Abstract
Introduction:
Matrix metalloproteinases (MMPs) play a significant role in the efficient tissue turnover and remodeling. This study focuses on the regulation of the MMPs by the protein kinases at the level of gene expression and their signaling pathways.
Materials and Methods:
Lipopolysaccharide-induced murine macrophage-like RAW 264.7 cell lines were obtained and maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum under optimal temperatures. Primers used were MMP-3 forward primer, MMP-3 reverse primer, and glyceraldehyde-3-phosphate dehydrogenase forward primer and glyceraldehyde-3-phosphate reverse primer. Total RNA was isolated, the sample was prepared, and electrophoresis was performed. The first strand of cDNA was synthesized and amplification of specific isolated gene using polymerase chain reactor (PCR). The amplified products were then separated on a 1.0% agarose gel in 1XTBE at 75 V for 3 h. The gel was stained with ethidium bromide, and the amplified product was visualized and photographed on Gel Doc system.
Results:
Real-time PCR showed only bands at expected size of 595 bp for internal control amplification of glyceraldehyde-3-dehydrogenase gene. Analysis was done with densitometry, and these values are compared with the negative control. Results showed a statistically significant rise in the relative levels of MMP-3-mRNA when compared with negative control at 1, 2, and 3 h.
Conclusion:
This study proved the significantly increased levels of MMP gene at different period, thereby it can be concluded that MMP-3 levels are higher in inflammatory conditions.
Keywords: Matrix metalloproteinase-3 gene, periapical abscess RAW 264-7 cell lines, pulpitis, real-time-polymerase chain reactor
INTRODUCTION
The regulation of extracellular matrix in both physiologic and pathologic conditions is carried out by different protease systems which include cysteine proteinase, aspartic proteinase, serine proteinase, and metalloproteinases.[1] Metalloproteinases consist of several superfamilies, of which metzincin is of prime important.[1] The matrix metalloproteinases (MMPs) belong to this family, and the hallmark of these is that they bind to the zinc at the catalytic site and have a conserved Met-turn motif.[2] These MMP family proteins have a dual role, in the pathogenesis of inflammation by causing tissue destruction and also by stimulating the protective immune responses.[3] MMPs are produced by odontoblasts and they have a wide role in dental caries and periapical inflammation.[1]
The aim of the study is to mainly evaluate the regulation of MMP-3 gene in inflammation. MMPs are classified into five main classes as collagenases, gelatinases, stromelysins, matrilysins, and membrane-type MMPs.[1] MMP-3 comes under stromelysins.[1] MMP-3 has been identified in the pulp, odontoblasts, predentin, and dentin.[4] Many other MMPs such as MMP-8, MMP-2, MMP-9, MMP-14, and MMP-20 are also identified as main MMPs in the pulp, odontoblasts, dentin, and predentin along with MMP-3.[5] The role of MMPs in mature odontoblasts are physiological secondary dentin formation, mineralization in intact and healthy teeth, degradation of matrix during dental injury, pulpal inflammation and tertiary dentinogenisis.[6] MMPs and tissue inhibitors of metalloproteinases (TIMPs) partially regulate the inflammatory pulpal tissue destruction.[1] MMP-3 seems to have a predominant role in pulpitis that other MMPs do not share. The concentration of MMP-3 was found to be higher in the acute pulpitis compared to the normal pulp tissue.[7] MMP-3 acts as a mediator in healing of dental pulp as an anti-inflammatory and regenerative factor.[8] They promote fibroblastic wound healing, reparative dentin formation, and helps in angiogenesis in pulpitis.[9] Significantly higher levels of MMP-1, MMP-2, and MMP-3 are seen in cases of acute pulpitis rather than in normal pulp tissue.[10] Expression of MMPs in adult tissues is quite low. However, its level seems to increase in pathological states with excessive destruction as in cases of chronic inflammation and destructive bone lesions.[11] Under normal conditions, the degradation and synthesis of the extracellular matrix are balanced. Hence, there is not much variation in the expressed MMP levels or expressed in low levels.[1] Its activity is seen more whenever active remodeling of tissues is needed and its level raises dramatically.[1] MMPs seem to have a role in periapical lesion and its inhibition increases the level of the lesion.[12] MMP-1 has been found to be one of the key enzymes in the periapical lesion by initiating the bone resorption in cases of periapical lesion.[13] The other MMPs found in periapical lesions are MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13.[14,15] These studies proved that MMPs are mainly involved in the defensive reaction against the microorganisms present in the pulp and periapical area, and the increase in the level of the lesion is mainly due to advanced infection leading to the spread.[1] Taking all these studies into consideration, it can be said that MMPs do play an important role in the spread of the pathology by degrading collagenous tissue. At the same time, they act as essential components in physiological tissue remodeling by promoting angiogenisis and reperative dentin remodelling..[1]
MATERIALS AND METHODS
Cell culture
Lipopolysaccharide (LPS)-induced murine macrophage-like RAW 264.7 cell lines were obtained from the cell bank. The cells were maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum, supplemented by penicillin (100 U/mL) and streptomycin (100 μg/mL), under a temperature of 37°C, and atmosphere of 5% of CO2 and 90% humidity.
Materials
The primer design is carried out with the aid of Primer Software. All the primers were synthesized from Sigma Genosys, Bangalore, India [Table 1]. M-MuLV Reverse Transcriptase and RNase Inhibitor were purchased from Fermentas (Thermo Scientific).
Table 1.
The primers used

Methods
Total RNA isolation
Add 0.1 ml of 1-bromo-3-chloropropane and close the sample vial tightly. Shake vigorously for 15 s and allow standing for 10 min at room temperature. Centrifuge the resulting mixture at 12,000 g for 15 min at 2°C–8°C. Transfer the aqueous phase to a fresh tube and add 0.5 ml of 2-propanol. Allow the sample to stand for 10 min at room temperature. Centrifuge at 12,000 g for 10 min at 2°C–8°C. Discard the supernatant and wash the pellet with 1 ml of 75% ethanol. Briefly, dry the RNA pellet for 5–10 min by air drying. Dissolve the pellet in 50 μl of diethyl pyrocarbonate-treated water. Quantity and purity of isolated RNA are confirmed by spectrophotometry. The integrity of isolated total RNA is confirmed by formaldehyde agarose gel electrophoresis.
Quantification of total RNA
For a 1-cm path length, the optical density at 260 nm (OD260) equals 1.0 for 40 μg/mL solution of RNA.
Calculations of RNA concentration = 40 μg/mL × OD260× dilution factor*
RNA sample preparation and electrophoresis
Add 5 μl of RNA sample (~8 μg of total RNA) to 13 μl of sample buffer. Add 1 μl of 10 mg/ml ethidium bromide. Mix gently and heat to 65°C for 10 min. Cool on ice. Add 3-μl loading dye and mix gently. Load the samples on gel. Run the gel at 5 V/cm until the bromophenol blue dye has migrated approximately 3/4 of length of the gel.
First-strand synthesis (cDNA synthesis)
Add the following components in sterile nuclease-free tube [Table 2].
Table 2.
The components used for the reaction setup

Mix gently and centrifuge briefly. Incubate the reaction mixture at 37°C for 1 h. Stop the reaction by heating the content to 70°C for 10 min.
Polymerase chain reactor amplification of specific gene
About 2 μl of reverse transcription reaction product from the above reaction was used for polymerase chain reactor (PCR) amplification [Table 3].
Table 3.
The components used for polymerase chain reactor amplification

PCR amplification was carried out on an Eppendorf Mastercycler® ep (Eppendorf AG, Germany). Cycling conditions used were as follows: initial denaturation at 94°C for 5 min, 34 cycles of 94°C for 40 s, 65°C for 60 s, 72°C for 60 s, and a final extension at 72°C for 10 min.
The amplified products were separated on a 1.0% agarose gel in 1XTBE at 75V for 3 h. The gel was stained with ethidium bromide, and the amplified product was visualized and photographed on Gel Doc system.
Polymerase chain reactor amplification of specific gene
Real-time PCR (RT-PCR) shows only bands at expected size of 595 bp for internal control amplification (GAPDH gene) [Figures 1 and 2].
Figure 1.
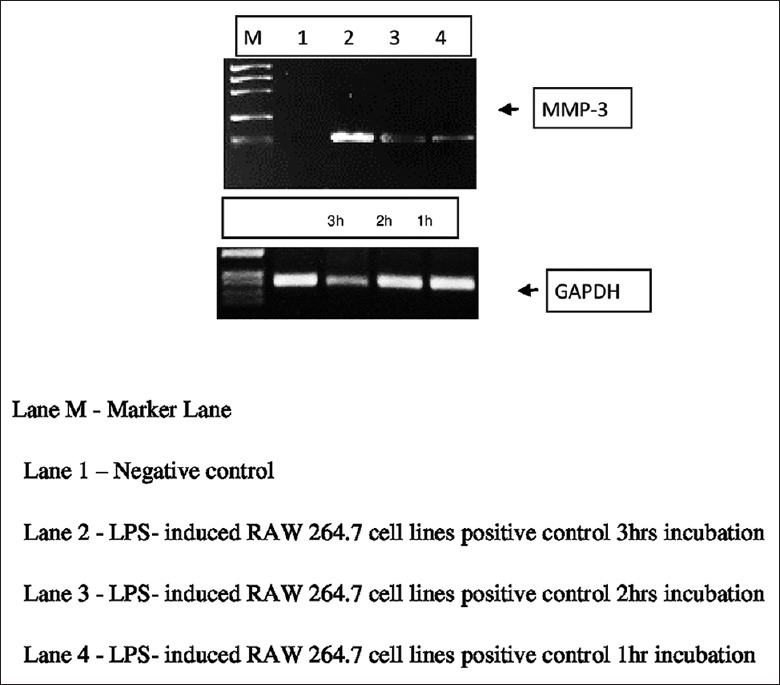
Representing the bands of matrix metalloproteinases-3 compared with GAPDH at three different intervals of time
Figure 2.
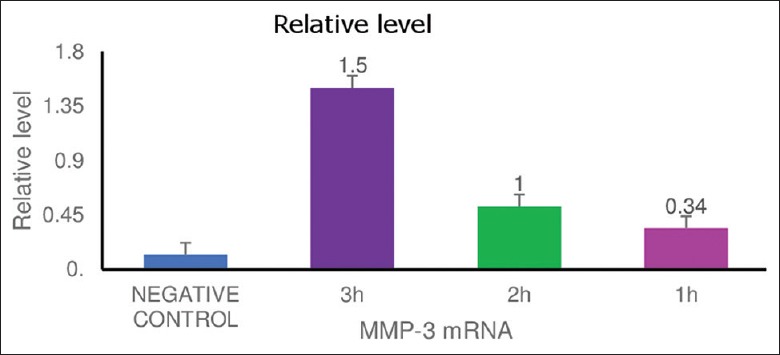
Comparing the relative levels of matrix metalloproteinases-3 with negative control at 1, 2, and 3 h intervals
The relative levels from different control were analyzed by densitometry, and the data are presented as mean intensity in relative arbitrary densitometric units ± standard error of the mean with n = 3. Values bearing different letters are statistically different with *P < 0.001 compared to negative control.
RESULTS
RT-PCR showed only bands at expected size of 595bp for internal control amplification of GAPDH gene. Results showed a statistical significant rise (p<0.05); in the relative levels of MMP-3-mRNA when compared with negative control at 1,2 and 3 hour intervals.
DISCUSSION
In this study, murine macrophage-like RAW 264.7 cell lines were obtained from the cell bank, and these were induced by LPS to stimulate the inflammatory conditions in these cell lines. RT-PCR amplification was carried out for a specific gene, and the amplified products were separated on a 1.0% agarose gel and stained with ethidium bromide, and the amplified product was visualized and photographed using Gel Doc system. Results of overexpression or downregulation of a micro-RNA on its target mRNA are often validated by reverse transcription and qualitative PCR analysis using an appropriate housekeeping gene as an internal control. Among many housekeeping genes, expressions of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and beta-actin have been used extensively for the normalization of gene expression data.[16] In this study, GAPDH is used as a negative control in comparison. The bands of mRNA were obtained at 1, 2, and 3 h intervals of time; these were compared with the negative control and were analyzed using densitometry. Figure 2 explains the relative levels of MMP-3 mRNA when compared with the negative control. The results show a statistically significant rise in the levels of MMP-3 gene at three different time intervals. MMPs have evolved as important regulatory enzymes in both pro- and anti-inflammatory pathways. MMP expression and activity are increased in any tissue injury and inflammatory disease process; there is growing evidence to prove that these proteinases function in inflammation, primarily to modulate the leukocyte influx, either through regulation of barrier function, chemokine/cytokine activity, or gradient formation. MMPs can both beneficial in regulating the host defense and also can be pathological in inflammatory disease. The physiological or a pathological role of an individual MMP is dependent on the substrate, cell type, and disease process. Sometimes, even a targeted inhibition may be of a limited value-added benefit or maybe even harmful also. Therefore, developing rational therapies require further identification of specific MMP substrates and characterization of the downstream consequences of MMP proteolytic activity.[17] MMP-3 plays an important role in the acute pulpal tissue destruction of the inflamed pulp.[7] Gene expression is not only confined to periapical inflammation but also in cases of chronic periodontitis increased levels of certain MMP genes causing the pathology.[18] The expression of these MMPs was also seen in oral potentially malignant disorders and seems to be associated with the early tissue changes of these oral potentially malignant disorders.[19] MMP-2 and MMP-3 have a major contribution toward the development of the periapical lesions and also the healing response. MMPs are strongly associated with the levels of inflammation and play an important role in the remodeling and resorption of bone.[20] They are secreted as proenzyme forms and require extracellular activation. They are seem to be regulated by endogenously secreted inhibitors such as TIMPs.[20] Clinical and radiographic evidence support a role of MMPs in osteoclastic bone resorption and bone metastasis. MMPs act in degradation of organic matrix, mainly type-1 collagen.[21] Their expression is frequently induced by interleukins (ILs) such as IL-1 and IL-6 which have a role in bone-resorbing activity.[22] MMPs seem to play an important role in periapical pathology development and also the elevated levels report to correlate with the nonhealing.[23,24] Due to their various roles in the bone remodeling, immune responses, caries, and dental development, we can generalize that variations in the MMP and TIMP genes may alter the level of bone destruction and remodeling and contribute to the formation of more extensive periapical lesions in teeth with deep caries. In summary, it can be suggested that markers in MMP-3 and MMP-2 genes could help to predict the host susceptibility to the developing periapical lesions and healing response.[25]
CONCLUSION
This study proved the significantly increased levels of MMP gene at different intervals of time, thereby it can be concluded that MMP-3 levels are higher in inflammatory conditions, and these increased levels are mainly due to the spread of the infection. Therefore, MMPs play an important role in the degradation of the collagenous structure and spread of the pathology. At the same time, they are the essential components in the physiologic tissue remodeling process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Dilution factor = 50.
REFERENCES
- 1.Jain A, Bahuguna R. Role of matrix metalloproteinases in dental caries, pulp and periapical inflammation: An overview. J Oral Biol Craniofac Res. 2015;5:212–8. doi: 10.1016/j.jobcr.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 3.Le NT, Xue M, Castelnoble LA, Jackson CJ. The dual personalities of matrix metalloproteinases in inflammation. Front Biosci. 2007;12:1475–87. doi: 10.2741/2161. [DOI] [PubMed] [Google Scholar]
- 4.Mazzoni A, Papa V, Nato F, Carrilho M, Tjäderhane L, Ruggeri A, Jr, et al. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J Dent. 2011;39:231–7. doi: 10.1016/j.jdent.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palosaari H, Ding Y, Larmas M, Sorsa T, Bartlett JD, Salo T, et al. Regulation and interactions of MT1-MMP and MMP-20 in human odontoblasts and pulp tissue in vitro. J Dent Res. 2002;81:354–9. doi: 10.1177/154405910208100513. [DOI] [PubMed] [Google Scholar]
- 6.Heidi P. Matrix Metalloprotienases (MMPs) and their Specific Tissue Inhibitors (TIMPs) in Mature Human Odontoblasts and Pulp Tissue: The Regulation of Expressions of Fibrillar Collagens, MMPs and TIMPs by Growth Factors, Transforming Growth Factor-[beta]1 (TGF-[beta]1) and Bone Morphogenic Protien-2 (BMP-2) Finland: Oulu University Press; 2003. pp. 17–82. [Google Scholar]
- 7.Shin SJ, Lee JI, Baek SH, Lim SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod. 2002;28:313–5. doi: 10.1097/00004770-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Muromachi K, Kamio N, Matsuki-Fukushima M, Narita T, Nishimura H, Tani-Ishii N, et al. Metalloproteases and CCN2/CTGF in dentin – Pulp complex repair. J Oral Biosci. 2015;57:86–90. [Google Scholar]
- 9.Zheng L, Amano K, Iohara K, Ito M, Imabayashi K, Into T, et al. Matrix metalloproteinase-3 accelerates wound healing following dental pulp injury. Am J Pathol. 2009;175:1905–14. doi: 10.2353/ajpath.2009.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding Y, Uitto VJ, Haapasalo M, Lounatmaa K, Konttinen YT, Salo T, et al. Membrane components of treponema denticola trigger proteinase release from human polymorphonuclear leukocytes. J Dent Res. 1996;75:1986–93. doi: 10.1177/00220345960750121101. [DOI] [PubMed] [Google Scholar]
- 11.Wahlgren J, Salo T, Teronen O, Luoto H, Sorsa T, Tjäderhane L, et al. Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. Int Endod J. 2002;35:897–904. doi: 10.1046/j.1365-2591.2002.00587.x. [DOI] [PubMed] [Google Scholar]
- 12.Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T, et al. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998;77:1622–9. doi: 10.1177/00220345980770081001. [DOI] [PubMed] [Google Scholar]
- 13.Hong CY, Lin SK, Kok SH, Cheng SJ, Lee MS, Wang TM, et al. The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J Oral Pathol Med. 2004;33:162–9. doi: 10.1111/j.0904-2512.2004.00045.x. [DOI] [PubMed] [Google Scholar]
- 14.Leonardi R, Caltabiano R, Loreto C. Collagenase-3 (MMP-13) is expressed in periapical lesions: An immunohistochemical study. Int Endod J. 2005;38:297–301. doi: 10.1111/j.1365-2591.2005.00943.x. [DOI] [PubMed] [Google Scholar]
- 15.Pezelj-Ribaric S, Anic I, Brekalo I, Miletic I, Hasan M, Simunovic-Soskic M, et al. Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps. Arch Med Res. 2002;33:482–4. doi: 10.1016/s0188-4409(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 16.Sikand K, Singh J, Ebron JS, Shukla GC. Housekeeping gene selection advisory: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin are targets of miR-644a. PLoS One. 2012;7:e47510. doi: 10.1371/journal.pone.0047510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar MS, Vamsi G, Sripriya R, Sehgal PK. Expression of matrix metalloproteinases (MMP-8 and -9) in chronic periodontitis patients with and without diabetes mellitus. J Periodontol. 2006;77:1803–8. doi: 10.1902/jop.2006.050293. [DOI] [PubMed] [Google Scholar]
- 19.Venugopal A, Uma Maheswari TN. Expression of matrix metalloproteinase-9 in oral potentially malignant disorders: A systematic review. J Oral Maxillofac Pathol. 2016;20:474–9. doi: 10.4103/0973-029X.190951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill PA, Murphy G, Docherty AJ, Hembry RM, Millican TA, Reynolds JJ, et al. The effects of selective inhibitors of matrix metalloproteinases (MMPs) on bone resorption and the identification of MMPs and TIMP-1 in isolated osteoclasts. J Cell Sci. 1994;107(Pt 11):3055–64. doi: 10.1242/jcs.107.11.3055. [DOI] [PubMed] [Google Scholar]
- 21.Everts V, Delaissé JM, Korper W, Niehof A, Vaes G, Beertsen W, et al. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J Cell Physiol. 1992;150:221–31. doi: 10.1002/jcp.1041500202. [DOI] [PubMed] [Google Scholar]
- 22.Kusano K, Miyaura C, Inada M, Tamura T, Ito A, Nagase H, et al. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: Association of MMP induction with bone resorption. Endocrinology. 1998;139:1338–45. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 23.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA, et al. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008;16:642–8. doi: 10.1111/j.1524-475X.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Min D, Bolton T, Nubé V, Twigg SM, Yue DK, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. 2009;32:117–9. doi: 10.2337/dc08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menezes-Silva R, Khaliq S, Deeley K, Letra A, Vieira AR. Genetic susceptibility to periapical disease: Conditional contribution of MMP2 and MMP3 genes to the development of periapical lesions and healing response. J Endod. 2012;38:604–7. doi: 10.1016/j.joen.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


