Abstract
Background
In recent years, the incidence of carbapenem-resistant Enterobacteriaceae (CRE) infections has increased rapidly. Since the CRE strain is usually resistant to most of antimicrobial agents, patients with this infection are often accompanied by a high mortality. Therefore, it instigates a severe challenge the clinical management of infection. In this study, we study the in vitro and in vivo bactericidal activity of ceftazidime-avibactam administrated either alone or in combination with aztreonam against KPC or NDM carbapenemase-producing Klebsiella pneumoniae, and explore a new clinical therapeutic regimen for infections induced by their resistant strains.
Methods
The microdilution broth method was performed to analyze the minimal inhibitory concentration (MIC). The time-kill curve assay of ceftazidime-avibactam at various concentrations was conducted in 16 strains of KPC-2 and 1 strain of OXA-232 carbapenemase–producing Klebsiella pneumoniae. The in vitro synergistic bactericidal effect of ceftazidime-avibactam combined with aztreonam was determined by checkerboard assay on 28 strains of NDM and 2 strains of NDM coupled with KPC carbapenemase–producing Klebsiella pneumoniae. According to calculating grade, the drugs with synergistic bactericidal effect were selected as an inhibitory concentration index. The in vitro bactericidal tests of ceftazidime-avibactam combined with aztreonam were implemented on 12 strains among them. Effect of ceftazidime-avibactam antibiotic against KPC carbapenemase–producing K. pneumoniae strain Y8 Infection was performed in the mouse model.
Results
The time-kill assays revealed that ceftazidime-avibactam at various concentrations of 2MIC, 4MIC and 8MIC showed significant bactericidal efficiency to the resistant bacteria strains. However, in 28 strains of NDM and 2 strains of NDM coupled with KPC carbapenemase- producing Klebsiella pneumoniae, only 7 strains appeared the susceptibility to ceftazidime-avibactam treatment, MIC50 and MIC90 were 64 mg/L and 256 mg/L, respectively. Antimicrobial susceptibility testing of ceftazidime-avibactam combined with aztreonam disclosed the synergism of two drugs in 90% (27/30) strains, an additive efficiency in 3.3% (1/30) strains, and irrelevant effects in 6.6% (2/30) strains. No antagonism was found. The subsequent bactericidal tests also confirmed the results mentioned above. Therapeutic efficacy of Ceftazidime-Avibactam against K. pneumoniae strain Y8 infection in mouse indicated 70% of infection group mice died within 4 days, and all mice in this group died within 13 days. Bacterial load testing results showed that there was no significant difference in the amount of bacteria in the blood between the infected group and the treatment group. However, the spleen and liver of treatment group mice showed lower CFU counts, as compare with infected group, indicating that ceftazidime-avibactam has a significant effect on the bacteria and led to a certain therapeutic efficacy.
Conclusion
This study indicated ceftazidime-avibactam therapy occupied significant bactericidal effects against KPC-2 and OXA-232 carbapenemase-producing Klebsiella pneumoniae. While combined with aztreonam, the stronger synergistic bactericidal effects against NDM carbapenemase-producing Klebsiella pneumoniae were achieved.
Keywords: Klebsiella pneumoniae, Carbapenemase, Ceftazidime-avibactam, Aztreonam, Time-kill curve assay
Background
Carbapenems are considered the most effective antibacterial agents against infections caused by multi-drug resistant gram-negative bacillus in clinical practice. However, with the broad use of carbapenems, and the emergence and widespread of carbapenem-resistant Enterobacteriaceae (CRE), in particular carbapenem-resistant Klebsiella pneumoniae (CR-KP), the clinical anti-infection treatment faces a drug-free dilemma [1–4]. Previous studies have shown that the most important resistant mechanism of CR-KP to carbapenems is production of carbapenemases including class A KPC carbapenemases, class B metallo-β-lactamases and class D OXA-48 family carbapenemases. Avibactam is a newly developed novel β-lactamase inhibitor in recent years and can efficiently inhibit class A and class D carbapenemases. As an inhibitor, avibactam can restore the antibacterial activity of ceftazidime to CR-KP. Nevertheless, it has less antibacterial efficiency against CR-KP with metallo-β-lactamase [5–7]. Knowing that aztreonam is stable against hydrolysis by class B metallo-β-lactamases, we hypothesized that supplement of aztreonam to the ceftazidime-avibactam would enhance the activity by “protecting” aztreonam from the “attack” of KPC type carbapenemase. This study aims to explore a new therapeutic regimen by administration of ceftazidime-avibactam alone or combined with aztreonam against carbapenemase-producing Klebsiella pneumoniae.
Materials and methods
Strains
A total of 47 non-repeated clinical strains of carbapenemase-producing Klebsiella pneumoniae were collected from 9 hospitals in 9 cities in China. Of these, 16 strains were blaKPC-2 positive, 1 was blaOXA-232 positive, 28 were blaNDM positive, and 2 were blaKPC2 coupled with blaNDM positive Klebsiella pneumoniae. All strains were identified by mass spectrometry and the gene type of carbapenemases was analyzed by PCR amplification using primers previously described and DNA sequencing [8–10]. E. coli ATCC 25922 were used as quality control strain for antimicrobial susceptibility testing. One K. pneumoniae clinical strain Y8 was used for the Infection in the mouse model.
Antimicrobial susceptibility testing
The minimal inhibitory concentrations were determined by microbroth dilution according to Clinical and Laboratory Standards Institute (CLSI) guidelines [11]. The in vitro synergistic bactericidal effects of ceftazidime- avibactam combined with aztreonam against blaNDM-positive strains were determined by checkerboard assay referred to the published reports [12, 13]. The calculation and interpretation of fractional inhibitory concentration (FIC) was referred to the document standards [14]. FIC = MIC drug A / MIC drug A plus drug B + MICdrug B/ MIC drug A plus drug B. FIC ≤ 0.5 was considered as a synergistic effect, 0.5 < FIC ≤ 1 was considered as an additive effect, 1 < FIC ≤ 2 was considered as an irrelevant effect, and > 2 was considered as an antagonism.
Time-kill assay
According to the minimal inhibitory concentration (MIC) of ceftazidime-avibactam to blaKPC-2 or blaOXA-232 producers, the bactericidal effects of ceftazidime-avibactam at various concentrations of 0.5MIC, 1MIC, 2MIC, 4MIC and 8MIC were studied by time-kill assay. In line with the results of antimicrobial susceptibility testing of ceftazidime-avibactam combined with aztreonam, and following the methods recommended in literatures [15, 16], 10 Klebsiella pneumoniae strains with blaNDM and 2 Klebsiella pneumoniae strains with blaNDM coupled with blaKPC-2 were randomly selected for synergistic bactericidal effects. The operation procedure is briefly described as follows: Mueller-hinton broth containing 1 × 105 CFU/mL bacteria is mixed with single or combined antimicrobial agents incubated overnight with consecutive shacking at 35 °C in an atmospheric environment. Meanwhile, the same broth without antibiotics was served as a growth control. Broth samples were serially diluted at times of 0, 2, 4, 6, 8 and 10 h and smeared on a mueller-Hinton plate respectively. After overnight incubation at 35 °C, the colonies were counted. If the reduction of bacterial survival amount in sample treated with combined antibiotics was ≥2 log10 CFU/mL than those in the sample treated by single drug, it was considered to have a synergistic bactericidal effect.
Effect of ceftazidime-avibactam against K. pneumoniae strain Y8 infection in the mouse model
Six-week-old BALB/c mice (female) were bought from Shanghai Laboratory Animal Company (SLAC), China. Animal experiments were performed in accordance with the Animal Ethics Committee of Shanghai Jiao Tong University. Groups of 10 mice were infected with 2.5 × 106 CFU of strain Y8 via the intraperitoneal (ip) route. Then mice were treated with PBS (infection group) or ceftazidime-avibactam (treatment group) (0.375 mg/g of body weight in 0.1 ml PBS) by subcutaneous injection 4 h post infection and given every 8 h for 10 days. The survival rates of mice were measured at desired time point to assess the therapeutic efficacy of ceftazidime-avibactam. Survival curves were monitored for 15 days.
Bacterial load in the blood and tissues of mice
To measure the efficacy of this drug, the bacterial load was measured in the blood and tissues of mice. Groups of 8 mice were infected with 2.5 × 106 CFU of strain Y8 via the ip and then treated with PBS or ceftazidime-avibactam by subcutaneous injection. The antibiotic dosage and administration were same as the survival experiment mentioned above. At 3 days post infection (dpi), mice in treatment and infected group were euthanized, and the blood and tissues of mice were removed to determine the bacterial burden through bacterial dilution-plate method.
Results
Antimicrobial susceptibility testing
All 16 Klebsiella pneumoniae strains with blaKPC-2 were susceptible to ceftazidime-avibactam with MIC range for 4–8 mg/L. However, all of strains were resistant to ceftazidime with MIC50 of 32 mg/L and MIC90 of > 256 mg/L. The resistance rate of imipenem was 93.8% with MIC50 and MIC90 for 64 mg/L and 128 mg/L, respectively. The resistance rate of meropenem was 93.8% with MIC50 and MIC90 for 64 mg/L and 256 mg/L, respectively (Table 1). The MIC of ceftazidime-avibactam to one OXA-232 carbapenemase-producing Klebsiella pneumoniae was 2 mg/L. The resistance rate of ceftazidime-avibactam to 30 blaNDM (including NDM plus KPC-2) positive Klebsiella pneumoniae strains was 76.7% with MIC range for 0.5~ 256 mg/L, MIC50 and MIC90 for 64 mg/L and 256 mg/L, respectively. The MIC range of aztreonam was 8~ > 256 mg/L with MIC50 and MIC90 were 128 mg/L and > 256 mg/L, respectively. Ceftazidime-avibactam combined with aztreonam showed synergistic effects to 90% (27/30) of strains with blaNDM, 3.3% (1/30) showed additive effects and 6.6%(2/30) showed unrelated effects. No antagonism was found for ceftazidime-avibactam combined with aztreonam (Table 2).
Table 1.
Minimal inhibitory concentration (MIC) of ceftazidime-avibactam against KPC-2 or OXA-232 carbapenemase-producing Klebsiella pneumoniae
| Strain no. | Bacteria | β-lactamase | MIC (mg/L) | Associated β-lactamase | |||
|---|---|---|---|---|---|---|---|
| CAZ-AVI | CAZ | IPM | MEM | ||||
| R16- Hefei | K. pneumoniae | KPC-2 | 8 | 128 | 64 | 64 | CTX-M-14, SHV-11, DHA-1 |
| R18- Hefei | K. pneumoniae | KPC-2 | 4 | 128 | 8 | 16 | SHV-28, DHA-1 |
| R19- Hefei | K. pneumoniae | KPC-2 | 4 | 128 | 16 | 16 | SHV-12, DHA-1 |
| R31- Beijing | K. pneumoniae | KPC-2 | 8 | 128 | 64 | 256 | SHV-11, DHA-1 |
| R35- Beijing | K. pneumoniae | KPC-2 | 8 | 128 | 128 | 256 | SHV-11, DHA-1 |
| R39-Fuzhou | K. pneumoniae | KPC-2 | 8 | > 256 | 32 | 64 | CTX-M-14, SHV-12, DHA-1 |
| R42- Fuzhou | K. pneumoniae | KPC-2 | 8 | > 256 | 32 | 64 | CTX-M-14, SHV-12, DHA-1 |
| R44- Fuzhou | K. pneumoniae | KPC-2 | 8 | > 256 | 64 | 128 | CTX-M-14, SHV-12, DHA-1 |
| R46- Fuzhou | K. pneumoniae | KPC-2 | 8 | > 256 | 64 | 64 | SHV-12, DHA-1 |
| R52- Fuzhou | K. pneumoniae | KPC-2 | 8 | > 256 | 64 | 128 | CTX-M-14, SHV-12, DHA-1 |
| R53- Fuzhou | K. pneumoniae | KPC-2 | 8 | > 256 | 64 | 128 | CTX-M-14, SHV-12, DHA-1 |
| R59- Hangzhou | K. pneumoniae | KPC-2 | 8 | 32 | 8 | 16 | CTX-M-14, SHV-11, DHA-1 |
| R60- Hangzhou | K. pneumoniae | KPC-2 | 4 | 32 | 4 | 4 | CTX-M-14, SHV-11, DHA-1 |
| JSD-Shanghai | K. pneumoniae | KPC-2 | 8 | 128 | 128 | 512 | CTX-M-14, SHV-11, DHA-1 |
| WJQ-Shanghai | K. pneumoniae | KPC-2 | 8 | > 256 | 128 | 256 | CTX-M-14, SHV-11, DHA-1 |
| LDX-Shanghai | K. pneumoniae | KPC-2 | 8 | > 256 | 32 | 64 | CTX-M-55, SHV-31, DHA-1 |
| PED-Shanghai | K. pneumoniae | OXA-232 | 2 | > 32 | 1 | 4 | CTX-M-15, SHV-1 |
Note: CAZ-AVI Ceftazidime-avibactam, CAZ Ceftazidime, IPM Imipenem, MEM Meropenem
Table 2.
Results of MIC and antimicrobial susceptibility testing of ceftazidime-avibactam single dosing and combined with aztreonam against NDM + KPC-2 carbapenemase-producing Klebsiella pneumoniae in 30 strains
| Strain no. | Bacteria | β-lactamase | MIC(mg/L) single dosing | MIC(mg/L) Combined dosing | FIC value | Associated β-lactamase | ||
|---|---|---|---|---|---|---|---|---|
| ATM | CAZ-AVI | ATM | CAZ-AVI | ATM + CAZ-AVI | ||||
| R078 Anhui | K. pneumoniae | NDM | 256 | 32 | 8 | 1 | 0.06 | SHV-28, DHA-1, CTX-M-15 |
| R080 Hainan | K. pneumoniae | NDM | 128 | 4 | 32 | 0.25 | 0.31 | SHV-11, DHA-1, CTX-M-14 |
| R081 Hainan | K. pneumoniae | NDM | 1024 | 8 | 64 | 2 | 0.31 | SHV-11, DHA-1, CTX-M-14 |
| R082 Hainan | K. pneumoniae | NDM | 256 | 64 | 16 | 1 | 0.08 | SHV-11, DHA-1, CTX-M-15, CTX-M-14 |
| R083 Hainan | K. pneumoniae | NDM | 128 | 64 | 32 | 0.5 | 0.26 | SHV-12, DHA-1, CTX-M-15 |
| R084 Hainan | K. pneumoniae | NDM | 512 | 64 | 16 | 1 | 0.05 | SHV-12, DHA-1 |
| R085 Hainan | K. pneumoniae | NDM | 1024 | 128 | 32 | 2 | 0.05 | SHV-12, DHA-1, CTX-M-15 |
| R086 Hainan | K. pneumoniae | NDM | 128 | 64 | 16 | 0.5 | 0.13 | SHV-12, DHA-1, CTX-M-15 |
| R088 Hainan | K. pneumoniae | NDM | 32 | 2 | 4 | 0.5 | 0.38 | SHV-11, DHA-1, CTX-M-14 |
| R093 Hebei | K. pneumoniae | NDM | 32 | 64 | 4 | 0.5 | 0.13 | SHV1, DHA-1, CTX-M-14 |
| R094 Hebei | K. pneumoniae | NDM | 32 | 64 | 8 | 0.25 | 0.25 | SHV-12, DHA-1, CTX-M-14 |
| R095 Hebei | K. pneumoniae | NDM | 32 | 2 | 8 | 0.25 | 0.38 | SHV-12, DHA-1, CTX-M-15 |
| R096 Henan | K. pneumoniae | NDM | 16 | 64 | 16 | 0.5 | 1.01 | SHV1, DHA-1, CTX-M-15 |
| R097 Henan | K. pneumoniae | NDM | 8 | 256 | 4 | 128 | 1.00 | SHV1, DHA-1, CTX-M-14 |
| R098 Henan | K. pneumoniae | NDM | 256 | 64 | 32 | 1 | 0.14 | DHA-1 |
| R100 Shanxi | K. pneumoniae | NDM | 8 | 32 | 4 | 32 | 1.50 | SHV-78, DHA-1, CTX-M-14 |
| R101 Shanxi | K. pneumoniae | NDM | 256 | 128 | 16 | 1 | 0.07 | SHV-78, DHA-1, CTX-M-14 |
| R102 Shanxi | K. pneumoniae | NDM | 32 | 256 | 4 | 1 | 0.13 | SHV-78, DHA-1, CTX-M-14 |
| R103 Shanxi | K. pneumoniae | NDM | 128 | 64 | 32 | 0.25 | 0.25 | SHV1, DHA-1, CTX-M-15 |
| R106 Sanxi | K. pneumoniae | NDM | 128 | 64 | 8 | 1 | 0.08 | SHV-12, DHA-1 |
| R110 Sanxi | K. pneumoniae | NDM | 128 | 256 | 8 | 1 | 0.07 | SHV-12, DHA-1 |
| R113 Sanxi | K. pneumoniae | NDM | 128 | 64 | 16 | 0.5 | 0.13 | SHV-12, DHA-1 |
| R122 Tianjin | K. pneumoniae | NDM | 32 | 1 | 4 | 0.25 | 0.38 | SHV-12, DHA-1, CTX-M-14 |
| R126 Tianjin | K. pneumoniae | NDM | 512 | 64 | 16 | 1 | 0.05 | SHV-12, DHA-1, CTX-M-14 |
| R127 Tianjin | K. pneumoniae | NDM | 256 | 128 | 32 | 1 | 0.13 | SHV2, DHA-1 |
| R128 Zhejiang | K. pneumoniae | NDM | 128 | 256 | 8 | 2 | 0.07 | SHV1, DHA-1 |
| R129 Zhejiang | K. pneumoniae | NDM | 4 | 0.5 | 0.5 | 0.125 | 0.38 | SHV-12, DHA-1, CTX-M-15 |
| R136 Zhejiang | K. pneumoniae | NDM | 256 | 64 | 16 | 1 | 0.08 | SHV-12, DHA-1 |
| R148 Tianjing | K. pneumoniae | KPC-2,NDM | 2048 | 8 | 256 | 2 | 0.38 | SHV-12, DHA-1, CTX-M-14 |
| R153 Henan | K. pneumoniae | KPC-2,NDM | 2048 | 128 | 128 | 8 | 0.13 | SHV-12, DHA-1 |
Time-kill assay
The results of time-kill assays showed that all blaKPC-2 or blaOXA-232 positive K. pneumoniae strains rebounded to grow 4 to 6 h at 0.5 MIC of ceftazidime-avibactam. At the concentration of 1 MIC ceftazidime-avibactam, 23.5% (4/17) of strains declined stably 2 h after dosing and no colonies were detected at 24 h, however, 76.5% (13/17) of strains rebounded to grow in 4–6 h. At the concentrations of 2MIC, 4MIC or 8MIC of ceftazidime-avibactam, it showed a significant bactericidal effectiveness for either blaKPC or blaOXA-232 positive K. pneumoniae and the colony growth was undetected after 24 h incubation for most of strains (Fig. 1 and Fig. 2). For blaNDM positive K. pneumoniae, ceftazidime-avibactam combined with aztreonam showed a significant bactericidal effectiveness and the colony growth was undetected after 10 h incubation (Fig. 3).
Fig. 1.
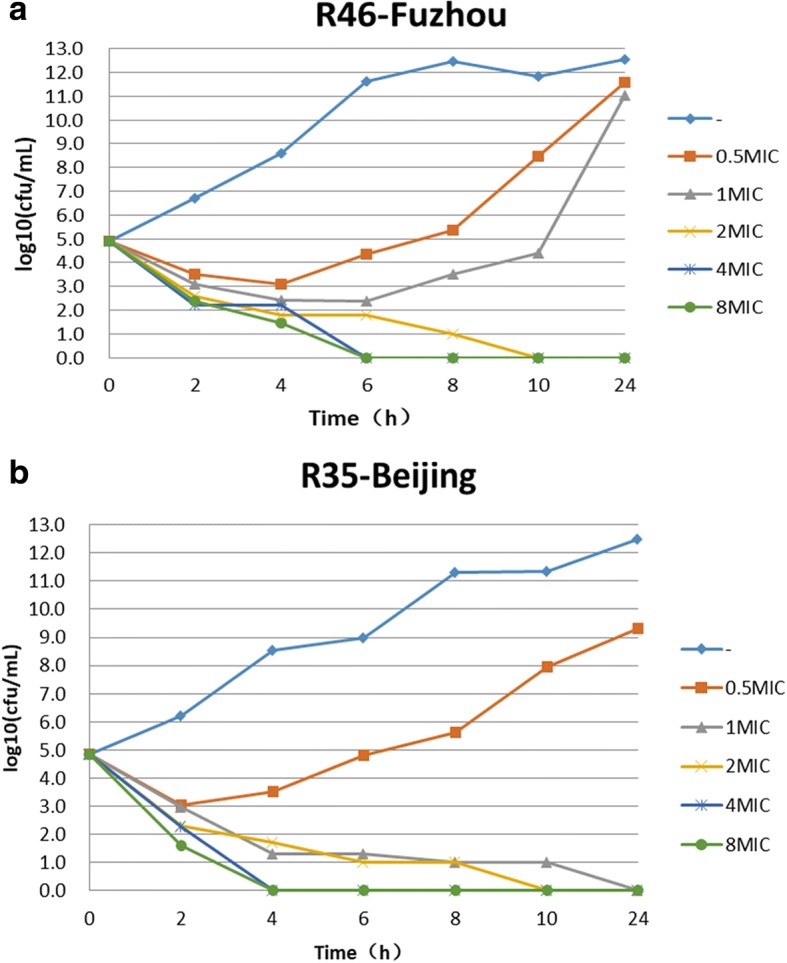
Bactericidal curve plots of ceftazidime-avibactam at various concentrations against KPC-2 carbapenemase-producing Klebsiella pneumoniae
Fig. 2.
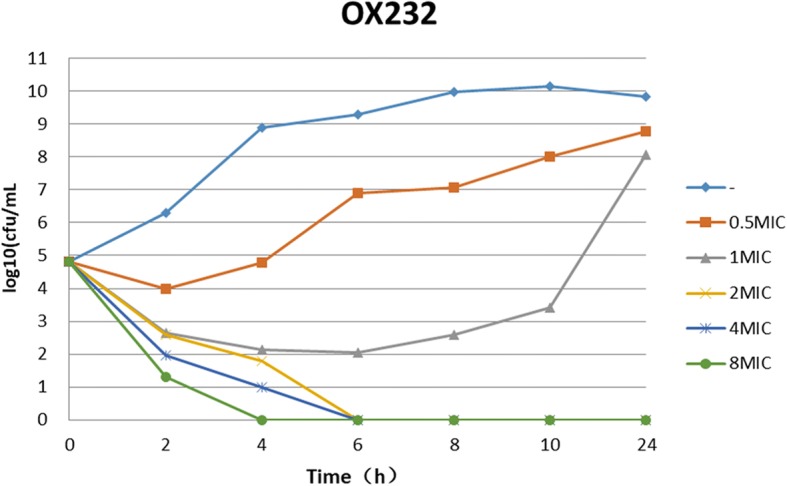
Bactericidal curve plots of ceftazidime-avibactam at various concentrations against OXA-232 carbapenemase-producing Klebsiella pneumoniae
Fig. 3.
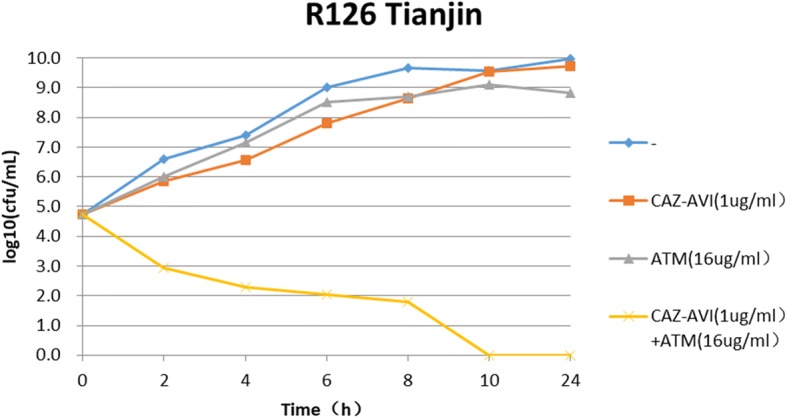
Bactericidal curve plots of ceftazidime-avibactam combined with aztreonam against NDM carbapenemase-producing Klebsiella pneumoniae
Therapeutic efficacy of ceftazidime-avibactam against K. pneumoniae strain Y8 infection in mouse
Mice were infected with 2.5 × 106 CFU of strain Y8 and treated with PBS or ceftazidime-avibactam for 10 days. 70% of infection group mice died within 4 days, and all mice in this group died within 13 days (Fig. 4). All treatment group mice survived at 10 dpi with the antibiotic applied every 8 h, whereas 100% of mice in this group died within 4 days after the antibiotic treatment stopped (Fig. 4).
Fig. 4.
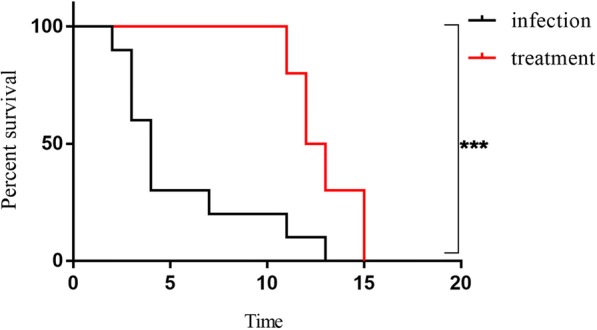
Therapeutic efficacy of ceftazidime-avibactam against K. pneumoniae strain Y8 infection in mouse. Mice were infected with 2.5 × 106 CFU of strain Y8 via the ip route and then treated with PBS or ceftazidime-avibactam by subcutaneous injection. Their survival was assessed daily for 15 days (n = 10). ∗P < 0.05; ∗∗∗P < 0.001
Bacterial load in the blood and tissues of mice
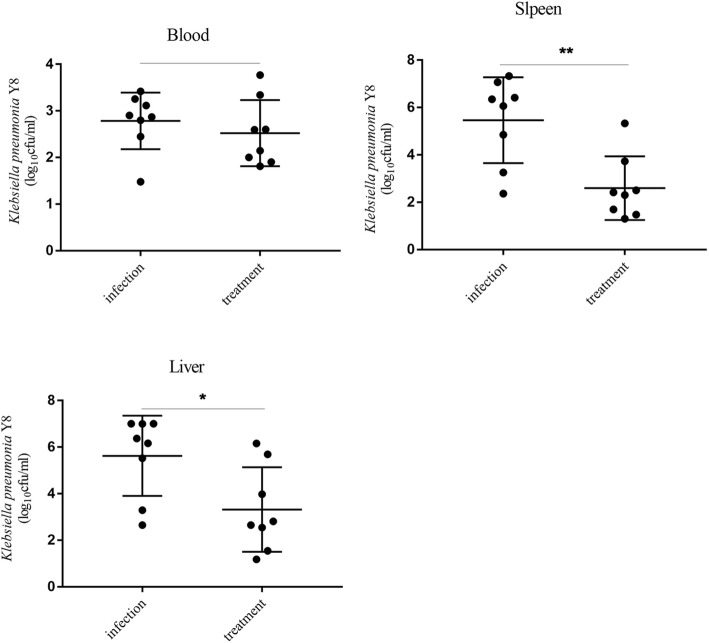
Mice were infected with 2.5 × 106 CFU of strain Y8 via the ip and then treated with PBS or ceftazidime-avibactam. The viable bacteria were quantified in blood, liver and spleen at 3 dpi. The results showed that there was no significant difference in the amount of bacteria in the blood between the infected group and the treatment group. However, the spleen and liver of treatment group mice showed lower CFU counts, as compare with that of infected group, indicating that the antibiotic has significant effect on the bacteria and ceftazidime-avibactam led to a certain therapeutic efficacy (Fig. 5).
Fig. 5.
Bacterial burden in blood, spleen and liver of mice at 3 days post infection (dpi). Mice were infected with 2.5 × 106 CFU of strain Y8 via the ip route and then treated with PBS or ceftazidime-avibactam by subcutaneous injection. At 3dpi, the viable bacteria of blood, spleen and liver was determined by plating serial dilutions on agar plates (n = 8). ∗P < 0.05; ∗∗P < 0.01
Discussion
In the recent decade, the prevalence and dissemination of CR-KP has posed a serious challenge in healthcare facilities in the world. The data from the Centers for Disease Control, USA disclosed the infection incidence of CR-KP increased from 1.2% in 2001 to 4.2% in 2011. In annual of 140,000 cases with Enterobacteriaceae infections, 9300 cases (6.6%) were infected by these multidrug-resistant bacteria [17]. According to CHINET surveillance data, the resistance rate of Klebsiella pneumoniae to carbapenems were significantly increasing from 3% in 2005 to 20% in 2017 [18]. Due to lack of effective antibacterial agents, infections due to CR-KP especially for hypervirulent Klebsiella pneumoniae usually accompany with high mortality [19, 20]. Carbapenemases are the major resistance mechanism of Klebsiella pneumoniae to carbapenems. Currently, the common carbapenemase among K. pneumoniae clinical strains include Ambler class A, Ambler class B and Ambler class D (eg. blaOXA-48, and blaOXA-232). blaKPC-2 is the most common carbapenemase in class A enzymes which can hydrolyze almost all of β-lactam antibiotics. blaNDM is the most common carbapenemase in class B metallo-β-lactamase which can hydrolyze all β-lactam antibiotics except aztreonam. Previous studies have shown the CR-KP strains isolated from children in China mainly produce blaNDM [21]. blaOXA-48 carbapenemase are predominantly existent in Klebsiella pneumoniae isolated in Tokyo and Europe [22]. In China, blaOXA-181 and blaOXA-232 have also been detected among Klebsiella pneumoniae clinical strains [23, 24].
Since CRE are usually extensively drug resistant, infections due to CRE are often associated with a high mortality. Therefore, a serious challenge of anti-infection therapy for CRE has been raised in clinical practice. Studies have revealed that compared to use carbapenems antibiotics alone, in the combination of carbapenems with other antibacterial agents such as tigecycline or polymyxin [25–28], amikacin [29] or fosfomycin [30] can considerably improve the outcomes of patient with CR-KP infection. Laurent adopted a combined pharmacotherapy regimen of dual carbapenems to provide a new approach in the treatment of CRE-induced infections [31]. As a novel β-lactam/β-lactamase inhibitor, studies have shown that ceftazidime-avibactam can successfully cure the infections due to Enterobacteriaceae with blaKPC [32–35]. Nevertheless, it is worth to be noticed that with the increasing application of ceftazidime-avibactam, the resistant strains and failed cases have been reported [6, 7, 36]. Our results showed that all of 16 blaKPC-2 positive and 1 of blaOXA-232 positive Klebsiella pneumoniae were susceptible to ceftazidime-avibactam with MIC50 and MIC90 for both 8 mg/L. Time-kill assays demonstrated that it showed a significant bactericidal effectiveness for either blaKPC or blaOXA-232 positive K. pneumoniae at the concentrations of 2MIC, 4MIC or 8MIC of ceftazidime-avibactam. 1 and Fig. 2). At 1 MIC of ceftazidime-avibactam, most of K. pneumoniae strains started to regrowth within 4–6 h. These results mean that the dosage of ceftazidime-avibactam is very important in the treatment of infections caused by blaKPC or blaOXA-232 positive K. pneumoniae [37].
In this study, 76.7% of blaNDM positive Klebsiella pneumoniae were resistance to ceftazidime-avibactam with MIC50 and MIC90 for 64 mg/L and 256 mg/L, respectively. Because avibactam can not inhibit the activity of metallo-β-lactamase, ceftazidime-avibactam monotherapy was ineffective for infections caused by blaNDM-1 positive Klebsiella pneumoniae [38]. According to the results of time-kill assay, ceftazidime-avibactam combined with aztreonam is necessary for the treatment of infection due to blaNDM positive K. pneumoniae based on the characteristics of weak hydrolysis capacity of metallo-β-lactamases to aztreonam. Eric Wenzler et al. also demonstrated that the combination of ceftazidime-avibactam and aztreonam had a synergistic bactericidal effect against class B metallo-β-lactamase-producing gram-negative bacteria [39]. Simultaneously, Benjamin Davido et al. reported that two cases infected either with metallo-β-lactamase-producing Klebsiella pneumoniae or Pseudomonas aeruginosa were successfully cured by combined administration of ceftazidime-avibactam and aztreonam [40]. The results of antimicrobial susceptibility testing indicated 90% (27/30) strains showed a synergistic effect for ceftazidime-avibactam combined with aztreonam. After combined with aztreonam, the MICs of ceftazidime-avibactam for 27 strains were reduced 4–256 times than ceftazidime-avibactam alone and all of them were susceptible to ceftazidime-avibactam with MIC ≤8 mg/L. Subsequently, the bactericidal curve tests performed on 12 strains of Klebsiella pneumoniae with a synergistic effect were also shown the consistent synergistic bactericidal effects. The reduction of bacterial colonies number was >2 log10 CFU/ mL compared with monotherapy and no colonies were detected after 24 h. In addition to strain of R148 showing the synergism 8 h after combined therapy, three blaNDM positive Klebsiella pneumoniae clinical strains of R96, R97 and R100 did not demonstrate the synergistic effects after combined use with aztreonam, implying the other mechanisms of drug resistance may exist and require the further investigation in the future.
Conclusions
In summary, as a compound preparation of novel enzyme inhibitor, ceftazidime-avibactam possesses visible advantages in the treatment of class A and class D type carbapenemase–producing Klebsiella pneumoniae clinical isolates. In addition, if combined with aztreonam, it can also play a synergetic bactericidal effects against infections caused by blaNDM positive Klebsiella pneumoniae clinical strains.
Acknowledgements
We thank all the laboratories for contributing data to this analysis.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81871690).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAZ
Ceftazidime
- CAZ-AVI
Ceftazidime-avibactam
- CRE
Carbapenem-resistant Enterobacteriaceae
- CR-KP
Carbapenem-resistant Klebsiella pneumoniae
- FIC
Fractional inhibitory concentration
- IPM
Imipenem
- MEM
Meropenem
- MIC
Minimal inhibitory concentration
Authors’ contributions
PH, and FPH designed the study. WXZ, YG, JYL and YYZ performed the experimental work. YY, DD, and DMZ collected the data. FPH analysed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by Huashan hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenxia Zhang, Email: aishrimp@126.com.
Yan Guo, Email: guoguoxy98@sina.com.
Jiayin Li, Email: 56583518@qq.com.
Yiyuan Zhang, Email: ericzyy@tom.com.
Yang Yang, Email: yangyang_hs@fudan.edu.cn.
Dong Dong, Email: dong10163298@163.com.
Demei Zhu, Email: zhu_demei@163.com.
Ping He, Email: hpatsh@sjtu.edu.cn.
Fupin Hu, Email: hufupin@fudan.edu.cn.
References
- 1.Ducomble T, Faucheux S, Helbig U, Kaisers UX, Konig B, Knaust A, Lubbert C, Moller I, Rodloff AC, Schweickert B, Eckmanns T. Large hospital outbreak of KPC-2-producing Klebsiella pneumoniae: investigating mortality and the impact of screening for KPC-2 with polymerase chain reaction. J Hosp Infect. 2015;89:179–185. doi: 10.1016/j.jhin.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Kim JO, Song SA, Yoon EJ, Shin JH, Lee H, Jeong SH, Lee K. Outbreak of KPC-2-producing Enterobacteriaceae caused by clonal dissemination of Klebsiella pneumoniae ST307 carrying an IncX3-type plasmid harboring a truncated Tn4401a. Diagn Microbiol Infect Dis. 2017;87:343–348. doi: 10.1016/j.diagmicrobio.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Ye L, Guo L, Zhao Q, Chen R, Luo Y, Chen Y, Tian S, Zhao J, Shen D, Han L. A nosocomial outbreak of KPC-2-producing Klebsiella pneumoniae in a Chinese hospital: dissemination of ST11 and emergence of ST37, ST392 and ST395. Clin Microbiol Infect. 2013;19:E509–E515. doi: 10.1111/1469-0691.12275. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Yu J, Chen F, Yu J, Simner P, Tamma P, Liu Y, Shen L. Emergence and establishment of KPC-2-producing ST11 Klebsiella pneumoniae in a general hospital in Shanghai, China. Eur J Clin Microbiol Infect Dis. 2018;37:293–299. doi: 10.1007/s10096-017-3131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zasowski EJ, Rybak JM, Rybak MJ. The beta-lactams strike Back: ceftazidime-avibactam. Pharmacotherapy. 2015;35:755–770. doi: 10.1002/phar.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields Ryan K., Nguyen M. Hong, Chen Liang, Press Ellen G., Kreiswirth Barry N., Clancy Cornelius J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrobial Agents and Chemotherapy. 2018;62(5):e02497–17. doi: 10.1128/AAC.02497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of Carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 2017;61(3):e02097–e02016. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Sun L, Ding B, Yang Y, Xu X, Liu W, Zhu D, Yang F, Zhang H, Hu F. Outbreak of NDM-1-producing Klebsiella pneumoniae ST76 and ST37 isolates in neonates. Eur J Clin Microbiol Infect Dis. 2016;35(4):611–618. doi: 10.1007/s10096-016-2578-z. [DOI] [PubMed] [Google Scholar]
- 9.Woodford N, Fagan EJ, Ellington MJ. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum (beta)-lactamases. J Antimicrob Chemother. 2006;57(1):154–155. doi: 10.1093/jac/dki412. [DOI] [PubMed] [Google Scholar]
- 10.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing[S]: Twenty-seventh Informational Supplement. PA: CLSI; 2017.
- 12.Bercot B, Poirel L, Dortet L, Nordmann P. In vitro evaluation of antibiotic synergy for NDM-1-producing Enterobacteriaceae. J Antimicrob Chemother. 2011;66:2295–2297. doi: 10.1093/jac/dkr296. [DOI] [PubMed] [Google Scholar]
- 13.Elemam A, Rahimian J, Doymaz M. In vitro evaluation of antibiotic synergy for polymyxin B-resistant carbapenemase-producing Klebsiella pneumoniae. J Clin Microbiol. 2010;48:3558–3562. doi: 10.1128/JCM.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2003;47:905–909. doi: 10.1128/AAC.47.3.905-909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paevskii SA. A means for determining the bactericidal activity of the tissues during the treatment of orthopedic patients by transosseous osteosynthesis methods. Klin Lab Diagn. 1993:25–9. [PubMed]
- 16.Norden CW, Wentzel H, Keleti E. Comparison of techniques for measurement of in vitro antibiotic synergism. J Infect Dis. 1979;140:629–633. doi: 10.1093/infdis/140.4.629. [DOI] [PubMed] [Google Scholar]
- 17.US Centers for Disease Control and Prevention. Antibiotic Resistance Theats in the United States. 2013. https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 18.Hu FP, Guo Y, Zhu DM, Wang F, Jiang XF, Xu YC, Zhang XJ, Zhang CX, Ji P, Xie Y, Kang M, Wang CQ, Wang AM, Xu YH, Shen JL, Sun ZY, Chen ZJ, Ni YX, Sun JY, Chu YZ, Tian SF, Hu ZD, Li J, Yu YS, Lin J, Shan B, Du Y, Han Y, Guo S, Wei LH, Wu L, Zhang H, Kong J, Hu YJ, Ai XM, Zhuo C, Su DH, Yang Q, Jia B, Huang W. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect. 2016;22(Suppl 1):S9–14. doi: 10.1016/j.cmi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Chew KL, Lin RTP, Teo JWP. Klebsiella pneumoniae in Singapore: Hypervirulent infections and the Carbapenemase threat. Front Cell Infect Microbiol. 2017;7:515. doi: 10.3389/fcimb.2017.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler PP, Volling C, Green K, Uleryk EM, Shah PS, McGeer A. Carbapenem resistance, initial antibiotic therapy, and mortality in Klebsiella pneumoniae bacteremia: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2017;38:1319–1328. doi: 10.1017/ice.2017.197. [DOI] [PubMed] [Google Scholar]
- 21.Javed H, Ejaz H, Zafar A, Rathore AW, Ikram ul H. Metallo-beta-lactamase producing Escherichia coli and Klebsiella pneumoniae: a rising threat for hospitalized children. J Pak Med Assoc. 2016;66:1068–1072. [PubMed] [Google Scholar]
- 22.Ma L, Wang JT, Wu TL, Siu LK, Chuang YC, Lin JC, Lu MC, Lu PL. Emergence of OXA-48-producing Klebsiella pneumoniae in Taiwan. PLoS One. 2015;10:e0139152. doi: 10.1371/journal.pone.0139152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Feng Y, Wu W, Xie Y, Wang X, Zhang X, Chen X, Zong Z. First report of OXA-181-producing Escherichia coli in China and characterization of the isolate using whole-genome sequencing. Antimicrob Agents Chemother. 2015;59(8):5022–5025. doi: 10.1128/AAC.00442-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin D, Dong D, Li K, Zhang L, Liang J, Yang Y, Wu N, Bao Y, Wang C. Hu F. clonal dissemination of OXA-232 Carbapenemase-producing Klebsiella pneumoniae in neonates. Antimicrob Agents Chemother. 2017;61(8):e00385–e00317. doi: 10.1128/AAC.00385-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect. 2014;20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Li P, Yin Y, Li F, Zhang Q. In vitro activity of tigecycline in combination with rifampin, doripenem or ceftazidime against carbapenem-resistant Klebsiella pneumoniae bloodstream isolates. J Antibiot (Tokyo) 2017;70:193–195. doi: 10.1038/ja.2016.93. [DOI] [PubMed] [Google Scholar]
- 28.Machuca I, Gutierrez-Gutierrez B, Gracia-Ahufinger I, Rivera Espinar F, Cano A, Guzman-Puche J, Perez-Nadales E, Natera C, Rodriguez M, Leon R, Caston JJ, Rodriguez-Lopez F, Rodriguez-Bano J, Torre-Cisneros J. Mortality associated with bacteremia due to Colistin-resistant Klebsiella pneumoniae with high-level Meropenem resistance: importance of combination therapy without Colistin and Carbapenems. Antimicrob Agents Chemother. 2017;61:e00406–17. [DOI] [PMC free article] [PubMed]
- 29.Hajjej Z, Gharsallah H, Naija H, Boutiba I, Labbene I, Ferjani M. Successful treatment of a Carbapenem-resistant Klebsiella pneumoniae carrying Bla OXA-48 , Bla VIM-2 , Bla CMY-2 and Bla SHV- with high dose combination of imipenem and amikacin. IDCases. 2016;4:10–12. doi: 10.1016/j.idcr.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albiero J, Sy SK, Mazucheli J, Caparroz-Assef SM, Costa BB, Alves JL, Gales AC, Tognim MC. Pharmacodynamic evaluation of the potential clinical utility of Fosfomycin and Meropenem in combination therapy against KPC-2-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2016;60:4128–4139. doi: 10.1128/AAC.03099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirel L, Kieffer N, Nordmann P. In vitro evaluation of dual carbapenem combinations against carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2016;71:156–161. doi: 10.1093/jac/dkv294. [DOI] [PubMed] [Google Scholar]
- 32.Gugliandolo A, Caio C, Mezzatesta ML, Rifici C, Bramanti P, Stefani S, Mazzon E. Successful ceftazidime-avibactam treatment of MDR-KPC-positive Klebsiella pneumoniae infection in a patient with traumatic brain injury: a case report. Medicine (Baltimore) 2017;96:e7664. doi: 10.1097/MD.0000000000007664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temkin E, Torre-Cisneros J, Beovic B, Benito N, Giannella M, Gilarranz R, Jeremiah C, Loeches B, Machuca I, Jimenez-Martin MJ, Martinez JA, Mora-Rillo M, Navas E, Osthoff M, Pozo JC, Ramos Ramos JC, Rodriguez M, Sanchez-Garcia M, Viale P, Wolff M, Carmeli Y. Ceftazidime-avibactam as salvage therapy for infections caused by Carbapenem-resistant organisms. Antimicrob Agents Chemother. 2017;61:e01964–16. [DOI] [PMC free article] [PubMed]
- 34.Holyk A, Belden V, Lee JJ, Musick W, Keul R, Britz GW, Lin J. Ceftazidime/avibactam use for carbapenem-resistant Klebsiella pneumoniae meningitis: a case report. J Antimicrob Chemother. 2018;73:254–256. doi: 10.1093/jac/dkx358. [DOI] [PubMed] [Google Scholar]
- 35.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, Kaye KS, Fowler VG, Jr., Paterson DL, Bonomo RA, Evans S, Antibacterial Resistance Leadership G. Colistin versus ceftazidime-avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66:163–71. [DOI] [PMC free article] [PubMed]
- 36.Barnes MD, Winkler ML, Taracila MA, Page MG, Desarbre E, Kreiswirth BN, Shields RK, Nguyen MH, Clancy C, Spellberg B, Papp-Wallace KM, Bonomo RA. Klebsiella pneumoniae Carbapenemase-2 (KPC-2), substitutions at ambler position Asp179, and resistance to ceftazidime-avibactam: unique antibiotic-resistant phenotypes emerge from beta-lactamase protein engineering. MBio. 2017;8:e00528–17. [DOI] [PMC free article] [PubMed]
- 37.Bensman TJ, Wang J, Jayne J, Fukushima L, Rao AP, D'Argenio DZ, Beringer PM. Pharmacokinetic-Pharmacodynamic target attainment analyses to determine optimal dosing of ceftazidime-avibactam for the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Antimicrob Agents Chemother. 2017;61:e00988–17. [DOI] [PMC free article] [PubMed]
- 38.Davido B, Senard O, de Truchis P, Salomon J, Dinh A. Monotherapy of ceftazidime-avibactam and ceftolozane-tazobactam: two effective antimicrobial agents against multidrug-resistant organisms except for NDM-1 isolates. Int J Infect Dis. 2017;62:124–125. doi: 10.1016/j.ijid.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Wenzler E, Deraedt MF, Harrington AT, Danizger LH. Synergistic activity of ceftazidime-avibactam and aztreonam against serine and metallo-beta-lactamase-producing gram-negative pathogens. Diagn Microbiol Infect Dis. 2017;88:352–354. doi: 10.1016/j.diagmicrobio.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Davido B, Fellous L, Lawrence C, Maxime V, Rottman M, Dinh A. Ceftazidime-avibactam and Aztreonam, an interesting strategy to overcome beta-lactam resistance conferred by Metallo-beta-lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61:e01008–17. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.