Abstract
Objectives:
The aim of this study was: (i) to formulate pit and fissure sealants (PFS) containing nano-hydroxyapatite (nHAP) filler; nHAP filler and silica co-filler; nHAP and nano-Amorphous Calcium Phosphate (nACP) co-filler, (ii) to evaluate physical properties; degree of conversion (DOC), curing depth (CD) and mechanical properties; microshear bond strength (MBS) of fortified PFS, and (iii) to assess remineralization potential and release of Ca2= and PO4 ions from newly synthesized sealants.
Materials and Methods:
Four PFS were prepared using monomers with mixture of 35.5 wt % BisGMA, 35.5 wt % triethylene glycol dimethacrylate and 28 wt % hydroxyethyl methacrylate. Bioactive nanofillers (nHAP and n-ACP) were added in various concentrations (0%–30%). Three commercial sealants were used as follows: unfilled (Clinpro; 3M ESPE), Fluoride releasing (Delton FS plus, Dentsply), ACP filled (Aegis, Bosworth). The samples (n = 35.5/gp) were tested for MBS, DOC, and CD. Remineralization potential was assessed by scanning electron microscopy (SEM). The concentrations of Ca2= and PO4 released from the sealant specimens were analyzed with Ultraviolet-visible Spectrophotometer. Data obtained was statistically analyzed (one-way analysis of variance, Tukey's test, P < 0.05).
Results:
10% hydroxyapatite (HAP) =20% ACP sealant showed significantly higher DOC. A remineralized region on the surface between fissure sealant and tooth enamel was observed by SEM in all three HAP filled bioactive sealants. Decreasing the solution pH significantly increased ion release from sealant filled with 10% nHAP = 20% nACP (P ≤ 0.001).
Conclusion:
Results suggested that admixture of nHAP and nACP to PFS showed remineralizing capability, without declining their mechanical and physical properties.
Keywords: Degree of conversion, depth of cure, hydroxyapatite, nanofiller, pit and fissure sealant, remineralization, shear bond strength
INTRODUCTION
An alarming increase in dental caries prevalence was seen in recent years on a global scale.[1] Caries is mainly considered a disease of pits and fissures. Manton and Messer[2] reported that pits and fissure caries constitute a greater proportion of coronal lesion compared to interproximal caries. Its early onset and high prevalence have recently been reviewed with new understanding and evolving evidence.[3] Highest caries experience is seen among individuals between the ages of 17 and 25 years. Pit and fissure caries account for 88% of total caries in children and adolescents.[4] There is plenty of evidence in the literature supporting the effectiveness of sealants in the prevention of pit and fissure caries.[5,6,7,8,9] Fissure sealant materials fall into two broad categories: resin-based sealants and glass ionomer sealants. Resin-based sealants are based on acrylic (methacrylate), may or may not contain filler particles or fluoride, and the setting reaction can be automatic (auto-polymerized) or light activated (light-polymerized).[10]
Sealant retention is critical to the effectiveness of resin-based sealants. Other concerns with their use are lesion progression under the sealed surface and fear of caries attack on the loss of sealant.[11,12] Therefore, consideration of the mechanical properties of fissure sealants is particularly important for their longevity, and there is a need for materials with the quintessential potential of remineralization. In the recent years, nano-sized hydroxyapatite (nHAP) and nano amorphous calcium phosphate (nACP) have been evaluated separately as a filler phase in bioactive polymeric composites.[13,14,15,16,17] The nanocomposites based on hydroxyapatite (HAP) filler were found to have the potential for stress-bearing restorations owing to their superior mechanical properties and demonstrated good bioactivity.[15] While ACP filled nanocomposites displayed a high level of Ca and PO4 release at a cariogenic pH4, when these ions would be most needed to inhibit caries. They also possessed flexural strength and an elastic modulus that matched or exceeded those of a commercial load-bearing composite.[17]
A 10% suspension of nHAP particles (10–20 nm diameter, 60–80 nm length) has been shown to be optimal for remineralization of early enamel caries.[18,19] nHAP has been used in toothpaste and pit and fissure sealants (PFS) for remineralization of incipient caries lesions in vitro.[20,21,22] Previous research has found that addition of ACP filler to fissure sealants makes the enamel more resistant to carious attack and leads to a reduction in secondary caries.[23,24] Nanoparticles having a higher surface to volume ratio are more effective than bigger filler particles as they have the potential to penetrate more in porosities beneath demineralized region as potential remineralizing substances. Owing to these convenient biologic properties of nHAP and n-ACP, it is desirable to benefit from these characteristics by adding them to repair materials like PFS. A literature search revealed no report on the synthesis of PFS containing both the bioactive nanofillers HAP and ACP. Therefore, the objective of the present study was:
To formulate PFS containing nHAP filler; nHAP filler and silica co-filler; nHAP and nACP co-filler
To evaluate and compare mechanical properties such as microshear bond strength (MBS) and physical properties like curing depth (CD) and degree of conversion (DOC), of fortified PFS with commercially available fluoride and ACP containing sealants
To evaluate the remineralization potential of fortified PFS.
MATERIALS AND METHODS
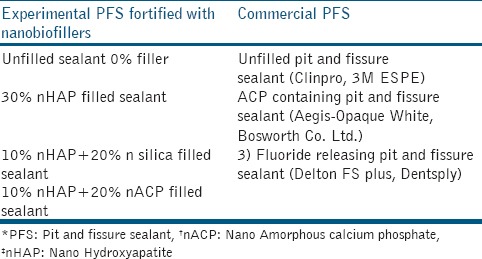
In the present pilot laboratory study, four PFS were prepared using monomers with a mixture of 35.5 wt % BisGMA, 35.5 wt % triethylene glycol dimethacrylate (TEGDMA) and 28 wt % hydroxyethyl methacrylate (HEMA). Bioactive nanofillers (nHAP and n-ACP) were added in various concentrations: (1) Unfilled 0% filler (2) 30% nHAP filler; (3) 10% nHAP + 20% silica filler; (4) 10% nHAP + 20% nACP filler. The total filler mass fraction was 30%. Commercially available ACP containing PFS (Aegis-Opaque White, Bosworth Co. Ltd.) which is 38% filled with ACP was used as another test material. Two commercial materials were used as comparative controls. Unfilled PFS served as a negative control (Clinpro, 3M ESPE). Fluoride-releasing PFS (Delton FS plus, Dentsply) which contains barium alumino fluoroboro silicate glass as filler served as positive control. This study comprised two control groups and five experimental groups. The commercially available base monomers, diluent monomers, and the polymerization initiator systems used to fabricate experimental resins are listed in Table 1. The various test materials used in the present study are shown inTable 2.
Table 1.
Composition of resin matrix used in all experimental pit and fissure sealants

Table 2.
Test materials
Synthesis and silanization of hydroxyapatite nanoparticles
Hydroxyapatite nanoparticles were synthesized by the wet chemical method. In a 2 L beaker, 500 ml of 0.4 mol diammonium hydrogen phosphate at pH-4.0 was vigorously stirred at room temperature. To this, 500 mL of 0.6 mol calcium nitrate tetrahydrate at pH = 7.4 was added drop-wise over a period of 4 h. The pH of the system was maintained at 10.8 by using 0.1 M sodium hydroxide throughout the stirring process, which continued overnight. A white precipitate that formed was vacuum dried and cleaned with distilled water and ethanol simultaneously for three or four times.
The prepared nHAP powder was characterized for phase composition, using X-ray diffractometer (X-ray diffraction [XRD], D8 Advance, Bruker Corporation). Powder XRD experiment was performed on a D8 Advance Diffractometer with Ni-filtered Cu K radiation (λ = 1.54 A°) generated at 40 KV and 40 mA. The particle size and morphology were studied using transmission electron microscope (TEM, CM 200, Philips) with an accelerating voltage of 200 kV.
1.5 g nHAP was silanized by stirring into 18.75 ml silane solution consisting of 4% mass methacryloyloxypropyltrimethoxy silane (MPS) and 2% mass n-propylamine in cyclohexane. (Sigma-Aldrich, Milwaukee, WI, USA). Stirring was done in magnetic stirrer for 60 min. The mixture was then heated to 90°C with continued stirring while still fluid until cyclohexane evaporated completely. The powder was heated to 120°C under vacuum for 2 h. After cooling under vacuum naturally to laboratory temperature (25°C ± 2°C), the mixture was washed with dichloromethane to remove residual MPS. The powder was filtered and redried at 120°C.
Two other fillers that were used to formulate the experimental sealants were purchased. Commercial ACP nanopowder (Aldrich, WI, USA) had an average particle size <150 nm as determined with Brunauer, Emmett and Teller (BET) surface area analyzer (SA 9600, Horiba Scientific, Germany). A commercial Silica nanopowder (Egoma Technologies Pvt., Ltd. Kanpur, India) was also used. The as-received silica nanopowder had an average particle size <100 nm as determined with BET surface area analyzer (SA 9600, Horiba Scientific, Germany).
Silanization of silica nanoparticles
0.6 g n-silica was silanized by stirring into 12 ml silane solution consisting of 0.06 g MPS and 0.012 g n-propylamine in 12 ml cyclohexane (Sigma-Aldrich, Milwaukee, WI, USA). Stirring was done at 30°C for 30 min, then kept at 60°C for another 30 min. The mixture was placed in a rotary evaporator at 60°C for the removal of the solvent and volatile by-products. The powder was heated at 100°C for 1 h in the rotary evaporator and finally dried at 80°C in a vacuum oven for 20 h.
Formulation and characterization of experimental resin-based Pit and fissure sealants
BisGMA 35.5 wt % was mixed with diluent comonomers TEGDMA 35.5 wt % and HEMA 28 wt % by hand spatulation. The initiator and co-initiator (CQ and EDMAB) were added into the monomer solution after covering the whole vial with an aluminum foil. The mixture was then stirred with magnetic stirrer (IKA, C-MAG HS7) for another 12 h in a dark ambiance. Silanized nHAP filler and n-silica filler were mixed into the matrix paste using sonicator (RK 106, Bandelin electronic) for 6 h and hand spatulation. nACP filler was not silanized and added into matrix paste as such. The sealant pastes thus formed were poured into appropriate plastic tubes and stored in a cool dry place.
Evaluation of mechanical and physical properties
Micro-shear bond strength test
To perform the MBS test, 35 healthy, nonhypoplastic, noncarious maxillary first premolars extracted for orthodontic reasons, were used. All the teeth were cleaned to remove blood debris, stains, and calculus and were divided into seven groups of five premolars each. The enamel surfaces were ground under running water with 600-grit silicon carbide paper (Phoenix Beta, Buehler, Germany) to create standardized flat surfaces. Each group of teeth was treated with 37% phosphoric acid etchant as per the manufacturer's instructions. Etching was done for 60 s, followed by rinsing with water for 20 s and air drying for 30 s till smooth chalky white surface was achieved. The middle segment of the buccal surface was chosen for the preparation of sealant core to standardize the site of bonding of specimen. A plastic tube of 0.8 mm internal diameter and 1 mm height was placed on the enamel surface. The respective sealants of each group were filled into the plastic mold; a clear cellophane sheet was placed over the resin sealant, pressed gently and photo-irradiated with light emitting diode for 40 s at power output of 1241 mW/cm2(Elipar FreeLight 2, 3M ESPE, USA). The plastic tubes were removed with a sharp blade, and the specimens were inspected under a light stereomicroscope at ×10 to discard those with any evident air bubbles or gaps at the interface. Specimens were then stored in distilled water (37°C/24 h) and were subjected for analysis in the Universal testing machine (Instron Microtensile Tester, 5848, Singapore). The samples were fixed on the mechanical jaw of the universal testing machine and 0.20-mm diameter stainless steel orthodontic wire was looped around the resin cylinder. Shear force was applied to each specimen at a crosshead speed of 1 mm/min until failure occurred. The load at which the sealant mold was debonded from the tooth surface was noted and the shear bond strength was calculated using the following formula: τ = 4 F/πd2
Where τ = Shear strength, F = Maximum force in breaking point, D = Sample diameter. The mode of fracture and morphology of all the samples were observed under a scanning electron microscope (SEM) (Zeiss EVO 50, Resolution 2.0 nm@ 30 kV, Acceleration Voltage 0.2–30 kV). The mode of fracture was either adhesive, cohesive, or a combination of both.
Measurement of depth of cure
The depth of cure was evaluated according to the ISO standards for dental resin 4049. Five specimens were made for each group by condensing the respective resin into a Teflon mould measuring 4 mm in diameter and 6 mm in depth. Mold was placed on the glass slab and was filled with resin sealant. Mylar strip was placed on top of the sealant. The specimens were light cured withlight emitting diode (LED) curing unit (Elipar FreeLight 2, 3M ESPE, USA) with an average power density output of 1241 mW/cm2 for 40 s. Specimens were removed from the mold, the soft uncured resin sealant was scraped using a plastic spatula. The height of each specimen was measured using a digital micrometer in three different areas and the average of the three measurements was recorded. The value was divided by two to obtain the ISO 4049 depth of cure.
Degree of conversion analysis
From the seven sealants four experimental and three commercial specimens (n = 5), 0.15 mm thick and 6 mm in diameter were prepared. A volume of 2 μ L of each sealant was collected with a micropipette and then compressed between two mylar strips and two glass slides to produce a thin film approximately 6 mm in diameter and 0.15 mm thick. Photo-activation was performed by positioning the light guide tip on the top surface of the glass slide using an LED light source for 40 s (Elipar Freelight2, 3M-ESPE, 1241 mW/cm2). Specimens were stored in dry, in dark containers, at 37°C (±1°C) for 24 h. The specimens were pulverized into a fine powder and were maintained in a dark room. 5 mg of the ground powder were thoroughly mixed with 100 mg of the KBr powder salt. This mixture was placed into a pelleting device, and then pressed in a press to obtain a pellet. The DOC was evaluated by Fourier transform infrared spectroscopy (FTIR). The spectrometer (Nicolet, Protege 460, Stifflers Surplus Inc. 2805 West Frye Road Chandler, AZ) was coupled to a horizontal attenuated total reflectance (ATR) device consisting of a diamond crystal of 2 mm in diameter (Thermo scientific). FTIR spectra for both uncured and cured specimens were analyzed. The measurements were recorded in the absorbance operating under the following conditions: 1/cm resolution and a 400–4000/cm wavelength. The percentage of unreacted carbon–carbon double bonds (% C = C) was determined from the ratio of the absorbance intensities of aliphatic C = C (peak at 1637/cm) against an internal standard aromatic C–C (peak at 1608/cm) before and after the curing of the specimen by means of following equation: DC% = 100 × (1−[Rcured/Runcured]).
Where R = peak at 1637/cm/peak at 1608/cm. Three readings were taken and the average was calculated.
Assessment of remineralization
A total of 35 healthy, nonhypoplastic, noncarious maxillary first premolars having occlusal surfaces with deep pits and fissures, were used. The selected teeth were cleaned of blood, saliva, calculus and other debris and treated with aqueous slurry of fine flour of pumice with the help of a rubber cup in a slow speed contra-angled handpiece. Before the placement of sealant, an acid-resistant varnish coating was applied on all the surfaces of the teeth except on the occlusal surface where sealant placement was done. The sample teeth were distributed at random into seven groups of five teeth each. The occlusal surface of the specimens was treated with 37% phosphoric acid gel (Dentsply) for 60 s; rinsed with distilled water for 20 s, and then dried with oil-free compressed air. After the enamel etching procedure, various sealants were applied to the fissure system following the manufacturer's instructions and polymerized using a light-cure unit for 40 s. Following sealant application, teeth in all the groups were subjected to the pH-cycling regimen (dynamic model) for a period of 10 days. Each cycle involved 3 h of demineralization twice a day with 2 h of remineralization in between. The purpose of pH-cycling was to simulate the drop of pH occurring in the oral environment every day. The temperature was kept at 37°C, by keeping all the groups with solutions in the incubator. After 10 days, the teeth were removed and prepared for SEM analysis. The root portions of teeth were cut using the diamond disc. The crowns were sectioned buccolingually to allow studying of the marginal topography of the fissure sealant and crystal changes along the depth of the fissure. The qualitative changes at the tooth surface and the sealant interface were observed under SEM (Zeiss EVO 50, Resolution 2.0 nm@ 30 kV, Acceleration Voltage 0.2–30 kV) under ×100, ×250, ×500, ×1000 and ×1500.
Calcium (Ca2+) and phosphate (PO4) ion release measurement
Two groups of materials were tested for Ca2+ and PO4 ion release: Experimental 10% nHAP + 20% nACP filled sealant and Commercial ACP containing PFS (Aegis-Opaque White, Bosworth Co. Ltd.). A total of 18 samples were made, 9 samples for each material. Teflon rings (5 mm inner diameter, 2 mm high) were filled with sealant materials and covered from both sides with Mylar strips and glass slides. Each sample was polymerized for 80 s (40 s each side) using LED light source (Freelight2, 3M-ESPE, 1241 mW/cm2). Three buffer solutions were prepared: 50 mmol/L lactic acid at pH 4: Dissolving 0.211 ml of lactic acid in 50 ml distilled water. 50 mmol/L acetic acid at pH 5.5: Dissolving 0.142 ml of acetic acid in 50 ml distilled water. 50 mmol/L HEPES at pH 7.4: Dissolving 0.6 g HEPES in 50 ml distilled water. 133 mmol/L Sodium Chloride solution was prepared and buffered to pH 4 with 50 mmol/L lactic acid, pH 5.5 with 50 mmol/L acetic acid and pH 7.4 with 50 mmol/L HEPES. The samples were kept dry at room temperature in a dark container for 24 h before the immersion of each sample in 8 mLof NaCl solution buffered to three different pH. For each solution, the concentrations of Ca2+ and PO4 released from the specimens were measured at 1, 7, 14, and 21 days. The dynamic approach was used in which, samples were consecutively transferred into a fresh solution after 1, 7, 14, and up to 21 days. At each time, aliquots of 2 mL were removed to analyze Ca2+ and PO4 concentrations via a spectrophotometric method according to established standards and calibration methods. The released ions were reported in cumulative concentrations.
Statistical analysis
Results of mechanical and physical properties were subjected to one-way analysis of variance (ANOVA), to compare the mean among the seven groups. Homogeneity of variance was tested with Levene's statistics and multiple comparisons Tukey's test was applied when homogeneity of variance assumption was satisfied. Dunnett T3 was applied if the unequal variance was there. Value of P < 0.05 was considered as statistically significant. For ion release experiment, two-way ANOVA was applied to compare the mean Ca2+ and PO4 concentration between the two groups at different pH level. Two factor repeated measures ANOVA (pH and time) was applied to test trend of Ca2+ and PO4 ion release at the different pH level within the two groups. Post hoc multiple comparison test was used to compare the measured data at a P ≤ 0.001.
RESULTS
Characterization of hydroxyapatite nanopowder
Figure 1 shows the XRD pattern of synthesized nHAP powder. The HAP nanopowder has the characteristic peaks at 2θ regions of 22°, 26°, 32°–34°, 48°–50°, which are consistent with the HAP phase. The structure and morphology of the samples were further confirmed by the TEM. The TEM analysis confirms the presence of the spherical shape morphology of the prepared hydroxyapatite nanoparticle with the particle size of 50 nm as shown in Figure 2.
Figure 1.
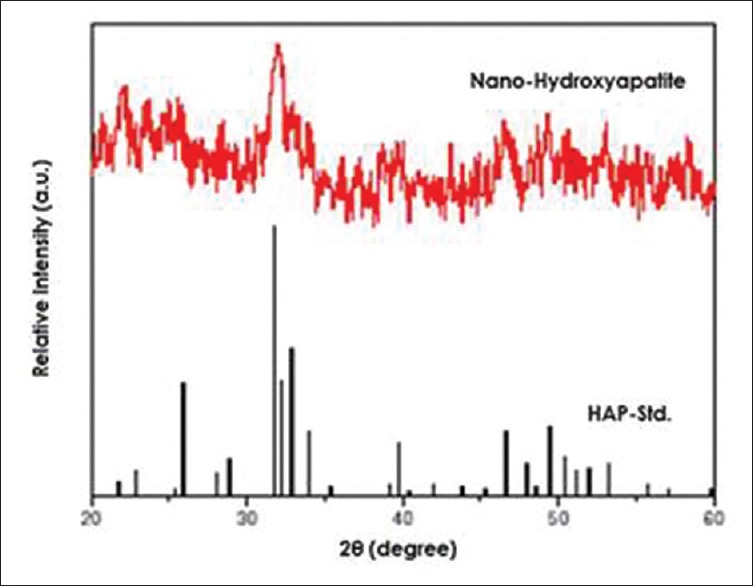
X-ray diffraction pattern of synthesized nano-hydroxyapatite powder
Figure 2.
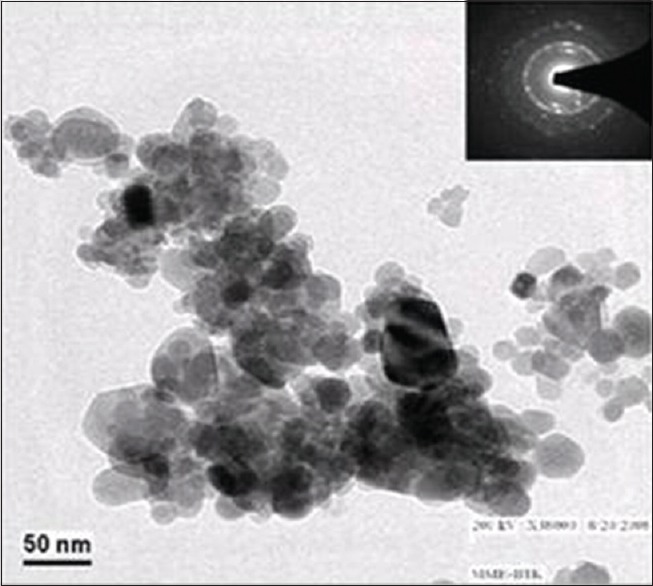
Transmission electron microscopy image of prepared hydroxyapatite nanoparticle
Mechanical properties of resin sealants (microshear bond strength)
The results of MBS test of all the resin sealants (experimental and control) are summarized in Figure 3. The average MBS values did not show a statistically significant difference (P > 0.05) between the commercial unfilled (Clinpro, 3M ESPE) fissure sealant (15.72 Mpa) and the prepared unfilled fissure sealant groups (15.35 MPa) (P > 0.05, P = 1.000). The lowest mean MBS was found in 10% HAP + 20% Silica group (5.48 ± 1.82 MPa) which was statistically significantly lower from other groups. Mean MBS of 10% HAP + 20% ACP sealant (14.04 MPa) was higher than 30% HAP sealant (11.93 MPa), but the difference was not statistically significant. There was no statistically significant difference in MBS of 10% HAP + 20% ACP and commercial sealants (Clinpro, Aegis, Delton FS-Plus).
Figure 3.
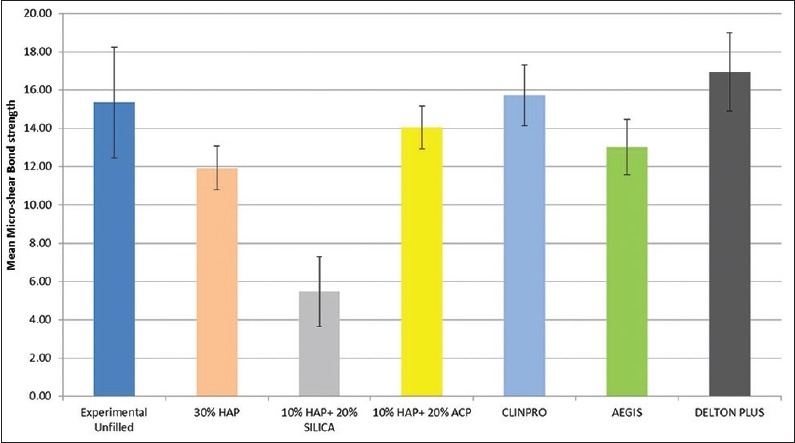
Mean microshear bond strength of experimental and commercial sealant groups
Physical properties of resin sealants (Depth of cure and degree of conversion)
The resin sealant's physical properties are plotted in Figures 4 and 5. There was no statistically significant difference (P > 0.05) in the average CD values between the commercial unfilled (Clinpro, 3M ESPE) fissure sealant (2.836 ± 0.05 mm) and the prepared unfilled fissure sealant groups (2.756 ± 0.07) (P > 0.05, P = 0.622). 10% HAP + 20% Silica group and 10% HAP + 20% ACP group showed significantly lower mean CDs than other groups with 10% HAP + 20% Silica (1.840 ± 0.38 mm) being the lowest. The mean CD of 10% HAP + 20% ACP (2.688 ± 0.07 mm) was not statistically significant from other commercial sealants except Aegis (P < 0.05, P = 0.036).
Figure 4.
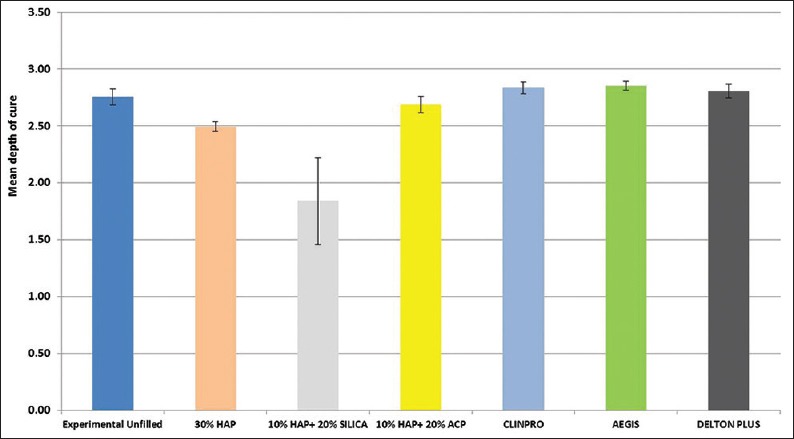
Mean depth of cure of experimental and commercial sealant groups
Figure 5.
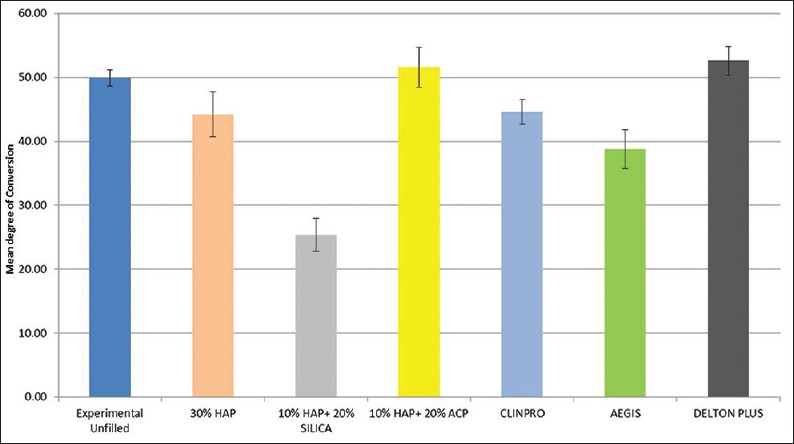
Mean degree of conversion of experimental and commercial sealant groups
The results of ANOVA test showed significantly higher DOC of experimental unfilled sealant group (49.90% ± 1.28%) compared to commercial unfilled (Clinpro, 3M ESPE) fissure sealant (44.56% ± 1.92%), (P < 0.05, P = 0.044). 10% HAP + 20% ACP sealant showed significantly higher DOC (51.62 ± 3.12%) than other two experimental sealant groups (10% HAP + 20% silica, P = 0.000), (30% HAP, P = 0.002) and two commercial sealant groups (Clinpro, P = 0.004), (Aegis, P = 0.000). The DOC of 10% HAP + 20% silica sealant (25.41 ± 2.54%) was significantly lower than all other groups.
SEM examination showed [Figure 6], a homogenous remineralized region (white zone) at the tooth surface sealant interface in all experimental sealant groups containing HAP nanofiller which was more remarkable in 30% nHAP filled sealant. Aegis and Delton FS plus showed a white irregular granular or globular zone in few areas of the tooth surface sealant interface. No such zone was observed in commercial unfilled (Clinpro, 3M ESPE) fissure sealant and the prepared unfilled fissure sealant groups.
Figure 6.
Interface between tooth and Sealant in scanning electron microscopy at ×1000, ×500, and ×250, respectively (a) 30% hydroxyapatite filled sealant; (b) aegis; (c) experimental unfilled sealant
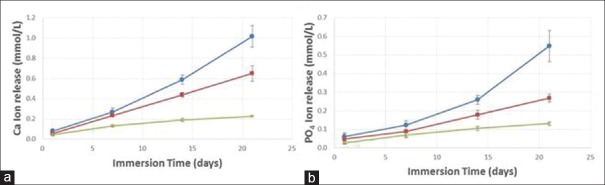
Results of Ca2+ and PO4 ion release from Group 1; experimental sealant, which was filled with 10% nHAP and 20% nACP are depicted in Figure 7a and b. Two-way ANOVA showed significant effects of solution pH and immersion time, with a significant interaction between the two variables (P ≤ 0.001). Decreasing the solution pH increased the ion release. The Ca2+ concentration at 21 days was (1.02 ± 0.108) mmol/L at pH of 4, significantly higher than (0.65 ± 0.077) mmol/L at pH of 5.5, and (0.23 ± 0.009) mmol/L at pH of 7.4 (P < 0.001). The PO4 was (0.55 ± 0.085) mmol/L at pH of 4, higher than (0.27 ± 0.021) mmol/L at pH of 5.5, and (0.13 ± 0.010) mmol/L at pH of 7.4 (P < 0.001).
Figure 7.
(a) Ca2= ion release from group 1; experimental sealant, which was filled with 10% nano-hydroxyapatite and 20% nano-Amorphous Calcium Phosphate, (b) PO4 ion release from group 1; experimental sealant, which was filled with 10% nano-hydroxyapatite and 20% nano-amorphous calcium phosphate
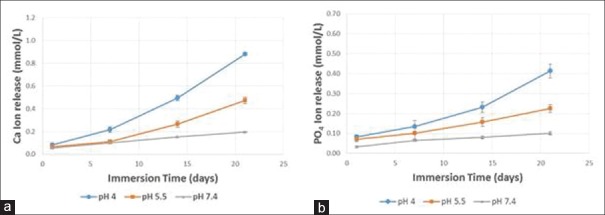
Results of Ca2+ and PO4 release for Group 2: commercial sealant with ACP is depicted in Figure 8a and b. Decreasing the solution pH increased the ion release. The Ca2+ concentration at 21 days was (0.88 ± 0.012) mmol/L at pH of 4, significantly higher than (0.47 ± 0.028) mmol/L at pH of 5.5, and 0.20 ± 0.004) mmol/L at pH of 7.4 (P < 0.001). The PO4 was (0.41 ± 0.035) mmol/L at pH of 4, higher than (0.23 ± 0.020) mmol/L at pH of 5.5, and (0.10 ± 0.007) mmol/L at pH of 7.4 (P < 0.001). The amount of Ca2+ release from the experimental sealant filled with 10% nHAP + 20% nACP was significantly higher than the corresponding release from Group 2 sealant (Aegis) at pH of 4 and 5.5. The amount of PO4 release from the experimental sealant filled with 10% nHAP + 20% nACP was significantly higher than the corresponding release from Group 2 sealant (Aegis) at ph 4.
Figure 8.
(a) Ca2= ion release for Group 2: commercial sealant with amorphous calcium phosphate, (b) PO4 ion release for Group 2: commercial sealant with amorphous calcium phosphate
DISCUSSION
A nanotechnology is a promising approach for the development of materials with better properties and anticaries potential. Reduction of the particle size from the micrometer scale to the nanometer scale and controlling their properties regarding shape and distribution have provided very bioactive calcium and phosphates, which probably have the potential to penetrate more in porosities beneath demineralized region as potential remineralizing substances.[25] The addition of such nanobiofillers to PFS is a new approach in tooth enamel remineralization. Combination of biocompatible reinforcing filler (nHAP) and ion releasing filler with sustained remineralization effect (n-ACP) in sealants would be a boon in preventive dentistry.
Amorphous calcium phosphate (ACP), as a bioactive nanofiller embedded in polymerized methacrylate matrices, responds to changes in the oral environment by releasing calcium and phosphate ions in a sustained manner, which can potentially migrate into the tooth structure to form apatite, thus counteracting the recurrent decay that frequently develops at tooth/conventional sealant interfaces.[23,26]
The addition of HA powders to restorative dental materials for remineralization effects and improvement of mechanical properties have been investigated due to its excellent biocompatibility and bioactivity. Recent research found nHAP containing toothpastes and mouthwashes effective in remineralizing primary carious lesions.[27] The incorporation of nanostructured hydroxyapatite in PFS was proposed by Haghgoo et al.[22] who found a reduction in the development of secondary caries. In this study, HAP and ACP nanobiofillers were admixed to make new PFS. The influence of their addition to resin sealant's mechanical and physical properties and remineralization potential was assessed. The resin system used was BisGMA as a base monomer and TEGDMA as diluents comonomer. HEMA was added as another co-monomer with a potential for adhesion.[23]
Microshear bond strength
In this research, microshear test was used to assess the bond strength, which is an easy, reliable and common method for measurement of composite material bond to dental structures. The simple test protocol of the microshear test allows for straightforward specimen preparation, without the need for sectioning procedures, which may induce early microcracking, to obtain specimens.[28,29] The results of the current study show that incorporation of nHAP and n-ACP into fissure sealant did not affect the bond strength of the sealant to enamel. There was no statistically significant difference in the mean MBS of 10% HAP +20% ACP sealant (14.04 MPa) and commercial control sealants (Clinpro 15.72MPa; Aegis 13.02MPa; Delton FS Plus 16.95 MPa). It was also higher than 30% HAP sealant (11.93 MPa), but the difference was not statistically significant. This improved adhesion strength is probably related to the similarity of nHAP to tooth components which facilitate its infiltration into the micropores of the tooth surface. Additionally, availability of a higher surface area for bonding to tooth structure due to the incorporation of nanoscale filler particles might have contributed to high bond strength. Nanofillers can improve adhesion at the interface between the restorative material and the tooth structure by providing structural reinforcement and functioning as a stress absorbing layer between dental composite and enamel. However, the amount of nanofiller and distribution of particles are the critical parameters which should be optimized in experiments. Park et al.[21] found that adding hydroxyapatite particles in various concentrations (1%, 5%, 10%, 15%, and 20%) to fissure sealant lead to an increase in bond strength to enamel compared to the control group. Similar results were obtained in Haghgoo et al.[22] study where nanoparticles of hydroxyapatite (1%, 3%, 5%, 10%, and 15%) were added to fissure sealant. Kim et al.[30] found that addition of 15 wt% nHAP to light cure glass ionomer leads to an increase in bond strength to dentine. In the present experiment, the exception was the sealant with 10% HAP + 20% Silica nanofillers which had statistically lower mean MBS (5.48 ± 1.82 MPa) than all other groups. This effect might be due to the tendency of silica to agglomeration. The agglomerated particles were identified as strength controlling flaws which initiated a crack and consequently lead to the fracture.
Depth of cure
The depth of cure is used as a simple method, to approximate the degree of cure of dental resins.[31] It was determined according to the ISO 4049 standardized technique. The depth of cure of composite resin is affected by the amount of light that reaches the photoinitiator. The penetration of light beam in filled systems is determined by the transparency of the matrix resin and fillers toward the irradiation wavelength and the difference between the refractive indices of the matrix and the filler. Hydroxyapatite is opaque against visible light hence when added to resin system affects curing reaction. Another limiting factor for depth of cure is light scattering that is related to filler particle size. Nanofillers maximize light scattering owing to their reduced size which is about half the wavelength emitted by the curing device. All the experimental filled sealants (30% HAP, 10% HAP +20% Silica and 10% HAP +20% ACP) in our study showed low CD values compared to commercial control groups although higher than minimum requirement for ISO 4049 standard which could be attributed to the nanoscale size of filler particles and opacity of Hydroxyapatite. Thirty percent HAP sealant of the present study showed the lower depth of cure than 10% HAP +20% ACP sealant which might be due to a higher concentration of hydroxyapatite in the former sealant. The previous investigation in which fissure sealants containing varying concentration of hydroxyapatite was formulated, showed that the CD of the fissure sealant was decreased slightly with increasing HA content.[21] Similar findings were also reported by Haghgoo et al.[22] and they suggest this due to the accumulation of nano-opaque particles in high thickness of the substance. A dramatic decrease in depth of cure was also seen with the increase in hydroxyapatite content in dental adhesives which was thought to be due to the opacity of hydroxyapatite nanorods.[32] In another experiment, in which light cure glass ionomer cement (GIC) was prepared by addition of hydroxyapatite particles both micrometer and nanometer scale, low CD were attained compared to GIC without hydroxyapatite incorporation.[30] The authors relate it to the light scattering effect of HAP particles.[33]
Degree of conversion
The dDOC is an important parameter in determining the physical, mechanical and biological properties of photo-activated composite resins.[34] Fourier Transform Infra-red Spectroscopy is one of the most widely used techniques for measurement of DOC in dental composites.[35,36] Several factors can influence the DOC such as light source used, power density, wavelength, irradiation time, light-tip size, photo-activation method, distribution, the quantity of inorganic fillers, the type and quantity of the photoinitiator, and color also strongly affect the DOC of the composite resins.[35] In this study, the addition of nHAP and n-ACP fillers to resin sealants did not influence the DOC when compared to other commercial sealants used in the study. 10% HAP + 20% ACP sealant showed significantly higher DOC than other two experimental sealant groups (10% HAP + 20% silica, P = 0.000), (30% n-HAP, P = 0.002) and two commercial sealant groups (Clinpro, P = 0.004), (Aegis, P = 0.000). Uncontrolled aggregation of silica nanofillers and their uneven distribution within polymer matrices adversely affected the DOC of this sealant group.
Remineralization assessment
In SEM evaluation, the qualitative changes at the tooth surface and sealant interface were examined, and presence of white zone at the interface was considered positive for remineralization.[21] A homogenous remineralized region (white zone) at the tooth surface sealant interface was observed in all experimental sealant groups containing HAP nanofiller which was more remarkable in 30% nHAP filled sealant. Aegis and Delton FS plus showed a white irregular granular or globular zone in few areas of the tooth surface sealant interface. No such intermediate layer was observed in samples of commercial unfilled (Clinpro, 3M ESPE) fissure sealant and the prepared unfilled fissure sealant groups.
A similar observation under the SEM was made by Park et al.,[21] They found that the white granular zone was more clearly visible, as the amount of Hydroxyapatite was increased. It was considered a remineralization zone as there was no such pattern seen in the control group. Such intermediate precipitate of nHAP particles was also seen in SEM evaluation by Haghoo et al.[22] The thickness of this interstitial layer was highest in sealant containing the maximum amount of HAP nanoparticles (15 wt %). Chaudhary et al.[37] evaluated the remineralization potential of Amorphous Calcium Phosphate (ACP) and Fluoride containing PFS using SEM and observed a white zone at the tooth surface-sealant interface.
Calcium (Ca) and phosphate (P) ion release
Both the experimental and commercial ACP containing sealants were “smart” in that they dramatically increased the Ca2+ and PO4 ion release when the pH was reduced from neutral to a cariogenic pH of 4. In the oral cavity, a local plaque pH of above 6 is the safe zone, pH of 6.0–5.5 is potentially cariogenic, and pH of 5.5–4 is the cariogenic or danger zone.[17] There was a 4–5-fold increase in ion release as the pH was lowered from 7.4–4, when these ions are most needed for caries inhibition. Ion release from resin-based materials containing calcium-phosphates is influenced by the percentage of bioactive particles in the material,[38] surface area of filler particles,[17] pH of the immersion medium,[39] and also to the hydrophilicity of the resin matrix.[40] The results of the present study are in agreement with the previous studies which have also found an increase in calcium and phosphate ion release from nanocomposites and adhesives containing nano ACP fillers when pH of the immersion solution was decreased.[17,41] Moreover in the present study, dynamic approach was used, where the solution was replenished with the same volume of fresh solvent at each time interval to simulate the oral environment. The higher amount of ion release seen in experimental sealant compared to commercial sealant Aegis might be due to the size difference of ACP particles which is not nanoscale in Aegis sealant. The PFS synthesized in the present experiment containing 10% nHAP and 20% nACP would serve as an effective remineralizing agent in cariogenic situations.
CONCLUSION
The synergistic use of ion releasing nanofillers and bioactive reinforcing fillers yielded PFS with improved mechanical properties and caries-inhibiting capabilities
This is a unique combination, not available in current dental materials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This work was supported in the form of contingency grant by Council of Scientific and Industrial Research, Human Resource Development group, CSIR Complex, Library Avenue, Pusa, New Delhi-110012. This study was presented as oral paper at I.A.D.R/A.A.D.R/C.A.D.R Annual General Meeting held in Boston, Massachusetts, USA, 11–14th March 2015.
REFERENCES
- 1.Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. Am J Dent. 2009;22:3–8. [PubMed] [Google Scholar]
- 2.Manton DJ, Messer LB. Pit and fissure sealants: Another major cornerstone in preventive dentistry. Aust Dent J. 1995;40:22–9. doi: 10.1111/j.1834-7819.1995.tb05608.x. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho JC. Caries process on occlusal surfaces: Evolving evidence and understanding. Caries Res. 2014;48:339–46. doi: 10.1159/000356307. [DOI] [PubMed] [Google Scholar]
- 4.Demirci M, Tuncer S, Yuceokur AA. Prevalence of caries on individual tooth surfaces and its distribution by age and gender in university clinic patients. Eur J Dent. 2010;4:270–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Llodra JC, Bravo M, Delgado-Rodriguez M, Baca P, Galvez R. Factors influencing the effectiveness of sealants – A meta-analysis. Community Dent Oral Epidemiol. 1993;21:261–8. doi: 10.1111/j.1600-0528.1993.tb00771.x. [DOI] [PubMed] [Google Scholar]
- 6.Mejàre I, Lingström P, Petersson LG, Holm AK, Twetman S, Källestål C, et al. Caries-preventive effect of fissure sealants: A systematic review. Acta Odontol Scand. 2003;61:321–30. doi: 10.1080/00016350310007581. [DOI] [PubMed] [Google Scholar]
- 7.Ahovuo-Saloranta A, Hiiri A, Nordblad A, Worthington H, Mäkelä M. Pit and fissure sealants for preventing dental decay in the permanent teeth of children and adolescents. Cochrane Database Syst Rev. 2004;3:CD001830. doi: 10.1002/14651858.CD001830.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Azarpazhooh A, Main PA. Pit and fissure sealants in the prevention of dental caries in children and adolescents: A systematic review. J Can Dent Assoc. 2008;74:171–7. [PubMed] [Google Scholar]
- 9.Ahovuo-Saloranta A, Hiiri A, Nordblad A, Mäkelä M, Worthington HV. Pit and fissure sealants for preventing dental decay in the permanent teeth of children and adolescents. Cochrane Database Syst Rev. 2013;3:CD001830. doi: 10.1002/14651858.CD001830.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Pardi V, Pereira AC, Ambrosano GM, Meneghim Mde C. Clinical evaluation of three different materials used as pit and fissure sealant: 24-months results. J Clin Pediatr Dent. 2005;29:133–7. doi: 10.17796/jcpd.29.2.e44h17387x324345. [DOI] [PubMed] [Google Scholar]
- 11.Beauchamp J, Caufield PW, Crall JJ, Donly K, Feigal R, Gooch B, et al. Evidence-based clinical recommendations for the use of pit-and-fissure sealants: A report of the American dental association council on scientific affairs. J Am Dent Assoc. 2008;139:257–68. doi: 10.14219/jada.archive.2008.0155. [DOI] [PubMed] [Google Scholar]
- 12.Simonsen RJ. Pit and fissure sealant: Review of the literature. Pediatr Dent. 2002;24:393–414. [PubMed] [Google Scholar]
- 13.Zhang H, Darvell BW. Mechanical properties of hydroxyapatite whisker-reinforced bis-GMA-based resin composites. Dent Mater. 2012;28:824–30. doi: 10.1016/j.dental.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Domingo C, Arcís RW, López-Macipe A, Osorio R, Rodríguez-Clemente R, Murtra J, et al. Dental composites reinforced with hydroxyapatite: Mechanical behavior and absorption/elution characteristics. J Biomed Mater Res. 2001;56:297–305. doi: 10.1002/1097-4636(200108)56:2<297::aid-jbm1098>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Liu WW, He XP, Mo AC, Yao QQ, Ye J, Jing N, et al. Investigation of the mechanical properties of a low-shrinkage liquid crystalline matrix combined with nano-hydroxyapatite. Int J Nanomedicine. 2011;6:1787–91. doi: 10.2147/IJN.S23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, et al. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28:561–72. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Gao S, Cheng L, Yu H. Combined effects of nano-hydroxyapatite and Galla Chinensis on remineralisation of initial enamel lesion in vitro. J Dent. 2010;38:811–9. doi: 10.1016/j.jdent.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Huang S, Gao S, Cheng L, Yu H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011;45:460–8. doi: 10.1159/000331207. [DOI] [PubMed] [Google Scholar]
- 20.Tschoppe P, Zandim DL, Martus P, Kielbassa AM. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Dent. 2011;39:430–7. doi: 10.1016/j.jdent.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Park SW, Dee YK, Kim YU. The effect of hydroxyapatite on the remineralization of dental fissure sealant. Key Eng Materials. 2005;17:284–6. [Google Scholar]
- 22.Haghgoo R, Ataie M, Hojjati ST, Kameli S, Imam SR. The effect of various amounts of nanohydroxyapatite on the mechanical properties and remineralization of a fissure sealant. J Dent Sch. 2012;30:184–91. [Google Scholar]
- 23.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75:1679–86. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 24.Silva KG, Pedrini D, Delbem AC, Ferreira L, Cannon M. In situ evaluation of the remineralizing capacity of pit and fissure sealants containing amorphous calcium phosphate and/or fluoride. Acta Odontol Scand. 2010;68:11–8. doi: 10.3109/00016350903260264. [DOI] [PubMed] [Google Scholar]
- 25.Chen MH. Update on dental nanocomposites. J Dent Res. 2010;89:549–60. doi: 10.1177/0022034510363765. [DOI] [PubMed] [Google Scholar]
- 26.Skrtic D, Antonucci JM, Eanes ED. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J Res Natl Inst Stand Technol. 2003;108:167–82. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4:034104. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]
- 28.El Zohairy AA, Saber MH, Abdalla AI, Feilzer AJ. Efficacy of microtensile versus microshear bond testing for evaluation of bond strength of dental adhesive systems to enamel. Dent Mater. 2010;26:848–54. doi: 10.1016/j.dental.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 29.McDonough WG, Antonucci JM, He J, Shimada Y, Chiang MY, Schumacher GE, et al. A microshear test to measure bond strengths of dentin-polymer interfaces. Biomaterials. 2002;23:3603–8. doi: 10.1016/s0142-9612(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Lee YK, Kim SO, Song JS, Choi BJ, Choi HJ. The effects of nano-sized hydroxyapatite on demineralization resistance and bonding strength in light-cured glass ionomer dental cement. J Korean Acad Pediatr Dent. 2010;37:24–34. [Google Scholar]
- 31.Guillot G, Nunes TG, Ruaud JP, Polido W. Aspects of the photopolymerization of a commercial dental resin studied by H-1 magnetic resonance imaging. Polymer. 2004;45:5525–32. [Google Scholar]
- 32.Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent Mater. 2010;26:471–82. doi: 10.1016/j.dental.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Arcís RW, López-Macipe A, Toledano M, Osorio E, Rodríguez-Clemente R, Murtra J, et al. Mechanical properties of visible light-cured resins reinforced with hydroxyapatite for dental restoration. Dent Mater. 2002;18:49–57. doi: 10.1016/s0109-5641(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 34.Costa SX, Galvao MR, Jacomass DP, Bernardi MI, Hernandes AC, Rastelli AN, et al. Continuous and gradual photo-activation methods: Influence on degree of conversion and crosslink density of composite resins. J Ther Anal Calorim. 2011;103:219–27. [Google Scholar]
- 35.Rastelli AN, Jacomassi DP, Bagnato VS. Degree of conversion and temperature increase of a composite resin light-cured with argon laser and blue LED. Las Phys. 2008;18:1570–5. [Google Scholar]
- 36.Moraes LG, Rocha RS, Menegazzo LM, de Araújo EB, Yukimito K, Moraes JC, et al. Infrared spectroscopy: A tool for determination of the degree of conversion in dental composites. J Appl Oral Sci. 2008;16:145–9. doi: 10.1590/S1678-77572008000200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhary P, Tandon S, Ganesh M, Mehra A. Evaluation of the remineralization potential of amorphous calcium phosphate and fluoride containing pit and fissure sealants using scanning electron microscopy. Indian J Dent Res. 2012;23:157–63. doi: 10.4103/0970-9290.100419. [DOI] [PubMed] [Google Scholar]
- 38.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 39.Xu HH, Weir MD, Sun L. Calcium and phosphate ion releasing composite: Effect of pH on release and mechanical properties. Dent Mater. 2009;25:535–42. doi: 10.1016/j.dental.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skrtic D, Antonucci JM. Dental composites based on amorphous calcium phosphate – Resin composition/physicochemical properties study. J Biomater Appl. 2007;21:375–93. doi: 10.1177/0885328206064823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C, Weir MD, Cheng L, Lin NJ, Lin-Gibson S, Chow LC, et al. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent Mater. 2014;30:891–901. doi: 10.1016/j.dental.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]