isotopes that are extracted into a heterogeneous ion beam. Isotope Separation On-Line (ISOL) mass-separates these isotopes to produce a homogeneous isobaric ion beam [27]. Isolation of mass 225 produces an ion beam containing 225Ra and 225Ac that is directed onto an aluminum target in which the isotopes are deposited at a depth of 20 nm (as determined by SRIM [28]). Etching of the aluminum post-implantation followed by separation of 225Ra and 225Ac on a solid phase extraction (DGA) resin provides both a primary 225Ac fraction for immediate studies, as well as a number of subsequent 225Ac batches isolated through the decay of 225Ra. When eluted at the optimal time (every 17.5 days) this generator produces 225Ac with an activity equal to 44.4% of the 225Ra activity present at the previous elution1. Since 2015, 225Ac production at ISAC has enabled radiolabeling and preclinical studies at TRIUMF. ISOL methods have also been applied at ISOLDE (CERN, Geneva) to produce 225Ac for radiopharmaceutical development [29].
Though ISOL provides isotopically pure 225Ac sources, yields remain insignificant compared to quantities available from 229Th generators. At TRIUMF, the maximum measured ISAC beam intensities of 1.3 × 108 ions/s for 225Ac and 1.6 × 108 ions/s for 225Ra could theoretically produce up to 370 MBq (10 mCi) of 225Ac per month2, ISAC does not operate as a dedicated medical isotope production facility and 225Ac production occurs within the overarching context of the laboratory’s research program. Total 225Ac production for 2016 was only 44.4 MBq (1.2 mCi).
In order to better accommodate medical isotope research, the European Organization for Nuclear Research (CERN) has launched the Medical Isotopes Collected from ISOLDE facility (MEDICIS), which plans to produce mass-separated isotope beams from offline targets starting in 2017 and with uranium targets for 225Ac production available in late 2018 [29, 30]. As a dedicated medical isotope production facility, MEDICIS suggests the capacity to produce up to about 112 MBq (3 mCi) of 225Ac per month3.
2.3. The Need for New 225Ac Production Methods
Estimates of current demand for 225Ac are less than 185 GBq (5 Ci) per year, however, this is likely significantly tempered by both supply constraints and cost. While predicting future demand is difficult, it can be estimated to grow by about 200 to 400 GBq per year (about 5 to 10 Ci per year) for each 225Ac-based therapy that is approved for clinical use4. Should efforts to develop 213Bi-radiopharmaceuticals also increase, 225Ac demands will be even higher.
While facilities like ISAC and MEDICIS can facilitate radiopharmaceutical development by providing access to medical isotopes that are otherwise challenging to obtain, their application will remain limited to medical isotopes in the development stages. Even with potential proposed upgrades to the MEDICIS facility that could enable monthly production of up to 1.7 GBq (45 mCi) of 225Ac [29], and potential upgrades to ISAC that could increase yields by a factor of ∼10005, these facilities are not expected to meet existing 225Ac demand, are not commercially viable production sources, and could not
supply enough 225Ac to support the widespread use of a clinically approved therapy. These facilities should instead be viewed as valuable research enablers whose utility comes from their ability to provide quick access to a range of high-purity medical isotopes so that the feasibility of a given isotope’s applications can be explored before having to build dedicated large-scale, isotope-specific production infrastructure.
While harnessing untapped 229Th supplies has the potential to more significantly impact 225Ac availability, in 2005 the U.S. Congress ordered the Department of Energy (DOE) to cease extraction of 229Th from 233U stockpiles and to instead begin down-blending (dilution with 238U to a non-weaponizable 233U concentration) and permanent disposal of the two tonnes of stockpiled 233U [15]. Petitions to recover 229Th before 233U disposition have been denied [20], and completion of these efforts is scheduled for 2018 [15]. From the high- and intermediate-purity 233U sources within the inventory [32], this represents the loss of 32.6 g (∼260 GBq or 7 Ci) of 229Th or a potential 1.5 TBq (40.5 Ci) of annual 225Ac production. Other estimates suggest this is a loss of 37 g (∼8 Ci) of 229Th [16] and suggest a loss of 2.2 TBq (60 Ci) of annual 225Ac production [20]. Without new 229Th sources, 225Ac production from current DOE 229Th generators could increase by only 20% if the current elution schedule is optimized for 225Ac production instead of unit cost [19]. While quantities of additional 229Th-containing 233U sources may exist in other countries (ex. Russia), quantities - to the best of our knowledge - are unknown.
Without the existence of significant additional 229Th sources, the use of 225Ac or 213Bi in multiple approved therapies will require the development of new 225Ac production methods. The remainder of this section aims to present a comprehensive list of alternative 225Ac production options. The potential of each method to meet projected 225Ac demand and the practical challenges associated with each method will be discussed. Possible production methods proposed in the literature are summarized in Table 1, while Table 2 summarizes other nuclear reactions capable of producing 225Ac but that are considered impractical at this time. When not derived from original sources, details of calculations are provided in footnotes.
Table 1.
Summary of current and potential future 225Ac production methods. Production values for current sources list current production levels, while values for potential sources list estimates of maximum possible production at sample of existing and operational facilities that have dedicated stations for large-scale medical isotope production. Details of calculations or references to cited values can be found in the text. Values listed for 226Ra targets assume a target mass of 1 g.
| Production Method | Facility | Capabilities |
Monthly 225Ac
Production [GBq (Ci)] |
|
|---|---|---|---|---|
|
Current Sources |
229Th generator | ORNL ITU IPPE |
0.704 g (150 mCi) of 229Th 0.215 g (46 mCi) of 229Th 0.704 g (150 mCi) of 229Th |
2.2 (0.06) 1.1 (0.03) 2.2 (0.06) |
| Potential | 232Th(p, x)225Ac | TRIUMF | 500 MeV, 120 µA | 11266.5 (304.05) |
| BNL | 200 MeV, 173 µA | 2675.84 (72.32) | ||
| INR Arronax |
160 MeV, 120 µA 70 MeV, 2×375 µA |
1002.0 (27.08) 462.1 (12.49) |
||
| LANL | 100 MeV, 250 µA | 444.0 (12.00) | ||
| iThemba LABS | 66 MeV, 250 µA | 127.7 (3.45) | ||
| Future | 226Ra(p, 2n)225Ac | 20 MeV, 500 µA cyclotron 15 MeV, 500 µA cyclotron |
3983.1 (107.65) 1157.4 (31.28) |
|
| Sources | ||||
| ISOL | TRIUMF (existing) TRIUMF (potential upgrades) |
0.37 (0.01) 190.6 (5.15) |
||
| 226Ra(γ, n)225Ra | medical linac ALTO |
18 MeV, 26 µA 50 MeV, 10 µA |
48.1 (1.3) 55.5 (1.5) |
|
| 226Ra(n, 2n)225Ra | fast breeder reactor | ∼37 (1) | ||
Table 2.
Possible yet impractical methods for 225Ac production.
| Production Method | Comments |
|---|---|
| 226Ra(p, pn)225Ra | Yields insignificant compared to 226Ra(p, n) 225Ac production (105× less according to FLUKA simulation) |
| 232Th(p,4n)229Pa | Low cross-section |
| natU(p, x)225Ac | Produces ∼ 10× less 225Ac and 225Ra compared to thorium spallation, creates fissile 239Pu and 235U, can handle less beam current than thorium spallation targets |
| 232Th(n, γ)233U | Would take decades for 229Th to build up |
| 230Th(γ, n)229Th | 230Th not available in sufficient quantities |
| Reactor production of 229Th | Potential target materials 228Ac, 228Ra, 228Th, and 230Th not available in sufficient quantities. Production yields from 226Ra irradiation (110 MBq/month/g, or 3 mCi/month/g) too low considering cost and difficulty of 226Ra source production. |
2.4. Potential for 225Ac Production in Nuclear Reactors
While the majority of medical isotopes today are sourced from nuclear reactors [33], the potential for reactor-based 225Ac production is limited. The parent isotope, 225Ra, can be produced in reactors via the 226Ra(n, 2n) 225Ra reaction, however, this reaction requires an intense source of high (>6.4 MeV) neutrons found only near the tail end of a typical breeder reactor neutron energy spectrum. Given that significantly more lower energy neutrons would be present, these irradiations would be dominated by the co-production of 227Ac, a long-lived (t1/2 = 21.8 y) and highly toxic actinium isotope, the presence of which in significant quantities may prevent the clinical approval of a pharmaceutical. To the best of our knowledge, this method has not been investigated experimentally or thoroughly modelled. However, rough estimates suggest this method could produce MBq to GBq (µCi to mCi) amounts of 225Ac per month per gram of 226Ra target material at a single reactor facility6.
The potential to increase 229Th stocks using reactors has also been investigated [36, 37]. Results suggest the irradiation of 226Ra targets at a single reactor could produce 100 MBq (2.7 mCi) of 229Th per month per gram of 226Ra target material [36]. Other results have suggested this value may be closer to 59 MBq (1.6 mCi) [37]. While larger specific yields were seen when using 228Ra and 227Ac target materials (352 and 600 MBq (9.5 and 16.2 mCi) of 229Th per month per gram, respectively), these isotopes cannot be supplied in sufficient quantities [36]. The 228Th(n,γ)229Th reaction is impractical for the same reason. For example, 2.5 thousand tonnes of natural thorium would have to be processed to produce a single gram of 228Ra (t1/2 = 5.8 y), which could potentially be used to slowly generate 228Th (t1/2 = 1.9 y).
Given the challenges and costs associated with safely handling large 226Ra sources (see Section 2.7) and the resulting low 225Ra or 229Th yields, reactor production of sufficient 225Ac quantities would likely be prohibitively expensive.
2.5. Potential for 225Ac Production Using Electron Accelerators
The use of the 226Ra(γ, n)225Ra reaction for 225Ac production has been experimentally explored [38] and modelled [39]. These works have explored irradiating old radium needles on electron linear accelerators (linacs) found in most modern cancer centres. These linacs typically use electron beams incident on tungsten targets to produce bremsstrahlung x-rays for external beam radiation therapy. Experimental results measured the production of 2.44 MBq (66 µCi) of 225Ac after a single-hour irradiation by 18 MV x-rays of a 20 mg of 226Ra source located 12.5 cm from the tungsten target and with an incident electron beam of 26 µA average current [38]. This scales to a potential 48 GBq (1.3 Ci) of 225Ac per month for a 1 g 226Ra source, which could be potentially increased by irradiation parameter optimization. This method has the advantage of producing 225Ac without contamination from other actinium isotopes. While co-production of 224Ra occurs for photons above 12 MeV, this should not impact the desired 225Ra/225Ac generator as 224Ra (t1/2 = 3.7 d) decays to inert 220Rn and does not result in the production of any Ac isotopes.
While many medical linacs capable of this 225Ac production method may exist, these facilities are used for patient care and, to our knowledge, none are currently equipped with the infrastructure required for safe large-scale isotope production and processing. Again, the 48 GBq (1.3 Ci) of 225Ac per month would likely be cost prohibitive given the challenges (see Section 2.7) associated with a 1 g 226Ra target. It has been estimated that linac-based 225Ac production could be increased by up to a factor of 16, although accompanied by an increase in target mass [40].
Given this low yield, sufficient 225Ac production via the 226Ra(γ, n)225Ra reaction would require the use of a facility with significantly higher electron beam current. Though none are dedicated medical isotope production facilities, some such facilities exist for which 225Ac production values determined by scaling experimental medical linac irradiation results by electron beam current can be found in Table 1. In addition, TRIUMF’s planned Advanced Rare IsotopE Laboratory (ARIEL) facility will use a 35 MeV, 10 mA electron beam to produce intense high-energy x-rays for radioisotope production by photofission [26]. While the ARIEL electron target is intended for operation as an ISOL facility for fundamental research - not a medical isotope production facility - scaling experimental results for 225Ra production on medical linacs to account for the higher current and different irradiation geometry suggests ARIEL could theoretically produce up to 74 TBq (2000 Ci) of 225Ac per month from a 1 g 226Ra target. However, how an isotope production target could survive a 100 kW beam is another unsolved problem - current designs for ARIEL ISOL targets only consider 50 kW. Other lower current electron accelerators, such as the existing 50 MeV, 10 µA ALTO electron accelerator (Orsay, France) [41], could theoretically produce up to 56 GBq (1.5 Ci) per month.
2.6. Potential for 225Ac Production using Low Energy Proton Accelerators
The promising use of the 226Ra(p,2n)225Ac reaction to produce 225Ac on low-energy proton accelerators was first demonstrated in 2005 by Apostolidis et al. [42]. This reaction has a high (710 mb) cross-section peak at 16.8 MeV and could thus be performed on the many low energy cyclotrons already in use worldwide for medical isotope production. An estimated >550 of these cyclotrons have an energy over 16 MeV, some of which operate at up to 500 µA [43]. Another advantage of this approach is that it would not coproduce 227Ac. While the (p, n) reaction is expected to produce some 226Ac (t1/2 = 29.4 h), measurements of co-production of 226Ac have not been reported from experiments found in the literature [42]. A simple FLUKA [44,45] simulation approximating the Apostolidis et al. experiment suggests an 226Ac activity at end of bombardment (EOB) equal to ∼11% the 225Ac activity. However, unlike with 227Ac contaminants, the ratio of expected 226Ac to 225Ac activity would decrease over time due to the differences in half-lives. The co-production of 225Ra via the 226Ra(p,pn)225Ra reaction is expected to be negligible at the optimal energies required for direct 225Ac production [46].
Given the high cross-section, large-scale production of 225Ac via the (p,2n) reaction would be capable of meeting long-term demand for 225Ac with only a single production site. Combining available cross-section data [42] with stopping power for 226Ra [28] suggests a single 20 MeV, 500 µA proton beam incident on a 226Ra target (∼1 g) could produce a theoretical maximum of 4 TBq (108 Ci) of 225Ac per month7.
The use of low energy proton irradiation of 232Th to produce 229Pa, which decays to 229Th, has also been explored [47]. The peak measured cross-section for this 232Th(p, 4n)229Pa reaction is 162 mb for a proton energy of 29.8 MeV. However, yields for this production method are low, with a potential to produce only 7.4 MBq (0.2 mCi) of 229Th per month on a 50 MeV, 500 µA cyclotron.
2.7. Challenges Associated with 226Ra Targets
All alternative 225Ac production methods discussed so far have involved the use of 226Ra as a target material. Cost-effective isotope production requires the use of stable or naturally occurring and long-lived target materials, and as the closest such isotope to 225Ac, 226Ra is one of only a few options - spallation reactions on naturally occurring 232Th and 238U being the only other possibilities. Other potential target materials such as 230Th, 228Th, 228Ac, and 228Ra are not available in large enough quantities to be of practical use.
Despite its potential, the use of 226Ra targets poses significant challenges due to the availability of the isotope and safety hazards that complicate the target manufacturing, irradiation, processing, and recycling. Part of the 238U decay chain, 226Ra ultimately decays to stable 206Pb and is typically found in equilibrium with most isotopes in its decay chain. 226Ra was the first radioactive isotope discovered and was produced in large quantities from the 1920s for use in a number of medical and industrial applications until production stopped in 1960 [48]. Due to its high radiotoxicity, reactivity with water and air, and decay to the noble alpha-emitting 222Rn gas, 226Ra sources typically contained radium salts encapsulated in platinum [49]. The internal production of helium from the five alpha decays in the 226Ra chain caused most of these sources to rupture, after which 222Rn gases are released and 226Ra salts can leak out. Even when sealed, the high energy gamma rays present from 226Ra progeny present external radiation hazards, with a dose rate of 8.1 mSv/h at 1 m from a 37 GBq (1 Ci, 1 g) 226Ra source.
While the use of 226Ra sources declined after the health effects of radiation exposure became known and safer reactor-based isotopes became available, many 226Ra sources remained in storage - primarily in hospitals - for decades. The hazards associated with the presence of 226Ra sources lead many governments to push for the elimination of 226Ra inventories and in 1996 the International Atomic Energy Agency (IAEA) established guidelines for the disposal of 226Ra sources in long-term geological repositories [49]. This limits the availability of large 226Ra quantities, with the IAEA estimating only a few kilograms of 226Ra exist among these sources worldwide [49]. Typical medical sources contained <100 mg of 226Ra, with some industrial sources containing up to 1000 mg quantities. For this reason, calculations in Table 1 assume 1 g as a reasonable upper limit on the size of potential 226Ra targets.
New 226Ra sources could be extracted from the waste of current uranium mining operations. Approximately 50 thousand tonnes of uranium ore is mined each year [50], from which 226Ra is separated and disposed of as waste. With the potential to extract 257 mg of 226Ra from each tonne of U3O8 [39], this amounts to about 12.85 kg of 226Ra waste per year.
Whether obtained using old sources or through uranium mine tailings, the manufacturing of 226Ra targets for 225Ac production would require infrastructure beyond what is typically used to make medical isotope production targets. 226Ra regulatory and safety issues - specifically those associated with 222Rn - would also require additional infrastructure during the target irradiation, processing, and the necessary recycling of irradiated 226Ra material. While 226Ra targets have the greatest potential for 225Ac production per gram of target material, difficulties and costs associated with these targets is a significant disadvantage of the 226Ra(p,2n)225Ac and 226Ra(γ, n)225Ra production methods.
2.8. Potential for 225Ac Production via High-Energy Proton Spallation of Thorium
An alternative 225Ac production method that avoids the use of 226Ra targets involves the irradiation of natural thorium targets with protons above 70 MeV. This 232Th(p, x)225Ac reaction produces 225Ac through a number of reaction pathways, though the total cross-section peaks ∼40 times lower than for 226Ra(p, 2n) 225Ac production (for details on cross-sections, the reader is referred to: [51]). Thorium metal is the preferred chemical form for post-irradiation processing of the target, and isolation of MBq to GBq (µCi to mCi) quantities of 225Ac from irradiated thorium metal has been demonstrated by both American and Russian research groups at Brookhaven National Laboratory (BNL, Brookhaven, NY), Los Alamos National Lab (LANL, Los Alamos, NM), and the Institute for Nuclear Research of the Russian Academy of Sciences (INR) [20, 22, 23, 51-58]. Unlike 226Ra (3.7×1010 Bq/g, or 1 Ci/g), 232Th (4.1×103 Bq/g, 110 nCi/g) is not prohibitively radioactive, poses fewer radiological hazards and is readily available as a target material. Tens of kilograms are known to exist in stockpiles within a number of countries, and more thorium metal is able to be produced in bulk quantities from thorium oxide or thorium nitrate, hundreds of tonnes of which are produced annually worldwide as a by-product of rare-earth mining [50]. This availability means recycling of irradiated 232Th target material may not be necessary. Another advantage of this method is that facilities already exist with demonstrated ability to perform target fabrication, irradiation, and processing. Examples of accelerator facilities capable of producing large amounts of 225Ac via proton spallation are listed in Table 1.
While spallation of naturally occurring 238U will also produce 225Ac, 232Th irradiation is preferred for a number of reasons. The 238U(p,x)225Ac reaction cross-section is ∼10 times lower (as modelled using FLUKA and GEANT4), and due to the higher density and lower melting point of uranium, thorium targets could more safely handle the higher heat load induced by higher beam currents. The co-production of fissile 239Pu and 235U is also avoided by 232Th irradiation.
The spallation of thorium produces a number of isotopes other than 225Ac. While this may provide an opportunity for recovery of other useful isotopes, it also complicates target processing by requiring the separation of dozens of elements. An overview of the different methods for isolating 225Ac from irradiated thorium is described in Section 3.2.
Concerns exist in the field that the amount of 227Ac coproduced from thorium spallation will prevent its use as a method for clinical-grade 225Ac production. An 227Ac to 225Ac activity ratio of 0.1-0.2% is typically found in irradiated targets at end of bombardment. However, potential exists for current target processing methods to be modified to produce 225Ac quantities that are free of 227Ac by isolation of an radium-actinium generator [59]. Most methods already isolate radium from the irradiated thorium matrix, and if this is done days after EOB, only 228Ra, 226Ra, 225Ra, 224Ra, and 223Ra will be present because of the length of their half-lives (t1/2 = 5.7 y, 1600 y, 14.9 d, 3.6 d, and 11.4 d, respectively). Of these, 228Ra and 225Ra beta-decay to actinium isotopes, while the others alpha-decay to radon isotopes. Use of this mixture as a radium-actinium generator will produce 225Ac free of 227Ac. While 228Ac (t1/2 = 6.2 h) will be present after 225Ra/225Ac separation, the ratio of 228Ac to 225Ac activity (∼0.88% at the time of optimal 225Ac elution [59]) will decrease with time. After sufficient 228Ac decay, 225Ac could then be removed from the 228Th (t1/2 = 1.9 y) produced by 228Ac decay to obtain a final 225Ac product with significantly reduced radioactive impurities when compared to the directly produced 225Ac fraction. While the total 225Ac yield from this method will be reduced by a factor of about 10, it does not prevent the use of the directly produced, 227Ac-containing batch of 225Ac from being used for research or for use in 225Ac/213Bi generators.
Only a few existing accelerators can produce proton beams with a current and energy sufficient for large-scale 225Ac production. A list of some of these facilities is given in Table 1 along with estimates of the maximum amount of 225Ac each could produce per month. These values only include directly produced 225Ac and exclude 225Ac produced from 225Ra generators that would increase production for each by roughly 10% to 20%. Without knowing details of each institutions’s target irradiation facilities, all are compared based on their maximal yield estimates that assume a target station capable of handling a thorium target thick enough to completely stop the proton beam (the same assumption was made for 226Ra (p,2n)225Ac maximal yields)8. As a result, practical yields will be lower. For example, while TRIUMF’s 500 MeV, 120 µA beam could theoretically produce 11.2 TBq (304 Ci) of 225Ac per month, 3 TBq (82 Ci) of monthly 225Ac production is a more practical limit given the existing target station’s size and cooling capacity. Similarly, practical estimates for production at BNL and LANL are 165 GBq (4.5 Ci) per month [58].
2.9. TRIUMF Perspective on 225Ac Production
Given the costs and challenges associated with 226Ra targets, the existing facilities already capable of thorium target spallation, and the large number of successful thorium target irradiations described in the literature, we believe that the development of 225Ac production via proton spallation of thorium is the fastest way to reliably meet the current global demand for 225Ac and support the widespread clinical use of any future therapies.
To this end, TRIUMF is working towards the development of routine 225Ac production via the irradiation of thorium metal targets on its primary beamline (BL1A), which delivers up to 500 MeV, 120 µA protons to the currently under-utilized legacy 500 MeV Isotope Production Facility (IPF). Since IPF is located directly before the BL1A beam dump, IPF routinely receives >100 µA of beam. Progress so far has included the low-level (2 µAh) irradiation of a prototype thorium oxide target in December 2016. The irradiation of thorium metal targets followed by processing on-site to isolate 370 MBq (10 mCi) of 225Ac is anticipated for late 2017, followed by 3700 MBq (100 mCi) production in 2018.
3. RADIOCHEMICAL ISOLATION OF 225Ac
The most established production method is the 229Th generator, which currently provides the main source of 225Ac and 225Ac/213Bi generators used in preclinical or clinical trials. In addition, radiochemical procedures for several alternative strategies for 225Ac production from thorium and radium targets are also discussed. Details on radiochemical aspects of 225Ac/213Bi generators are beyond the scope of this review, and the reader is referred to a review by Mogenstern et al. for additional insight into this area [24].
3.1. Isolation of 225Ac from 229Th Generators
Separation of 225Ac and 225Ra from 229Th (Fig. 1) is routinely performed at Oak Ridge National Laboratory (ORNL, TN, USA) [16], Institute for Transuranium Elements (JRC-ITU, Karlsruhe, Germany) [17, 61], and Leipunskii Institute for Physics and Power Engineering (IPPE, Obninsk, Russia) [62]. At ORNL, 229Th (5.6 BGq, 150 mCi) stock is divided into several batches and separation occurs every three weeks. A four-step chemical recovery procedure is used, including the combination of anion and cation exchange columns in nitric and hydrochloric media [16]. An anion exchange resin is first used to remove the bulk thorium mass. A 5×60 cm2 (1.2 L) column filled with MP1 (250±50 mesh) resin, allows the sorption of up to 30 g of thorium in 8 M HNO3, while 225Ac and 225Ra pass through the column. This process is repeated using a second MP1 column (2×10 cm2, 30 mL) in order to separate residual thorium quantities. The fraction containing Ac and Ra is evaporated to dryness and re-dissolved in 10 M HCl and then loaded onto the next anion exchange (MP1) resin, which allows purification of actinium and radium from iron and uranium micro-impurities. Final separation of 225Ac from radium is performed on two cation exchange (AG50x4) columns in nitric acid media. Average recovery yield of 225Ac is 80% over one campaign with radionuclidic purity of over 99%. The use of isolated 225Ra as an additional 225Ac source occurs by storing 225Ra on a cation exchange resin for 225Ac accumulation and subsequent separation.
At ITU, a similar strategy using anion exchange column for 225Ac and 225Ra separation from 229Th is employed [17]. Due to the smaller 229Th (1.7 GBq, 46 mCi) stock compared to ORNL, the production of clinically relevant 225Ac quantities requires separation once every nine weeks after maximum 225Ac buildup is achieved. Separation from thorium is performed on two anion exchange (AG1X8) columns (0.5 L and 80 mL) in 8 M HNO3, where 225Ac and 225Ra first pass through without sorption and 229Th is later eluted from the resin in 0.05 M HNO3. The 229Th fraction is evaporated to near dryness and re-dissolved in 8 M HNO3 and mixed with anion exchange resin for storage until the next elution. The Ra and Ac fraction is subject to further purification from residual thorium via solid phase extraction chromatography, using three columns filed with diamyl, amylphosphonate (UTEVA) resin. Separation of Ra from Ac is then performed inoctyl(phenyl)-N,N-diisobutylcarbamoyl-methylphosphine oxide (RE-resin) resin in nitric acid. The reported recovery yield for 225Ac is higher than 95% with a de-contamination factor from thorium of about 102. The same group also reports alternative separation of 225Ra and 225Ac via N,N,N’,N”-tetrakis-2-ethylhexyldiglycolamide (DGA) based resin in nitric acid [61].
At IPPE, separation of 225Ac and 225Ra from 229Th stock (5.6 BGq, 150 mCi) occurs on an anion exchange column in nitric acid followed by Ra/Ac separation on a cation exchange column in diluted nitric acid [18]. The final 225Ac fraction is additionally purified with a combination of cation and anion exchange columns. A portion of the stock of 229Th is loaded on an anion exchange column (Dowex 1X8, 0.5 L) in 8 M HNO3. As previously described, Ac and Ra pass through the column, while 229Th is retained. The column is additionally washed with 1 L of 8 M HNO3 for complete Ac/Ra elution. 229Th is then stripped with 2.2 L of 0.05 M HNO3 and converted to 8 M HNO3 (250-300 mL) for future use. The eluted Ac and Ra fractions are evaporated to dryness and re-dissolved in 0.5 M HNO3 and passed through a cation exchange column (Dowex 50X8, 6 mL) on which 225Ac is retained while 225Ra passes through. The column is further washed with 1.5 M HNO3 to remove residual 225Ra isotopes. Actinium is desorbed with 8 M HNO3 and converted to 10 M HCl for further purification. Finer purification of the 225Ac solution is performed using anion and cation exchange columns in hydrochloric and nitric acids, respectively. The final actinium product is eluted in 10-12 mL of 8 M HNO3. 225Ac separation yield varies from 85-95% with 229, 228Th impurities below 10-5 of 225Ac by activity.
Additionally, several alternative methods were previously reported for isolation of research quantities of 225Ac and 225Ra from 229Th [63,64]. One purifies 225Ac from 229Th by chelation with citric acid at various pH on a cation exchange column [63]. The final separation scheme includes sorption of Ac, Ra, and Th on a cation exchange (Aminex A-5, 4×40 mm2) column after loading with 0.25 M citrate (pH <1). Th was further eluted with ammonia citrate (pH 2.3) and Ac and Ra were eluted with ammonia citrate pH 4.5 and 4 M HNO3, respectively. Another separation strategy based on the combination of cation and anion exchange chromatography with mixed HNO3-methanol solution was also tested [64]. This strategy provides a tandem system for consequent separation of 225Ac from 229Th followed by loading of the 225Ac fraction on a generator for 213Bi.
3.2. 225Ac Isolation from Thorium Targets
As described in Section 2.8, the irradiation of thorium metal with protons above 100 MeV can produce large quantities of 225Ac. The main challenges associated with the chemical isolation of 225Ac from these targets are a limited number of facilities capable of handling targets containing large 225Ac quantities (>10 GBq, or >0.3 Ci) and the challenging chemistry required for separating actinium from multiple grams of thorium and several hundreds of co-produced fission products. As discussed in Section 2.8, another drawback is the co-production of other actinium isotopes, most of which have relatively short half-lives (≤30 hours). One exception is the longest-lived Ac isotope, 227Ac (t1/2 = 21.8 y), co-produced at a rate of 0.1-0.2% when compared to 225Ac activity. While the production of 225Ac without 227Ac is possible via the isolation of a 225Ra/225Ac generator (see Section 2.8), this further complicates the required chemical processing.
Strategies similar to those described in Section 3.1 that use anion exchange columns to retain thorium while eluting Ac and Ra can also be applied to the processing of thorium targets. However, this method requires a large volume of resin to remove the many grams of thorium used as target material - compared to the mg quantities used in 229Th generators - as well as additional purification steps to remove other spallation products. While several strategies were developed and tested for separation of actinium and radium from thorium [54, 65], none of these provide actinium with the minimal impurities required for radiolabeling of biomolecules and clinical application.
More recently, two alternative separation methods were developed and tested for isolation of 225Ac suitable for radiopharmaceutical application. Both methods are practically suitable for masses of thorium of up to 100 grams - larger 232Th targets will require larger volumes of extraction and chelation agents. The first, described by Aliev et al. [23], uses a three-step procedure that includes liquid-liquid and solid phase extraction chromatography [23, 57, 66]. First thorium metal target was dissolved in nitric acid with a small portion of HF. Further, two extractions with Di-(2- ethylhexyl)phosphoric acid (HDEHP) were performed, where the bulk thorium mass was extracted into in the organic phase while actinium, radium and most spallation products were retained in aqueous phase. Following phase separation, the aqueous phase is passed through N,N,N’,N”-tetrakis-2-ethylhexyldiglycolamide (DGA) column for separation of actinium and lathanides from fission products and radium in nitric acid media. The Ac and lanthanide fraction is then processed using TRU resin in 3 M HNO3. Reported recovery yields for actinium are higher than 85% and the process was adopted to a hot cell environment for remote manipulation [23]. A modified strategy has also been applied to co-extract 223Ra along with 225Ac [67].
The second method was the result of an multi-institutional collaboration within the US DOE that included Los Alamos, Oak Ridge and Brookhaven National Laboratories [22, 68, 69]. This two-step procedure first removes the bulk thorium mass by chelating thorium and spallation products in 1 M oxalic acid at pH 2. At these conditions, all cations with charge 4+ and higher form negatively charged complexes, while cations with lower charge form positively charged complexes. Therefore, positively charged actinium and radium complexes are retained on the cation exchange resin while the bulk mass of thorium and majority of the fission products pass through the column without absorption. A similar strategy had also been previously used for 229Th/225Ac generators [63]. Ac and Ra are then eluted in 6 M HNO3 and directly loaded onto a solid phase extraction chromatography column (N,N,N’,N”- tetrakis-2-ethylhexyldiglycolamide (DGA branched). At loading conditions, 2+ charged cations (e.g. Ra and Ba) pass through the column without sorption and actinium and lanthanides are retained. Actinium can be separated from lanthanides by specifically eluting with 10 M HNO3 from the DGA column, while lanthanides are retained under such conditions. Reported recovery yield of actinium is higher than 98% with high radionuclidic and radiochemical purity suitable for radiolabeling or generator application.
This strategy has been further improved by additional steps for the co-extraction of other valuable medical radionuclides - including 225, 224, 223Ra, 230Pa, 103Ru and 111Ag - without disturbing the actinium purification process [70].
3.3. 225Ac Isolation from 226Ra Targets
Irradiation of radium with low energy protons and photons also results in 225Ac production (see Sections 2.5 and 2.6). From a radiochemical separation standpoint, 225Ac needs to be isolated from macroscopic amounts of radium isotopes and their daughters. Previously published work suggests the use of a two-step procedure for 225Ac purification [42]. Irradiated 226Ra targets are dissolved in 0.01 M HCl, followed by purification of Ac from the bulk Ra mass (plus small Po and Pb quantities) by sorption of 225Ac on di(2-ethylhexyl) orthophosphoric acid (HDEHP) resin (LN-resin) in 0.01 M HCl. The column is washed with 0.1 M HCl and actinium was eluted with 2 M HCl. The second purification step uses 4,4’(5’)-di-t-butylcyclohexano 18-crown-6 (crown ether) resin (Sr-resin) for removal of residual amounts of radium and its daughters. The actinium fraction was passed through the Sr-resin column in 2 M HCl. No radiochemical yields or specific values for radiochemical purities have been reported for this method reported, though radiochemical purity is stated to be similar to 225Ac derived from 229Th generators.
3.4. Status at TRIUMF
At TRIUMF, several production routes for 225Ac are currently in use and under consideration. The collection of 225Ra and 225Ac ion beams at ISAC (see Section 2.2), is followed by a radiochemical separation consisting simply of one DGA column in nitric acid. However, chemical purity of 225Ac is still under evaluation due to the possible stable impurities originating from the implantation target. As mentioned in Section 2.9, 225Ac production via irradiation of sealed thorium metal targets is also planned at TRIUMF’s 500 MeV Isotope Production Facility, located on beamline 1A. While isolation of actinium and radium isotopes can be performed with one of the previously described strategies, any potential future full utilization of the high proton beam energy will require irradiation of large targets (>100 g). Therefore, alternative separation strategies are under evaluation. The upcoming ARIEL facility [26, 71] will also provide additional opportunity for irradiation of thorium targets with protons (480 MeV, 10-100 µA) as well irradiation of radium targets with photons. Design of radium targets and radiochemical separation of actinium from bulk radium are currently under consideration by our Life Sciences group.
4. TARGETING DESEASE with 225AC
4.1. Chemistry of 225Ac and Radiolabeling Challenges
The lack of any stable actinium isotopes has restrained the advancement of actinium chemistry, and as a consequence the chemistry of Ac(III) is virtually unknown [72, 73]. Only very recently have some studies begun to elucidate the fundamental coordination chemistry of this highly radioactive element [74, 75]. Actinium isotopes are typically +3 ions with a documented ionic radius of 112 pm (CN 6) [76]; its large size is likely suited to large polydentate chelators of high denticities, since most commonly used chelates for Ac(III) range between 8-12 coordinate [72]. Actinium is similar to other actinides and rare earth elements, and can undergo hydrolysis in solution in the absence of a chelating agent to form [Ac(OH)3−x]x- ; the sub-picomolar concentrations of 225Ac will cause the hydroxide species in turn to form radiocolloids that bind to surfaces such as reaction vessels [77].
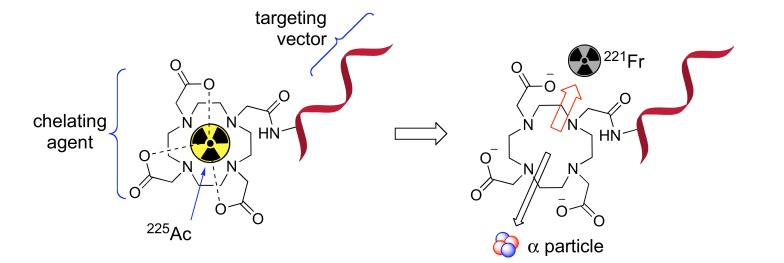
The emission of multiple alpha-particles in the 225Ac decay chain (Fig. 1) makes 225Ac a particular effective isotope to kill cancer cells, yet also makes the directed delivery of the nuclide and its decay daughters a challenge. Due to the conservation of momentum, the emission of an energetic alpha particle (energies shown in Fig. (1)) imparts a recoil energy to the daughter nucleus often >100 keV, 1000 times larger than the binding energy for any chemical bond [14]. This results in release of the daughter nuclide from the original delivery vector (Fig. 2). The subsequent redistribution of the alpha- emitting daughter nuclides in vivo can cause substantial harm to untargeted healthy tissues and reduce the therapeutic effect. Consequently, renal toxicity induced by 213Bi is considered to be a major constraint to the application of 225Ac in a large number of clinical trials [78]. There are three main strategies for limiting the toxicity of recoil daughters in the literature: fast uptake and internalization of the alpha emitters in the target tissue, encapsulation of the nuclide in a nanoparticle, or local administration of radioactivity directly into the target site via injection [14]. Herein, a literature review of the first two strategies is included.
Fig. (2).
Depiction of the recoil effect associated with α-decay of 225Ac. Daughter isotope 221Fr is released from the chelating agent due to the 100 keV recoil energy associated with the alpha emission of 225Ac. 221Fr and its decay daughters are consequently free to migrate within in the body.
4.2. Chelating Agents for 225Ac(III)
The discovery of a chelating agent that binds Ac(III) with sufficient stability and that also controls the release of its daughter nuclides remains a challenge. Moreover, limited global availability of 225Ac and the absence of a stable surrogate nuclide has limited the study of this isotope to a handful of institutions around the world that have secured a reliable 225Ac supply. The majority of initial 225Ac chelation studies have focused on screening a variety of commercially available polydentate macrocyclic or acyclic ligands for their ability to bind 225Ac and form stable complexes in vitro or in vivo. Despite the unique coordination preferences of the large +3 actinide, very few studies investigating new ligands specifically designed to coordinate Ac(III) can be found throughout the literature. A brief summary of ligands tested with 225Ac can be found in Table 3.
Table 3.
Tested 225Ac chelates coupled with targeting vectors for in vitro or in vivo application and their radiolabeling yields (RCY) and stabilities. Ligands rating system: red = not suitable for use; orange = adequate labeling, but likely unstable complex, or needs improvement; green = sufficient labeling and complex stability in vitro and in vivo, recommended for use.
| Chelate (and Corresponding Tested Bifunctional Analogues) |
Donor
Set (CN#) |
Grade |
Radiolabeling
Conditions & RCY |
In vitro or In vivo Stability | Ref. |
|---|---|---|---|---|---|
| DOTA 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid | N4O4 CN = 8 |
Green- orange | 0.02 M ligand, NH4Ac pH 6, 37 ◦C, 2 h, RCY = 99% |
In vitro human stability, 37 ◦C remained 90% intact after 10 days. |
[79] |
| DOTPA 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrapropionic acid | N4O4 CN = 8 |
Red | 0.02 M ligand, NH4Ac pH 6, 37 ◦C, 2 h, RCY = 0% |
n/a | [79] |
| DOTMP 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylene-phosphinic acid | N4O4 CN = 8 |
Red | 0.02 M ligand, NH4Ac pH 6, 37 ◦C, 2 h, RCY = 78% |
In vitro human serum stability - rapid decom- plexation | [79] |
| TETPA 1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetrapropionic acid |
N4O4, CN = 8 |
Red | 0.02 M ligand, NH4Ac pH 6, 37 ◦C, 2 h, RCY = 0% |
n/a | [79] |
| DTPA diethylenetriaminepentaacetic acid | N3O5, CN = 8 |
Red | 0.02 M ligand, NH4Ac pH 6, 37 ◦C, 2 h, RCY = 0% |
n/a | [79] |
Early studies by Davis et al. Deal et al. and McDevitt et al. screened a library of ligands for their ability to coordinate 225Ac and tested the resultant complex’s in vitro or in vivo stability [77, 79, 80]. In the study presented by Davis et al., ligands EDTA (ethylenediaminetetraacetic acid, N2O4), CHX-A”-DTPA (cyclohexyl-diethylenetriaminepen- taacetic acid, N3O5), and PEPA (1,4,7,10,13-pentaazacyc- lopentadecane-N,N’,N”,N”’,N””-pentaacetic acid, N5O5) were radiolabeled with 225Ac with radiochemical yields (RCY) of 80-90%. Biodistribution profiles over the course of 8 days for each of the purified 225Ac-complexes were assessed by injecting 92 kBq (2.5 µCi) of each complex, and compared to the 225Ac-acetate biodistribution as a control. Since uncomplexed 225Ac accumulates predominantly in the liver with small amounts in the bone, kidney, and heart, high liver uptake of an 225Ac-chelate indicates an unstable complex in vivo. CHX-A”-DTPA and PEPA reduced liver uptake of the 225Ac-complex by more than 5.5 times compared to 225Ac-acetate, and although their data suggests 225Ac-CHX-A”-DTPA to be the most effective tested chelator complex with regard to its in vivo stability, improvements can still be made to further reduce non-target tissue accumulation [77]. As such, CHX-A”-DTPA provides inadequate chelation of Ac(III). Another important finding of the initial in vivo study was that the maximum tolerated dose of 225Ac-CHX-A”-DTPA was less than 185 kBq (5 µCi), since at doses of 185 kBq (5 µCi) or higher, severe tissue damage was observed as early as 1 hr post-injection (p.i.), which ultimately lead to death causing 100% mortality by day 8 p.i. [77].
In search of a better 225Ac chelator, Deal et al. developed a novel dodecadentate (coordination number, CN = 12) chelator with an extended macrocyclic ring to accommodate the large Ac3+ ion, HEHA (1,4,7,10,13,16-hexaazacyclohexadecane-N,N’,N”,N”’,N””,N””’-hexaacetic acid, N6O6), and compared its in vivo stability to 225Ac labeled EDTA, CHX-A”-DTPA, DOTA, and PEPA [80]. 225Ac-HEHA demonstrated the highest complex stability evidenced by the low uptake of the complex in all tissues; essentially all radioactivity was excreted within 1 hour. Despite this perceived in vivo stability, the authors suggested the predicted -3 charge at physiological pH of the 225Ac-HEHA complex may be mediating the fast excretion of the complex, giving it the appearance of stability since the radiometal ion doesn’t have time to dissociate within the time frame of excretion [80]. Given these initial promising results, efforts towards the preparation of a bifunctional HEHA analogue were undertaken [81]. A C-functionalized isothiocyanate HEHA derivative was successfully prepared and conjugated to three monoclonal antibodies (mAbs): BL-3, T101, and CC49. One-step labeling of 225Ac to HEHA-mAb incubated for 30 min at 37 °C, pH 7.0 produced moderate to high RCYs of 60-85% with specific activities of 7.4-14.8 MBq/mg (200-400 µCi/mg) (for ligand:mAb ratios > 1.0); these bioconjugates were sufficient for animal therapy studies. In vitro serum stability revealed the 225Ac-HEHA-BL-3 mAb conjugate to be only 50% stable in serum after 24 h, suggesting that the HEHA system may very well not be a suitable chelator for sequestering 225Ac [81].
McDevitt et al. screened the 225Ac radiolabeling efficiency and in vitro stability of several polydentate chelators including DTPA (diethylenetriaminepentaacetic acid, N3O5), DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, N4O4), TETA (1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid, N4O4), DOTPA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrapropionic acid, N4O4), TETPA (1,4,8,11- tetraazacyclotetradecane-1,4,8,11-tetrapropionic acid, N4O4), and DOTMP (1,4,7,10- tetraazacyclododecane-1,4,7,10-tetramethylene-phosphinic acid, N4O4) [79]. Of the six ligands tested, only DOTA and DOTMP showed any complexation of 225Ac after 2 h at 37 °C with RCYs of >99 and 78%, respectively. Subsequent in vitro stability assays in serum suggested that the 225Ac-DOTA complex was robust, remaining >90% intact after 10 days, while the 225Ac-DOTMP complex rapidly dissociated.
The initial promising in vitro stability of 225Ac-DOTA motivated the authors to prepare conjugates of DOTA with antibodies HuM195 (anti-CD33), mJ591 and huJ591 (anti-PSMA), B4 (anti-CD19), and 3F8 (anti-GD2). The elevated temperatures required to achieve adequate labeling yield of Ac-DOTA were not amenable to antibody conjugates since such reaction conditions would denature proteins and result in loss of function. Consequently, a two-step labeling process was employed which required 225Ac radiolabeling of the bifunctional DOTA-NCS ligand first, followed by mAb conjugation (pH 8.7, 37 °C for 52 min). Despite low overall radiochemical yields of only 9.8 ± 4.5%, reasonable specific activity (4.1 ± 2.6 GBq/g, or 0.11 ± 0.07 Ci/g) was achieved which would allow for preclinical therapeutic studies. Low yields were attributed to the first 225Ac labeling step of DOTA-NCS which required heating and, consequently, degradation of the isothiocyanate linker resulting in poor mAb conjugation in the following step.
Attempts to increase the 2-step labeling yields of 225Ac-DOTA-mAb conjugates via the modification of a more robust DOTA-linker chelate system have yielded some improvements [82]. Antczak et al. established a 2-step labeling protocol for thiol-based DOTA-linkers which provided a marked improvement compared to the DOTA- NCS 2-step method, with chelation yields of 95-99% and labeling yield up to 40%. In the same study, the authors incorporated an enzymatically cleavable linker which could minimize the toxicity associated with long-circulating mAbs to normal tissues by allowing the release of a small molecular weight radiometal-chelate complex from the mAb to promote fast clearance of the therapeutic nuclide. However, constructs resulted in high accumulation of 225Ac in the liver in small animal models, indicative of 225Ac release from the chelate [82].
Perhaps the most noteworthy improvement to 225Ac radiolabeling to date was presented by Maguire et al. [83], which offered for the first time an efficient 1-step radiolabeling procedure for 225Ac-DOTA-antibody constructs. Typical radiolabeling proceeded in 2 M tetramethyl ammonium acetate buffer (pH 7.5) with the addition of L- ascorbic acid as radioprotectant to the addition of DOTA-antibody construct and 225Ac3+ with a typical final reaction pH of 5.8. Heating to 37 °C for 2 hours allowed a 10-fold increase in radiochemical yield (80%) compared to previous 2-step methods (6-12%), and resulted in the preparation of bioconjugates with up to 30-fold higher specific activities (120 GBq/g compared to 3.7-14.8 GBq/g) [83]. The highest specific activity achieved was equivalent to 1 actinium for every 25 antibodies. These results will likely have great implications in preclinical and clinical uses of 225Ac labeled antibodies, since the ease of synthesis can more easily be translated in a clinical setting and the significant reduction of 225Ac losses during labeling can save the cost of this rare and valuable isotope.
4.3. Bioconjugates for 225Ac Labelling
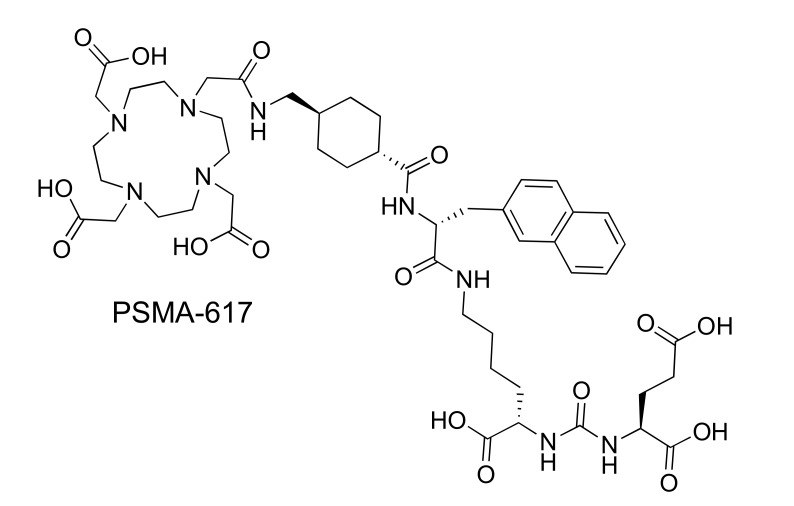
Despite often requiring elevated temperatures and extended reaction times, promising stability in serum and efficient labeling of 225Ac-DOTA complexes has cemented the commercial chelator and its bifunctional analogues as the most exploited chelator for 225Ac thus far. With the relatively limited scope of chelates tested with 225Ac to date (see Table 3), and very few examples of specifically tailored Ac-chelates in the literature, it seems apparent there is much room for improvement in the field of Ac-chelation, particularly as more powerful spectroscopic and computational techniques evolve that will continue to help elucidate the unique chemical differences between Ac3+ and other +3 actinides and lanthanides [74]. Nonetheless, 225Ac labeled DOTA-small molecule [10], peptide [84-86], and antibody [78, 87, 88] conjugates have been tested in a variety of in vitro and in vivo preclinical studies, and a select few have seen clinical successes. Specifically, the 225Ac-labeled humanized anti-CD33 (HuM195) mAb is in clinical trials for the treatment of patients with advanced myeloma [89]. Most recently a brief communication by Kratochwil et al. [10] reported remarkable treatment success in two patients with metastasized castration-resistant rostate cancer (mCRPC) who had extensive pretreatments and showed resistance to other therapies including beta-emitting radiopharmaceuticals. The small molecule 225Ac-PSMA-617 (Fig. 3) was administered bi-monthly at doses of 100 kBq/kg (2.7 µCi/kg); patient 2 saw complete remission after 3 treatment cycles.
Fig. (3).
DOTA-urea conjugate PSMA-617 used for targeting of prostate-specific membrane antigen.
4.4. 225Ac Labelled Nanoparticles
To circumvent the inevitable loss of 225Ac daughters after alpha decay from an actinium-chelate complex, researchers have sought to encapsulate the highly potent alpha-emitter into a nanoparticle structure. It is hypothesized that the 225Ac3+ ion and its decay daughters can be retained within the cavity of the nanoparticle structure, while the alpha particles are released and able to deposit their therapeutic dose at the intended target site. However, the use of nanoparticles as a platform to affix radionuclides or other biomolecular targeting vectors comes with several limitations. The biodistribution of nanoparticles is dominated by their large size and ability to take advantage of the enhanced permeability and retention (EPR) effect of cancer cells, where “leaky” vessels of poorly vascularized tumours allow for the uptake and retention of large macromolecules [72]. Moreover, the relatively large particles are often primarily excreted through the hepatic pathway which can cause unwanted high liver uptake. The accumulation of a highly toxic alpha-emitter in the liver may cause damage to the organ. Much of the available literature describing 225Ac-labeled nanoparticles provides in vitro data only [90-95]. Nonetheless, a brief overview of some strategies to prepare 225Ac radiolabeled nanoparticles is found below.
Work by Matson et al. [94] investigated the encapsulation of 225Ac 3+ ions in single-walled carbon nanotubes (SWNTs) by co-encapsulation of Gd3+ in an ion cluster. Although the Gd3+ ions remained inside the SWNTs, continual leakage of the 225Ac3+ ions was seen when challenged with serum. McLaughlin et al. [90] employed a multilayered nanoparticle structure which can contain the recoiling daughters of the in vivo alpha generator at the centre cavity, while coupling the outer layer to antibodies and without preventing the release of emitted alpha-particles. The shells included a radiation resistant lanthanide phosphate crystal doped with 225Ac and layered with a magnetic GdPO4 layer, plus a gold outer shell for the attachment of targeting vectors. Polymer vesicles (polymersomes) composed of poly(butadiene-b-ethylene oxide) have also been used to encapsulate 225Ac [92]. Preliminary in vitro studies in cells showed that smaller particles were absorbed by the cells and gathered around the cell nucleus. However, experiments and simulation indicated that larger polymerases are needed to attain satisfactory retention of recoiling daughters [92]. PEGylated liposomes loaded with 225Ac and labeled with mouse antihuman PSMA J951 antibody or with the A10 PSMA aptamer were tested in vitro for their targeting, internalization, and cytotoxicity on a prostate cancer cell line [91, 95]. These studies demonstrated anti- PSMA targeted liposome loaded with 225Ac can selectively bind, become internalized, and kill PSMA-expressing cells. Similarly, a 225Ac-loaded lipid-based nanocarrier was labeled with a PSMA targeting antibody or small molecule urea-based agent, and the targeting selectivity and cytotoxicity were compared to the radiolabeled antibody on its own [95]. It was found that the loaded lipid vesicles improved the killing efficacy 3-fold compared to the same levels of activity per cell when delivered by the PSMA-targeting antibody.
4.5. Assessing the Biodistribution of the 225Ac Decay Chain
While this discussion so far has been limited to the biodistribution of 225Ac itself, assessment of the biodistribution of each alpha-emission in the decay chain is necessary when evaluating the performance of 225Ac-radiopharmaceuticals. As previously mentioned in Section 4.1, the retention or redistribution of 221Fr, 217At, and 213Bi at the target site impacts the efficacy and toxicity of the radiopharmaceutical. While the half-life of 217At is short enough that its biodistribution can be assumed to be effectively identical to 221Fr, the short 221Fr half-life makes accurately determining its biodistribution - and also independently determining the biodistribution of its 213Bi granddaughter - a challenge using conventional ex vivo counting methods. Speedy harvesting and counting of organs is essential, since while successive measurements of the same ex vivo tissue samples over time can be used to estimate the amount of 221Fr or 213Bi present at the time of sacrifice, the uncertainty in these estimates increases the longer after sacrifice the first measurements are made [14].
Imaging-based methods can also be used to assess the biodistribution of the radionuclides in vivo, and quantitative SPECT imaging of 225Ac progeny isotopes has been demonstrated on small-animal SPECT/CT systems for 213Bi alone [96], and for both 221Fr and 213Bi simultaneously, via their 218 keV and 440 keV gamma lines, respectively [97]. Unfortunately, quantitative imaging of the high energy 213Bi photopeak (440 keV) requires the use of a high energy collimator not available on most imaging systems. However, qualitative SPECT imaging of 213Bi has been performed clinically, as has qualitative 221Fr SPECT in preclinical settings [11, 12, 90, 98, 99]. The use of Cerenkov imaging has also been demonstrated in vivo for the 225Ac decay chain [86], though this imaging modality is incapable of quantitative biodistribution measurements and cannot distinguish between individual 225Ac decay chain components.
While quantitative SPECT imaging of 221Fr and 213Bi with sub-millimeter spatial resolution has the potential to assess the retention of 225Ac progeny within the tumour and determine uptake within whole organs [97], the short range (<100 µm) of alpha particles mean that information regarding the sub-organ biodistribution - a level of detail not provided by current in vivo imaging modalities - is necessary for alpha-particle dosimetry [100, 101]. While ex vivo imaging using alpha-cameras can determine 225Ac biodistributions with spatial resolutions sufficient for dosimetry [102-106], alpha particle dosimetry itself faces additional challenges that currently limit the translation of preclinical dosimetric data to biological outcomes in the clinic [100, 101], a discussion of which is beyond the scope of this review.
4.6. Progress at TRIUMF
By leveraging our existing infrastructure TRIUMF has established ∼37 MBq (1 mCi) annual production of 225Ac via our ISOL facility (Section 2.2). This 225Ac has enabled TRIUMF to conduct a variety of preclinical radiolabeling, complex stability, and imaging studies [97]. In particular, the apparent lack of chelating agents available to complex 225Ac under conditions commensurate for “kit” type preparation of radiopharmaceuticals has motivated our researchers to search for alternate ligands which can quantitatively sequester the radioactive metal under mild, room temperature conditions with low ligand concentrations while forming thermodynamically stable and kinetically inert radiometal-chelate complexes. To this end, we are currently screening a wide variety of macrocyclic and acyclic chelates for their potential as ligands in 225Ac radiopharmaceutical development. Ligand candidates which display promising 225Ac radiolabeling efficiencies and high in vitro stability will be conjugated to selected antibody or peptide targeting vectors, and small animal in vivo studies can be conducted.
Conclusion
With some recent and remarkable clinical results in the treatment of late-stage cancers using beta- and alpha-emitting radiopharmaceuticals, the community has experienced a surge in interest in certain therapeutic isotopes. Supply challenges, especially for isotopes such as 225Ac, have limited the development of existing and emerging targeted radiotherapeutics, and pose a significant challenge in the discovery of new agents. That said, there exists a substantial amount of untapped production potential across a number of facilities.
Discussed within are the different 225Ac production routes which span the spectrum of neutron (reactor), electron (gamma) and proton-induced reactions. At this time, the most promising short-term approach to greater availability of 225Ac would be to irradiate thorium metal with high energy (>70 MeV) protons. By enable existing facilities with access to protons in this energy range to produce 225Ac, it will allow for a focus on addressing unanswered questions on a specific activity, radiochemical and/or radionuclidic purity. There exists the possibility of exploiting the co-production of 225Ra to assemble and distribute 225Ra/225Ac generators - a route that could help reduce the amount of 227Ac in the 225Ac used for radiopharmaceutical preparation. 225Ac produced during the irradiation itself could be used for manufacturing 225Ac/213Bi generator. Beyond this, safety challenges will continue in the handling and processing of irradiated Th targets given their extensive radionuclide content, which can be mitigated by leveraging the experience and repurposing equipment from facilities designed to handle large quantities of radioactive material from reactor fuel and waste processing.
Given the widespread availability of lower energy (16-24 MeV) medical cyclotrons, there also exists the tantalizing possibility of large-scale, decentralized production across the fleet of the hundreds of medical cyclotrons in operation across the globe today. The key challenge, in this case, would be more on the procurement of sufficient quantities of 226Ra, not to mention the safety implications of a failed irradiation in a hospital-based setting.
Adequate radiopharmaceuticals will also require the optimization of chelate design, with several advances having been recently reported. Enhanced supply will allow for the study and identification of compounds with the most suited pharmacokinetic profiles for therapy with minimized bystander organ dose or side effects. With clinical interest increasing, the most efficient way forward would be to enable increased production quantities by building or enabling existing facilities to produce isotopes such as 225Ac at the bulk-scale. By using these facilities to supply sufficient and routine quantities of 225Ac to advance existing clinical trials for compounds in clinical development - and should the early results seen to date represent results anticipated from a larger patient pool - there will undoubtedly be an increase in demand for more alpha-therapeutics toward more indications.
Consent for Publication
Not applicable.
| Chelate (and Corresponding Tested Bifunctional Analogues) |
Donor Set (CN#) |
Grade |
Radiolabeling Conditions & RCY |
In vitro or In vivo Stability | Ref. |
| PEPA 1,4,7,10,13-pentaazacyclopentadecane-N,N’,N”,N”’,N””-pentaacetic acid | N5O5, CN = 10 |
Red | 0.01 M ligand, NH4OAc pH 5.8, 40 ◦C, 30 min, RCY = 80% |
inadequate stability in vivo | [80] |
| HEHA 1,4,7,10,13,16-hexaazacyclohexadecane-N,N’,N”,N”’,N””,N””’-hexaacetic acid | N6O6, CN = 12 |
Orange | M ligand, NH4OAc pH 5.8, 40 ◦C, 30 min, RCY = >95%, or >98% after 2 h |
Rapid whole body clearance in vivo, facilitated by -3 charge. Low liver uptake suggests stability over short time in body |
[80, 81] |
| CHX-A”-DTPA | N3O5, CN = 8 |
Red | 0.01 M ligand, NH4OAc pH 5.8, 40 ◦C, 30 min, RCY = >95% |
In vivo decom- plexation indicated by high liver uptake | [77, 80] |
| EDTA ethylenediaminetetraacetic acid | N2O4, CN = 6 |
Red | 0.01 M ligand, NH4OAc pH 5, 40 ◦C, 30 min, RCY = 80-90% | In vivo decom- plexation indicated by high liver uptake | [77] |
Acknowledgements
The authors would like to acknowledge the following individuals for their input into this work: Dr. John D’Auria, Dr. Peter Kunz, Keith Ladouceur, and Dr. Tom Ruth.
Footnotes
1 MBq of 225Ra produces 1/(1-0.444) = 0.80 MBq of 225Ac from the generator if it is eluted every 17.5 days. Calculations in this publication convert 225Ra production values to 225Ac production values using this conversion factor.
For three 10-day implantations, 225Ac yield = 3×[1.3×108() + 0.8×1.6×108()] = 370 MBq (10 mCi).
While no specific monthly production estimate is explicitly stated by Dos Santos Augusto et al. [29], the reported estimated activity from one target is 28 MBq (0.75 mCi) from targets exchanged on a “weekly basis”, which is 112 MBq (3 mCi) monthly.
Assuming four rounds of 1 µCi/kg doses for 10 000 patients per year per therapy, and one half-life for processing and transport.
10× from increasing proton beam current from 10 to 100 µA, 6× from increasing target thickness from 10 to 60 g/cm2, 2× more efficient beam extraction, and 8.39× from replacing uranium carbide targets with thorium targets [31].
Assuming two 15-day irradiations per month, 1 gram 226Ra target, average cross-section of 2 barns over the 6.4 to 16.4 MeV range [34], and average neutron flux of 1012 n/s/cm2 over the same energy range [35] produces 52 GBq (1.4 Ci) of 225Ra or 44 GBq (1.2 Ci) of 225Ac per month.
Activity produced by a target completely stopping the incident proton beam can be calculated using energy-dependent values for stopping power, S(E), and cross-section, σ(E), using the following equation: A(t) =, given target density ρ, proton fluence ϕ and initial energy E0, irradiation time time t, and product isotope decay constant λ. For this equation, a 226Ra target density of 5 g/cm2 was assumed, as well as an irradiation schedule of three 10-day irradiations to get a monthly production value. The integral was performed using fitted data in MATLAB.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.McDevitt M.R., Sgouros G., Finn R.D., Humm L.J., Jurcic J.G., Larson S.M., Scheinberg D.A. Radioimmunotherapy with alpha-emitting nuclides. Eur. J. Nucl. Med. Mol. Imaging. 1998;25(9):1341–1351. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 2.Couturier O., Supiot S., Degraef-Mougin M., Faivre-Chauvet A., Car-lier T., Chatal J.F., Davodeau F., Cherel M. Cancer radioimmunotherapy with alpha-emitting nuclides. Eur. J. Nucl. Med. Mol. Imaging. 2005;32(5):601–614. doi: 10.1007/s00259-005-1803-2. [DOI] [PubMed] [Google Scholar]
- 3.Mulford D.A., Scheinberg D.A., Jurcic J.G. The promise of targeted {alpha}-particle therapy. J. Nucl. Med. 2005;46(1):S199–S204. [PubMed] [Google Scholar]
- 4.Brechbiel M.W. Targeted alpha-therapy: Past, present, future? Dalton Trans. 2007;43:4918–4928. doi: 10.1039/b704726f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilbur S.D. Chemical and radiochemical considerations in radiolabeling with α-emitting radionuclides. Curr. Radiopharm. 2011;4:214–217. doi: 10.2174/1874471011104030214. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.S., Brechbiel M.W. An overview of targeted alpha therapy. Tumour Biol. 2012;3:573–590. doi: 10.1007/s13277-011-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baidoo K.E. Res. 2013;19(3):530–537. doi: 10.1158/1078-0432.CCR-12-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgqvist J., Frost S., Pouget J.P., Albertsson P. The potential and hurdles of targeted alpha therapy - clinical trials and beyond. Front. Oncol. 2014;3:324. doi: 10.3389/fonc.2013.00324. [Abstract]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miederer M., Scheinber D.A., McDevitt M.R. Realizing the potential of the Actinium-225 radionuclide generator in targeted alpha particle therapy applications. Adv. Drug Deliv. Rev. 2008;60(12):1371–1382. doi: 10.1016/j.addr.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kratchowil C., Bruchertseifer F., Giesel F.L., Weis M., Verburg F.A., Mottaghy F., Kopka K., Apostolidis C., Haberkorn U., Morgenstern A. 225Ac-PSMA-617 for PSMA-targeted -radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016;57(12):1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 11.Jurcic J.G., Rosenblat T.L. Targeted alpha-particle immunotherapy for acute myeloid leukemia. Am. Soc. Clin. Oncol. Educ. Book. 2014;•••:126–131. doi: 10.14694/EdBook_AM.2014.34.e126. [DOI] [PubMed] [Google Scholar]
- 12.Kratochwil C., Giesel F.L., Bruchertseifer F., Mier W., Apostolidis C., Boll R., Murphy K., Haberkorn U., Morgenstern A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in- human experience. Eur. J. Nucl. Med. Mol. Imaging. 2014;41(11):2106–2119. doi: 10.1007/s00259-014-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen B.J., Singla A.A., Rizvi S.M., Graham P., Bruchertseifer F., Apostolidis C., Morgenstern A. Analysis of patient survival in a Phase I trial of systemic targeted α-therapy for metastatic melanoma. J. Immunother. 2011;3(9):1041–1050. doi: 10.2217/imt.11.97. [DOI] [PubMed] [Google Scholar]
- 14.Kruijiff R., Wolterbeek H., Denkova A. A Critical review of alpha radionuclide therapy-how to deal with recoiling daughters? Pharm. 2015;8(2):321–336. doi: 10.3390/ph8020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez R. Managing the Uranium-233 Stockpile of the United States. Sci. Glob. Secur. 2013;21(1):53–69. [Google Scholar]
- 16.Boll R.A., Malkemus D., Mirsadeh S. Production of actinium-225 for alpha-particle mediated radioimmunotherapy. Appl. Radiat. Isot. 2005;62(5):667–679. doi: 10.1016/j.apradiso.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Apostolids C., Molinet R., Rasmussen G., Morgenstern A. Produciton of Ac-225 from Th-229 for targeted alpha therapy. Anal. Chem. 2005;77(19):6288–6291. doi: 10.1021/ac0580114. [DOI] [PubMed] [Google Scholar]
- 18.Kotovskii A.A., Nerozin N.A., Prokof’ev I.V., Shapovalov V.V., Yakovshchits Y.A., Bolonkin A.S., Dunin A.V. Isolation of actinium-225 for medical purposes. Radiochem. 2015;57(3):285–291. [Google Scholar]
- 19.NSAC Isotopes Subcommittee . Meeting Isotope Needs and Capturing Opportunities for the Future: The 2015 Long Range Plan for the DOE-NP Isotope Program. Technical report. US Department of Energy; 2015. [Google Scholar]
- 20.NorthStar Medical Radioisotopes . Production of Actinium-225 via high Energy proton Induced Spallation of Thorium-232. Technical report. NorthStar Medical Radioisotopes; 2011. [Google Scholar]
- 21.Zhuikov B.L. Successes and problems in the development of medical radioisotope production in Russia. Phys. Uspekhi. 2016;59(5):481–486. [Google Scholar]
- 22.Radchenko V., Engle J.W., Wilson J.J., Maassen J.R., Nortier F.M., Taylor W.A., Birnbaum E.R., Hudston L.A., John K.D., Fassbender M.E. Application of ion exchange and extraction chromatography to the separation of actinium from proton-irradiated thorium metal for analytical purposes. J. Chromatogr. A. 2015;1380:55–63. doi: 10.1016/j.chroma.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 23.Aliev R.A., Ermolaev S.V., Vasiliev A.N., Ostapenko V.S., Lapshina E.V., Zhuikov B.L., Zakharov N.V., Pozdeev V.V., Kokhanyuk V.M., Myasoedov B.F., Kalmykov S.N. Isolation of Medicine-Applicable Actinium-225 from Thorium Targets Irradiated by Medium-Energy Protons. Solvent Extr. Ion Exch. 2014;32(5):468–477. [Google Scholar]
- 24.Morgenstern A. Bismuth-213 and actinium-225-generator performance and evolving therapeutic applications of two generator-derived alpha-emitting radioisotopes. Curr. Radiopharm. 2012;5(3):221–227. doi: 10.2174/1874471011205030221. [DOI] [PubMed] [Google Scholar]
- 25.International Atomic Energy Agency . Technicial Meeting on Alpha emitting radionuclides and radiopharmaceuticals for therapy. Technical report. Vienna: International Atomic Energy Agency; 2013. [Google Scholar]
- 26.Dilling J., Kreuken R., Merminga L., editors. ISAC and ARIEL: The TRIUMF Radioactive Beam Facilities and the Scientific Program. Springer; 2014. [Google Scholar]
- 27.Laxdal R.E., Morton A.C., Schaffer P. Radioactive Ion Beams and Radiopharmaceuticals. Rev. Accel. Sci. Technol. 2013;6:37–57. [Google Scholar]
- 28.Ziegler J.F., Ziegler M.D., Biersack J.P. SRIM- The stopping and range of ions in matter (2010). Nucl. Instrum. Methods Phys. Res. Sect. B. 2010;268(11):1818–1823. [Google Scholar]
- 29.dos Santos Augusto R., Buehler L., Lawson Z., Marzari S., Stachura M., Stora T. CERN-MEDICIS (Medical Isotopes Collected from ISOLDE): A New Facility. Appl. Sci. (Basel) 2014;4(2):256–281. [Google Scholar]
- 30.Buehler L., Prior J., Stora T. CERN MEDICIS to produce radioisotopes for health. CERN Cour. 2016;56(8):28–32. [Google Scholar]
- 31.Garcia F.H. Calculation of rates for radioactive isotope beam production at TRIUMF. 2016. [Google Scholar]
- 32.Forsber C.W., Lewic L.C. 1999.
- 33.IAEA . IAEA-TECDOC-1340: Manual for reactor produced radioisotopes. Vienna: Technical Report International Atomic Energy Agency; 2003. [Google Scholar]
- 34.Chadwick M.B., Herman M., Oblǒzinsky P., Dunn M.E., Danon Y., Kahler A.C., Smith D.L., Pritychenko B., Arbanas G., Arcilla R., Brewer R., Brown D.A., Capote R., Carlson A.D., Cho Y.S., Derrien H., Guber K., Hale G.M., Hoblit S., Holloway S., Johnson T.D., Kawano T., Kiedrowski B.C., Kim H., Kunieda S., Larson N.M., Leal L., Lestone J.P., Little R.C., McCutchan E.A., MacFarlane R.E., MacInnes M., Mattoon C.M., McKnight R.D., Mughabghab S.F., Nobre G.P.A., Palmiotti G., Palumbo A., Pigni M.T., Pronyaev V.G., Sayer R.O., Sonzogni A.A., Summers N.C., Talou P., Thompson I.J., Trkov A., Vogt R.L., van der Marck S.C., Wallner A., White M.C., Wiarda D., Young P.G. ENDF/B-VII.1 Nuclear Data for Science and Technology: Cross Sections, Covariances, Fission Product Yields and Decay Data. Nucl. Data Sheets (N.Y. N.Y.) 2011;112(12):2887–2996. [Google Scholar]
- 35.Stacey M.W. Nuclear Reactor Physics. 2nd ed. Weinheim, Germany: Wiley-VCH; 2007. [Google Scholar]
- 36.Hogle S., Boll R.A., Murphy K., Denton D., Owens A., Haverlock T.J., Garland M., Mirzadeh S. Reactor production of Thorium-229. Appl. Radiat. Isot. 2016;114:19–27. doi: 10.1016/j.apradiso.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Kuznetsov R.A., Butkalyuk P.S., Tarasov V.A., Baranov Y., Butkalyuk I.L., Romanov E.G., Kupriyanov V.N., Kazakova E.V. Yields of activation products in 226Ra irradiation in the high-flux SM reactor. Radiochemistry. 2007;54(4):383–387. [Google Scholar]
- 38.Melville G., Meriarty H., Metcalfe P., Knittel T., Allen B.J. Production of Ac-225 for cancer therapy by photon-induced transmutation of Ra-226. Appl. Radiat. Isot. 2007;65(9):1014–1022. doi: 10.1016/j.apradiso.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Vansant P.D. 2013. [Google Scholar]
- 40.Melville G., Allen B.J. Cyclotron and linac production of Ac-225. Appl. Radiat. Isot. 2009;67(4):549–555. doi: 10.1016/j.apradiso.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Lesrel J., Arianer J., Arianer M., Bajeat O., Buhour J-m., Byzl H., Carrey F., Chabot M., Corbin T., Croizet H., Curaudeau J., Doizon F., Ducourtieu M., Dufour J-m., Grialou D., Joly C., Kaminski M., Lefort H., Lesellier B., Magneney G., Mottet L., Planat C., Raynaud M., Richard Y., Said A., Semsoum A., Taquin F., Vogel C., Bienvenu G., Cayla J-n., Lal M.D. Commissioning of the Alto 50 MeV Electron Linac.; Proc. EPAC 2006; 2006. pp. 3–5. [Google Scholar]
- 42.Apostolidis C., Molinet R., McGinley J., Abbas K., Mollenbeck J., Morgenstern A. Cyclotron production of Ac-225 for targeted alpha therapy. Appl. Radiat. Isot. 2005;62(3):383–387. doi: 10.1016/j.apradiso.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Schaffer P., Bénard F., Bernstein A., Buckley K., Celler A., Cockburn N., Corsaut J., Dodd M., Economou C., Eriksson T., Frontera M., Hanemaayer V., Hook B., Klug J., Kovacs M., Prato F.S., McDiarmid S., Ruth T.J., Shanks C., Valliant J.F., Zeisler S., Zetterberg U., Zavodszky P.A. Direct Production of 99mTc via 100Mo(p,2n) on Small Medical Cyclotrons. Phys. Procedia. 2015;66:383–395. [Google Scholar]
- 44.Ferrari A., Sala P.R., Fassò A., Ranft J. FLUKA: a multi-particle transport code. Technical Report. Geneva: CERN; 2005. [Google Scholar]
- 45.Böhlen T.T., Cerutti F., Chin M.P.W., Fassò A., Ferrari A., Ortega P.G., Mairani A., Sala P.R., Smimov G., Vlachoudis V. The FLUKA Code: Developments and Challenges for High Energy and Medical Applications. Nucl. Data Sheets (N.Y. N.Y.) 2014;120:211–214. [Google Scholar]
- 46.ENDF Evaluated Nuclear Data File. https://www-nds.iaea.org/exfor/endf00a.htm
- 47.Jost C.U., Griswold J.R., Bruffey S.H., Mirzadeh S., Stracener D.W., Williams C.L. Measurement of cross sections for the 232Th(P,4n) 229Pa reaction at low proton energies. AIP Conf. Proc. 2013;1525:520–524. [Google Scholar]
- 48.IAEA . 1991. [Google Scholar]
- 49.IAEA . 1996. [Google Scholar]
- 50.Englert M., Krall L., Ewing R.C. Is nuclear fission a sustainable source of energy? MRS Bull. 2012;37(4):417–424. [Google Scholar]
- 51.Griswold J.R., Medvedev D.G., Engle J.W., Copping R., Fitzsimmons J.M., Radchenko V., Cooley J.C., Fassbender M.E., Denton D.L., Murphy K.E., Owens A.C., Bimbaum E.R., John K.D., Nortier F.M., Stracener D.W., Heilbron L.H. Mausner. L. F.; Mirzadeh, S. Large scale accelerator production of 225Ac: Effective cross sections for 78-192MeV protons incident on 232Th targets. Appl. Radiat. Isot. 2016;118:366–374. doi: 10.1016/j.apradiso.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 52.Engle J.W., Mashnik S.G., Weidner J.W., Wolfsberg L.E., Fassbender M.E., Jackman K., Couture A., Bitteker L.J., Ullman J.L., Gulley M.S., Pillai C., John K.D., Bimbaum E.R., Nortier F.M. Cross sections from proton irradiation of thorium at 800 MeV. Phys. Rev. C Nucl. Phys. 2013;88(1):014604. [Google Scholar]
- 53.Ermolaev S.V., Zhuikov B.L., Kokhanyuk V.M., Matshuko V.L., Kalmykov S.N., Aliev R.A., Tananev I.G., Myasoedev B.F. Production of actinium, thorium and radium isotopes from natural thorium irradiated with protons up to 141 MeV. Radiochim. Acta. 2012;100(4):223–229. [Google Scholar]
- 54.Filosofov D.V., Rakhimov A.V., Bozhikov G.A., Karaivanov D.V., Lebedev N.A., Norseev Y.V., Sadikov I.I. Isolation of radionuclides from thorium targets irradiated with 300-MeV protons. Radiochemistry. 2013;55(4):410–417. [Google Scholar]
- 55.Weidner J.W., Mashnik S.G., John K.D., Ballard B., Bimbaum E.R., Bitteker L.J., Couture A., Fassbender M.E., Goff G.S., Gritzo R., Hemez F.M., Runde W., Ullman J.L., Wolfsberg L.E., Nortier F.M. 225Ac and 223Ra production via 800 MeV proton irradiation of natural thorium targets. Appl. Radiat. Isot. 2012;70(11):2590–2595. doi: 10.1016/j.apradiso.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Weidner J.W., Mashnik S.G., John K.D., Hemez F., Ballard B., Bach H., Bimbaum E.R., Bitteker L.J., Couture A., Dry D., Fassbender M.E., Gulley M.S., Jackman K.R., Ullman J.L., Wolfsberg L.E., Nortier F.M. Proton-induced cross sections relevant to production of 225Ac and 223Ra in natural thorium targets below 200 MeV. Appl. Radiat. Isot. 2012;70(11):2602–2607. doi: 10.1016/j.apradiso.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Zhuikov B.L., Kalmykov S.N., Ermolaev S.V., Aliey R.A., Kokhanyuk V.M., Matshuko V.L., Taranaev I.G., Myasoedov B.F. Production of 225Ac and 223Ra br irradiation of Th with accelerated protons. Radiokhimiya. 2011;53(1):73–80. [Google Scholar]
- 58.Griswold J.R. 2016. [Google Scholar]
- 59.Robertson A.K.H., Ladouceur K., Nozar M., Moskven L., Ramogida C.F., D’Auria J., Sossi V., Schaffer P. 2016. [Google Scholar]
- 60.Experimental Nuclea Readction Data
- 61.Zielinska B., Apostolidis C., Bruchertseifer F., Morgenstern A. An Improved Method for the Production of Ac-225/Bi-213 from Th-229 for Targeted Alpha Therapy. Solvent Extr. Ion Exch. 2007;25(3):339–349. [Google Scholar]
- 62.Zhuikov B.L. Production of medical radionuclides in Russia: Status and future - a review. Appl. Radiat. Isot. 2014;84:48–56. doi: 10.1016/j.apradiso.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 63.Tsoupko-Sitnikov V., Norseey Y., Khalkin V. Generator of actinium-225. J. Radioanal. Nucl. Chem. 1996;205:75–83. [Google Scholar]
- 64.Guseva L.I., Dogadkin N.N. Development of a Tandem Generator System Bi for Repeated Production of Short-Lived α-Emitting Radionuclides. Radiochem. Original Russian Text. 2009;51(2):169–174. [Google Scholar]
- 65.Moskvin L.N., Tsaritsyna L.G. Isolation of actinium and radium from a thorium target irradiated by 600 MeV protons. Sov. At. Energy. 1968;24(4):475–476. [Google Scholar]
- 66.Ostapenko V., Vasiliev A., Lapshina E., Ermolaev S., Aliey R., Totskiy Y., Zhuikov B., Kalmykov S. Extraction chromatographic behavious of actinium and REE on DGA, Ln and TRU resins in nitric acid solutions. J. Radioanal. Nucl. Chem. 2015;306(3):707–711. [Google Scholar]
- 67.Vasiliev A.N., Ostapenko V.S., Lapshina E.V., Ermolaev S.V., Danilov S.S., Zhuikov B.L., Kalmykov S.L. Recovery of Ra-223 from natural thorium irradiated by protons. Acta. 2016;104(8):539–547. [Google Scholar]
- 68.Mastren T., Radchenko V., Ownes A., Copping R., Boll R., Griswold J.R., Mirzadeh S., Wyant L.E., Brugh M., Engle J.W., Nortier F.M., Bimbaum E.R., John K.D., Fassbender M.E. Simultaneous Separation of Actinium and Radium Isotopes from a Proton Irradiated Thorium Matrix. Sci. Rep. 2017;7(1):2–8. doi: 10.1038/s41598-017-08506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radchenko V., Mastren T., Meyer C.A.L., Ivanov A.S., Bryantsev V.S., Copping R., Denton D., Engle J.W., Griswold J.R., Murphy K., Wilson J.J., Owens A., Wyant L., Bimbaum E.R., Fitzsimmons J., Medvedev D., Culter C.S., Mausner L.F., Nortier M.F., John K.D., Mirzadeh S., Fassbender M.E. Radiometric evaluation of diglycolamide resins for the chromatographic separation of actinium from fission product lanthanides. Talanta. 2017;175:318–324. doi: 10.1016/j.talanta.2017.07.057. [DOI] [PubMed] [Google Scholar]
- 70.Radchenko V., Engle J.W., Wilson J.J., Maasen J.R., Nortier M.F., Bimbaum E.R., John K.D., Fassbender M.E. Formation cross-sections and chromatographic separation of protactinium isotopes formed in proton-irradiated thorium metal. Radiochimica. 2016;104(5):291–304. [Google Scholar]
- 71.Merminga L., Ames F., Baartman R., Bricault P., Bylinski Y., Chao Y.C., Dawson R., Kaltchev D., Koscielniak S., Laxdal R., Mammarella F., Marchetto M., Minor G., Mitra A., Rao Y.N., Trinczek M., Trudel A., Verzilov V., Zvyagintsev V. ariel: triumf’s advanced rare isotope laboratory. Proceedings of. 2011;IPAC2011:1917–1919. [Google Scholar]
- 72.Ramogida C.F., Orvig C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. (Camb.) 2013;49(42):4720–4739. doi: 10.1039/c3cc41554f. [DOI] [PubMed] [Google Scholar]
- 73.Price E.W., Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014;43(1):260–290. doi: 10.1039/c3cs60304k. [DOI] [PubMed] [Google Scholar]
- 74.Ferrier M.G., Batista E.R., Berg J.M., Bimbaum E.R., Cross J.N., Engle J.W., La Pierre H.S., Kozimor S.A., Pacheco J.S.L., Stein B.W., Chantal S., Steiber E., Wilson J.J. Spectroscopic and computational investigation of actinium coordination chemistry. Nat. Commun. 2016;7:1–8. doi: 10.1038/ncomms12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrier M.G., Stein B.W., Batista E.R., Berg J.M., Bimbaum E.R., Engle J.W., John K.D., Kozimor S.A., Pacheco J.S.L., Redman L. N Synthesis and Characterization of the Actinium Aquo Ion. ACS Cent. Sci. 2017;3(3):176–185. doi: 10.1021/acscentsci.6b00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shannon R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalogenides. Acta Crystallogr. 1976;A32:751–767. [Google Scholar]
- 77.Davis I.A., Glowienka K.A., Boll R.A., Deal K.A., Brechbiel M.W., Stabin M., Bochsler P.N., Mirzadeh S., Kennel S.J. Comparison of 225actinium chelates: Tissue distribution and radiotoxicity. Nucl. Med. Biol. 1999;26(5):581–589. doi: 10.1016/s0969-8051(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 78.Jaggi J.S., Kappel B.J., McDevitt M.R., Sgouros G., Flombaum C.D., Cabassa C., Scheinberg D.a. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005;65(11):4888–4895. doi: 10.1158/0008-5472.CAN-04-3096. [DOI] [PubMed] [Google Scholar]
- 79.R.M. McDevitt, M.R.; Ma, D.; Simon, J.; Frank, R.K.; Scheinberg, D.A. Design and synthesis of 225Ac radioimmunopharmaceuticals. Appl. Radiat. Isot. 2002;57(6):841–847. doi: 10.1016/s0969-8043(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 80.Deal K.A., Davis I.A., Mirzadeh S., Kennel S.J., Brechbiel M.W. Improved in vivo stability of actinium-225 macrocyclic complexes. J. Med. Chem. 1999;42(15):2989–2992. doi: 10.1021/jm990141f. [DOI] [PubMed] [Google Scholar]
- 81.Chappell L.L., Deal K.A., Dadachove E., Brechbiel M.W. Synthesis conjugation and radiolabeling of a novel bifunctional chelating agent for (225)Ac radioimmunotherapy applications. Bioconjug. Chem. 2000;11(4):510–519. doi: 10.1021/bc990153f. [DOI] [PubMed] [Google Scholar]
- 82.Antczak C., Jaggi J.S., LeFave C.V., Curcio M.J., McDevitt M.R., Scheinberg D.A. Influence of the linker on the biodistribution and catabolism of actinium-225 self-immolative tumor-targeted isotope generators. Bioconjug. Chem. 2006;17(6):1551–1560. doi: 10.1021/bc060156+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maguire W.F., McDevitt M.R., Smith-Jones P.M., Scheinberg D.A. Efficient 1-Step Radiolabeling of Monoclonal Antibodies to High Specific Activity with 225Ac for -Particle Radioimmunotherapy of Cancer. J. Nucl. Med. 2014;55(9):1492–1498. doi: 10.2967/jnumed.114.138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Essler M., Gӓrtner F.C., Neff F., Blechert B., Senekowitsch-Schmidtke R., Bruchertseifer F., Morgenstern A., Seidl C. Therapeutic efficacy and toxicity of 225Ac-labelled vs. 213Bi-labelled tumour-homing peptides in a preclinical mouse model of peritoneal carcinomatosis. Eur. J. Nucl. Med. Mol. Imaging. 2012;39(4):602–612. doi: 10.1007/s00259-011-2023-6. [DOI] [PubMed] [Google Scholar]
- 85.Graf F., Fahrer J., Maus S., Morgenstern A., Bruchertseifer F., Venkatachalam S., Fottner C., Weber M.M., Huelsenbeck J., Schrekenberger M., Kaina B., Miederer M. DNA double strand breaks as predictor of efficacy of the alpha-particle emitter Ac-225 and electron emitter Lu-177 for somatostatin receptor targeted radiotherapy. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pandya D.N., Hantgan R., Budzevich M.M., Kock N.D., Morse D.L., Batista I., Mintz A., Li K.C., Wadas T.W. Preliminary therapy evaluation of 225-Ac-DOTA-c (RGDyK) demonstrates that Cerenkov radiation derived from 225Ac daughter decay can be detected by optical imaging for in vivo tumor visualization. Theranostics. 2016;6(5):698–709. doi: 10.7150/thno.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McDevitt M.R., Ma D., Lai L.T., Simon J., Borchardt P., Frank R.K., Wu K., Pellegrini V., Curcio M.J., Miederer M., Bander N.H., Scheinberg D.A. Tumor Therapy with Targeted Atomic Nanogenerators. Science. 2001;294(5546):1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 88.Ballangrud, ͦA. M.; Yang, W. H.; Palm, S.; Enmon, R.; Borchardt, P.E.; Pellegrini, V.A.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Alpha-particle emitting atomic generator (actinium-225)-labelled trastuzumab (Herceptin) targeting of breast cancer spheroids: Efficacy versus HER2/neu expression. Clin. Cancer Res. 2004;10(13):4489–4497. doi: 10.1158/1078-0432.CCR-03-0800. [DOI] [PubMed] [Google Scholar]
- 89.2012 https://clinicaltrials.gov/
- 90.McLaughlin M.F., Woodward J., Boll R.A., Wall J.S., Rondinone A.J., Kennel S.J., Mirzadeh S., Robertson J.D. Gold Coated Lanthanide Phosphate Nanoparticles for Targeted Alpha Generator Radiotherapy. PLoS One. 2013;8(1):2–9. doi: 10.1371/journal.pone.0054531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bandekar A., Zhu C., Jindal R., Bruchertseifer F., Morgenstern A., Sofou S. Anti-Prostate-Specific Membrane Antigen Liposomes Loaded with Ac-225 for Potential Targetted Antivascular alpha-Particle Therapy of Cancer. J. Nucl. Med. 2014;55(1):107–114. doi: 10.2967/jnumed.113.125476. [DOI] [PubMed] [Google Scholar]
- 92.Wang G., de Kruijff R.M., Rol A., Thijssen L., Mendes E., Morgenstern A., Bruchertseifer F., Stuart M.C.A., Wolterbeek H.T., Denkova A.G. Retention studies of recoiling daughter nuclides of 225Ac in polymer vesicles. Appl. Radiat. Isot. 2014;85:45–53. doi: 10.1016/j.apradiso.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 93.Fitzsimmons J., Atcher R., Cutler C. Development of a prelabeling approach for a targeted nanochelator. J. Radioanal. Nucl. Chem. 2015;305(1):161–167. [Google Scholar]
- 94.Matson M.L., Villa C.H., Ananta J.S., Law J.J., Scheinberg D.A., Wilson L.J. Encapsulation of Alpha-Particle-Emitting 225Ac3+ Ions Within Carbon Nanotubes. J. Nucl. Med. 2015;56(6):897–900. doi: 10.2967/jnumed.115.158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu C., Bandekar A., Sempkowski M., Banerjee R.B., Pomper M.G., Bruchertseifer F., Morgenstern A., Sofou S. Nanoconjugation of PSMA-Targeting Ligands Enhances Perinuclear Localization and Improves Efficacy of Delivered Alpha-Particle Emitters against Tumor Endothelial Analogues. Mol. Cancer Ther. 2016;15(1):106–113. doi: 10.1158/1535-7163.MCT-15-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Swart J., Chan H.S., Goorden M.C., Morgenstern A., Bruchertseifer F., Beekman F.J., de Jong M., Konijnenberg M.W. Utilizing High-Energy γ-Photons for High-Resolution 213 Bi SPECT in Mice. J. Nucl. Med. 2016;57(3):486–492. doi: 10.2967/jnumed.115.157685. [DOI] [PubMed] [Google Scholar]
- 97.Robertson A.K.H., Ramogida C., Rodriguez-Rodriguez C., Blinder S., Kunz P., Sossi V., Schaffer P. Multi-isotope SPECT imaging of the 225Ac decay chain: feasibility studies. Phys. Med. Biol. 2017 doi: 10.1088/1361-6560/aa6a99. [DOI] [PubMed] [Google Scholar]
- 98.Cordier D., Forrer F., Bruchertseifer F., Morgenstern A., Apostolidis C., Good S., Müller-Brand J., Mӓcke H., Reubi J.C., Merlo A. Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213 Bi-DOTA-[Thi8, Met(O2)11]-substance P: a pilot trial. Eur. J. Nucl. Med. Mol. Imaging. 2010;37(7):1335–1344. doi: 10.1007/s00259-010-1385-5. [DOI] [PubMed] [Google Scholar]
- 99.Woodward J., Kennel S.J., Stuckey A., Osborne D., Wall J., Rondinone A.J., Standaert R.F., Mirzadeh S. LaPO4 nanoparticles doped with actinium-225 that partially sequester daughter radionuclide. Bioconjug. Chem. 2011;22(4):766–776. doi: 10.1021/bc100574f. [DOI] [PubMed] [Google Scholar]
- 100.Sgouros G., Hobbs R.F., Song H. Modelling and dosimetry for alpha-particle therapy. Curr. Radiopharm. 2011;4(3):261–265. doi: 10.2174/1874471011104030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sgouros G., Roeske J.C., McDevitt M.R., Palm S., Allen B.J., Fisher D.R., Brill A.B., Song H., Howell R.W., Akabani G. MIRD Pamphlet No. 22: Radiobiology and Dosimetry of Alpha-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010;51(2):311–328. doi: 10.2967/jnumed.108.058651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bӓck T., Jacobsson L. The alpha-camera: A quantitative digital autoradiography technique using a charge-coupled device for ex vivo high-resoultion bioimaging of alpha-particles. Nucl. Med. (Stuttg.) 2010;51(10):1616–1623. doi: 10.2967/jnumed.110.077578. [DOI] [PubMed] [Google Scholar]
- 103.Bӓck T., Chouin S., Lindegren S., Jensen H., Albertsson P., Palm S. Image-based small-scale 3D-dosimetry in targeted alpha therapy using voxel dose-point kernels and alpha camera imaging of serial tissue sections. In Soc. Nucl. Med. Annu. Meet. Abstr. 2015;55:50. [Google Scholar]
- 104.Chouin S., Lindegren S., Jensen H., Albertsson P., Bӓck T. Quantification of activity by alpha-camera imaging and small-scale dosimetry within ovarian carcinoma micrometastases treated with targeted alpha therapy. Q. J. Nucl. Med. Mol. Imaging. 2012;56(6):487–495. [PubMed] [Google Scholar]
- 105.Miller B.W., Gregory S.J., Fuller E.S., Barrett H.H., Barber H.B., Furenlid L.R. The iOID camera: An ionizing-radiation quantum imaging detector. Nucl. Instruments Methods Phys. 2014;767:146–152. doi: 10.1016/j.nima.2014.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller B.W., Frost S.H.L., Frayo S.L., Kenoyer A.L., Santos E., Jones J.C., Green D.J., Hamlin D.K., Wilbur D.S., Fisher D.R., Orozco J.J., Press O.W., Pagel J.M., Sandmaier B.M. Quantitative single particle digital autoradiography with α-particle emitters for targeted radionuclide therapy using the iOID camera. Med. Phys. 2015;42(7):4094–4105. doi: 10.1118/1.4921997. [DOI] [PMC free article] [PubMed] [Google Scholar]