Abstract
Cigarette smoking has been identified as a major risk factor for osteoporosis decades ago. Several studies have shown a direct relationship between cigarette smoking, decreased bone mineral density, and impaired fracture healing. However, the mechanisms behind impaired fracture healing and cigarette smoking are yet to be elucidated. Migration and osteogenesis of mesenchymal stem/stromal cells (MSCs) into the fracture site play a vital role in the process of fracture healing. In human nicotine, the most pharmacologically active and major addictive component present in tobacco gets rapidly metabolized to the more stable cotinine. This study demonstrates that physiological concentrations of both nicotine and cotinine do not affect the osteogenic differentiation of MSCs. However, cigarette smoke exposure induces oxidative stress by increasing superoxide radicals and reducing intracellular glutathione in MSCs, negatively affecting osteogenic differentiation. Although, not actively producing reactive oxygen species (ROS) nicotine and cotinine inhibit catalase and glutathione reductase activity, contributing to an accumulation of ROS by cigarette smoke exposure. Coincubation with N-acetylcysteine or L-ascorbate improves impaired osteogenesis caused by cigarette smoke exposure by both activation of nuclear factor erythroid 2-related factor 2 (Nrf2) signaling and scavenging of ROS, which thus might represent therapeutic targets to support fracture healing in smokers.
1. Introduction
Smoking is the most common method of consuming tobacco which is the most popular substance smoked. For that, tobacco is combusted and the smoke with the active substances is inhaled. Smoking cigarettes represents a major health risk, which increases morbidity and mortality. It affects the whole human body and is linked to various health disorders. Deleterious effects of cigarette smoking on bone integrity have been shown, with a positive correlation between the number of cigarettes smoked per day and the years of exposure. Furthermore, smoking affects patients submitted to orthopedic surgery negatively by delaying the fracture healing, increasing the frequency of complications, and prolonging the hospital stay [1, 2].
Over 150 of the 6000 molecular species present in cigarette smoke have been identified as toxic compounds [3, 4]. It is still not completely elucidated which of these compounds are responsible for the negative effects of smoking on bone metabolism and fracture healing. Nicotine is the most pharmacologically active component present in tobacco which directly and indirectly affects cellular metabolism. Several studies have shown dose-dependent positive effects of nicotine on proliferation and differentiation of mesenchymal stem/stromal cells (MSCs) [5, 6]. However, the concentration of nicotine in these in vitro studies was much lower than the concentration found in blood samples from smokers [5]. Other studies revealed negative effects of nicotine on MSC proliferation as well as differentiation [7, 8]. Therefore, the effect of nicotine on the osteogenic differentiation of MSCs still remains unclear.
Cotinine is the most important metabolite of nicotine. 70–80% of nicotine is converted to cotinine in the liver. This metabolite is also present in the blood from smokers, with an average of 250–300 ng/ml cotinine which reaches higher blood levels than nicotine (50–100 ng/ml), which might be due to the longer half-life of cotinine (nicotine 2 h, cotinine 16 h) [9].
Recently, we reported that oxidative stress induced by cigarette smoke extract (CSE) [10] could be one of the responsible factors for the impaired osteogenic differentiation of SCP-1 cells. Coincubation with the antioxidant resveratrol protected the SCP-1 cells from the CSE deleterious effect [11]. However, the underlying mechanisms are not completely understood.
Nuclear factor erythroid-2-related factor-2 (Nrf2) signaling is known as a major mechanism in the cellular defense against oxidative stress which is activated in response to stress conditions [12]. In an unstressed condition, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH associating protein 1 (Keap-1) [13] which favors its proteasomal degradation. Under stress conditions, Keap-1 changes its structure by stabilizing its thiol groups, which interferes with its binding to Nrf2. Free in the cytoplasm, Nrf2 is activated [14] and translocates into the nucleus, where it binds to the antioxidant response element (ARE) in the promoter region of genes, e.g., antioxidative enzymes and genes involved in glutathione (GSH) homeostasis, regulating their expression. Some studies in mice have shown that disruption of Nrf2 impairs the induction of cellular defense pathways and increases the negative effects of oxidative stress induced by cigarette smoke [15]. Moreover, upregulating Nrf2 signaling by knockdown of Keap-1 increases antioxidative defense and diminishes lung injury caused by smoking [16]. However, there are controversial findings on the roles of antioxidant signaling pathways on bone metabolism under oxidative stress. On the one hand, it was shown that MC3T3 cells exposed to H2O2 activation of Nrf2 signaling negatively affect osteogenic differentiation—a mechanism inhibited by N-acetylcysteine (NAC) [17]. On the other hand, deletion of Nrf2 in bone tissue leads to a poor bone mineral density not only due to increased osteoclast activity but also because of a lack of functional osteoblasts [18, 19].
Up to now, it is not known if and how nicotine and cotinine affect the osteogenic differentiation of MSCs. Therefore, the aim of the present study was to evaluate the effect of nicotine and cotinine on MSCs during osteogenic differentiation and, furthermore, to investigate which type of reactive oxygen species (ROS) is induced by CSE, nicotine, or cotinine and how these substances affect the cell response to oxidative stress.
2. Materials and Methods
Anti-acetylated-α-tubulin, anti-SOD-1, and anti-rabbit HRP-labeled secondary antibodies were obtained from Santa Cruz (SC-23950, SC-11407, and SC-2004; Heidelberg, Germany). Anti-GAPDH antibody was obtained from Sigma-Aldrich (G9545; Munich, Germany). Anti-phospho-p38 MAPKinase and anti-catalase antibodies were obtained from Cell Signaling (Frankfurt am Main, Germany). Anti-phospho-Nrf2 antibody was obtained from Abcam (ab76026; Cambridge, United Kingdom). Alexa Fluor 448-labeled secondary antibody was obtained from Invitrogen (Karlsruhe, Germany). N-Acetylcysteine (NAC) and nicotine were obtained from Carl Roth (Karlsruhe, Germany). L-Ascorbic acid was obtained from Sigma-Aldrich (Darmstadt, Germany). Cotinine was obtained from Alfa Aesar (Karlsruhe, Germany).
2.1. Generation of Cigarette Smoke Extract (CSE)
CSE was freshly prepared for every experiment. In total, the smoke of two commercial cigarettes (Marlboro, Philip Morris, New York City, USA) containing 0.8 mg nicotine and 10 mg tar each was continuously bubbled through a 50 ml prewarmed SCP-1 differentiation medium (0% FCS) in a standard gas wash bottle, as described before [11]. The CSE was normalized by its optical density at 320 nm (OD320), with an OD320 of 0.7 considered 100% CSE [20]. After sterile filtration (0.22 μm pore filter), the CSE was diluted with SCP-1 differentiation medium to receive 5% V/V CSE, which corresponds to exposures associated with smoking up to 10 cigarettes/day [21].
2.2. Culture and Osteogenic Differentiation of SCP-1 Cells
Human immortalized mesenchymal stem cells (SCP-1 cells, provided by Dr. Matthias Schieker [22]) were cultured in MEM alpha medium (10% V/V FCS, 100 U/ml penicillin, and 100 mg/ml streptomycin) in a water-saturated atmosphere of 5% CO2 at 37°C [23]. SCP-1 cells were osteogenically differentiated for 21 days in MEM alpha medium (1% V/V FCS, 100 U/ml penicillin, 100 mg/ml streptomycin, 200 μM L-ascorbate-2-phosphate, 10 mM β-glycerol-phosphate, 25 mM HEPES, 1.5 mM CaCl2, and 100 nM dexamethasone). The medium was changed every 3–4 days.
2.3. Resazurin Conversion Assay
Cell viability (mitochondrial activity) was measured by Resazurin conversion assay. Briefly, cells were covered with 0.0025% W/V Resazurin in PBS. After 30 min incubation at 37°C, the resulting Resorufin fluorescence was measured (excitation = 544 nm/emission = 590 nm) as described [24, 25]. The incubation time was optimized based on the high metabolic activity of SCP-1 cells.
2.4. Sulforhodamine B (SRB) Staining to Assess Total Protein Content
Total protein content was determined by SRB staining of ethanol-fixed (1 h at −20°C) cells. Cells were stained with 0.4% W/V SRB (in 1% V/V acetic acid) for 20 min at ambient temperature. Cells were washed 4–5 times with 1% acetic acid to remove unbound SRB. Bound SRB was resolved with 10 mM unbuffered TRIS solution (pH ~10.5). Resulting absorption (λ = 565 nm) was determined with a plate reader [26].
2.5. Alkaline Phosphatase (AP) Activity Assay
AP activity was measured as an early osteogenic marker. Briefly, cells were incubated with AP reaction buffer (0.2% W/V 4-nitrophenyl-phosphate, 50 mM glycine, 1 mM MgCl2, 100 mM TRIS, and pH 10.5) for 30 min at 37°C. Formed 4-nitrophenol was determined photometrically (λ = 405 nm) as described and normalized to relative cell numbers by SRB staining. Changes in AP activity are displayed relative to untreated cells [24, 25].
2.6. Alizarin Red Staining
Matrix mineralization was measured as a late osteogenic marker. Cells were fixed with ice-cold ethanol for 1 h. After washing with tap water, cells were incubated with 0.5% W/V Alizarin Red solution (pH 4.0) for 30 min at ambient temperature. After 3 additional washing steps, the resulting staining (red) was assessed microscopically. After resolving the stain with 10% W/V cetylpyridinium chloride, Alizarin Red staining was quantified photometrically at λ = 562 nm [24, 25].
2.7. Immunofluorescence Staining
Cells were fixed with 4% V/V paraformaldehyde solution and permeabilized with 0.2% V/V Triton-X-100 for 10 min each. Unspecific binding sites were blocked with 5% W/V BSA for 1 h. Incubation with primary antibodies (1 : 100) was performed overnight at 4°C, followed by incubation with ALEXA488 labeled secondary antibodies (1 : 2.000) for 1 h. Images were taken with a fluorescence microscope (EVOS FL AF 4301, Life Technologies, Darmstadt, Germany). The excitation and emission wavelengths were used as specified by the manufacturer. Pictures were analyzed using the ImageJ software (line tool) (National Institute of Health, Bethesda, USA) by 4 independent investigators in a blinded fashion. Based on the microscopic pictures taken, cilia length was determined by the maximum intensity projection method [27].
2.8. Determination of ROS Levels
To measure the formation of ROS, different fluorescent probes were used [28]:
For the most unspecific 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) assay, cells were incubated with 10 μM DCFH-DA for 25 min at 37°C. After washing twice with PBS, cells were stimulated with CSE according to the experimental setup. As positive control, SCP-1 cells were stimulated with 0.01% V/V (882 μM) H2O2. After 0, 5, 10, and 15 min, the increase in fluorescence (excitation = 485 nm/emission = 520 nm) was detected by a plate reader, representing levels of ·O2 −, H2O2, HO·, and ONOO− [29]. To trap the ROS, cells were coincubated with either 25 μM α-tocopherol (·O2 − i), 10 mM sodium-pyruvate (H2O2i), 250 mM DMSO (HO· i), or 100 μM uric acid (ONOO− i) [28]
To determine ·O2 − levels, cells were incubated with 10 μM dihydroethidium (DHE) for 25 min at 37°C. After washing twice with PBS, cells were stimulated with CSE according to the experimental setup. As negative control (assay specificity), SCP-1 cells were stimulated with 0.01% V/V (882 μM) H2O2. After 0, 5, 10, and 15 min, the increase in fluorescence (excitation = 544 nm/emission 590 nm) was detected by a plate reader. The slope of the linear part of the curve, resembling the product formation rate, was calculated. Cellular localization of the fluorescence was confirmed by fluorescence microscopy
2.9. Determination of Total Glutathione
The total GSH measurement was performed according to the Ellman assay: after stimulation, protein precipitation of cellular lysates was carried out with 3% W/V m-phosphoric acid. The protein samples were reneutralized with 5 mM EDTA in 0.1 M potassium phosphate buffer (pH = 7.4), and the total GSH was determined. For the determination, 20 μl of sample was incubated for 30 seconds with 120 μl of a mixture (1 : 1) of 1.68 mM 5,5′-dithiobis-(2-nitrobenzoic acid) and 2.5 U/ml glutathione reductase in 0.1 M potassium phosphate buffer. Then, 60 μl of NADPH 0.8 mM was added and absorbance was measured at λ = 412 nm for 15 min [30].
2.10. Western Blot Analysis
Cells were lysed in freshly prepared ice-cold RIPA buffer. 30 μg total protein was separated by SDS page and transferred to nitrocellulose membranes. Membranes were blocked with 5% W/V BSA for 1 h. After overnight incubation with primary antibodies (1 : 1.000) at 4°C, membranes were incubated with the corresponding peroxidase-labeled secondary antibodies (1 : 10.000) for 2 h at ambient temperature. For signal development, membranes were incubated for 1 min with ECL substrate solution. Chemiluminescent signals were quantified using the ImageJ software [24].
2.11. Catalase Activity Assay
The catalase activity was measured with the fluorometric catalase activity kit OxiSelect (Cell Biolabs, San Diego, CA, USA) according to the manufacturer's instructions. Fluorescence was measured at 544 nm (λex) and 590 nm (λem) [31].
2.12. Superoxide Dismutase Activity Assay
In order to measure the SOD activity, SOD from HepG2 cells and a commercially available kit (Sigma-Aldrich, Taufkirchen, Germany) were used according to the manufacturer's protocol. Absorbance was measured at 450 nm every 5 min over 30 min [31].
2.13. Glutathione Peroxidase (GPx) Activity Assay
The measurement of GPx activity was performed using cumene hydroperoxide as a substrate for GPx. 5 μl of 1 U/ml GPx was mixed with 5 μl of each sample, 15 μl of 4 mM NADPH, and 75 μl of GPx assay solution (1.33 U/ml glutathione reductase, 1.33 mM L-glutathione reduced in 0.05 mM potassium phosphate buffer (pH = 7.0) containing 1.1 mM EDTA and 1.1 mM NaN3) and incubated at RT for 5 min. Then, 10 μl of 15 mM cumene hydroperoxide solution was added and the decrease in absorbance at λ = 340 nm was measured within a 15 min time interval [30, 32].
2.14. Glutathione Reductase (GR) Activity Assay
The GR activity was measured by the increase in the absorbance due to the reduction of 5′-dithiobis-(2-nitrobenzoic acid) to 5-thio-(2-nitrobenzoic acid). 2.5 μl of 1 U/ml GR and 2.5 μl of each sample were mixed with 185 μl reaction mixture (0.8 mM DTNB, 0.1 mM NADPH, and 1 M EDTA (1 M) in 0.2 M potassium phosphate buffer (pH = 7.5)) and 10 μl of 20 mM L-glutathione (oxidized). Then, the increase in absorbance at λ = 412 nm was measured of a time interval of 15 min [30, 33].
2.15. Statistics
Results are expressed as the bar chart (mean ± SEM) of at least 4 independent experiments (N ≥ 4) measured as triplicate or more (n ≥ 3). Data sets were compared by the Kruskal-Wallis H test followed by Dunn's multiple comparison test (GraphPad Software Inc., La Jolla, CA, USA). p < 0.05 was taken as the minimum level of significance.
3. Results
3.1. Effect of Nicotine and Its Primary Metabolite Cotinine on Osteogenic Differentiation of SCP-1 Cells
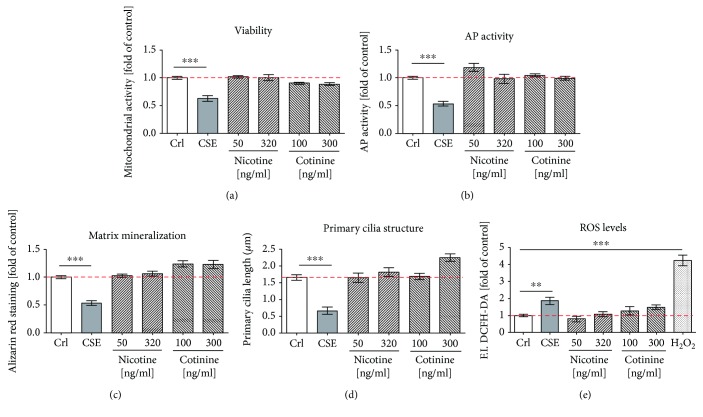
In order to determine which components present in cigarette smoke are responsible for impaired osteogenic differentiation of MSCs exposed to CSE [11], SCP-1 cells were treated with nicotine and its first metabolite cotinine. Nicotine and cotinine were applied to the cells at concentrations ranging from 50 ng/ml to 320 ng/ml and 100 ng/ml to 300 ng/ml, respectively. These concentrations were chosen based on reported blood levels of nicotine and cotinine from smokers and the calculated amount from our CSE. Nicotine concentration in arterial blood from smokers ranks between 20 and 60 ng/ml and rises up to 100 ng/ml after smoking one cigarette [9]. The average of cotinine in blood from smokers is 250–300 ng/ml [9]. The theoretical concentration of nicotine and cotinine in our CSE is 160 ng/ml and 150 ng/ml, respectively [11]. Therefore, SCP-1 cells were osteogenically differentiated for 21 days, in the presence of nicotine and cotinine, with concentrations up to 320 ng/ml and 300 ng/ml, respectively. Based on our previous work, 5% CSE was used as control. After osteogenic differentiation, effects on the mitochondrial activity (an indirect indicator of viability and proliferation) were measured by Resazurin conversion (Figure 1(a)). Mitochondrial activity of SCP-1 cells exposed to nicotine and cotinine was not significantly affected (Figure 1(a)). The differentiation status of the SCP-1 cells was evaluated by AP activity and matrix mineralization: AP activity, an early marker of osteogenic differentiation [34], after 14 days and the production of matrix mineralization, a late marker of osteogenic differentiation [34], after 21 days. Similar to the viability of the cells, the AP activity and the matrix production were not affected by nicotine and cotinine (Figures 1(b) and 1(c)).
Figure 1.
Nicotine and cotinine do not affect osteogenic differentiation of SCP-1. SCP-1 cells were osteogenically differentiated with nicotine (50 and 320 ng/ml) or its primary metabolite cotinine (100 and 300 ng/ml). Cell viability by Resazurin conversion (a) and AP activity (b) was measured on day 14. (c) Matrix mineralization was evaluated by Alizarin red after 21 days. (d) Primary cilium length was measured on day 21. (e) DCFH-DA assay was used to detect ROS in SCP-1 cells exposed to nicotine and cotinine. 0.01% V/V H2O2 was used as a positive control. Each experiment was conducted at least four times independently with triplicate. The statistical significance was determined by the Kruskal-Wallis H test followed by Dunn's posttest. Data are represented as the mean ± SEM, and the significance was represented as ∗∗∗ p < 0.001 vs the control group.
Primary cilia, a microtubule-based organelle, have been shown to play an important role in the initiation of osteogenic differentiation of MSCs and also in the maintenance of function in the differentiated cells [35]. Therefore, the primary cilium structure was assessed by immunofluorescence staining of acetylated α-tubulin on SCP-1 cells differentiated in the presence of nicotine or cotinine for 21 days. Primary cilia on SCP-1 cells exposed to CSE showed a reduction of 62.5% in the length. However, nicotine and cotinine exposure did not affect the primary cilia structure of SCP-1 cells (Figure 1(d)). Representative immunofluorescence staining pictures of primary cilia are shown in Supplementary Figure 1.
Since the production of ROS is one of the best known negative consequences of cigarette smoking, we were interested in evaluating the effect of nicotine and cotinine in ROS production by SCP-1 cells. After exposure to CSE, the production of ROS by SCP-1 cells significantly increased; however, ROS levels did not increase after nicotine exposure. Surprisingly, its first metabolite showed a slight (not significant) increase in ROS production by SCP-1 cells, which seemed to be dose-dependent, concluding that nicotine and its first metabolite, cotinine, are not the direct effectors inducing ROS production in SCP-1 cells exposed to CSE. However, this data does not exclude that both substances might interfere with the cells' antioxidative defense mechanisms and thus indirectly favor the accumulation of ROS in the presence of CSE.
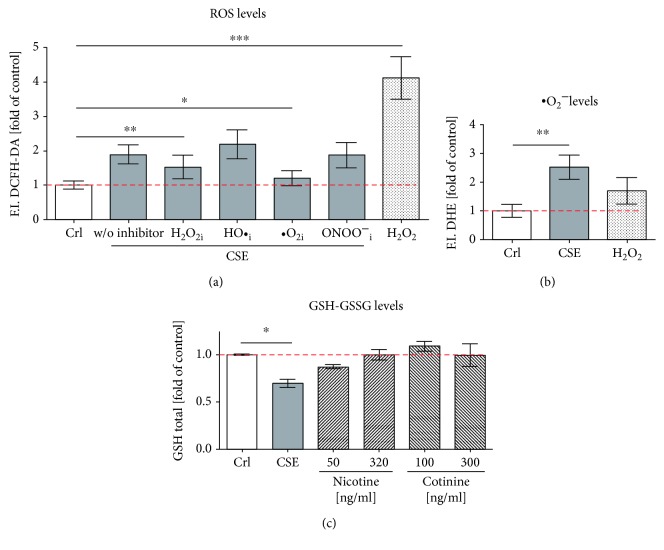
3.2. CSE Induced Oxidative Stress by Increasing ·O2 − and Reducing GST Activity in SCP-1 Cells
In order to better identify the ROS formed by CSE exposure, a DCFH-DA assay with several radical scavengers was performed [28, 31]. Exposure to CSE significantly (2 fold) induced ROS levels, measured by DCFH-DA assay. Incubation with 25 μM α-tocopherol, which traps ·O2 −, significantly reduced ROS levels in SCP-1 cells exposed to CSE. However, scavengers of H2O2 (10 mM sodium-pyruvate), HO· (250 mM DMSO), and ONOO− (100 μM uric acid) could not significantly reduce ROS levels in SCP-1 cells exposed to CSE (Figure 2(a)). In order to confirm that ·O2 − is induced by CSE in SCP-1 cells, a dihydroethidium (DHE) assay was performed. CSE exposure, but not nicotine and cotinine, significantly increased (2.5 fold) the level of ·O2 − (Figure 2(b)). Since GSH is the master antioxidant present in mammalian cells to prevent damage caused by ROS, the total GSH was measured by Ellman assay. SCP-1 cells exposed to CSE significantly decreased total GSH levels; nevertheless, nicotine and cotinine did not affect total GSH levels (Figure 2(c)). Thus, increased levels of ·O2− and decreased total GSH affect the antioxidant capacity of SCP-1 cells exposed to CSE.
Figure 2.
CSE induces oxidative stress with an increase in ·O2 and GSH reduction in SCP-1. SCP-1 cells were exposed to 5% CSE, and intracellular ROS and GSH levels were measured with different fluorescent probes: (a) DCFH-DA assay was used to detect ·O2 −, H2O2, HO·, and ONOO−. To trap different ROS, SCP-1 cells were coincubated with 25 μM α-tocopherol (·O2 i), 10 mM sodium-pyruvate (H2O2i), 250 mM DMSO (HO·i), or 100 μM uric acid (ONOO− i); (b) DHE assay was used to detect ·O2 −, and (c) Ellman assay was used to detect total GSH levels. Results were normalized to control SCP-1 cells (Crl). 0.01% V/V H2O2 was used as positive control (a) or negative control (b) of the assays. Each experiment was conducted at least four times independently with triplicate. The statistical significance was determined by the Kruskal-Wallis H test followed by Dunn's posttest. Data are represented as the mean ± SEM, and the significance was represented as ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001 vs the control group.
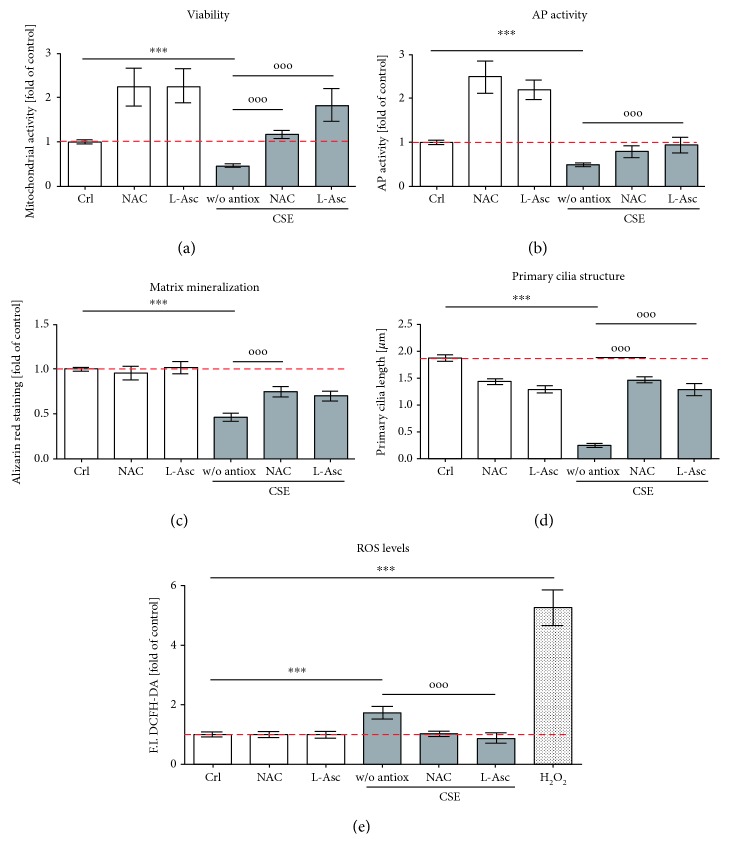
3.3. Antioxidants Rescued CSE-Impaired Osteogenesis in SCP-1 Cells
The overproduction of ROS as well as the decrease of intracellular GSH, beyond the antioxidant scavenging capacity of the cells, causes oxidative stress that disrupts the primary cilia structure, which in turn impairs osteogenic differentiation of SCP-1 cells [11]. Previous studies have demonstrated the positive effects of NAC and L-ascorbate on osteogenesis [17, 36–42]. Therefore, to protect SCP-1 cells from oxidative stress generated by CSE, the effect of NAC and L-ascorbate during osteogenic differentiation with CSE was evaluated. In order to determine the concentrations of NAC and L-ascorbate that were not toxic to the cells, SCP-1 cells were osteogenically differentiated in the presence of NAC (1 mM–30 mM) or L-ascorbate (200 μM, 1 mM) for 14 days. According to the mitochondrial activity and the total protein staining of the cells, the concentrations of 1 mM NAC and 200 μM L-ascorbate were used in the following experiments (Supplementary Figure 2). NAC and L-ascorbate significantly increased the mitochondrial activity of SCP-1 cells after 14 days of coincubation with CSE (Figure 3(a)). Their addition upregulated the AP activity and the matrix mineralization of SCP-1 cells differentiated with CSE after 14 and 21 days, respectively (Figures 3(b) and 3(c)). NAC and L-ascorbate restored the primary cilium structure on SCP-1 cells that was altered by CSE exposure (Figure 3(d)). Moreover, treatment with NAC or L-ascorbate significantly decreased ROS levels in SCP-1 cells exposed to CSE (Figure 3(e)). These results suggest that antioxidant treatment enhances primary cilium integrity and improves the osteogenic differentiation of SCP-1 cells exposed to CSE by decreasing oxidative stress.
Figure 3.
Antioxidants rescue CSE-impaired osteogenesis in SCP-1. SCP-1 cells were osteogenically differentiated with coincubation of antioxidants NAC 1 mM or L-Asc 200 μM and CSE 5%. After 14 days of treatment, (a) the viability of the cells was measured by Resazurin conversion. The differentiation status of the cells was evaluated by (b) AP activity at day 14 and (c) Alizarin red staining at day 21. (d) Primary cilium length was measured at day 21. (e) DCFH-DA assay was used to detect ROS in SCP-1 cells exposed to 5% CSE and coincubation of antioxidants NAC 1 mM or L-Asc 200 μM. 0.01% V/V H2O2 was used as positive control. Each experiment was conducted at least four times independently with triplicate. The statistical significance was determined by the Kruskal-Wallis H test followed by Dunn's posttest. Data are represented as the mean ± SEM, and the significance was represented as ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001 vs control and °p < 0.05, °°p < 0.01, and °°°p < 0.001 vs CSE.
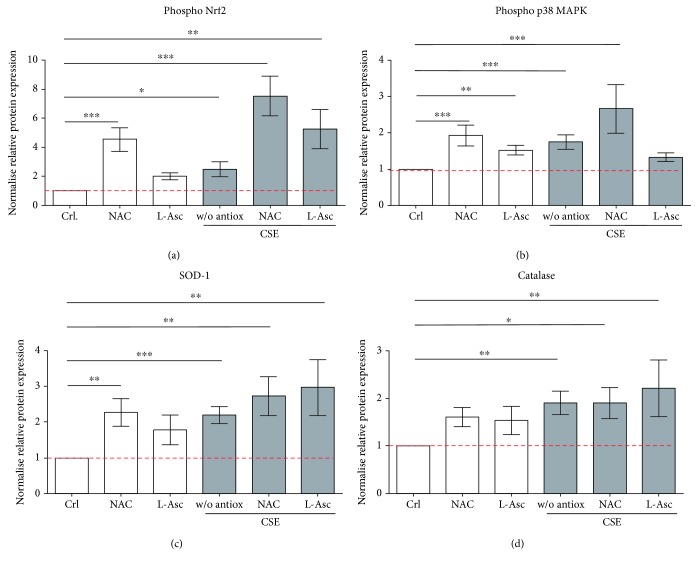
3.4. Nrf2 Signaling Was Activated by NAC and L-Ascorbate in SCP-1 Cells during Osteogenic Differentiation with CSE
Several studies have shown that redox-sensitive transcription factor Nrf2 plays an important role in cellular defense against oxidative stress by inducing the transcription of antioxidative enzymes [43, 44]. Therefore, the protective effect of the Nrf2 signaling pathway on SCP-1 cells, which were osteogenically differentiated and treated with antioxidants, was investigated. Western blot for phospho-Nrf2 and phospho-p38 MAPKinase was performed from SCP-1 cells differentiated with 5% CSE and 1 mM NAC or 200 μM L-ascorbate. CSE exposure significantly increased the active form of Nrf2 in response to the oxidative stress (Figure 4(a)). Costimulation with NAC or L-ascorbate increased the levels of phospho-Nrf2 compared with SCP-1 cells exposed to CSE alone (Figure 4(a)), suggesting that the NAC and L-ascorbate protective effect is through an Nrf2-dependent mechanism and not only due to their radical scavenging properties. Besides, NAC coincubation with CSE significantly increased the levels of phospho-p38 MAPKinase (Figure 4(b)). Therefore, NAC-dependent activation of Nrf2 could be via the activation of protein kinases such as p38, causing phosphorylation and subsequent release of Nrf2 from its inhibitory protein Keap-1 (Figure 5). However, coincubation of CSE with L-ascorbate had no effect on phospho-p38 MAPKinase levels (Figure 4(b)), suggesting that L-ascorbate might react with the thiol residues in Keap-1, increasing the cellular availability of Nrf2 (Figure 5). Since the activation of the transcription factor Nrf2 was induced by antioxidant treatment, it was interesting to investigate the expression of SOD-1 and catalase, the target enzymes of this pathway. SOD-1 catalyzes the dismutation of two molecules of ·O2 − to H2O2 and molecular oxygen O2 for further processing. Catalase catalyzes the reduction of H2O2 to water and O2, completing the detoxification process initiated by SOD-1 [45]. Similarly, to Nrf2 activation, SOD-1 and catalase protein expression was upregulated by NAC and L-ascorbate coincubation with CSE (Figure 4(c) and 4(d)). Thus, an increase of SOD-1 and catalase initiated by Nrf2 might be involved in the protective effect of NAC and L-ascorbate during osteogenic differentiation of SCP-1 cells exposed to CSE. Representative Western blot pictures are shown in Supplementary Figure 3.
Figure 4.
Nrf2-related signaling was activated by NAC and L-ascorbate in SCP-1 during osteogenic differentiation with CSE. SCP-1 cells were osteogenically differentiated with coincubation of antioxidants NAC 1 mM or L-Asc 200 μM and CSE 5%. After 14 days of treatment, phosphorylated Nrf2 (a), p38 MAPKinase (b), SOD-1 (c), and catalase (d) protein expression levels were detected by Western blot. GAPDH was used as internal control. Each experiment was conducted at least three times independently with triplicate. The statistical significance was determined by the Kruskal-Wallis H test followed by Dunn's posttest. Data are represented as the mean ± SEM, and the significance was represented as ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001 vs the control group.
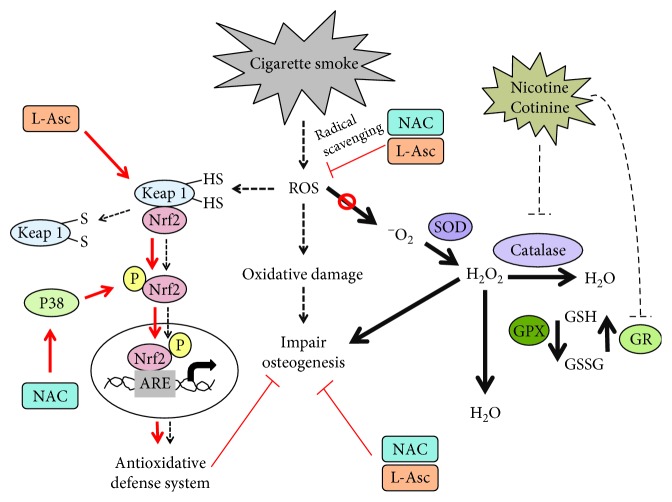
Figure 5.
NAC and L-ascorbate enhance the osteogenic differentiation in SCP-1 cells exposed to CSE by activation of Nrf-2 signaling and through radical scavenging. Proposed mechanisms for oxidative stress impair osteogenic differentiation under CSE exposure and potential roles of antioxidant. High-level oxidative stress generated by CSE resulted in oxidative damage and impaired SCP-1 cells' osteogenic differentiation. ROS induced through CSE exposure can oxidize the Cys residues on Keap-1, leading to the conformational change and releasing Nrf2. Phospho-Nrf2 can translocate to the nucleus and activates the antioxidant response element (ARE) leading to an activation of antioxidant genes. However, activation of Nrf2 in CSE exposure cells may not be enough to protect the cells from the oxidative stress generated by CSE. NAC activates upstream p38 MAPKinase, which is required to activate Nrf2 and transactivate antioxidant genes that may reduce oxidative stress induced by CSE. L-Asc might act with thiol residues of Keap-1, increasing the levels of Nrf2 available. CSE inhibited catalase activity being not able to process H2O2. GR activity is also affected by CSE to a decrease of total GSH. NAC and L-Asc treatment decreased CSE-induced ROS production by increasing the biosynthesis of GSH via Nrf2 signaling and also by radical scavenging. CSE decreased the enzymatic activity of SOD and catalase, leading to accumulation of ·O2 − and H2O2 in the cells. Additionally, CSE decreased total GSH and decreased GR activity causing that there is no GSH availed. Therefore, GPx cannot catalyze the reduction of H2O2 to H2O. Nicotine and cotinine, despite not affecting the osteogenic differentiation of the cells, evidenced negative inhibitory effects on the enzymatic activity of catalase and GR. Nicotine and cotinine imbalance the antioxidative system contributing in part to the negative effects in the osteogenic differentiation of SCP-1 cell exposure to CSE.
3.5. CSE, Nicotine, and Cotinine Generated an Imbalance in the Antioxidative System
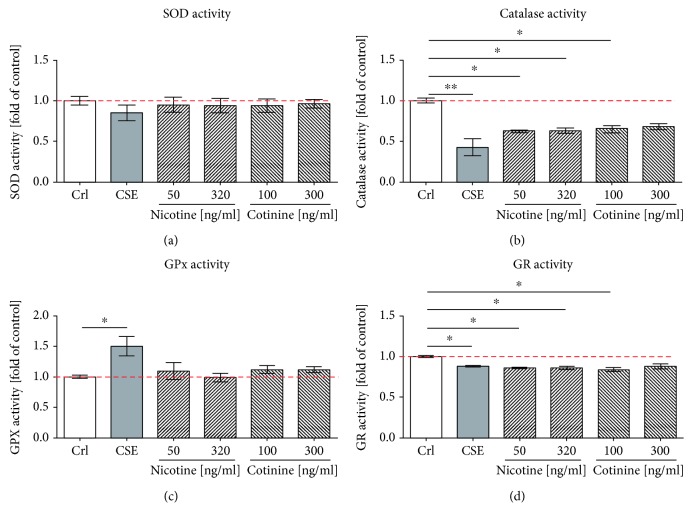
Since CSE exposure activated Nrf2 signaling and induced the protein expression of antioxidative enzymes, it was interesting to evaluate the effect of CSE on the activity of enzymes involved in ·O2 − detoxification and GSH recycling. Thus, we investigated the effect of CSE, nicotine, and cotinine on the activity of the isolated enzymes involved in mitochondrial antioxidative defense, namely, SOD, catalase, GPx, and GR. Interestingly, the presence of CSE significantly decreased the activity of catalase (Figure 6(a)) and slightly decreased the enzymatic activity of SOD (Figure 6(b)). This might explain the observed accumulation of ·O2 − and H2O2 in the CSE-treated cells. However, GPx can also catalyze the reduction of H2O2 to H2O via oxidation of reduced GSH into its disulfide form. The presence of CSE significantly increased GPx enzymatic activity (Figure 6(c)). At the same time, the presence of CSE significantly decreased the total GSH (Figure 2(c)) and significantly decreased the GR activity (Figure 6(d)), causing that there is no GSH availed in cells exposed to CSE. Nicotine and cotinine, despite not affecting the osteogenic differentiation of the cells, evidenced significant inhibitory effects on the enzymatic activity of catalase and GR. Therefore, an imbalance in the antioxidative system induced by the most addictive substance, its first metabolite, and other molecules present in cigarette smoke affects the osteogenic differentiation of SCP-1 cells.
Figure 6.
CSE generated an imbalance in the antioxidative system. Enzyme activities SOD (a), catalase (b), GPx (c), and GR (d) were determined with and without exposure to 5% CSE, 50 ng/ml or 320 ng/ml nicotine, and 100 ng/ml or 300 ng/ml cotinine. The enzymatic activity was expressed as the fold of control. Each experiment was conducted at least three times independently with triplicate. The statistical significance was determined by the Kruskal-Wallis H test followed by Dunn's posttest. Data are represented as the mean ± SEM, and the significance was represented as ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001 vs the control group.
4. Discussion
Cigarette smoke contains more than 150 of 6000 molecular substances that are considered toxic compounds [3, 4]. Nicotine is known as the major active and addictive compound associated with smoking. The role of nicotine on osteogenic differentiation is still unclear, as different effects have been shown dependent on the concentration and exposure time. On the one hand, it has been shown that nicotine induces apoptosis in human osteoblasts via increased ROS levels [8]. Moreover, reduced matrix formation was observed with SaOS-2 cells exposed to nicotine for 14 days [7]. On the other hand, it has been reported that nicotine increases osteoblast activity in bone marrow stromal cells [5]. In our experiments, blood nicotine concentrations did not affect the osteogenic differentiation of SCP-1 cells; besides, its first metabolite cotinine also did not influence the osteogenic differentiation of SCP-1 cells. These results point out the negative role of ROS induced by CSE during osteogenic differentiation. In the clinic, smokers often show delayed fracture healing, increased frequency of complications, and prolonged hospital stays [1, 2]. This led to the assumption that devices that administer nicotine, e.g., e-cigarettes, tobacco heat systems, nicotine sprays, or tapes, could be a less harmful alternative for smoking in orthopedic patients.
In line with this assumption, our results clearly show that only CSE exposure, but not nicotine and cotinine, increases the levels of ·O2 − while reducing the total GSH available in the cells. From these results, we endorse previous suggestions that an imbalance in the antioxidative system could be responsible for the impaired osteogenesis observed in SCP-1 cells after CSE exposure [46–48].
Since we observed a significant reduction in total GSH levels and a significant increase in ROS levels upon CSE exposure, we decided to use NAC as a precursor for GSH to support the osteogenic differentiation of SCP-1 cells exposed to CSE. NAC is a derivative of the amino acid L-cysteine, in which the thiol (sulfhydryl) group exhibits antioxidative effects by scavenging free radicals [49]. Previous studies have shown the beneficial effects of NAC on osteogenic differentiation [37–39]. Moreover, NAC has been shown to reverse the negative effects of H2O2 on osteogenic differentiation of MC3T3-E1 cells [17]. We used L-ascorbate as a potential treatment strategy due to the fact that smokers present lower levels of L-ascorbate in the blood than nonsmokers [50, 51]. Therefore, smokers require a higher daily intake of L-ascorbate to reach similar blood levels than nonsmokers due to an increased metabolic demand and a defective L-ascorbate recycling [51, 52]. Furthermore, L-ascorbate is known for stimulating proliferation of osteoblasts as well as for possessing free radical scavenging properties [41, 42]. Besides its antioxidative properties, L-ascorbate has been shown to support bone formation by stimulating the production of collagen [40]. Indeed, concentrations of 1 mM NAC and 200 μM L-ascorbate improved the differentiation of SCP-1 cells coincubated with CSE, resulting in an increased AP activity on day 14 of differentiation and an increased amount of mineralized matrix formed after 21 days of differentiation. The concentrations of L-ascorbate and NAC used in this study were higher than those found in blood plasma that can be obtained by oral supplementation (L-ascorbate 3 g every 4 h administered orally produces a peak plasma concentration of 200 μM [53]; NAC 600 mg oral administration produces a peak plasma concentration of 15 μM [54]). However, intravenous doses of L-ascorbate and NAC can produce plasma concentrations up to 800 μM [53] and 121 μM [55], respectively, suggesting the possibility of implementing intravenous applications of antioxidant treatment in smokers during the surgical reposition of fractures or even for a limited time during the process of fracture healing.
However, ROS levels are necessary to induce cellular responses. ROS induced by CSE exposure can oxidize the Cys residues on Keap-1, causing its conformational change and releasing Nrf2 for activation. The active phospho-Nrf2 can then translocate to the nucleus and, upon binding to the antioxidant response element (ARE), lead to the transcription of antioxidant enzymes like SOD, catalase, GPx, and others [56]. The impaired osteogenic function observed in the CSE-exposed SCP-1 cells suggests that a mechanism in this process fails or that the activation of Nrf2 in CSE-exposed cells may not be enough to protect the cells from the oxidative stress generated by CSE.
Addition of antioxidants like NAC and L-ascorbate partly restored the osteogenic differentiation of CSE-exposed SCP-1 cells. NAC and L-ascorbate treatment decreased CSE-induced ROS production. However, despite a strong activation of p38 MAPKinase signaling, which is required for the activation of Nrf2 [57, 58], NAC treatment could not significantly increase protein levels of activated Nrf2 or antioxidative enzymes, when compared to CSE exposure alone. Similarly, addition of L-ascorbate which might react with the thiol residues in Keap-1, increasing the Nrf2 availability, could not further increase the cellular levels of phospho-Nrf2 and antioxidative enzymes, when compared to CSE exposure alone.
In response to the oxidative stress induced by CSE exposure, we have shown that CSE can induce the antioxidant signaling pathways via its transcription factor phospho-Nrf2 and increase the protein expression of SOD-1 and catalase, suggesting that the cells react adequately to the stress stimuli. SOD and catalase are strongly upregulated during osteogenic differentiation of MSCs [59], suggesting key regulatory roles of these enzymes. In low amounts, H2O2 is involved in many cellular processes such as activation of signaling pathways involved in cell migration, proliferation, and differentiation [60]. Additionally, H2O2 has been shown to induce osteogenic differentiation of vascular smooth muscle cells through the increase of Runt-related transcription factor 2 (Runx2) a key transcription factor for osteogenesis [61]. However, with increasing amounts, H2O2 exerts damage to cellular macromolecules including proteins and DNA consequently, causing cell death [62–64].
Even so, SOD and catalase protein expression was induced upon stimulation with CSE, an impaired osteogenic function was still observed, suggesting that the function of the antioxidative enzymes during the differentiation with CSE fails. We could show that CSE as well as nicotine and cotinine strongly inhibits catalase activity, suggesting that the inhibitory effect of CSE on the enzymatic activity of catalase and SOD, contributed in part from nicotine and cotinine. This supposedly generated a feedback where the cells produce more antioxidative enzymes. However, in the presence of nicotine and cotinine, these enzymes cannot perform their function properly; thus, both substances seem to indirectly participate in the observed ROS accumulation by CSE. SOD being less affected converts ·O2 − and H2O2 which then accumulates in the cells as catalase is not able to further process the H2O2. Nicotine and cotinine, despite having no effect on osteogenic differentiation, also decreased catalase activity contributing to the negative effect observed with CSE, supporting previous results that demonstrated lower enzymatic activity of antioxidant enzymes in blood of smokers [65]. As GPx activity is not negatively affected by CSE, nicotine, or cotinine, it is conceivable that upregulation of GPx could compensate for the inhibition of catalase in the CSE-exposed cells. However, similar to catalase, GR activity is also affected by CSE, nicotine, and cotinine. Thus, these substances interfere with glutathione recycling, limiting the substrate for GPx and thus leading to a decrease in total GSH.
The observation that nicotine and cotinine inhibit catalase and GR function critically challenges the assumption that devices administering nicotine could be a less harmful alternative for smoking in orthopedic patients, as the trauma itself and the associated surgical intervention represent (oxidative) stress the body has to cope with.
5. Conclusions
In summary, our study shows for the first time that nicotine and cotinine do not directly affect osteogenic differentiation of MSCs; however, these compounds negatively affect the function of the antioxidative enzymes. Therefore, the most addictive compound present in cigarette smoke and its first metabolite contribute in part to the negative effects on osteogenic differentiation observed in MSC following CSE exposure.
Acknowledgments
We would like to thank Hanna Scheffler for her excellent technical assistance. This work was partially supported by the Elsbeth Bonhoff Foundation (D.30.21114).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
Romina H. Aspera-Werz and Sabrina Ehnert contributed equally to this work.
Supplementary Materials
Supplementary Figure 1: nicotine and cotinine not affected hMSC primary cilia structure. Immunostained images of primary cilia in SCP-1 cells osteogenically differentiated at day 14 without CSE (a), with 5% CSE (b), 320 ng/ml nicotine (c), and 300 ng/ml cotinine (d) exposed are shown. Primary cilium is shown by acetylated α-tubulin (green) and nucleus by Hoechst staining.
Supplementary Figure 2: toxicity test of NAC and L-Asc in hMSC. SCP-1 cells were osteogenically differentiated with increasing concentrations of NAC (a, b) and L-Asc (c, d). The toxicity was measure by Resazurin conversion (mitochondrial activity) (a–c) and SRB staining (total protein measure) (b–d) after 14 days. Each experiment was conducted at least four times independently with triplicate. The statistical significance was determined by the Kruskal-Wallis H test followed by Dunn's posttest. Data are represented as the mean ± SEM, and the significance is represented as ∗∗∗ p < 0.001 vs the control group.
Supplementary Figure 3: protein expression analysis of antioxidative enzymes. Representative Western blot pictures from phosphorylated Nrf2, phosphorylated p38 MAPKinase, SOD-1, catalase, and GAPDH are shown. SCP-1 cells were osteogenically differentiated with coincubation of antioxidants NAC 1 mM or L-Asc 200 μM and CSE 5%. After 14 days of treatment, protein expression level was detected.
References
- 1.Abate M., Vanni D., Pantalone A., Salini V. Cigarette smoking and musculoskeletal disorders. Muscle, Ligaments and Tendons Journal. 2013;3(2):63–69. doi: 10.11138/mltj/2013.3.2.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis J. A., Johnell O., Oden A., et al. Smoking and fracture risk: a meta-analysis. Osteoporosis International. 2005;16(2):155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 3.Rothem D. E., Rothem L., Soudry M., Dahan A., Eliakim R. Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. Journal of Bone and Mineral Metabolism. 2009;27(5):555–561. doi: 10.1007/s00774-009-0075-5. [DOI] [PubMed] [Google Scholar]
- 4.Pappas R. S. Toxic elements in tobacco and in cigarette smoke: inflammation and sensitization. Metallomics. 2011;3(11):1181–1198. doi: 10.1039/c1mt00066g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daffner S. D., Waugh S., Norman T. L., Mukherjee N., France J. C. Nicotine increases osteoblast activity of induced bone marrow stromal cells in a dose-dependent manner: an in vitro cell culture experiment. Global Spine Journal. 2012;2(3):153–158. doi: 10.1055/s-0032-1326946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B. S., Kim S. J., Kim H. J., et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sciences. 2012;90(3-4):109–115. doi: 10.1016/j.lfs.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka H., Tanabe N., Suzuki N., et al. Nicotine affects mineralized nodule formation by the human osteosarcoma cell line saos-2. Life Sciences. 2005;77(18):2273–2284. doi: 10.1016/j.lfs.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Marinucci L., Balloni S., Fettucciari K., Bodo M., Talesa V. N., Antognelli C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H2O2 and entailing glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: implication for smokers-related osteoporosis. Free Radical Biology & Medicine. 2018;117:6–17. doi: 10.1016/j.freeradbiomed.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz N. L., Hukkanen J., Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of Experimental Pharmacology. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kode A., Rajendrasozhan S., Caito S., Yang S. R., Megson I. L., Rahman I. Resveratrol induces glutathione synthesis by activation of nrf 2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2008;294(3):L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 11.Sreekumar V., Aspera-Werz R., Ehnert S., et al. Resveratrol protects primary cilia integrity of human mesenchymal stem cells from cigarette smoke to improve osteogenic differentiation in vitro. Archives of Toxicology. 2018;92(4):1525–1538. doi: 10.1007/s00204-017-2149-9. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q. Role of nrf 2 in oxidative stress and toxicity. Annual Review of Pharmacology and Toxicology. 2013;53(1):401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo S., Fei H. D., Ji F., Chen F. L., Xie Y., Wang S. G. Activation of nrf 2 by mind4-17 protects osteoblasts from hydrogen peroxide-induced oxidative stress. Oncotarget. 2017;8(62):105662–105672. doi: 10.18632/oncotarget.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massague J., Weis-Garcia F. Serine/threonine kinase receptors: mediators of transforming growth factor beta family signals. Cancer Surveys. 1996;27:41–64. [PubMed] [Google Scholar]
- 15.Rangasamy T., Cho C. Y., Thimmulappa R. K., et al. Genetic ablation of nrf 2 enhances susceptibility to cigarette smoke-induced emphysema in mice. The Journal of Clinical Investigation. 2004;114(9):1248–1259. doi: 10.1172/JCI200421146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blake D. J., Singh A., Kombairaju P., et al. Deletion of keap 1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. American Journal of Respiratory Cell and Molecular Biology. 2010;42(5):524–536. doi: 10.1165/rcmb.2009-0054OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee D., Kook S. H., Ji H., et al. N-Acetyl cysteine inhibits H2O2-mediated reduction in the mineralization of MC3T3-E1 cells by down-regulating Nrf2/HO-1 pathway. BMB Reports. 2015;48(11):636–641. doi: 10.5483/BMBRep.2015.48.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y. X., Xu A. H., Yang Y., Li J. Role of nrf 2 in bone metabolism. Journal of Biomedical Science. 2015;22(1):p. 101. doi: 10.1186/s12929-015-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y. X., Li L., Corry K. A., et al. Deletion of nrf 2 reduces skeletal mechanical properties and decreases load-driven bone formation. Bone. 2015;74:1–9. doi: 10.1016/j.bone.2014.12.066. [DOI] [PubMed] [Google Scholar]
- 20.Braun K. F., Ehnert S., Freude T., et al. Quercetin protects primary human osteoblasts exposed to cigarette smoke through activation of the antioxidative enzymes ho-1 and sod-1. The Scientific World JOURNAL. 2011;11:2357. doi: 10.1100/2011/471426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su Y., Han W., Giraldo C., De Li Y., Block E. R. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. American Journal of Respiratory Cell and Molecular Biology. 1998;19(5):819–825. doi: 10.1165/ajrcmb.19.5.3091. [DOI] [PubMed] [Google Scholar]
- 22.Böcker W., Yin Z., Drosse I., et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing htert after lentiviral gene transfer. Journal of Cellular and Molecular Medicine. 2008;12(4):1347–1359. doi: 10.1111/j.1582-4934.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehnert S., Freude T., Ihle C., et al. Factors circulating in the blood of type 2 diabetes mellitus patients affect osteoblast maturation - description of a novel in vitro model. Experimental Cell Research. 2015;332(2):247–258. doi: 10.1016/j.yexcr.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Ehnert S., Zhao J., Pscherer S., et al. Transforming growth factor β1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of ski-related novel protein N (SnoN): possible mechanism for the failure of BMP therapy? BMC Medicine. 2012;10(1):p. 101. doi: 10.1186/1741-7015-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehnert S., Baur J., Schmitt A., et al. TGF-β1 as possible link between loss of bone mineral density and chronic inflammation. PLoS One. 2010;5(11, article e14073) doi: 10.1371/journal.pone.0014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skehan P., Storeng R., Scudiero D., et al. New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 27.Dummer A., Poelma C., DeRuiter M. C., Goumans M. J. T. H., Hierck B. P. Measuring the primary cilium length: improved method for unbiased high-throughput analysis. Cilia. 2016;5(1):p. 7. doi: 10.1186/s13630-016-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco R., Panayiotidis M. I., Cidlowski J. A. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. The Journal of Biological Chemistry. 2007;282(42):30452–30465. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalyanaraman B., Darley-Usmar V., Davies K. J. A., et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Biology & Medicine. 2012;52(1):1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W., Burkhardt B., Fischer L., et al. Age-dependent changes of the antioxidant system in rat livers are accompanied by altered mapk activation and a decline in motor signaling. EXCLI Journal. 2015;14:1273–1290. doi: 10.17179/excli2015-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehnert S., Fentz A. K., Schreiner A., et al. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of ∗o2(−) and h2o2. Scientific Reports. 2017;7(1, article 14544) doi: 10.1038/s41598-017-14983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weydert C. J., Cullen J. J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nature Protocols. 2010;5(1):51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith I. K., Vierheller T. L., Thorne C. A. Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid) Analytical Biochemistry. 1988;175(2):408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- 34.Huang W., Yang S., Shao J., Yi-Ping L. Signalling and transcriptional regulation in osteoblast commitment and differentiation. Frontiers in Bioscience. 2007;12(8-12):p. 3068. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tummala P., Arnsdorf E. J., Jacobs C. R. The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cellular and Molecular Bioengineering. 2010;3(3):207–212. doi: 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehnert S., Döbele S., Braun K. F., et al. N-Acetylcyteine and flavonoid rich diet: the protective effect of 15 different antioxidants on cigarette smoke-damaged primary human osteoblasts. Advances in Bioscience and Biotechnology. 2012;3(8, article 25825):11. doi: 10.4236/abb.2012.38139. [DOI] [Google Scholar]
- 37.Yamada M., Tsukimura N., Ikeda T., et al. N-Acetyl cysteine as an osteogenesis-enhancing molecule for bone regeneration. Biomaterials. 2013;34(26):6147–6156. doi: 10.1016/j.biomaterials.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 38.Ji H., Liu Y., Zhao X., Zhang M. N-Acetyl-l-cysteine enhances the osteogenic differentiation and inhibits the adipogenic differentiation through up regulation of wnt 5a and down regulation of pparg in bone marrow stromal cells. Biomedicine & Pharmacotherapy. 2011;65(5):369–374. doi: 10.1016/j.biopha.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Jun J. H., Lee S. H., Kwak H. B., et al. N-Acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. Journal of Cellular Biochemistry. 2008;103(4):1246–1255. doi: 10.1002/jcb.21508. [DOI] [PubMed] [Google Scholar]
- 40.Ganta D. R., McCarthy M. B., Gronowicz G. A. Ascorbic acid alters collagen integrins in bone culture. Endocrinology. 1997;138(9):3606–3612. doi: 10.1210/endo.138.9.5367. [DOI] [PubMed] [Google Scholar]
- 41.Alcain F. J., Buron M. I. Ascorbate on cell growth and differentiation. Journal of Bioenergetics and Biomembranes. 1994;26(4):393–398. doi: 10.1007/BF00762780. [DOI] [PubMed] [Google Scholar]
- 42.Takamizawa S., Maehata Y., Imai K., Senoo H., Sato S., Hata R. Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin c derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biology International. 2004;28(4):255–265. doi: 10.1016/j.cellbi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen T., Nioi P., Pickett C. B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. Journal of Biological Chemistry. 2009;284(20):13291–13295. doi: 10.1074/jbc.r900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venugopal R., Jaiswal A. K. Nrf 1 and nrf 2 positively and c-fos and fra 1 negatively regulate the human antioxidant response element-mediated expression of nad (p)h:quinone oxidoreductase 1 gene. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ighodaro O. M., Akinloye O. A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine. 2017 doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 46.Bai X. C., Lu D., Bai J., et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by erk and nf-kappab. Biochemical and Biophysical Research Communications. 2004;314(1):197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 47.Lee D. H., Lim B. S., Lee Y. K., Yang H. C. Effects of hydrogen peroxide (h2o2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biology and Toxicology. 2006;22(1):39–46. doi: 10.1007/s10565-006-0018-z. [DOI] [PubMed] [Google Scholar]
- 48.Romagnoli C., Marcucci G., Favilli F., et al. Role of gsh/gssg redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like saos-2 cells. The FEBS Journal. 2013;280(3):867–879. doi: 10.1111/febs.12075. [DOI] [PubMed] [Google Scholar]
- 49.Aruoma O. I., Halliwell B., Hoey B. M., Butler J. The antioxidant action of n-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biology & Medicine. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-X. [DOI] [PubMed] [Google Scholar]
- 50.Kelly G. The interaction of cigarette smoking and antioxidants. Part iii: ascorbic acid. Alternative Medicine Review. 2003;8(1):43–54. [PubMed] [Google Scholar]
- 51.Smith J. L., Hodges R. E. Serum levels of vitamin c in relation to dietary and supplemental intake of vitamin c in smokers and nonsmokers. Annals of the New York Academy of Sciences. 1987;498:144–152. doi: 10.1111/j.1749-6632.1987.tb23758.x. [DOI] [PubMed] [Google Scholar]
- 52.Schectman G. Estimating ascorbic acid requirements for cigarette smokers. Annals of the New York Academy of Sciences. 1993;686:335–345. doi: 10.1111/j.1749-6632.1993.tb39197.x. [DOI] [PubMed] [Google Scholar]
- 53.Padayatty S. J., Sun H., Wang Y., et al. Vitamin c pharmacokinetics: implications for oral and intravenous use. Annals of Internal Medicine. 2004;140(7):533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 54.Borgström L., Kågedal B., Paulsen O. Pharmacokinetics of n-acetylcysteine in man. European Journal of Clinical Pharmacology. 1986;31(2):217–222. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- 55.Olsson B., Johansson M., Gabrielsson J., Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized n-acetylcysteine. European Journal of Clinical Pharmacology. 1988;34(1):77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- 56.Osburn W., Kensler T. Nrf 2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutation Research. 2008;659(1-2):31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma L., Liu J., Zhang X., Qi J., Yu W., Gu Y. P 38 mapk-dependent nrf 2 induction enhances the resistance of glioma cells against tmz. Medical Oncology. 2015;32(3):p. 69. doi: 10.1007/s12032-015-0517-y. [DOI] [PubMed] [Google Scholar]
- 58.Du Z. X., Yan Y., Zhang H. Y., et al. Proteasome inhibition induces a p 38 mapk pathway-dependent antiapoptotic program via nrf 2 in thyroid cancer cells. The Journal of Clinical Endocrinology and Metabolism. 2011;96(5):E763–E771. doi: 10.1210/jc.2010-2642. [DOI] [PubMed] [Google Scholar]
- 59.Chen C. T., Shih Y. R. V., Kuo T. K., Lee O. K., Wei Y. H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26(4):960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 60.Rhee S. G. Cell signaling. H2o2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 61.Byon C. H., Javed A., Dai Q., et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor runx 2 by akt signaling. The Journal of Biological Chemistry. 2008;283(22):15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duan J., Duan J., Zhang Z., Tong T. Irreversible cellular senescence induced by prolonged exposure to h2o2 involves DNA-damage-and-repair genes and telomere shortening. The International Journal of Biochemistry & Cell Biology. 2005;37(7):1407–1420. doi: 10.1016/j.biocel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Miranda D. D. C., Arcari D. P., Pedrazzoli J., et al. Protective effects of mate tea (Ilex paraguariensis) on H2O2-induced DNA damage and DNA repair in mice. Mutagenesis. 2008;23(4):261–265. doi: 10.1093/mutage/gen011. [DOI] [PubMed] [Google Scholar]
- 64.Barbouti A., Doulias P.-T., Nousis L., Tenopoulou M., Galaris D. Dna damage and apoptosis in hydrogen peroxide-exposed jurkat cells: bolus addition versus continuous generation of h2o2. Free Radical Biology and Medicine. 2002;33(5):691–702. doi: 10.1016/S0891-5849(02)00967-X. [DOI] [PubMed] [Google Scholar]
- 65.Raddam Q. N., Zeidan M. M., Asaad N. K., Abdulrahman M. A. Smoking effects on blood antioxidants level: lactate dehydrogenase, catalase, superoxide dismutase and glutathione peroxidase in university students. Journal of Clinical & Experimental Pathology. 2017;7(6) doi: 10.4172/2161-0681.1000331. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: nicotine and cotinine not affected hMSC primary cilia structure. Immunostained images of primary cilia in SCP-1 cells osteogenically differentiated at day 14 without CSE (a), with 5% CSE (b), 320 ng/ml nicotine (c), and 300 ng/ml cotinine (d) exposed are shown. Primary cilium is shown by acetylated α-tubulin (green) and nucleus by Hoechst staining.
Supplementary Figure 2: toxicity test of NAC and L-Asc in hMSC. SCP-1 cells were osteogenically differentiated with increasing concentrations of NAC (a, b) and L-Asc (c, d). The toxicity was measure by Resazurin conversion (mitochondrial activity) (a–c) and SRB staining (total protein measure) (b–d) after 14 days. Each experiment was conducted at least four times independently with triplicate. The statistical significance was determined by the Kruskal-Wallis H test followed by Dunn's posttest. Data are represented as the mean ± SEM, and the significance is represented as ∗∗∗ p < 0.001 vs the control group.
Supplementary Figure 3: protein expression analysis of antioxidative enzymes. Representative Western blot pictures from phosphorylated Nrf2, phosphorylated p38 MAPKinase, SOD-1, catalase, and GAPDH are shown. SCP-1 cells were osteogenically differentiated with coincubation of antioxidants NAC 1 mM or L-Asc 200 μM and CSE 5%. After 14 days of treatment, protein expression level was detected.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.