Abstract
Autophagy, a lysosomal degradation pathway, plays a crucial role in cellular homeostasis, development, immunity, tumor suppression, metabolism, prevention of neurodegeneration and lifespan extension. Thus, pharmacological stimulation of autophagy may be an effective approach for preventing or treating certain human diseases and/or aging. We sought to establish a method for developing new chemical compounds that specifically induce autophagy. To do this, we developed two assays to identify compounds that target a key regulatory node of autophagy induction – specifically, the binding of Bcl-2 (a negative regulator of autophagy) to Beclin 1 (an allosteric modulator of the Beclin 1/VPS34 lipid kinase complex that functions in autophagy initiation). These assays use either a split-luciferase assay to measure Beclin 1/Bcl-2 binding in cells or an AlphaLISA assay to directly measure direct Beclin 1/Bcl-2 binding in vitro. We screened two different chemical compound libraries, comprising ~300K compounds, to identify small molecules that disrupt Beclin 1/Bcl-2 binding and induce autophagy. Three novel compounds were identified that directly inhibit Beclin 1/Bcl-2 interaction with an IC50 in the micromolar range and increase autophagic flux. These compounds do not demonstrate significant cytotoxicity and they exert selectivity for disruption of Bcl-2 binding to the BH3 domain of Beclin 1 compared to the BH3 domain of the pro-apoptotic Bcl-2 family members, Bax and Bim. Thus, we have identified candidate molecules that serve as lead templates for developing potent and selective Beclin 1/Bcl-2 inhibitors that may be clinically useful as autophagy-inducing agents.
INTRODUCTION
Macroautophagy (hereafter referred to as autophagy) is a catabolic pathway by which cells sequester unwanted or damaged cellular proteins or organelles through a double membraned structure called the autophagosome. This process is mediated by a set of evolutionarily conserved genes, the autophagy-related (ATG) genes,1–2 which function in nucleation of the autophagosomal membrane, elongation of the autophagic membrane, sequestration of cytoplasmic constituents, closure of the double membrane, fusion with the lysosome, and degradation of the sequestered contents.
Autophagy plays significant physiological roles in cellular survival and stress adaptation,3 metabolism,4 development,5–6 immunity,7 protein and organellar homeostasis,8 and protection against aging.9 Moreover, several lines of evidence indicate a link between autophagy and mammalian diseases including diabetes, infection, cancer, neurodegenerative diseases, and aging.3, 9–10 Whole-body or tissue-specific genetic disruption of autophagy in mice leads to multiple pathologies, including tissue abnormalities, aberrant inflammation, impaired immunity, neurodegeneration, and susceptibility to tumorigenesis.11 In humans, mutations or polymorphisms in autophagy genes are associated with susceptibility to infection, cancer, inflammatory diseases, asthma, cerebral palsy, frontotemporal dementia, amyotrophic lateral sclerosis (ALS), Huntington’s disease, and Parkinson’s disease.11–14 Moreover, gain-of-function mutations or enforced expression of autophagy genes in mice have beneficial effects including improved metabolism and tissue function, extended lifespan, neuroprotection, and decreased tumorigenesis.11, 15–19 Thus, the development of autophagy-inducing agents may be a promising therapeutic approach to prevent and/or treat certain diseases in clinical medicine.11, 20–22
Several drugs that are currently in clinical trials or clinical use are known to induce autophagy11, 23; however, the effects of these drugs are pleiotropic and their actions are not limited to the autophagy pathway. Specifically, many drugs enhance autophagy through the modulation of upstream signaling pathways (such as mTOR inhibition, AMPK activation, calcium channel inhibition, and cAMP signaling) but they also regulate diverse downstream biological functions, thereby resulting in non-autophagy-related effects that may limit clinical utility.21 Therefore, to maximize benefits and minimize toxicity, there is an urgent need for autophagy-inducing agents that selectively target rate-limiting steps in autophagy execution rather than upstream signaling.
One of the key mechanisms that regulates autophagy initiation is the binding of Bcl-2 to Beclin 1, a scaffold protein that is an essential determinant of the activity of the Beclin 1-VPS34 class III phosphatidylinisotol 3 kinase complex.24–25 During basal conditions, autophagy levels are constrained by the binding of Bcl-2 (or its related family member, Bcl-xL) to Beclin 1. In response to stressful stimuli (such as nutrient starvation,24, 26 JNK activation,26 ceramide,27 or immunological signaling28), the disruption of the Beclin 1/Bcl-2 complex leads to autophagy upregulation in vitro. This disruption can be mediated by multi-site phosphorylation of the non-structured loop of Bcl-2,26 regulatory phosphorylation of the BH3 domain (an amphipathic alpha helix) of Beclin 1 that reduces its affinity for Bcl-2,29–30 or BH3-only proteins that competitively disrupt Beclin 1/Bcl-2 binding.31 Genetically engineered mice with mutations in the Bcl-2 phosphorylation sites required for disruption of Beclin 1/Bcl-2 binding are deficient in starvation- and exercise-induced autophagy in vivo, and fail to experience beneficial metabolic effects of long-term exercise training.32
Conversely, knock-in mice with a point mutation in Beclin 1 (F123A in human protein, F121A in mouse protein) that reduces its binding affinity in vitro for Bcl-2 and Bcl-xL results in increased constitutive autophagy in multiple tissues, including brain, heart, muscle, liver, mammary gland and kidney.17–19 These mice demonstrate increased longevity and diminished aging-related phenotypes, particularly age-related renal and cardiac pathological changes and age-related spontaneous tumorigenesis. The Beclin 1 knock-in mutation also decreases the accumulation of amyloid oligomers and improves cognitive function and survival in a mouse model of Alzheimers-like disease18 and decreases the incidence of breast cancer in a mouse model of HER2-driven tumorigenesis.19
Thus, there is compelling genetic evidence that Beclin 1/Bcl-2 interaction serves as an important checkpoint for autophagy induction in vivo. Importantly, the long-term disruption of this complex is not only safe in mice, but it also improves healthspan, extends lifespan, and protects against neurodegenerative diseases and cancers. These in vivo findings, taken together with the extensive in vitro data about the role of Beclin 1/Bcl-2 in autophagy regulation,25, 30 provide a strong rationale for the development of new autophagy-inducing strategies that target Beclin 1/Bcl-2 binding.
An important challenge of this approach is posed by the overlapping modality by which the BH3 domain of the autophagic protein Beclin 1 and that of pro-apoptotic proteins bind to the conserved hydrophobic grooves of Bcl-2/Bcl-xL.33–34 BH3 mimetics (e.g. ABT-737, ABT-263, ABT-199) have been developed that disrupt Bcl-2 (and/or Bcl-xL) binding of Beclin 1 (and thereby induce autophagy)31, 35 but they also disrupt binding between anti-apoptotic Bcl-2 family members and pro-apoptotic BH3 domains (and thereby induce apoptosis).36 These compounds were optimized for their apoptosis-inducing activity and use as potential cancer chemotherapeutic agents. However, from the standpoint of treating infectious diseases and neurodegeneration and preventing aging (as well as other pathophysiological contexts where upregulation of autophagy may be beneficial), it would be desirable to develop agents that selectively disrupt Beclin 1/Bcl-2 binding but not Bcl-2 family member/pro-apoptotic family member binding to selectively induce autophagy (a pro-survival pathway) without inducing apoptosis. This may be technically feasible since the binding affinity of the BH3 domain of Beclin 1 to Bcl-xL is considerably lower than that of pro-apoptotic BH3 family members,34, 37–38 thus providing a potential therapeutic window for selective disruption of Beclin 1 binding to Bcl-2/Bcl-xL. Furthermore, detailed biochemical and biophysical analyses of BH3 peptide binding to Bcl-2 family members indicate that differences in the precise binding modalities of different BH3 domains exist that might be exploited therapeutically.33–34, 39 Therefore, we sought to identify novel autophagy-inducing drugs that target the Beclin 1/Bcl-2 interaction without perturbing the binding of Bcl-2 to pro-apoptotic BH3 domain-containing molecules.
We employed a high-throughput screening (HTS) platform using either a cell-based split-luciferase or in vitro AlphaLISA assays to identify novel Beclin 1/Bcl-2 binding inhibitors. Using chemical libraries comprising ~300,000 compounds from the UT Southwestern Medical Center and the Broad Institute of MIT and Harvard, we identified three small molecule compounds that both disrupt Beclin 1/Bcl-2 interaction in the cell-based split-luciferase assay and directly inhibit Beclin 1/Bcl-2 interaction in the in vitro AlphaLISA assay with micromolar IC50 values. These three compounds induce autophagic flux in cells at concentrations that do not decrease cell viability. Our biochemical data indicate that these compounds inhibit the interaction between the BH3 domain of Beclin 1 and Bcl-2 without affecting the interaction between the BH3 domain of the pro-apoptotic protein, Bax, and Bcl-2 or between the BH3-only protein, Bim, and Bcl-2, suggesting that these compounds are selective Beclin 1/Bcl-2 inhibitors. Overall, this screening program identified compounds that selectively disrupt Beclin 1/Bcl-2 interaction and which may serve as good starting points for the future development of more selective and potent Beclin 1/Bcl-2 inhibitors.
RESULTS AND DISCUSSION
Development of Beclin 1/Bcl-2 Split-luciferase Assay
To identify novel compounds that disrupt Beclin 1/Bcl-2 interaction, we developed two new HTS assays designed in parallel to (1) identify compounds that have cell penetration activity and function in a cell-based assay to disrupt Beclin 1/Bcl-2 interaction; and (2) identify compounds that disrupt Beclin 1/Bcl-2 binding in vitro and therefore can be verified to function on-target in the cell-based assay.
The first HTS employed a cell-based split-luciferase assay, which is a proximity-based enzyme complementation reporter system. The split-luciferase approach relies on the reconstitution of two fragments of firefly luciferase, NLuc (amino acids 2–416) and CLuc (amino acids 398–550) expressed with proteins of interest as fusion partners. Upon the binding of interacting partners, two non-functional fragments of the luciferase are brought into proximity, forming an active luciferase protein. To measure Beclin 1/Bcl-2 interaction with the split-luciferase assay, we created HeLa cell lines expressing N-terminal NLuc-tagged Beclin 1 (NLuc-Beclin 1) and CLuc-tagged Bcl-2 (CLuc-Bcl-2) as split-luciferase reporters (Figure 1A and B). Both reporters were expressed under the control of a tetracycline-inducible promoter to avoid toxicity or clonal adaptations that might potentially occur as a result of constitutive overexpression of Beclin 1 or Bcl-2. Renilla luciferase was constitutively expressed as an internal control. The interaction of Beclin 1/Bcl-2 was measured as relative luminescence units (RLU), which was the calculated ratio of split-luciferase and Renilla luciferase signals:
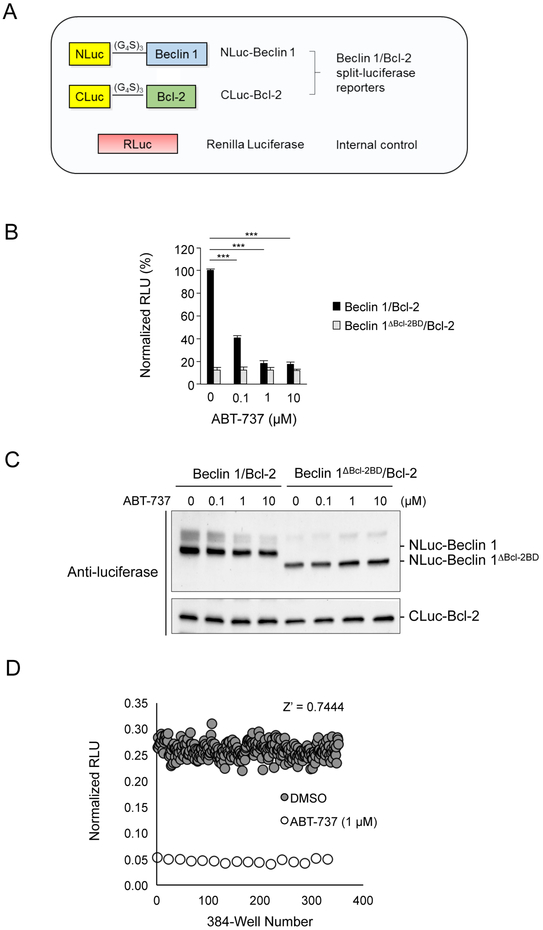
Figure 1.
Development of Beclin 1/Bcl-2 split-luciferase assay. (A) Schematic diagram showing constructs used in Beclin 1/Bcl-2 split-luciferase assay. (B) Split-luciferase activity measuring the Beclin 1 and Bcl-2 (black bars) or Beclin 1ΔBcl−2BD and Bcl-2 (gray bars) interaction in the presence of increasing concentrations of the BH3 mimetic compound, ABT-737 (treatment duration, 4 h). Results are shown as normalized relative luminescence units (RLU), which is the ratio of split-luciferase activity to Renilla luciferase control activity. The value for Beclin 1/Bcl-2 interaction in the absence of ABT-737 was arbitrarily set as 100%. Results shown are mean ± s.d. from a representative experiment. ***p<0.001; Student’s t-test. Similar results were obtained in three independent experiments. (C) Western blot analysis of NLuc-Beclin 1 and CLuc-Bcl-2 expression with anti-luciferase antibody in cell lysates used in experiment shown in (B). (D) Uniformity assay showing Z’ factor test to validate the Beclin 1/Bcl-2 split-luciferase in a 384-well plate HTS format. DMSO (gray circles, 240 wells) or ABT-737 (white circles, 16 wells) were used as neutral or positive controls, respectively.
The expression of Beclin 1 and Bcl-2 split-luciferase reporters in HeLa cells yielded measurable luminescence activity that was inhibited in a dose-dependent manner by the positive control compound, ABT-737, a potent BH3 mimetic40 (Figure 1B). To confirm that Beclin 1/Bcl-2 binding is required for the measured split-luciferase activity, we also created cell lines expressing an NLuc-Beclin 1 reporter carrying a deletion of the Bcl-2-binding domain of Beclin 1 (lacking amino acids 81–151; referred to as “Beclin 1ΔBcl−2BD”) and CLuc-Bcl-2. The expression of Beclin 1ΔBcl−2BD/Bcl-2 split-luciferase reporters resulted in 10- to 20-fold reduction of luminescence activity and the baseline activity of Beclin 1ΔBcl−2BD/Bcl-2 split-luciferase reporters was not affected by ABT-737 (Figure 1B). Thus, the full-length Beclin 1/Bcl-2 split-luciferase activity likely represents a specific interaction between the BH3 domain of Beclin 1 and Bcl-2 rather than spontaneous re-association of luciferase fragments. Overall, these data demonstrate that the HeLa cell Beclin 1/Bcl-2 split-luciferase assay is robust and sensitive, providing a wide dynamic range for measuring Beclin 1/Bcl-2 interaction.
We validated the Beclin 1/Bcl-2 split-luciferase assay for HTS with a Z’ factor test. The uniformity of the Beclin 1/Bcl-2 split-luciferase assay in a 384-well HTS format was examined (Figure 1D). DMSO (240 wells) and ABT-737 (16 wells) were used as neutral or positive controls, respectively. The calculated Z’ value was 0.7444, indicating that Beclin 1/Bcl-2 split-luciferase assay is well-suited for HTS.
Development of Beclin 1/Bcl-2 AlphaLISA Assay
We also developed a high-throughput Beclin 1/Bcl-2 AlphaLISA assay to directly measure the in vitro interaction between Beclin 1 and Bcl-2 (Figure 2A). AlphaLISA is a bead-based proximity assay that is capable of measuring protein-protein interaction in homogeneous solution.41 Oxygen singlet molecules are generated by Alpha donor beads upon irradiation at 680 nm and travel to acceptor beads in proximity. Oxygen singlets excite donor beads and result in luminescence emission at 615 nm. The half-life of oxygen singlet molecules is extremely short such that efficient energy transfer occurs only within a radius of 200 nm. Thus, a measurable Alpha luminescence signal requires chemical energy transfer between the donor and acceptor beads, and a pair of interacting molecules immobilized on the beads stabilizes bead association to produce measurable Alpha signals.
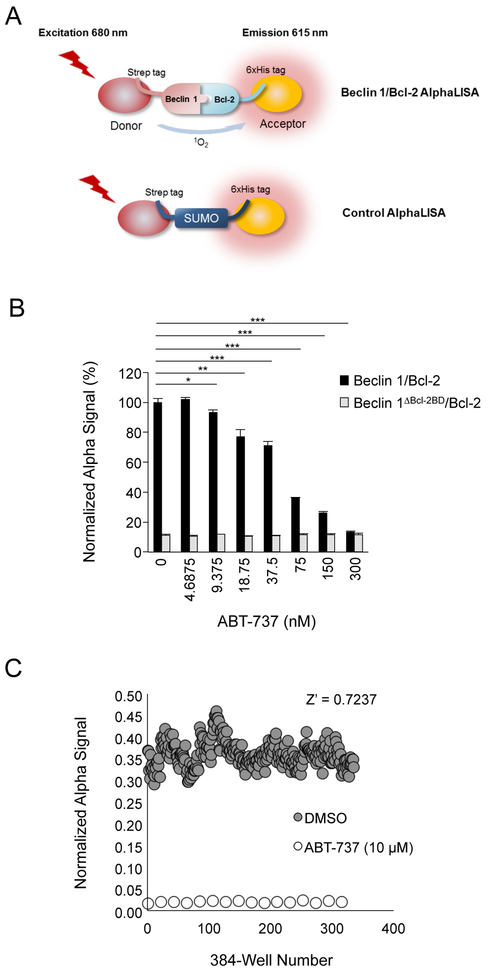
Figure 2.
Development of Beclin 1/Bcl-2 AlphaLISA assay. (A) Schematic diagram showing constructs used in Beclin 1/Bcl-2 AlphaLISA assay. (B) AlphaLISA assay activity measuring the Beclin 1 and Bcl-2 (black bars) or Beclin 1ΔBcl−2BD and Bcl-2 (gray bars) interaction in the presence of increasing concentrations of ABT-737 (treatment duration, 4 h). Results shown are mean ± s.d. from a representative experiment. **p<0.05; **p<0.01; ***p<0.001; Student’s t-test. Similar results were obtained in three independent experiments. (C) Uniformity assay showing Z’ factor test to validate the Beclin 1/Bcl-2 AlphaLISA asay in a 384-well plate HTS format. DMSO (gray circles, 240 wells) or ABT-737 (white circles, 16 wells) were used as neutral or positive controls, respectively.
We used purified recombinant Beclin 1 and Bcl-2 proteins for AlphaLISA, which were optimized for enhanced solubility and expression (Supporting Information Supplementary Figure 1). Human Beclin 1 was expressed as a fusion protein with StrepII-SUMO at the N-terminus, and three residues on the aromatic finger of Beclin 1 were mutated (F359D/F360D/W361D) to improve solubility and protein stability.42 These mutations are in the BARA domain, which is far from the BH3 motif recognized by Bcl-2. A recombinant Bcl-2-binding deficient mutant of Beclin 1 (StrepII-SUMO-Beclin 1ΔBcl−2BD) was used as a negative control. The Bcl-2 construct was composed of amino acids 1–218 and truncated at the transmembrane domain for the addition of a C-terminal 6xHis tag (Bcl-2–6xHis). For a normalization control, we included a parallel AlphaLISA assay with purified SUMO protein (with an N-terminal StrepII tag and a C-terminal 6xHis tag). The purpose of a normalization control is to eliminate inner filter (false-positive) hits that reduce luminescence signal by interfering with the AlphaLISA assay in ways that are unrelated to the Beclin 1/Bcl-2 interaction. With the normalization control, the interaction of Beclin 1 and Bcl-2 was measured as follows:
The incubation of StrepII-SUMO-Beclin 1 and Bcl-2–6xHis with AlphaLISA beads produced a strong Alpha signal (Figure 2B). Deletion of the Bcl-2-binding domain of Beclin 1 almost completely abolished the Alpha signal, indicating that the Alpha signal is specific to Beclin 1/Bcl-2 interaction (Figure 2B). Furthermore, the strong Alpha signal of Beclin 1/Bcl-2, but not the weak Beclin 1ΔBcl−2BD/Bcl-2 signal, was inhibited in a dose-dependent manner by the BH3 mimetic, ABT-737, which was used as a positive control for the screen.
These data demonstrate that this assay is suitable for measuring pharmacological inhibition of Beclin 1/Bcl-2 binding in vitro. To further evaluate the Beclin 1/Bcl-2 AlphaLISA assay as an HTS platform, we performed a Z’ factor test using a 384-well plate format. DMSO (240 wells) and ABT-737 (16 wells) were used as a neutral or positive control, respectively. The resulting Z’ value was 0.7237, indicating that the Beclin 1/Bcl-2 AlphaLISA assay is a robust HTS assay (Figure 2C).
Primary and Secondary Screens for Disruptors of Beclin 1/Bcl-2 Binding
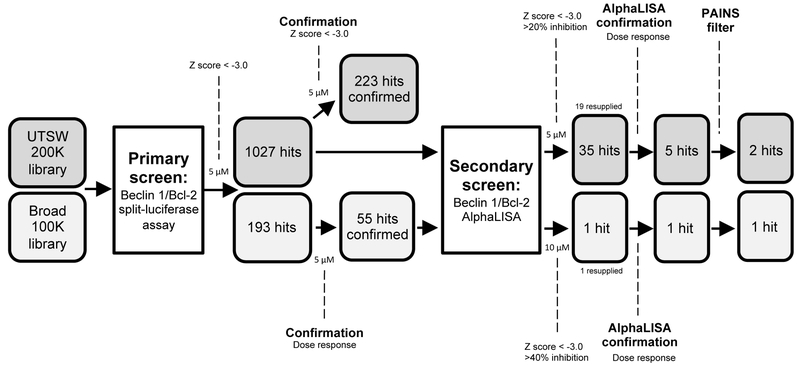
Using the Beclin 1/Bcl-2 split-luciferase assay, we performed a primary HTS with chemical libraries comprising ~200,000 compounds from UT Southwestern Medical Center (UTSW) and 100,000 compounds derived from diversity-oriented synthesis (DOS) at the Broad Institute of MIT and Harvard.43 The UTSW chemical library is composed of 75,000 compounds purchased from ChemBridge Corporation, 100,000 compounds purchased from Chemical Diversity Labs, 22,000 compounds from ComGenex, 1200 purchased from TimTek, 1100 purchased from Prestwick, and 450 drugs from the NIH clinical collection. Compounds purchased from TimTek are “natural product-like” synthetic compounds, and the Prestwick compounds are off-patent drugs. The NIH clinical collection is composed of compounds that have been tested in phase I clinical trials. The UTSW chemical library also contains approximately 30,000 natural products isolated from unique marine bacteria by Dr. John MacMillan (UC Santa Cruz). The compounds in the library satisfy a relaxed set of Lipinsky’s rules, with 99% having a molecular weight less than 550 g/mol (average 250–300 g/mol). All library compounds were screened at a concentration of 5 μM in a 384-well plate HTS format. During screening, we noticed that a large number of library compounds strongly increased Renilla Luciferase activity but did not affect split-luciferase activity. Thus, to eliminate such false-positive hits, we applied a Z-score cut-off of −3.0 on both split-luciferase activity and normalized activity (RLU). Of the identified 1027 hits from the UTSW library and 193 hits from the Broad library, 233 and 55 hits were subsequently confirmed in a repeat HTS assay, respectively (Figure 3).
Figure 3.
HTS algorithm for identifying compounds that disrupt Beclin 1/Bcl-2 interaction. Compounds that demonstrated Z-score ≤ −3.0 in primary and confirmation Beclin 1/Bcl-2 split-luciferase HTS screens were considered as initial hits. A selection of cherry-picked compounds (1027 from UTSW library and 55 from Broad library) was subjected to secondary screening using the Beclin 1/Bcl-2 AlphLISA HTS assay. In the secondary screen, compounds that demonstrated >20% (UTSW library) or >40% (Broad library) inhibition with a Z-score ≤ −3.0 were further analyzed in dose-response studies. A pan assay interference compound (PAINS) filter was applied to remove potential promiscuous inhibitors.
To identify compounds that directly inhibit Beclin 1/Bcl-2 interaction, a selection of cherry-picked compounds (1027 from the UTSW library and 55 from the Broad library) was subjected to a secondary screen with the Beclin 1/Bcl-2 AlphLISA assay. In the secondary HTS screen, we identified 35 (UTSW library) and one (Broad library) compound which demonstrated >20% (UTSW library) or >40% (Broad library) inhibition with a Z-score ≤ −3.0. We resupplied 19 compounds for additional dose-response AlphaLISA (natural product fractions and compounds that were unavailable were excluded). Six of the resupplied compounds were found to inhibit Beclin 1/Bcl-2 interaction in vitro in a dose-dependent manner (Supporting Information Supplementary Figure 2). After removing pan-assay interference (PAINS)44 compounds, there were two compounds (SW063058 and SW076956) from the UTSW library and one compound (BRD1991) from the Broad library chosen for further hit validation and biological investigations (Figure 3 and 4A).
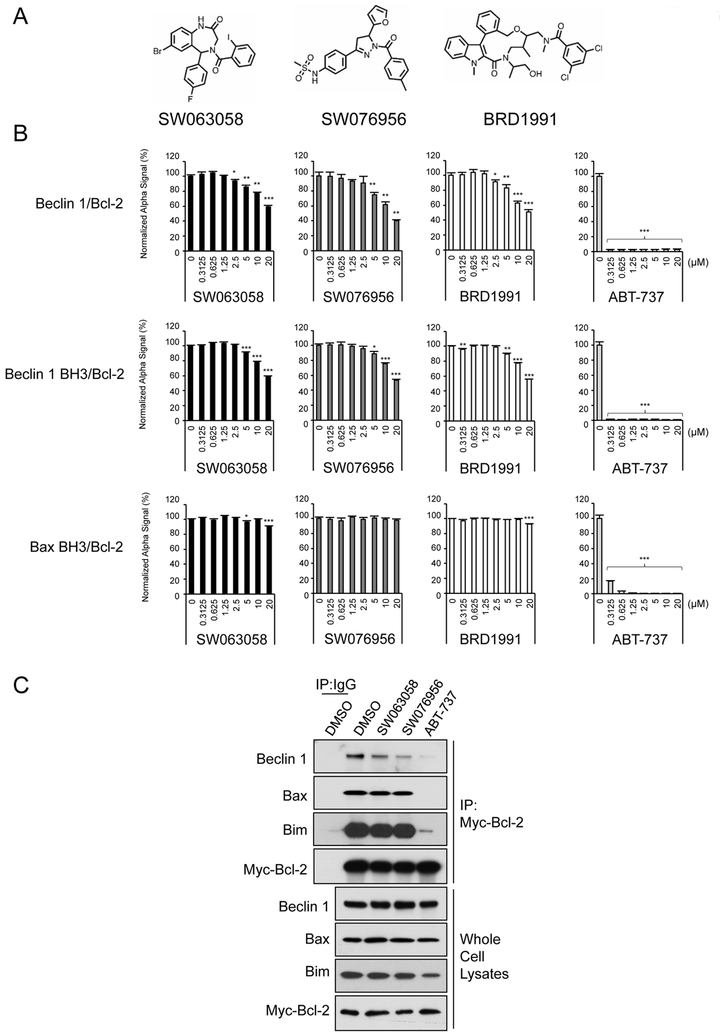
Figure 4.
Hit validation and assessment of selectivity. (A) Structure of three compounds chosen for further validation and biological assays. (B) Dose-response curves of the effects of SW063058, SW076956 and BRD1991 on inhibition of Beclin 1/Bcl-2 interaction. Serially-diluted compounds (2-fold, 7 points) were tested simultaneously in the Beclin 1/Bcl-2, Beclin 1 BH3 peptide/Bcl-2, or Bax BH3 peptide/Bcl-2 AlphaLISA. Results shown are mean ± s.d. from a representative experiment. Similar results were observed in three independent experiments. p-values were calculated by pairwise comparisons to DMSO control. *p<0.05; **p<0.01; ***p<0.001; Student’s t-test. (C) Co-immunoprecipitation of Beclin 1, Bax or Bim with Myc-Bcl-2 in HeLa/Myc-Bcl-2 cells treated with 20 μM indicated compound for 12 h prior to analysis. Immunoprecipitation was performed with an anti-Myc antibody followed by western blot analysis of indicated protein.
Hit Validation and Assessment of Selectivity
We confirmed that these three candidate compounds showed a dose-dependent inhibition of Beclin 1/Bcl-2 binding at an IC50 in the micromolar range using the AlphaLISA (Figure 4B). To assess selectivity for disruption of binding of the BH3 domain of Beclin 1 to Bcl-2 as compared to that of a pro-apoptotic Bcl-2 family member, we used an AlphaLISA with purified recombinant Bcl-2 and peptides spanning either the BH3 domain (amino acids 105–130) of Beclin 1 or the BH3 domain (amino acids 49–84) of Bax. Our results indicate that, at least within tested ranges, all three candidate molecules decreased the binding of the BH3 domain of Beclin 1 but not the BH3 domain of Bax to Bcl-2 (Figure 4B). In contrast, ABT-737 inhibited the binding of the BH3 domains of both Beclin 1 and Bax to Bcl-2 with nanomolar efficiency. Although it is still possible that SW063058, SW076956 and BRD1991 might disrupt the binding of Bcl-2 to Bax or other pro-apoptotic BH3 domain-containing molecules at a higher concentration (>20 μM), these data clearly demonstrate a window for selective inhibition of Beclin 1/Bcl-2 interaction. The window for selectivity may be further improved by subsequent structure-activity relationship (SAR) studies.
For the two compounds for which we had sufficient supply for large-scale tissue culture experiments (SW063058 and SW076956), we examined their effects on Beclin 1, Bax, and Bim co-immunoprecipitation with Bcl-2 (Figure 4C). Using previously described HeLa cells that stably express Myc-tagged Bcl-226, we observed that 12 h treatment with SW063058 or SW076956 decreased Beclin 1/Myc-Bcl-2 interaction, but not Bax/Myc-Bcl-2 or Bim/Myc-Bcl-2 interaction. The co-immunoprecipitation experiments in cells showed the same trends as the AlphaLISA in vitro binding experiments; SW076956 is more active than SW063058 in disrupting Beclin 1/Bcl-2 interaction (but not as active as the BH3 mimetic ABT-737) and SW063058 and SW076956, but not ABT-737, selectively disrupt Beclin 1/Bcl-2 versus Bax/Bcl-2 binding. Moreover, ABT-737, but not SW063058 or SW076956, disrupt coimmunoprecipitation between Bcl-2 and the BH3-only protein, Bim.
Functional Assays of Disruptors of Beclin 1/Bcl-2 binding
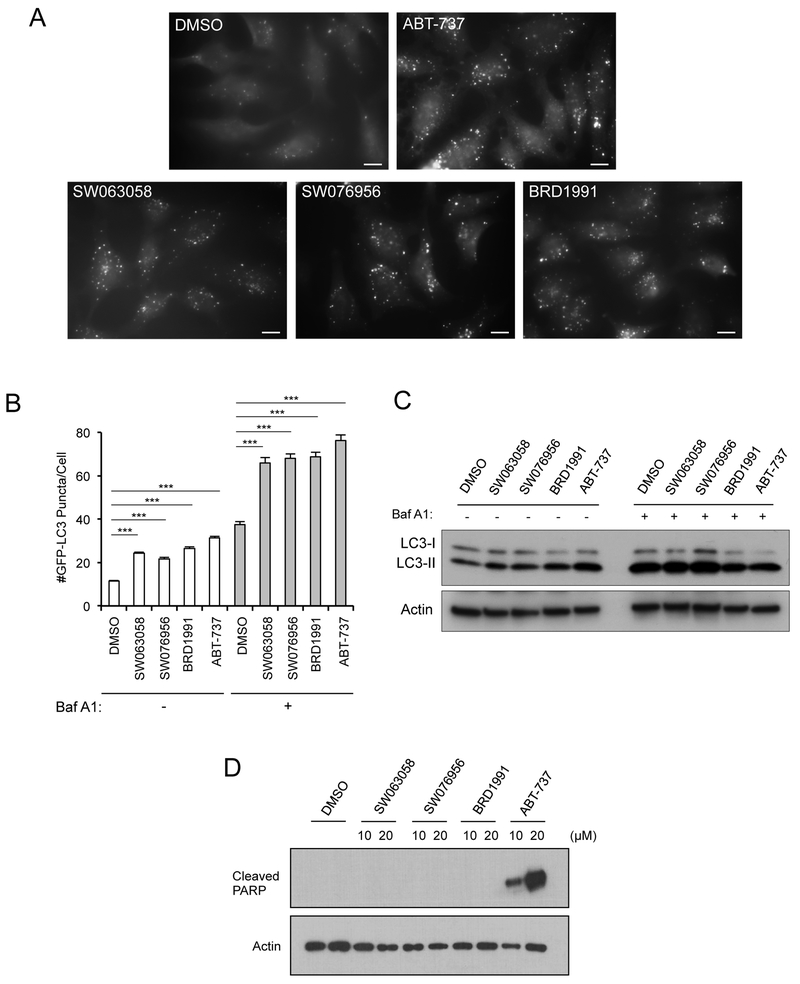
The selective activity of SW063058, SW076956, and BRD1991 for disrupting Beclin 1/Bcl-2 binding (versus Bax/Bcl-2 binding) indicated that these compounds may induce autophagy without cell death. We used well-established assays to assess their autophagic activity,45 including (i) quantitation of GFP-LC3 puncta (which label autophagosomes) in the presence and absence of the lysosomal inhibitor, bafilomycin A1 (Baf A1), and (ii) western blot analysis of the conversion of LC3-I to the lipidated, autophagosomal-associated protein, LC3-II in the presence and absence of Baf A1. HeLa cells stably expressing GFP-LC3 treated with SW063058, SW076956, or BRD1991 had increased numbers of GFP-LC3 puncta as compared to DMSO control 24 h after treatment with 20 μM compound (Figure 5A and 5B). Shorter durations of lower concentrations of compounds (10 μM) also produced similar results (Supplementary Figure 3). The numbers of GFP-LC3 puncta increased further in the presence of Baf A1, indicating that these compounds induce complete autophagic flux, rather than a block in autophagolysosomal maturation. In support of these findings, we also found that these compounds induce an increase in LC3-II that further increases in the presence of Baf A1, confirming an increase in autophagic flux (Figure 5C). Thus, these compounds induce autophagic flux in HeLa cells.
Figure 5.
Effects of Beclin 1/Bcl-2 binding disruptors on autophagy and cell death. (A-B) Representative images (A) or quantitation (B) of GFP-LC3-positive puncta in HeLa/GFP-LC3 cells treated with DMSO or indicated compound. 20 μM compound +/− 100 nM Baf A1, 24 h. Bars represent mean ± s.e.m. of triplicate samples (>100 cells analyzed per sample). Similar results were observed in three independent experiments. ***p<0.001; Mann–Whitney U test. Scale bars, 1 μm. (C) Western blot analysis of LC3 in HeLa cells treated with indicated concentration of indicated compound for 24 h in the presence or absence of 100 nM Baf A1. (D) Effects of indicated compounds at indicated concentrations on HeLa cell apoptosis as assessed by western blot detection of cleaved PARP.
We assessed whether the induction of autophagy by SW063058 and SW076956 requires Beclin 1 and Bcl-2 using siRNA to knockdown each protein, respectively, in HeLa, cells (Supplementary Information Figure 4A). Consistent with its role as an essential autophagy protein, Beclin 1 knockdown resulted in decreased autophagosome numbers in baseline conditions (as demonstrated by numbers of GFP-LC3 puncta) and, unlike in cells subjected to control siRNA, there was no further increase in GFP-LC3 puncta upon treatment with SW063058, SW076956 or ABT-737 (Supplementary Information Figure 4B). Consistent with its role as an autophagy inhibitor, Bcl-2 knockdown resulted in increased numbers of GFP-LC3 puncta in baseline conditions and, unlike in cells subjected to control siRNA, there was no further increase in GFP-LC3 puncta upon treatment with SW063058, SW076956 or ABT-737. We confirmed that Beclin 1 knockdown decreased and Bcl-2 knockdown increased, respectively, autophagic flux, by demonstrating increased levels of the autophagic substrate, p62, in cells with Beclin 1 knockdown and decreased levels of the autophagic substrate, p62, in cells with Bcl-2 knockdown (Supplementary Information Figure 4C). In contrast to cells treated with control siRNA, SW063058, SW076956 or ABT-737 failed to alter p62 levels in cells with either Beclin 1 or Bcl-2 knockdown. Taken together, these data demonstrate that both Beclin 1 and Bcl-2 are required for the autophagy-inducing effects of SW063058, SW076956 and ABT-737.
At the same concentrations that induce autophagic flux (10 and 20 μM) as measured in the GFP-LC3 assay (Figure 5B and Supplementary Figure 3), SW063058 and SW076956 did not exert cytotoxicity in HeLa cells whereas BRD1991 and ABT-737 exerted mild cytotoxicity at 20 μM as measured by CellTiter-Glo assays (Supplementary Information Figure 5A). Consistent with a previous report indicating that the overexpression of Bcl-2 sensitizes cells to ABT-737-induced apoptosis46, treatment with ABT-737, but not SW063058, SW076956, or BRD1991, resulted in a more pronounced dose-dependent increase in cell death in HeLa cells that overexpress Bcl-2 (Supplementary Information Figure 5B). We confirmed the CellTiter-Glo results using a more specific assay to detect apoptotic death, the cleavage of the caspase 3 substrate, PARP.47 A dose-dependent increase in PARP cleavage was observed with ABT-737 treatment (Supplementary Figure 5C, Figure 5D), with cleaved PARP detected with 10 or 20 μM of compound. At these same concentrations, SW063058, SW076956, and BRD1991 failed to induce PARP cleavage. Altogether, these data indicate that there is a window in which our newly identified disruptors of Beclin 1/Bcl-2 binding induce autophagy without triggering apoptosis or other forms of cell death.
Pharmacokinetic Properties
Given the potential utility of these hits as starting points for further SAR to develop specific autophagy inducers, we analyzed and quantified cell penetration and measured some metabolic stability properties. Using mass spectrometry, we measured the intracellular drug concentration in HeLa cells at different time points after the incubation with 1 μM of either SW063058, SW076956 or BRD1991. All three candidate compounds enter the cell within one hour, but only SW076956 and BRD1991 showed gradual intracellular accumulation over time (Supporting Information Supplementary Figure 6). In measuring in vitro ADME properties for these hit compounds (Supporting Information Supplementary Table 1), microscale thermodynamic solubility showed that the compounds had solubility ranging from 0.72 μM to 30 μM in PBS and 43.3 μM to 202 μM in AlphaLISA buffer. Plasma stability was greater than 90% in murine and human plasma. Murine and human microsomal stability showed that 36% of parental compound SW063058 was unmetabolized after one hour of human microsome extract exposure while all other combinations of compounds exposed to murine or human microsomes were present at concentrations of less 2% of parental compounds after 1 h. Depending on the scaffold chosen to elaborate, these properties will need to be taken into account in moving a series forward.
NMR Chemical Shift Perturbation Analysis
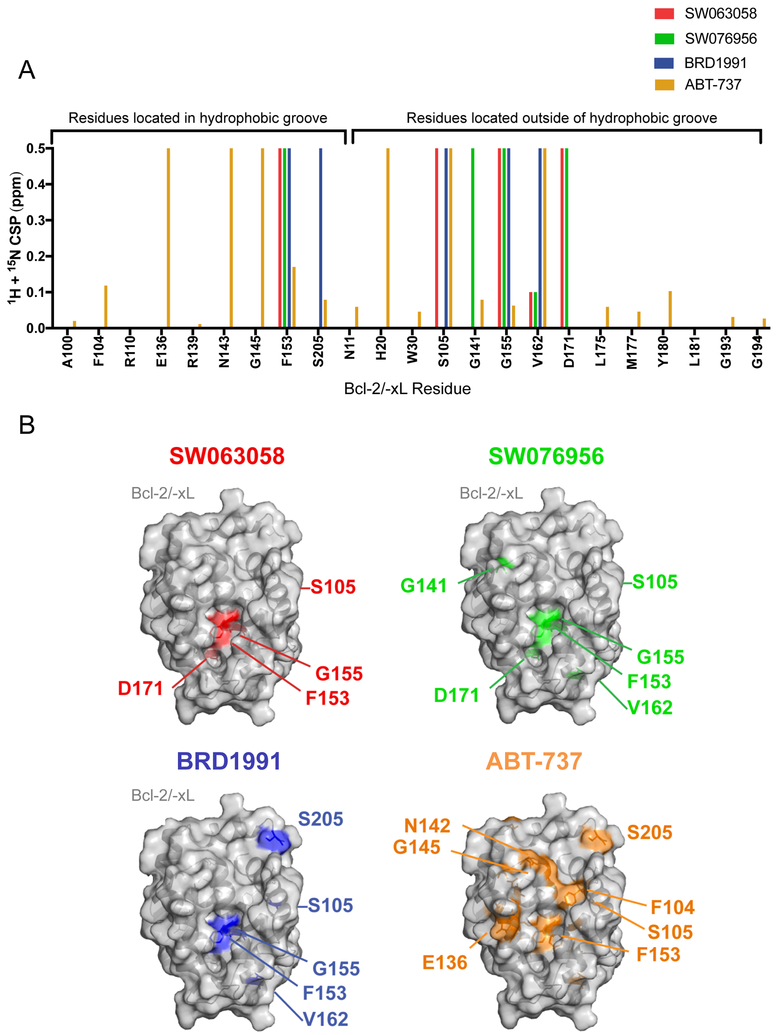
To examine whether SW063058, SW076956 and BRD1991 occupy the hydrophobic pocket of Bcl-2, we carried out a chemical shift perturbation analysis. The incubation of purified Bcl-2/-xL chimera protein (in which the unstructured loop of Bcl-2 was replaced with a short loop from Bcl-xL)48 with the candidate molecules resulted in chemical shift changes for several residues, suggesting that the candidate molecules bind to Bcl-2 (Figure 6 and Supporting Information, Supplementary Figure 7). Notably, a chemical shift change was observed for F153 located inside the P2 hydrophobic binding pocket of Bcl-2, indicating that these candidate compounds may bind within the BH3 pocket. Other chemical shift changes of residues not in the vicinity of the P2 pocket (G141, V162, and D171) may be indicative of allosteric conformational changes of Bcl-2/-xL chimera upon ligand binding. Overall, the chemical shift profiles of SW063058, SW076956 and BRD1991 are similar but showed some distinct features, as compared to known BH3 mimetics, including ABT-737 (Figure 6) and ABT-199 and ABT-263 (data not shown). For example, while SW063058, SW076956 and BRD1991 all resulted in a chemical shift in F153 inside the hydrophobic groove (as did the BH3 mimetics), they did not result in chemical shifts in other residues in the groove such as F104 and E136; in addition, SW063058, SW076956 and BRD1991 led to chemical shifts in G155, a residue essential for Bcl-2 binding to Beclin 1,49 whereas ATB-737 and other BH3 mimetics did not (Figure 6 and data not shown). These data suggest that the structural determinants for the binding of Bcl-2 to our newly identified Beclin 1/Bcl-2 binding disruptors may be different from that of known BH3 mimetics.
Figure 6.
Effects of ligand binding on chemical shifts of Bcl-2/-xL protein. (A) Graph showing quantitation of chemical shift perturbations at indicated select amino acid residues for indicated compounds and control BH3 mimetic, ABT-737. (B) Structure of Bcl-2/-xL (gray) determined by X-ray crystallography NMR (PDB:2XA0)38 with chemical shift perturbations observed upon ligand binding for SW063058, SW076956, BRD1991and ABT-737 mapped onto the structures. See Supporting Information Supplementary Figure 6 for 15N-1H TROSY spectrum of Bcl-2/-xL.
Conclusions
We have developed two new HTS approaches for identifying compounds that disrupt the Beclin 1/Bcl-2 interaction. These screening tools resulted in the identification of three validated hits, SW063058, SW076956 and BRD1991, that selectively disrupt Beclin 1/Bcl-2 binding as compared to Bax/Bcl-2 and Bim/Bcl-2 binding and induce autophagic flux at concentrations that showed minimal cytotoxicity. Moreover, NMR chemical shift perturbation data suggest that these newly identified compounds may bind to the P2 pocket of Bcl-2 via a modality that is distinct from currently available BH3 mimetics.
A previous screen was recently reported with a smaller number of compounds to identify small molecules that function as Beclin 1 BH3 mimetics and induce both autophagy and apoptosis.50 In contrast, our goal was to identify compounds that only induce autophagy by exerting selectivity for disrupting the binding of the BH3 domain of Beclin 1 but not the BH3 domain of Bax to Bcl-2. We identified three potential tool compounds that meet these criteria. Although they are currently biologically active in the micromolar range, the properties identified in this study – especially their selectivity for disrupting the interaction between Bcl-2 and the Beclin 1 BH3 motif, their ability to induce autophagy without cytotoxicity, and their unique NMR chemical shift perturbation profiles (compared to known BH3 mimetics) – render them strong hit compounds for the future design of analogs aimed at increasing potency, increasing selectivity for disrupting Beclin 1/Bcl-2 versus Beclin 1/Bax (or other pro-apoptotic BH3-containing proteins) interaction, and optimizing ADME properties. The cell-based and in vitro based Beclin 1/Bcl-2 binding assays developed in this study will be useful both for rapid assessment/optimization of such analogs as well as for screening new chemical libraries that become available.
Overall, our high-throughput screening program has generated novel compounds that serve as excellent starting points for the future development of effective Beclin 1/Bcl-2 selective inhibitors, and we expect that further structure-activity relationship studies will produce autophagy modulators that have potential efficacy for the treatment of a wide spectrum of human diseases.
METHODS
Cell Lines
All cell lines were cultured and maintained according to ATCC instructions. HeLa/GFP-LC351 and HeLa/Myc-Bcl-226 cells were previously described by our laboratory. Beclin 1/Bcl-2 split-luciferase cells were generated by stable transfection of HeLa tet-on cells (Clontech) with the tetracycline-inducible plasmid pTRE2pur vector expressing NLuc-Beclin 1 (or Beclin 1ΔBcl−2BD mutant) and the pTRE2-RLuc-hyg3 vector expressing CLuc-Bcl-2. Stable clones were selected in selection medium (DMEM supplemented with DMEM supplemented with 10% Tet-system-approved FBS, 1 mM glutamine, 100 μg/mL G418, 200 μg/ mL hygromycin, and 0.5 μg/ mL puromycin). The previously described Beclin 1ΔBcl−2BD mutant24, 49 contains a deletion in the Beclin 1 BH3 domain spanning from amino acids 88–150 which interferes with its binding to Bcl-2. Stable cell lines were maintained DMEM supplemented with 10% Tet-system-approved FBS, 1 mM glutamine, 100 μg/ mL G418, 100 μg/ mL hygromycin, and 0.5 μg/ mL puromycin.
Recombinant Proteins and Peptides
The human beclin 1 gene was cloned into the expression vector ppSUMO-Strep as a fusion with N-terminal StrepII tag and SUMO protein. Three aromatic residues located at the “aromatic finger” of human Beclin 142 were mutated to aspartate (F359D/F360D/W361D) to improve protein solubility. E. coli BL21 Star (DE3) pLysS containing StrepII-SUMO-Beclin 1, StrepIISUMO-Beclin 1ΔBcl−2BD or StrepII-SUMO-6xHis expression plasmids were grown at 37°C until logarithmic phase and incubated in 0.1 mM IPTG at 16°C overnight. Soluble StrepII-SUMOBeclin 1 or StrepII-SUMO-Beclin 1ΔBcl−2BD protein was affinity-purified by StrepTactin sepharose (IBA Biosciences) and further purified by size exclusion chromatography with a HiLoad 16/60 Superdex 200 column (GE Healthcare) equilibrated with Modified Buffer W (100 mM Tris-HCl, pH 8.0, 300 mM NaCl, 5% glycerol, 0.1% CHAPS, and 1 mM DTT) (See Supporting Information Supplementary Figure 1).
For the purification of Bcl-2, the expression plasmid pET21c-Bcl-2 (spanning amino acid 1–218), which encodes human Bcl-2 lacking the transmembrane domain with a C-terminal 6xHis tag was used (gift from Sangita Singha, North Dakota State University). BL21 Star (DE3) pLysS harboring the expression plasmid was grown at 37°C until logarithmic phase, and IPTG was added to a final concentration of 0.1 mM and incubated at 16°C overnight. Soluble Bcl-2–6xHis protein was affinity purified by Ni-NTA agarose (Qiagen) with lysis buffer (50 mM phosphate, pH 8.0, 300 mM NaCl, 0.1% CHAPS and 5% glycerol). Imidazole was used at 10 mM for binding, 20 mM for washing, and 250 mM for elution. The affinity purified Bcl-2–6xHis was further purified by size exclusion chromatography with a HiLoad 16/60 Superdex 200 column equilibrated with Modified Buffer W.
NMR experiments used a chimeric version (Bcl-2/-xL) of Bcl-2 that substitutes the Bcl-xL loop to improve the 2D spectra.48 DNA was optimized for expression in E. coli (GeneArt, Life Technologies) and cloned into a pET28a vector with an N-terminal His6-MBP tag to aid overexpression. A TEV cleavage site was engineered between the MBP tag and Bcl-2/-xL proteins for removal of the MBP-tag. The protein was expressed and labeled as described52 and protein purification was done as described.53 Protein purity was estimated to be ≥95% by SDS-PAGE, with image analysis by ImageJ, and quantified using an ε280 of 43,430 M−1 cm−1. Aliquots were concentrated to ≥100 μM and frozen at −8°C until needed.
Biotinylated L-amino acid peptides were synthesized by the University of Texas Southwestern Medical Center (UTSW) Protein Chemistry Technology Core. Peptides were purified to >95% by HPLC and confirmed by mass spectrometry. The Beclin 1 BH3 peptide consists of an N-terminally linked biotin, a YGGGGS linker, and 16 amino acids (a.a. 105–130) derived from human Beclin 1 (Biotin-YGGGGSDGGTMENLSRRLKVTGDLFDIMSGQT). Bax BH3 peptide consists of an N-terminally linked biotin, a YGGGGS linker, and 36 amino acids (a.a. 49–84) derived from human Bax (Biotin-YGGGGSPVPQDASTKKLSECLKRIGDELDSNMELQRMIAAVD). Biotin-6xHis control peptide consists of an N-terminally linked biotin, a YGGGGS linker, and a 6xHis tag at C-terminus (Biotin-YGGGGSHHHHHH).
Beclin 1/Bcl-2 Split-Luciferase Screen
Beclin 1/Bcl-2 split-luciferase cells were suspended in induction medium (DMEM supplemented with Tet-approved FBS, 1 mM glutamine, 100 units/mL of Penicillin/Streptomycin, and 1 μg/ mL doxycycline) and plated at 1.2 × 104 cells/well in 384-well plates (Corning #3570) and incubated at 37°C overnight.
DMSO (neutral control), ABT-737 (positive control), or the library compounds were added to a final concentration of 5 μM and incubated at 37°C. Compounds were from either the UTSW (200K) or the Broad Institute of MIT and Harvard diversity-oriented synthesis (100K) collection (provided courtesy of Stuart Schreiber). After 5 h of incubation, the medium was removed by centrifugation and the activity of Beclin 1/Bcl-2 split-luciferase reporters was measured with Dual-Glo® Luciferase Assay System (Promega). Twenty microliters of 1x FL buffer (1:1 dilution of Luciferase Reagent with PBS) was first added to the well, incubated at room temperature for 10 min, and firefly luminescence units were measured by an Envision plate reader (Perkin Elmer). Ten microliters of 1x RL buffer (Stop & Glo Reagent) was then added to the sample, the sample was incubated at room temperature for 10 min, and the activity of Renilla luciferase was measured again. The Beclin 1/Bcl-2 interaction was measured as relative luminescence units (RLU), calculated as the ratio of split-luciferase and Renilla luciferase signals.
The screen was performed in a 384-well format. Each assay plate contained 320 library compounds in column 3 to 22. DMSO was included as a neutral control in column 2 and 23, and ABT-737 was used as a positive control in column 1. For analysis of the primary screen data, numerical readouts obtained from EnVision plate reader were quality controlled and processed using the Assay Analyzer module of the Genedata Screener® Suite. To remove systemic variation bias such as edge effect or plate effect, normalized values (RLU) were corrected with a proprietary pattern detection algorithm in the Assay Analyzer software.54 Z-scores were calculated from the split-luciferase activity and the corrected normalized activity (RLU) for each compound.54 The percent activity of a compound at the tested concentration was defined as:
Compounds having Z-scores less than −3.0 in the split-luciferase activity and the corrected normalized activity (RLU) were advanced for study in the confirmation assays.
The confirmation assay for compounds from the UTSW library was performed in triplicate using compound concentrations of 5 μM. The confirmation assays for compounds from the Broad Institute library were performed at 5 μM with a 2-fold 8-point serial dilution. The values of percent activity in the triplicates for each compound were then condensed to a single value as “condensed activity”, the representative single value of the triplicates, using the “Robust Condensing” method in Genedata Screener®. In general, the triplicates were pre-condensed into a pair of values (X and Y) as follows:
A lower | X− Y | value indicated better data quality. For data points where | X− Y | ≤ 30%, the median of X and Y was used as the condensed activity, which is also the median of the triplicate measurements. Otherwise, a condensing function Max (X,Y) was used to estimate the condensed activity. A robust Z-Score was then calculated for each compound using the following equation:
Compounds having Z-scores less than −3.0 (UTSW library) or dose-response inhibition of split-luciferase activity (Broad Institute library) were considered confirmed.
Beclin 1/Bcl-2 AlphaLISA screen
All AlphaLISA assays were performed in triplicate in a 384-well format with BB buffer (phosphate-buffered saline supplemented with 0.5% BSA and 1 mM DTT). For the Beclin 1/Bcl-2 AlphaLISA, purified StrepII-SUMO-Beclin 1 was incubated with Bcl-2–6xHis proteins at 300 nM and 60 nM, respectively. For internal control AlphaLISA assays, StrepII-SUMO-6xHis protein (internal control) was used at 20 nM. Samples containing Beclin 1/Bcl-2 or SUMO proteins were added to AlphaPlates-384 (Perkin Elmer #6005350) by Multidrop and incubated with DMSO (neutral control), ABT-737 (positive control), or library compound at 5 μM (UTSW library) or 10 μM (Broad library) at room temperature for 3 h to allow protein-protein interaction. Following the initial incubation, Strep-Tactin Alpha donor (AS106D) and anti-6xHis AlphaLISA acceptor beads (AL128M) were added to a final concentration of 40 μg/mL each and incubated at room temperature in the dark for an additional 1 h. All samples were evaluated in triplicate. Alpha signals were measured by an Envision plate reader (Perkin Elmer). Beclin 1/Bcl-2 Alpha signal was normalized with internal control Alpha signal. The normalized value represents the binding activity of Beclin 1 and Bcl-2. The percent activity of a compound was calculated as the following formula:
The values of percent activity of each compound in triplicates were converted into condensed activity and a robust Z-score was calculated as described in the previous section. Compounds with a Z score less than −3.0 and percent activity >20% (UTSW library) or >40% (Broad Institute library) and were selected for further confirmation with dose-response experiments.
For dose-response AlphaLISA experiments, compounds were serially diluted (2-fold, 7 point) with DMSO and added to BB buffer containing purified recombinant proteins or biotinylated peptides at a final concentration range of 10 μM to 156 nM. Protein or peptide concentrations used in dose-response AlphaLISA experiments were as follows: Beclin1/Bcl-2 – 300 nM/60 nM; SUMO (internal control for Beclin 1/Bcl-2 AlphaLISA) – 20 nM; Beclin 1 BH3/Bcl-2 −1000 nM/60 nM; Bax BH3/Bcl-2 – 100 nM/20 nM; Biotin-6xHis (internal control for BH3/Bcl-2 AlphaLISA) – 10 nM.
GFP-LC3 Puncta Analyses
HeLa/GFP-LC3 cells51 were imaged using a Zeiss AxioImager Z2 microscope equipped with a Photometrics CoolSnap HQ2 camera using a Zeiss PLAN APOCHROMAT 20X/0.8 NA air objective. GFP-LC3 puncta were quantified on images captured using the Zeiss Z2 microscope by an observer blinded to experimental conditions.
Co-immunoprecipitation
For Bcl-2 co-immunoprecipitation, HeLa/Myc-Bcl-2 cells26 treated with DMSO or chemical compound were collected in lysis buffer (25 mM HEPES-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1x cOMPLETE protease inhibitor cocktail [Roche] and 1x HALT phosphatase inhibitor cocktail [Thermo Fisher]), incubated on ice for 10 min, and centrifuged at 20,000 rcf for 10 min. The lysates were pre-cleared with protein G beads, and then incubated with anti-Myc antibody agarose beads conjugates (Santa Cruz sc-40 AC) overnight at 4°C. Beads were washed three times with lysis buffer and then boiled in Laemmli buffer + β-mercaptoethanol for 5 min.
Western Blot Analyses
For western blot analyses, cells were lysed with Laemmli buffer containing 2.5% β- mercaptoethanol, boiled for 5 min, separated on SDS-PAGE denaturing gels (Bio-Rad) and transferred to PVDF membranes (Bio-Rad). Membranes were blocked with 5% dry milk and signals were visualized with Supersignal® West Pico or Femto Chemiluminescent Substrate kit (Pierce).
The following antibodies were used for western blot analyses: goat polyclonal anti-luciferase (Promega G7451, 1:1000 dilution), rabbit anti-Beclin 1 (Santa Cruz sc-11427, 1:500 dilution), HRP-conjugated anti-Myc (Santa Cruz SC-40 HRP, 1:100 dilution), rabbit anti-Bax (Cell Signaling Technology #5023, 1:500 dilution), rabbit anti-LC3 (Novus NB100–2220, 1:2000 dilution), mouse anti-p62 (Abnova #H00008878-M01, 1:20000 dilution), rabbit anti-Bim (Cell Signaling Technology #2933, 1:1000 dilution), rabbit anti-cleaved PARP (Cell Signaling Technology #9451, 1:1000 dilution) and HRP-conjugated anti-actin (Santa Cruz sc-47778-HRP, 1:5000 dilution).
Dicer-Substrate siRNA Treatment
Beclin 1 (IDT #hs.Ri.BECN1.13.1 5’-AGUACAUGUUUACAAUAC) and Bcl-2 (IDT #hs.Ri.BCL2.13.3 5’-CCCUGUGGAUGACUGAG) siRNA experiments were performed using reverse transfection at a final concentration of 10 nM dicer-substrate siRNA using RNAiMAX (Invitrogen) according to the manufacturer’s instructions. At 48 and 72 h after siRNA transfection, protein knockdown was assessed by western blot analysis.
Measurement of Intracellular Drug Concentration
HeLa cells were plated at 5×105 in a 6-cm plate and incubated overnight before treatment with indicated compound at 1 μM for 1, 2, 4, and 24 h. Treated cells were washed with PBS twice, trypsinized, and resuspended in ice-cold PBS. Samples were snap-frozen in liquid nitrogen and analyzed by LC-MS.
Cell Viability Assays
HeLa cells were seeded in 96-well opaque plates at 5 × 103 cells/well 24 h before chemical compound treatment. Compounds in DMSO were serially diluted and added to the cells at final concentrations of 20, 10, 5, and 2.5 μM. At the indicated time point, cells were lysed by CellTiter-Glo reagents for 10 min before luminescence measurement. Apoptosis was assessed by western blot analysis to detect cleaved PARP 24 h after compound administration to HeLa cells.
Analytical Assays
Solubility was either measured in PBS buffer by the Analytical Group of the Broad Institute or in AlphaLISA reaction buffer by the Preclinical Pharmacology Core Laboratory at UT Southwestern Medical Center. To measure solubility in PBS buffer, each compound was prepared in triplicate at 100 μM in both 100% DMSO and PBS with 1% DMSO. Compounds were allowed to equilibrate at room temperature with a 750 rpm vortex shake for 18 h. After equilibration, samples were analyzed by UPLC-MS (Waters, Milford, MA) with compounds detected by SIR detection on a single quadrupole mass spectrometer. The DMSO samples were used to create a two-point calibration curve to which the response in PBS was fit. To measure solubility in AlphaLISA reaction buffer, each compound was prepared in triplicate at 1 mM in PBS containing 0.5% BSA and 1 mM DTT in glass vials. The vials were shaken vigorously (250 rpm) on an orbital shaker for 18 h. The samples were placed in teflon eppendorf tubes and centrifuged at 16,100xg for 10 min. The supernatant was collected and analyzed by a Qtrap 3200 LC-MS/MS system.
Plasma stability was determined at 37°C at 5 h in both human and mouse plasma. Each compound was prepared in duplicate at 5 μM in plasma diluted 50/50 (v/v) with PBS pH 7.4 (0.95% acetonitrile, 0.05% DMSO). Compounds were incubated at 37°C for 5 h with a 350-rpm orbital shake with time points taken at 0 h and 5 h. Samples were analyzed by UPLC-MS (Waters, Milford, MA) with compounds detected by SIR detection on a single quadrupole mass spectrometer.
Microsomal stability was determined at 37°C at 60 min in both human and mouse microsomes. Each compound was prepared in duplicate at 1 μM with 0.3 mg/mL microsomes in PBS pH 7.4 (1% DMSO). Compounds were incubated at 37°C for 60 min with a 350-rpm orbital shake with time points taken at 0 min and 60 min. Samples were analyzed by UPLC-MS (Waters, Milford, MA) with compounds detected by SIR detection on a single quadrupole mass spectrometer.
NMR Spectroscopy Experiments
The NMR data were acquired using a Bruker Avance III HD 600 MHz spectrometer equipped with a cryogenic QCI cryoprobe at the Broad Institute. The 2D 15N-1H HSQC and TROSY spectra were collected at 25°C in the absence or presence of compound. The NMR samples were comprised of 90% H2O/10% D2O in 25 mM HEPES (pH 7.5), 150 mM NaCl and 0.5 mM TCEP. For the chemical shift perturbation experiments with ABT-737, ABT-199, ABT-263, SW063058, SW076956 and BRD1991, aliquots were titrated into the 15N-labeled Bcl-2/-xL and 2D 15N-1H HSQC and TROSY spectra were collected at 25°C. The chemical shift assignments for Bcl-2/-xL were performed as previously described.48 All of the NMR data were analyzed using SPARKY software.
Supplementary Material
Supplementary Figure 1. Purification of recombinant Beclin 1 and Bcl-2. All proteins were affinity purified, fractions were analyzed by SDS-PAGE (Left), and further separated by gel filtration (Right). StrepII-SUMO-Beclin 1 (A), StrepII-SUMO-Beclin 1ΔBcl-2BD (B), and Bcl-2–6xHis (C) are shown. FT: flow through. (D) Purified recombinant proteins used in AlphaLISA experiments.
Supplementary Figure 2. Chemical structures of compounds that showed dose-dependent inhibition of Beclin 1/Bcl-2 interaction as measured by AlphaLISA.
Supplementary Figure 3. GFP-LC3-positive puncta in HeLa/GFP-LC3 cells treated with DMSO or indicated compound (10 μM compound +/− 100 nM Baf A1, 5 h). Bars represent mean ± s.e.m. of triplicate samples (>100 cells analyzed per sample). Similar results were observed in three independent experiments. *p<0.05; ***p<0.001; Mann-Whitney U test.
Supplementary Figure 4. Effects of Beclin 1 and Bcl-2 knockdown on autophagy induction by Beclin 1/Bcl-2 binding disruptors. (A) Western blot showing indicated protein levels in HeLa cells 72 h after treatment with indicated siRNA. NC, negative control. (B) Quantitation of GFPLC3-positive puncta in HeLa/GFP-LC3 treated with indicated siRNA and then 48 h later cells treated with DMSO or indicated compound (20 μM compound +/− 100 nM Baf A1, 24 h. Bars represent mean ± s.e.m. of triplicate samples (>100 cells analyzed per sample). Similar results were observed in three independent experiments. n.s., not significant; *p<0.05, ***p<0.001 for comparison of compound to DMSO control in each siRNA group; Mann-Whitney U test. ###p<0.001 for comparison of DMSO control in beclin 1 or Bcl-2 siRNA groups compared to DMSO in NC siRNA group: Mann-Whitney U test. (C) Western blot detection of p62 degradation in HeLa cells treated with indicated siRNA and indicated compound (24 h, 20 μM). Similar results were observed in three independent experiments.
Supplementary Figure 5. Effects of Beclin 1/Bcl-2 binding disruptors on cell death. Effects of indicated compounds at indicated concentrations on HeLa (A) and HeLa/Bcl-2 (B) viability as measured by Celltiter Glo assay 24 h after treatment. Bars represent mean ± s.d. of triplicate samples. Similar results were observed in three independent experiments. p-values were calculated by pairwise comparisons to DMSO control. *p<0.05; ***p<0.001; Student's t-test. (C). Dose-response of ABT-737 induction of PARP cleavage in HeLa cells at 24 h after treatment with indicated concentration. See also main text Figure 5D.
Supplementary Figure 6. Intracellular concentrations of compound SW063058, SW076956 or BRD1991 after indicated duration of treatment in HeLa cells. (A) Numerical data showing average intracellular concentration of indicated compound at indicated time point. (B) Graphical representation of data shown in (A). Bars represent mean ± s.d. of triplicate samples.
Supplementary Figure 7. 15N-1H TROSY spectrum of Bcl-2/-xL acquired at 14.1 T (1H Larmor frequency of 600 MHz). Chemical shift assignments for select amino acids that showed chemical shift perturbations upon ligand binding are labeled on the spectrum. See Figure 6A in main text for quantitative information of chemical shift perturbations of amino acids upon binding of SW063058, SW076956, BRD1991 and ABT-737.
Primary data from HTS assays.
Supplementary Table 1. Solubility, metabolic stability, and plasma protein binding properties of SW063058, SW076956 and BRD1991.
ACKNOWLEDGMENTS
The authors thank Sangita Sinha and Stuart Schreiber for providing critical reagents; Xuewu Zhang for helpful discussions; Guijun Shang and Hua Chen for assistance with protein purification; Noelle Williams for the measurement of intracellular drug concentration, Hanspeter Niederstrasser, Hong Chen, Melissa McCoy for HTS screening; and Haley Smith for assistance with manuscript preparation. This work was supported by NIH grants U19 AI109725 (J.P. and B.L.) RO1 CA109608 (B.L.), and Cancer Prevention Research Institute of Texas (CPRIT) grant RP120718 (B.L). The UTSW HTS facility is supported by an NIH NCI grant P30 CA142543.
Footnotes
CONFLICT OF INTEREST STATEMENT
B.L. is a scientific founder of Casma Therapeutics, Inc.
REFERENCES
- 1.Yang Z; Klionsky DJ, Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010, 22 (2), 124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizushima N; Yoshimori T; Ohsumi Y, The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 2011, 27, 107–32. [DOI] [PubMed] [Google Scholar]
- 3.Levine B; Kroemer G, Autophagy in the pathogenesis of disease. Cell 2008, 132 (1), 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KH; Lee MS, Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol 2014, 10 (6), 322–37. [DOI] [PubMed] [Google Scholar]
- 5.Levine B; Klionsky DJ, Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004, 6 (4), 463–77. [DOI] [PubMed] [Google Scholar]
- 6.Mizushima N; Levine B, Autophagy in mammalian development and differentiation. Nat Cell Biol 2010, 12 (9), 823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang RC; Levine B, Calcipotriol induces autophagy in HeLa cells and keratinocytes. J Invest Dermatol 2011, 131 (4), 990–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizushima N; Komatsu M, Autophagy: renovation of cells and tissues. Cell 2011, 147 (4), 728–41. [DOI] [PubMed] [Google Scholar]
- 9.Rubinsztein DC; Marino G; Kroemer G, Autophagy and aging. Cell 2011, 146 (5), 682–95. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N; Levine B; Cuervo AM; Klionsky DJ, Autophagy fights disease through cellular self-digestion. Nature 2008, 451 (7182), 1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine B; Packer M; Codogno P, Development of autophagy inducers in clinical medicine. J Clin Invest 2015, 125 (1), 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang P; Mizushima N, Autophagy and human diseases. Cell Res 2014, 24 (1), 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi AM; Ryter SW; Levine B, Autophagy in human health and disease. N Engl J Med 2013, 368 (19), 1845–6. [DOI] [PubMed] [Google Scholar]
- 14.Xu J; Xia L; Shang Q; Du J; Zhu D; Wang Y; Bi D; Song J; Ma C; Gao C; Zhang X; Sun Y; Zhu L; Wang X; Zhu C; Xing Q, A variant of the autophagy-related 5 gene is associated with child cerebral palsy. Front Cell Neurosci 2017, 11 (407), doi: 10.3389/fncel.2017.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pyo JO; Yoo SM; Ahn HH; Nah J; Hong SH; Kam TI; Jung S; Jung YK, Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun 2013, 4, 2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decressac M; Mattsson B; Weikop P; Lundblad M; Jakobsson J; Bjorklund A, TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A 2013, 110 (19), E1817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández AF; Sebti S; Wei Y; Zou Z; Shi M; McMillan KL; He C; Chiang W; Ting T; Marciano DK; Schiattarella GG; Bhagat G; Moe OW; Hu M; Levine B, Disruption of the beclin 1/BCL2 autophagy regulatory complex promotes longevity in mice. Nature 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocchi A; Yamamoto S; Ting T; Fan Y; Sadleir K; Wang Y; Zhang W; Huang S; Levine B; Vassar R; He C, A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer’s disease. PLoS Genet 2017, 13 (8), e1006962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vega-Rubin-de-Celis S; Zou Z; Fernandez AF; Xiao G; Kim M; Levine B, Increased autophagy blocks HER2-mediated breast tumorigenesis. Proc Natl Acad Sci USA 2018, 115 (7), 4176–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinsztein DC; Codogno P; Levine B, Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 2012, 11 (9), 709–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galluzzi L; Bravo-San Pedro JM; Levine B; Green DR; Kroemer G , Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov 2017, 16 (7), 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menzies FM; Fleming A; Caricasole A; Bento CF; Andrews SP; Ashkenazi A; Fullgrabe J; Jackson A; Jimenez Sanchez M; Karabiyik C; Licitra F; Lopez Ramirez A; Pavel M; Puri C; Renna M; Ricketts T; Schlotawa L; Vicinanza M; Won H; Zhu Y; Skidmore J; Rubinsztein DC, Autophagy and neurodegeneration: Pathogenic mechanisms and therapeutic opportunities. Neuron 2017, 93 (5), 1015–1034. [DOI] [PubMed] [Google Scholar]
- 23.Williams A; Sarkar S; Cuddon P; Ttofi EK; Saiki S; Siddiqi FH; Jahreiss L; Fleming A; Pask D; Goldsmith P; O’Kane CJ; Floto RA; Rubinsztein DC, Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol 2008, 4 (5), 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattingre S; Tassa A; Qu X; Garuti R; Liang XH; Mizushima N; Packer M; Schneider MD; Levine B, Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122 (6), 927–39. [DOI] [PubMed] [Google Scholar]
- 25.Decuypere JP; Parys JB; Bultynck G, Regulation of the autophagic bcl-2/beclin 1 interaction. Cells 2012, 1 (3), 284–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y; Pattingre S; Sinha S; Bassik M; Levine B, JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 2008, 30 (6), 678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pattingre S; Bauvy C; Carpentier S; Levade T; Levine B; Codogno P, Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. The Journal of biological chemistry 2009, 284 (5), 2719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi CS; Kehrl JH, MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. The Journal of biological chemistry 2008, 283 (48), 33175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zalckvar E; Berissi H; Mizrachy L; Idelchuk Y; Koren I; Eisenstein M; Sabanay H; Pinkas-Kramarski R; Kimchi A, DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep 2009, 10 (3), 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine B; Liu R; Dong X; Zhong Q, Beclin orthologs: integrative hubs of cell signaling, membrane trafficking, and physiology. Trends in cell biology 2015, 25 (9), 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiuri MC; Le Toumelin G; Criollo A; Rain JC; Gautier F; Juin P; Tasdemir E; Pierron G; Troulinaki K; Tavernarakis N; Hickman JA; Geneste O; Kroemer G, Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 2007, 26 (10), 2527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He C; Bassik MC; Moresi V; Sun K; Wei Y; Zou Z; An Z; Loh J; Fisher J; Sun Q; Korsmeyer S; Packer M; May HI; Hill JA; Virgin HW; Gilpin C; Xiao G; Bassel-Duby R; Scherer PE; Levine B, Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 2012, 481 (7382), 511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng W; Huang S; Wu H; Zhang M, Molecular basis of Bcl-xL’s target recognition versatility revealed by the structure of Bcl-xL in complex with the BH3 domain of Beclin-1. J Mol Biol 2007, 372 (1), 223–35. [DOI] [PubMed] [Google Scholar]
- 34.Oberstein A; Jeffrey PD; Shi Y, Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem 2007, 282 (17), 13123–32. [DOI] [PubMed] [Google Scholar]
- 35.Pedro JM; Wei Y; Sica V; Maiuri MC; Zou Z; Kroemer G; Levine B, BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy 2015, 11 (3), 452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opydo-Chanek M; Gonzalo O; Marzo I, Multifaceted anticancer activity of BH3 mimetics: Current evidence and future prospects. Biochem Pharmacol 2017, 136, 12–23. [DOI] [PubMed] [Google Scholar]
- 37.Sinha S; Colbert CL; Becker N; Wei Y; Levine B, Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy 2008, 4 (8), 989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ku B; Liang C; Jung JU; Oh BH, Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res 2011, 21 (4), 627–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su M; Mei Y; Sanishvili R; Levine B; Colbert CL; Sinha S, Targeting gamma-herpesvirus 68 Bcl-2-mediated down-regulation of autophagy. J Biol Chem 2014, 289 (12), 8029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oltersdorf T; Elmore SW; Shoemaker AR; Armstrong RC; Augeri DJ; Belli BA; Bruncko M; Deckwerth TL; Dinges J; Hajduk PJ; Joseph MK; Kitada S; Korsmeyer SJ; Kunzer AR; Letai A; Li C; Mitten MJ; Nettesheim DG; Ng S; Nimmer PM; O’Connor JM; Oleksijew A; Petros AM; Reed JC; Shen W; Tahir SK; Thompson CB; Tomaselli KJ; Wang B; Wendt MD; Zhang H; Fesik SW; Rosenberg SH, An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435 (7042), 677–81. [DOI] [PubMed] [Google Scholar]
- 41.Eglen RM; Reisine T; Roby P; Rouleau N; Illy C; Bosse R; Bielefeld M, The use of AlphaScreen technology in HTS: current status. Curr Chem Genomics 2008, 1, 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang W; Choi W; Hu W; Mi N; Guo Q; Ma M; Liu M; Tian Y; Lu P; Wang FL; Deng H; Liu L; Gao N; Yu L; Shi Y, Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res 2012, 22 (3), 473–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreiber SL, Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 2000, 287 (5460), 1964–9. [DOI] [PubMed] [Google Scholar]
- 44.Baell J; Walters MA, Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513 (7519), 481–3. [DOI] [PubMed] [Google Scholar]
- 45.Mizushima N; Yoshimori T; Levine B, Methods in mammalian autophagy research. Cell 2010, 140 (3), 313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merino D; Khaw SL; Glaser SP; Anderson DJ; Belmont LD; Wong C; Yue P; Robati M; Phipson B; Fairlie WD; Lee EF; Campbell KJ; Vandenberg CJ; Cory S; Roberts AW; Ludlam MJ; Huang DC; Bouillet P, Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 2012, 119 (24), 5807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholson DW; Ali A; Thornberry NA; Vaillancourt JP; Ding CK; Gallant M; Gareau Y; Griffin PR; Labelle M; Lazebnik YA; et al. , Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376 (6535), 37–43. [DOI] [PubMed] [Google Scholar]
- 48.Petros AM; Medek A; Nettesheim DG; Kim DH; Yoon HS; Swift K; Matayoshi ED; Oltersdorf T; Fesik SW, Solution structure of the antiapoptotic protein bcl-2. Proc Natl Acad Sci U S A 2001, 98 (6), 3012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang XH; Kleeman LK; Jiang HH; Gordon G; Goldman JE; Berry G; Herman B; Levine B, Protection against fatal Sindbis virus encephalitis by Beclin, a novel Bcl-2-interacting protein. J Virol 1998, 72 (11), 8586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J; Lan L; Lewin SJ; Rogers SA; Roy A; Wu X; Gao P; Karanicolas J; Aube J; Sun B; Xu L , Identification of novel small molecule Beclin 1 mimetics activating autophagy. Oncotarget 2017, 8 (31), 51355–51369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shoji-Kawata S; Sumpter R; Leveno M; Campbell GR; Zou Z; Kinch L; Wilkins AD; Sun Q; Pallauf K; MacDuff D; Huerta C; Virgin HW; Helms JB; Eerland R; Tooze SA; Xavier R; Lenschow DJ; Yamamoto A; King D; Lichtarge O; Grishin NV; Spector SA; Kaloyanova DV; Levine B, Identification of a candidate therapeutic autophagy-inducing peptide. Nature 2013, 494 (7436), 201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marley J; Lu M; Bracken C, A method for efficient isotopic labeling of recombinant proteins. J Biomol NMR 2001, 20 (1), 71–5. [DOI] [PubMed] [Google Scholar]
- 53.Ranaghan MJ; Durney MA; Mesleh MF; McCarren PR; Garvie CW; Daniels DS; Carey KL; Skepner AP; Levine B; Perez JR, The autophagy-related Beclin-1 protein requires the coiled-coil and BARA domains to form a homodimer with submicromolar affinity. Biochem 2017, 56 (51), 6639–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Z; Liu D; Sui Y, Quantitative assessment of hit detection and confirmation in single and duplicate high-throughput screenings. J Biomol Screen 2008, 13 (2), 159–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Purification of recombinant Beclin 1 and Bcl-2. All proteins were affinity purified, fractions were analyzed by SDS-PAGE (Left), and further separated by gel filtration (Right). StrepII-SUMO-Beclin 1 (A), StrepII-SUMO-Beclin 1ΔBcl-2BD (B), and Bcl-2–6xHis (C) are shown. FT: flow through. (D) Purified recombinant proteins used in AlphaLISA experiments.
Supplementary Figure 2. Chemical structures of compounds that showed dose-dependent inhibition of Beclin 1/Bcl-2 interaction as measured by AlphaLISA.
Supplementary Figure 3. GFP-LC3-positive puncta in HeLa/GFP-LC3 cells treated with DMSO or indicated compound (10 μM compound +/− 100 nM Baf A1, 5 h). Bars represent mean ± s.e.m. of triplicate samples (>100 cells analyzed per sample). Similar results were observed in three independent experiments. *p<0.05; ***p<0.001; Mann-Whitney U test.
Supplementary Figure 4. Effects of Beclin 1 and Bcl-2 knockdown on autophagy induction by Beclin 1/Bcl-2 binding disruptors. (A) Western blot showing indicated protein levels in HeLa cells 72 h after treatment with indicated siRNA. NC, negative control. (B) Quantitation of GFPLC3-positive puncta in HeLa/GFP-LC3 treated with indicated siRNA and then 48 h later cells treated with DMSO or indicated compound (20 μM compound +/− 100 nM Baf A1, 24 h. Bars represent mean ± s.e.m. of triplicate samples (>100 cells analyzed per sample). Similar results were observed in three independent experiments. n.s., not significant; *p<0.05, ***p<0.001 for comparison of compound to DMSO control in each siRNA group; Mann-Whitney U test. ###p<0.001 for comparison of DMSO control in beclin 1 or Bcl-2 siRNA groups compared to DMSO in NC siRNA group: Mann-Whitney U test. (C) Western blot detection of p62 degradation in HeLa cells treated with indicated siRNA and indicated compound (24 h, 20 μM). Similar results were observed in three independent experiments.
Supplementary Figure 5. Effects of Beclin 1/Bcl-2 binding disruptors on cell death. Effects of indicated compounds at indicated concentrations on HeLa (A) and HeLa/Bcl-2 (B) viability as measured by Celltiter Glo assay 24 h after treatment. Bars represent mean ± s.d. of triplicate samples. Similar results were observed in three independent experiments. p-values were calculated by pairwise comparisons to DMSO control. *p<0.05; ***p<0.001; Student's t-test. (C). Dose-response of ABT-737 induction of PARP cleavage in HeLa cells at 24 h after treatment with indicated concentration. See also main text Figure 5D.
Supplementary Figure 6. Intracellular concentrations of compound SW063058, SW076956 or BRD1991 after indicated duration of treatment in HeLa cells. (A) Numerical data showing average intracellular concentration of indicated compound at indicated time point. (B) Graphical representation of data shown in (A). Bars represent mean ± s.d. of triplicate samples.
Supplementary Figure 7. 15N-1H TROSY spectrum of Bcl-2/-xL acquired at 14.1 T (1H Larmor frequency of 600 MHz). Chemical shift assignments for select amino acids that showed chemical shift perturbations upon ligand binding are labeled on the spectrum. See Figure 6A in main text for quantitative information of chemical shift perturbations of amino acids upon binding of SW063058, SW076956, BRD1991 and ABT-737.
Primary data from HTS assays.
Supplementary Table 1. Solubility, metabolic stability, and plasma protein binding properties of SW063058, SW076956 and BRD1991.