Abstract
Background
As laparoscopic liver resection is becoming a commonly used method for hepatic surgery, postoperative pain management is emerging as one of the trickiest problems after surgery. The ideal method of pain management is controversial and the optimal strategy for postoperative pain management after surgery remains unclear. The present study evaluated the postoperative analgesic efficacy of parecoxib and fentanyl, and the benefit of a new intravenous parecoxib infusion pump with patient-controlled analgesia after laparoscopic liver resection.
Material/Methods
This controlled, prospective, randomized, double-blind trial compared VAS scores among 3 groups of patients: a fentanyl group (FEN group) using a fentanyl citrate pump, an intravenous parecoxib group (IVPA group) receiving intravenous parecoxib, and a parecoxib pump group (PUPA group) receiving parecoxib sodium by analgesia pump. We enrolled 124 patients planned for laparoscopic liver resection. The primary outcome was VAS score at rest and with movement. Secondary outcomes were adverse effects (including nausea), sedation, pruritus, and quality of life.
Result
For all time intervals, the VAS scores were significantly lower in the PUPA group. VAS scores at rest and with movement in the PUPA group were the lowest among the 3 groups, while the scores in the FEN group were the highest. More adverse effects were detected in the FEN group, and no significant differences in adverse effects were found between the intravenous group and the parecoxib pump group.
Conclusions
Use of the intravenous infusion parecoxib pump for patient-controlled analgesia provides superior analgesic efficacy and fewer adverse effects for patients after laparoscopic liver resection.
MeSH Keywords: Analgesia; Anti-Inflammatory Agents, Non-Steroidal; Laparoscopy
Background
Postoperative analgesia is an important issue in general surgery due to its close association with patients’ early recovery. Although laparoscopic liver resection uses a smaller incision and causes less pain compared with open surgery, postoperative pain still affects the prognosis of some patients. Use of analgesia drugs is the most common method for analgesia; among these drugs, fentanyl holds the dominant position. Because fentanyl has no ceiling effect, increasing the drug dose can strengthen the effect, no matter how severe the pain is [1]. Nonetheless, increasing the dose can increase risk of adverse effects like nausea, emesis, and respiratory inhibition. Intrathecal fentanyl has been proved to be effective in laparoscopic abdominal surgery. However, the delayed onset effect limits its application. Therefore, an alternative method is needed to solve this problem.
Parecoxib sodium is the first COX-2 inhibitor that can be administered by intravenous and intramuscular injection. Numerous clinical trials have demonstrated the excellent efficacy of parecoxib in orthopedics, general surgery, and gynecology [2–4]. Research shows that parecoxib plays an important role in the perioperative and postoperative period, especially in patients about to undergo abdominal surgery. Moreover, increasing the dose rarely causes adverse effects. Therefore, parecoxib is a promising drug for postoperative pain management.
Effective control and management of postoperative pain is the primary concern of patients and is also important to surgeons because of potential adverse effects of the physiologic response to pain after surgery. Severe pain leads to reduced patient satisfaction and increased morbidity and mortality, and it places a burden on patient and health system finances. Inadequately controlled pain negatively affects quality of life, function, and functional recovery [5]. Therefore, postoperative pain management is one of the most important issues throughout the perioperative period, and researchers are devoting themselves to developing optimal methods to control postoperative pain and thus enhance recovery after surgery. PCA (patient-controlled analgesia) was initially developed due to insufficient pain relief from conventional intramuscular delivery of opioids [6]. PCA has now become widely prescribed for pain management [7]. It offers greater analgesic efficacy and potentially fewer adverse events than intermittent administration because of the ability of patients to titrate generally smaller on-demand doses according to their own needs rather than receiving analgesics intravenously several times a day.
Thus, we hypothesized that using a new patient-controlled analgesic system with an intravenous infusion pump would significantly improve postoperative pain management compared to use of a conventional intravenous infusion pump for patient-controlled analgesia. This controlled, prospective, randomized trial was performed to evaluate the benefit of a new type of intravenous infusion pump for patient-controlled analgesia and also the advantage of parecoxib as analgesic compared with fentanyl for postoperative analgesia after laparoscopic liver resection.
This study is registered on the ClinicalTrial.gov. The number is NCT02408146.
Material and Methods
Patients
We enrolled 124 patients aged 18–75 years old who were scheduled for laparoscopic liver resection. Informed consent was obtained from each patient prior to biopsy or surgery, and ethics approval for the use of human subjects was obtained from the Research Ethics Committee of the First Affiliated Hospital of Harbin Medical University. Patients who had contraindications to designated drugs (morphine, fentanyl, and parecoxib), chronic pain, and history of drug dependence or sleep apnea syndrome are excluded from this study. Patients were randomly separated into 3 groups – a fentanyl (FEN) group, an intravenous parecoxib (IVPA) group, and a parecoxib pump (PUPA) group – by use of a computerized randomization table. The patients, anesthesiologist, surgeon, research nurse, and the stuff in the ward or the surgical unit were blind to the group assignment of the patients.
Preoperative treatment
Patients did not get any premedication while they were in the ward, but all received midazolam 0.05 mg per kilogram intravenously in the operating room. Patients’ monitoring was standardized and consisted of oximetry, PaCO2, 3-lead electrocardiogram, blood pressure monitoring, and continuous CVP. All patients had a peripheral intravenous drip, urinary catheter (no longer than 3 days), and a nasogastric tube.
Anesthesia
All patients received intravenous-inhalation combined anesthesia. Induction was begun with sufentanil 0.4 μg/kg bolus and was maintained by an infusion of 0.2 μg/kg per hour. Sufentanil was stopped 30 min before the surgery finished. Induction was maintained with propofol 1.0 to 2.0 mg per kilogram, and rocuronium 0.6 mg per kilogram when needed. After tracheal intubation, anesthesia was maintained with a mixture of air and oxygen (0.5 L/0.5 L) and desflurane 0.6 to 1 minimum alveolar concentration. Additional boluses of sufentanil 0.05 μg/kg were monitored at 5-min intervals if current arterial pressure was over 20% higher than the baseline value. If arterial pressure was under 20% of baseline value, phenylephrine infusion was added to maintain it.
Surgical technique
Liver resection used 4 ports (10 mm and 3×5 mm) based on the location of the liver. After anesthesia, doctors use carbon dioxide to fill suffused patients’ abdominal cavity with a maximal pressure of no more than 15 mmHg. Patients were placed in a 30-degree Fowler position during liver resection. The specimen after resection was removed through an incision about 7 cm long.
Postoperative treatments
After the surgery, the FEN group received patient-controlled analgesics (fentanyl citrate 20 μg/mL·kg + ondansetron hydrochloride 2 mg per mL +saline solution) with a conventional intravenous infusion pump for 3 days after liver resection. The pump infused continuously at the basic flow rate of 2 mL per h, at which 0.5 mL of bolus is injected into patients if the patients press the button while they are suffering from postoperative pain. The lock-out time for each bolus injection is 15 min. The IVPA group patients received oral celecoxib before surgery, then received 40 mg parecoxib sodium intravenously guttae twice a day for the next 3 days after surgery. For the IVPA group, we used a targeted-controlled infusion (TCI) pump for pain management. TCI utilizes a computer-controlled infusion pump to deliver analgesic according to the set-up by the administering physician. The PUPA group received oral celecoxib every day before surgery, and received 40 mg parecoxib sodium intravenously guttae as soon as the surgery finished, then the patients wore the new intravenous infusion pump for patient-controlled analgesia (parecoxib sodium 1 mg/mL + normal saline) for 3 days after surgery. The pump infuses continuously with the basic flow rate of 2 mL per h, at which 0.5 mL of bolus will be injected into patients if the patients press the button while they are suffering from postoperative pain. The lock-out time is 15 min for each bolus injection. When the patients received the pump, the analgesic inside the pump and the dosage of the medicine were already set by the researcher. These methods were all initiated before patients were sent back to the ward. Therefore, it the medical staff were blind to group assignments.
While using the PCA pump, patients’ respiration (respiratory rate and oxygen saturation) was monitored at every hour for the first 24 h and every 2 h thereafter. If blood saturation was lower than 95%, supplemental oxygen by nasal prongs was provided for the patients.
Clinical outcome
The primary outcome was pain scores. Pain scores were evaluated with a VAS of 0 to 10, which is measured at rest and with movement like walking, coughing, and deep breathing. We also used the Bruggeman comfort scale to assess postoperative pain.
The secondary outcomes were sedation, nausea, and respiration. Sedation, nausea, and pruritus were measured as well at 2, 4, 8, 16, 24, 48, and 72 h after patients were back in the ward. All the data were collected and recorded by the same research nurse. We also recorded whether pain affected patients’ movement and sleep, divided into 5 levels: no effect, affecting sleep, unable to sleep, unable to eat, and limited self-care ability.
Rescue medication
Rescue medication consisted of diphenhydramine 25 mg through intravenous drip every 8 h for pruritus, metoclopramide 10 mg IV every 8 h if patients suffer had nausea or vomiting, and, if the therapy above is ineffective, ondansetron 4 mg IV every 8 h. If patients in the ward could not bear the pain after surgery and other analgesics were ineffective, dolantin (50 mg) was used once.
Statistics analyze
Data are expressed as mean ± standard deviation for continuous variables and median and interquantile range for categorical variables. To ensure statistical was done correctly, distribution of data was examined. VAS was analyzed as a nonparametric variable for its non-normal distribution. VAS score was analyzed by t test and one-way ANOVA. The secondary outcome was assessed as categorical variables and were analyzed by Mann-Whitney U test. All patients’ characteristics were analyzed by t test, Shapiro-Wilk test and D’Agostino test. All statistical analysis was performed with SPSS19.0 (SPSS, Inc., Chicago, IL) and GraphPad Prism6.
Result
Patients and characteristics
In this study, 124 patients participated in and completed the study. All of the 3 groups had similar demographic characteristics and their baseline laboratory values had no significant difference. There was no significant difference among the groups with regard to age, sex, ASA level, height, or weight (Table 1). All variables except ASA levels were normally distributed as assessed by Shapiro-Wilk test and D’Agostino test. There were no significant differences in duration of surgery, time to extubation, urine output, or blood loss among the 3 groups. All patients were extubated in the operating room and no patient was transferred to the ICU for further treatment.
Table 1.
Patient characteristics for each group.
| Variables | Fentanyl | IVPA | PUPA |
|---|---|---|---|
| Age (years) | 50 (15) | 52 (11) | 51 (13) |
| Sex (% male) | 47.5 | 47.6 | 52.4 |
| Weight (kg) | 71 (14) | 76 (15) | 73 (16) |
| Height (cm) | 165 (7) | 167 (9) | 168 (7) |
| ASA (II/III/IV) | 14/20/6 | 12/21/9 | 14/22/6 |
| Starting Hb value (g/L) | 136 (11) | 133 (12) | 130 (10) |
| Starting platelet count, 109 pl/L | 245 (44) | 254 (47) | 251 (46) |
Values are mean (SD), otherwise absolute number. All the P values of the statistical are nonsignificant. All the varieties except ASA level follow the normal distribution. w <0.919
VAS scores
The primary outcome of this study was the pain score as evaluated by VAS. The data (Figure 1) demonstrated that VAS scores at rest in the IVPA and PUPA groups were significantly lower than in the FEN group (P<0.001) at all time intervals, and VAS of the PUPA group was lower compared with the IVPA group. Similar results (Figure 2) were achieved for VAS score with movement (Table 2). We conclude that patients using parecoxib as analgesic suffered less pain compared to patients using fentanyl, and we found that using the PCA pump helps relieve postoperative pain.
Figure 1.
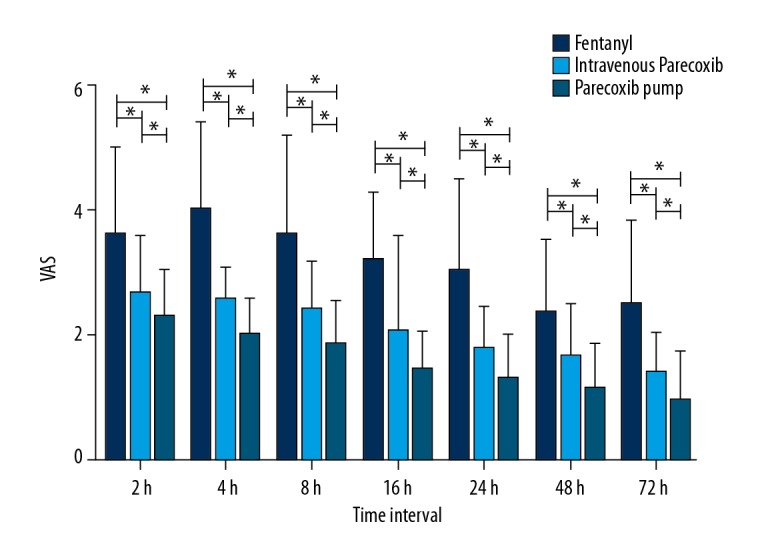
VAS score at rest for each time interval. * Difference is significant. The VAS score of patients in the IVPA and PUPA groups was significantly lower than that in the FEN group (P<0.001) at all time intervals (P<0.001). There is no difference in VAS scores between the IVPA and PUPA groups. The P values are 0.508 at 2 h, 0.698 at 4 h, 0.998 at 8 h, 0.527 at 16 h, 0.638 at 24 h, 0.785 at 48 h, and 0.769 at 72 h.
Figure 2.

VAS score with movement for each time interval. * Difference is significant. The VAS score of patients in the IVPA and PUPA groups was significantly lower than that in the FEN group (P<0.001) at all time intervals (P<0.001). There is no VAS score difference in the IVPA and PUPA groups. The P values are 0.899 at 2 h, 0.376 at 4 h, 0.462 at 8 h, 0.541 at 16 h, 0.884 at 24 h, 0.577 at 48 h, and 0.798 at 72 h.
Table 2.
VAS scores after surgery during 72 h.
| Variables | Time points | Group FEN | Group IVPA | Group PUPA |
|---|---|---|---|---|
| Rest VAS | 2 h | 3.600±1.392 | 2.690±0.890 | 2.310±0.749 |
| 4 h | 4.000±1.414 | 2.548±0.539 | 2.000±0.584 | |
| 8 h | 3.625±1.580 | 2.381±0.803 | 1.881±0.670 | |
| 16 h | 3.200±1.091 | 2.048±0.739 | 1.452±0.663 | |
| 24 h | 3.050±1.449 | 1.762±0.701 | 1.333±0.687 | |
| 48 h | 2.350±1.189 | 1.667±0.853 | 1.119±0.739 | |
| 72 h | 2.500±1.359 | 1.381±0.670 | 0.929±0.808 | |
| Movement VAS | 2 h | 4.175±1.500 | 3.214±0.891 | 2.738±0.828 |
| 4 h | 4.200±1.436 | 2.952±0.705 | 2.595±0.767 | |
| 8 h | 3.825±1.615 | 2.786±0.708 | 2.405±0.767 | |
| 16 h | 3.300±1.159 | 2.500±0.796 | 1.905±0.617 | |
| 24 h | 3.450±1.431 | 2.262±0.656 | 1.738±0.828 | |
| 48 h | 2.875±1.418 | 2.238±1.037 | 1.619±0.909 | |
| 72 h | 2.550±1.260 | 1.833±0.928 | 1.381±0.764 |
Data are expressed as mean ±SD. All the P value of group FEN and group IVPA are <0.001. All the P value of group FEN and group PUPA are <0.001. All the P value of group PUPA and group IVPA are <0.001. The statistics were analyzed by t test and one-way ANOVA test.
Adverse effects
We also investigated adverse effects, which was selected as the secondary outcome. The adverse effects we measured included sedation, nausea, pruritus, and respiratory rate. We found that nausea scores in the FEN group were significantly higher than in the IVPA group and PUPA group at 2, 4, and 8 h (Table 3). Moreover, the sedation score of the FEN group was markedly higher than that in the IVPA and PUPA groups at 2 h, 4 h, 8 h, and 16 h. Hence, we found that patients using parecoxib as analgesic had fewer adverse effects.
Table 3.
Median evaluation of adverse effects for each group.
| Hours | Sedation (1–5) | Nausea (0–5) | Pruritus (0–2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FEN | IVPA | PUPA | FEN | IVPA | PUPA | FEN | IVPA | PUPA | |
| 2 | 3 (2) | 1 (1) | 1 (1) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 4 | 2 (2) | 1 (1) | 1 (1) | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 8 | 3 (2) | 1 (0) | 1 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 16 | 2 (2) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 24 | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 48 | 1 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 72 | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Data are expressed as median (interquartile range). Mann-Whitney U test was used to analyze data. Scoring is according to the Sedation Score (Ramsey).
Application of dolantin
Some patients suffer unbearable pain that requires dolantin to relieve; therefore, we also accessed the effect of dolantin in each group. Eight patients in the FEN group required dolantin, while only 1 in the IVPA group and 2 in the PUPA group required dolantin, suggesting the better efficacy of parecoxib for analgesia compared to FEN (Table 4).
Table 4.
Number of times dolantin was used.
| Groups | Times dolantin used |
|---|---|
| Group FEN | 8 |
| Group IVPA | 2 |
| Group PUPA | 1 |
Values are absolute numbers.
Quality of life
Quality of life during the perioperative period is another important factor that can be affected by postoperative pain. In this study, we detected significant differences in sleeping quality among the 3 group (Table 5). In the FEN group, 20 patients reported that their sleeping quality was affected by pain and 10 patients complained that they could not sleep due to unbearable pain. Thirteen patients in the IVPA group and 15 patients in the PUPA group reported that their sleep quality was slightly affected by pain, and only 6 patients in the IVPA group and 7 patients in the PUPA group patients reported that they could not sleep. No patients complained that they could not asleep at all. Altogether, our results demonstrate that parecoxib has better analgesic efficacy than FEN.
Table 5.
Patients’ movement situation affected by the pain.
| Group | No effect | Affecting sleep | Unable to sleep | Unable to eat | Limited self-care ability |
|---|---|---|---|---|---|
| Group FEN | 5 | 20 | 10 | 3 | 2 |
| Group IVPA | 21 | 13 | 6 | 1 | 1 |
| Group PUPA | 18 | 15 | 7 | 1 | 1 |
Values are absolute numbers.
Discussion
Laparoscopic surgery is becoming a common therapeutic option for liver cancer. Although laparoscopic surgery is less painful than normal open surgery, postoperative pain after laparoscopic surgery still is a problem that affects 17% to 41% of patients [8]. Moreover, the postoperative pain leads to delay of early ambulation after surgery, thereby resulting in delayed recovery from surgery. Therefore, various strategies were developed to solve this problem, including use of intrathecal morphine and other oral or intravenous analgesics [9]. However, the efficacy of these analgesics differs, and they may be used according to pain properties, such as type, location, and duration. There have been few studies on analgesia in laparoscopic liver resection, and there is debate about the best strategies for postoperative analgesia; therefore, there is an urgent need to develop novel methods to solve this problem.
Opioid analgesics are the most widely used drugs for controlling postoperative pain, but they may cause nausea, vomiting, and addiction [10]. Some researchers have attempted to use other analgesics, such as paracetamol, ketoprofen, and parecoxib, to improve postoperative pain management, but they found that these analgesics have the same problems as opioids [11–14].
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been proved to have effects similar to those of opioids [15]. NSAIDs are a well-established class of drugs that have been used for the blockage of pain and inflammation. They can maintain a constant level of prostaglandin inhibition over the course of prolonged surgery and during the postoperative period. Thus, we speculate that NSAIDs could be an alternative for opioids due to having fewer adverse effects.
Non-selective NSAIDs can inhibit COX-1 and COX-2, which are isoenzymes that play an important role in prostaglandin synthesis. Specific inhibition of COX-2 provides anti-inflammatory effects, while inhibition of COX-1 results in gastrointestinal adverse effects. Some researchers have confirmed that selective COX-2 inhibitors may be associated with better gastrointestinal tolerance and comparable cardiovascular events [16]. Therefore, it is inappropriate to apply orally administered NSAIDs in cases of postoperative nausea and vomiting or where the oral route of administration is inaccessible after surgery. Some researchers have confirmed that selective COX-2 inhibitors may be associated with better gastrointestinal tolerance and comparable cardiovascular events [17]. Therefore, using the new selective COX-2 inhibitor, parecoxib, intravenously, could avoid some adverse effects. Nevertheless, these adverse effects may not be problematic with the short-term administration of parecoxib in patients who have normal renal function and normal cardiac function, as shown in our study. Parecoxib is a first COX-2 inhibitor for parenteral administration, and can be given either intravenously or intramuscularly, providing potential advantages over oral medication. Studies have shown that it is the only parenterally administered selective COX-2 inhibitor that has proven analgesic effects [18].
We performed a randomized, double-blind study to investigate whether parecoxib has advantage over fentanyl, and to assess the advantages of PCA pumps. We used a patient-controlled analgesia pump with a lock time that is 15 min. With this PCA pump we can control the dose of parecoxib and fentanyl to prevent overdose, which can affect the conscious state of patients and can cause more serious adverse effects. All of the above considerations result in longer recovery time after laparoscopic surgery (Figure 3).
Figure 3.

The remaining 124 patients were included in randomization and data analysis.
Our results show that the intravenous parecoxib group and the parecoxib pump group felt less pain after surgery. VAS at rest or with movement in the IVPA and PUPA groups were lower than in the group fentanyl, with less adverse effects. Sedation and nausea were more frequent in the group receiving fentanyl. Some researchers have found that overuse of opioids can be associated with troublesome complications, including depression, hemodynamic instability, nausea, vomiting, prolonged sedation, respiratory depression, and ileus [19–21]. Moreover, the sedation caused by opioids impede treatment of patients after surgery [22]. The VAS scores and adverse effects suggest that parecoxib has more beneficial effects than fentanyl in patient undergoing laparoscopic liver resection.
Opioid-induced hyperalgesia (OIH) is a problem that may arise in patients treated with opioids, in which patients receiving opioids to control pain somewhat paradoxically become more sensitive to pain as a direct result of opioid therapy. In our study, induction was begun with sufentanil 0.4 μg per kilogram bolus followed by an infusion of 0.2 μg/kg per hour. Thus, our patients had an opportunity to have OIH. Two controlled studies reported aggravated postoperative pain despite increased postoperative opioid consumption in patients receiving a high rather than a low systemic opioid dose during surgery [23,24]. Some studies found that N-methyl-D-aspartate receptors (NMDA) is involved in this mechanism. The activation of NMDA may cause the phenomenon of OIH [25,26]. Parecoxib, as a COX-2 inhibitor, can inhibit PGE2 generation. Therefore, parecoxib can indirectly inhibit the NMDA by reducing the amount of PGE2. This mechanism could partly explain why the VAS scores of the PUPA and IVPA groups were lower. Our results suggest that parecoxib can alleviate postoperative hyperalgesia after opioid anesthesia.
Conclusions
We found that use of an intravenous infusion pump for patient-controlled analgesia provided superior analgesic efficacy after laparoscopic liver resection. Using the parecoxib PCA pump can significantly reduce the VAS scores both at rest and with movement. It can also reduce the adverse effects. Patients using the parecoxib pump had higher satisfaction with pain management after laparoscopic liver resection.
Acknowledgments
We thank the nurses in the Department of General Surgery of the First Affiliated Hospital of Harbin Medical University for collecting data. We thank Wang Chunxu for her constructive suggestions.
Abbreviation
- VAS
visual analogue scale
- ASA
America Society of Anesthesiologists
- FEN
fentanyl group
- PUPA
parecoxib pump group
- IVPA
intravenously parecoxib group
- PCA
patient-controlled analgesia
- NSAID
nonsteroidal anti-inflammatory drugs
- CVP
central venous pressure
Appendix.
Sedation Score (Ramsey)
1 for wide awake. 2 for drowsy. 3 for dozing. 4 for mostly sleeping. 5 for needs stimulation to rouse.
Nausea Score
0 for no nausea. 1 for mild nausea. 2 for discomforting nausea. 3 for distressing nausea. 4 for horrible nausea. 5 for worst possible nausea.
Itchiness Score
0 for no itchiness. 1 for mild itchiness. 2 for discomforting itchiness.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (81773194), the First Affiliated Hospital Youth Innovating Talents Training Plan Fund for Regular Undergraduate Colleges (UNPYSCT-2017066), the Harbin Medical University Doctoral Research Innovation Fund (2018B003), the Heilongjiang Province Postdoctoral Science Foundation (LBH-Z17135), the China Postdoctoral Science Foundation Funded Project (2018M631948), the National Natural Science Foundation of China (81803001), and the National Natural Science Foundation of China (81772588)
References
- 1.Stanley TH. The fentanyl story. J Pain. 2014;15:1215–26. doi: 10.1016/j.jpain.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Barton SF, Langeland FF, Snabes MC, et al. Efficacy and safety of intravenous parecoxib sodium in relieving acute postoperative pain following gynecologic laparotomy surgery. Anesthesiology. 2002;97:306–14. doi: 10.1097/00000542-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Akaraviputh T, Leelouhapong C, Lohsiriwat V, Aroonpruksakul S. Efficacy of perioperative parecoxib injection on postoperative pain relief after laparoscopic cholecystectomy: A prospective, randomized study. World J Gastroenterol. 2009;15:2005–8. doi: 10.3748/wjg.15.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd R, Derry S, Moore RA, McQuay HJ. Intravenous or intramuscular parecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009;(15):Cd004771. doi: 10.1002/14651858.CD004771.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet (London, England) 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 6.Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101:S44–61. doi: 10.1213/01.ANE.0000177102.11682.20. [DOI] [PubMed] [Google Scholar]
- 7.Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66:2321–37. doi: 10.2165/00003495-200666180-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: A critical assessment of the evidence. Anesthesiology. 2006;104:835–46. doi: 10.1097/00000542-200604000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Hong RA, Robbins CB, et al. Intrathecal morphine and oral analgesics provide safe and effective pain control after posterior spinal fusion for adolescent idiopathic scoliosis. Spine. 2018;43:E98–104. doi: 10.1097/BRS.0000000000002245. [DOI] [PubMed] [Google Scholar]
- 10.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–20. [PubMed] [Google Scholar]
- 11.Cakan T, Inan N, Culhaoglu S, et al. Intravenous paracetamol improves the quality of postoperative analgesia but does not decrease narcotic requirements. J Neurosurg Anesthesiol. 2008;20:169–73. doi: 10.1097/ANA.0b013e3181705cfb. [DOI] [PubMed] [Google Scholar]
- 12.Korkmaz Dilmen O, Tunali Y, Cakmakkaya OS, et al. Efficacy of intravenous paracetamol, metamizol and lornoxicam on postoperative pain and morphine consumption after lumbar disc surgery. Eur J Anaesthesiol. 2010;27:428–32. doi: 10.1097/EJA.0b013e32833731a4. [DOI] [PubMed] [Google Scholar]
- 13.Toygar P, Akkaya T, Ozkan D, et al. [Does iv paracetamol have preemptive analgesic effect on lumber disc surgeries?]. Agri. 2008;20:14–19. [in Turkish] [PubMed] [Google Scholar]
- 14.Fletcher D, Negre I, Barbin C, et al. Postoperative analgesia with i.v. propacetamol and ketoprofen combination after disc surgery. Can J Anaesth. 1997;44:479–85. doi: 10.1007/BF03011934. [DOI] [PubMed] [Google Scholar]
- 15.Malan TP, Jr, Gordon S, Hubbard R, Snabes M. The cyclooxygenase-2-specific inhibitor parecoxib sodium is as effective as 12 mg of morphine administered intramuscularly for treating pain after gynecologic laparotomy surgery. Anesth Analg. 2005;100:454–60. doi: 10.1213/01.ANE.0000143355.52418.CF. [DOI] [PubMed] [Google Scholar]
- 16.Warner TD, Giuliano F, Vojnovic I, et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–68. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–28. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 18.Abdulla S, Eckhardt R, Netter U, Abdulla W. Randomized, double-blind, placebo-controlled study to assess the efficacy of nonopioid analgesics on pain following arthroscopic knee surgery. Pain Res Treat. 2012;2012 doi: 10.1155/2012/305821. 305821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan TJ, Joshi GP, Zhao SZ, et al. Presurgical intravenous parecoxib sodium and follow-up oral valdecoxib for pain management after laparoscopic cholecystectomy surgery reduces opioid requirements and opioid-related adverse effects. Acta Anaesthesiol Scand. 2004;48:1194–207. doi: 10.1111/j.1399-6576.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 20.Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88:959–63. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 21.Anastase DM, Cionac Florescu S, Munteanu AM, et al. Analgesic techniques in hip and knee arthroplasty: From the daily practice to evidence-based medicine. Anesthesiol Res Pract. 2014;2014 doi: 10.1155/2014/569319. 569319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baratta JL, Gandhi K, Viscusi ER. Perioperative pain management for total knee arthroplasty. J Surg Orthop Adv. 2014;23:22–36. doi: 10.3113/jsoa.2014.0022. [DOI] [PubMed] [Google Scholar]
- 23.Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: Intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–17. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Chia YY, Liu K, Wang JJ, et al. Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Can J Anaesthes. 1999;46:872–77. doi: 10.1007/BF03012978. [DOI] [PubMed] [Google Scholar]
- 25.Lee M, Silverman SM, Hansen H, et al. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–61. [PubMed] [Google Scholar]
- 26.Leal Pda C, Clivatti J, Garcia JB, Sakata RK. Opioid-induced hyperalgesia (OIH) Rev Bras Anestesiol. 2010;60:639–47. 355–59. doi: 10.1016/S0034-7094(10)70080-5. [DOI] [PubMed] [Google Scholar]


