Abstract
Tuberous Sclerosis Complex (TSC) is a disease caused by autosomal dominant mutations in the TSC1 or TSC2 genes, and is characterized by tumor susceptibility, brain lesions, seizures and behavioral impairments. The TSC1 and TSC2 genes encode proteins forming a complex (TSC), which is a major regulator and suppressor of mammalian target of rapamycin complex 1 (mTORC1), a signaling complex that promotes cell growth and proliferation. TSC1/2 loss of heterozygosity (LOH) and the subsequent complete loss of TSC regulatory activity in null cells causes mTORC1 dysregulation and TSC-associated brain lesions or other tissue tumors. However, it is not clear whether TSC1/2 heterozygous brain cells are abnormal and contribute to TSC neuropathology. To investigate this issue, we generated induced pluripotent stem cells (iPSCs) from TSC patients and unaffected controls, and utilized these to obtain neural progenitor cells (NPCs) and differentiated neurons in vitro. These patient-derived TSC2 heterozygous NPCs were delayed in their ability to differentiate into neurons. Patient-derived progenitor cells also exhibited a modest activation of mTORC1 signaling downstream of TSC, and a marked attenuation of upstream PI3K/AKT signaling. We further show that pharmacologic PI3K or AKT inhibition, but not mTORC1 inhibition, causes a neuronal differentiation delay, mimicking the patient phenotype. Together these data suggest that heterozygous TSC2 mutations disrupt neuronal development, potentially contributing to the disease neuropathology, and that this defect may result from dysregulated PI3K/AKT signaling in neural progenitor cells.
Keywords: Human iPSC, Neuronal differentiation, mTOR, signal transduction, brain development, developmental brain disorder
1. Introduction
Tuberous Sclerosis Complex (TSC) is a multisystem disorder affecting around 1:6000 live births annually worldwide (Osborne et al., 1991; Sahin et al., 2016). Most mutations responsible for TSC occur in the TSC1 or TSC2 genes. Individuals diagnosed carry heterozygous mutations of either of these genes and approximately two-thirds are de novo mutations (Northrup et al., 1993). Gross neuroanatomical lesions include cortical tubers, subependymal nodules (SENs) and subependymal giant cell astrocytomas (SEGAs). Many other brain areas show histopathological abnormalities. Most (90%) TSC patients develop epilepsy. Autism (25–50%), intellectual disability (>50%), and learning disorders (>80%) affect a large proportion of cases (Hunt and Shepherd, 1993; Khwaja and Sahin, 2011; Lewis et al., 2004; Smalley et al., 1992). Together with other frequent behavioral manifestations, these abnormalities are referred to as TSC-associated neuropsychiatric disorders (TAND) (de Vries et al., 2015). Despite the heavy burden posed by TAND on patients and their family, there is currently no treatment for these conditions. Furthermore, the genetic and molecular mechanisms leading to neuropsychiatric disorders in TSC have not been elucidated. In this study we have generated human iPSC-derived neural progenitor cells as an in vitro system to investigate whether heterozygous mutations in the TSC2 gene are sufficient to alter neuronal development, possibly setting the stage for the emergence of TAND.
Previous studies of TSC have largely focused on homozygous loss of function cellular or animal models of either TSC1 or TSC2 (Crowell et al., 2015; Meikle et al., 2007; Scheidenhelm and Gutmann, 2004; Zeng et al., 2008). These models mimic neuroanatomical lesions in TSC resulting from loss-of-heterozygosity (LOH) where the non-mutated allele receives a somatic mutation or is otherwise inactivated causing a focal brain lesion (Henske et al., 1997; Qin et al., 2010; Sepp et al., 1996). The complete loss of function of TSC1 (Hamartin) or TSC2 (Tuberin) in these abnormal brain regions leads to excessive cell growth and lesion formation due to elevated levels of mTORC1 activity. mTORC1 is a regulatory kinase complex that promotes growth by affecting multiple processes including protein synthesis and metabolism (Jewell and Guan, 2013; Kenerson et al., 2002). TSC1 and TSC2 proteins form a heteromeric complex that regulates mTORC1 by limiting the activity of the small GTP-binding protein Rheb, an important activator of mTORC1. The TSC2 component of the TSC complex is directly responsible for this regulatory activity due to the presence of a GTPase activating protein (GAP) domain near its C-terminus, which maintains Rheb in an inactive, GDP-bound state. Rheb activity, and downstream mTORC1 activity, is thus normally curtailed by the TSC complex (Huang and Manning, 2009; Inoki et al., 2003). Due to this function TSC1 and TSC2 play a critical role in regulating cell growth and have been shown to be prominent tumor suppressors. mTORC1 activity is typically elevated in TSC lesions and cortical tubers of individuals with TSC and can be readily detected by measuring levels of phosphorylated mTORC1 targets such as S6 Kinase (or its downstream target, the ribosomal protein S6) and 4E-BP1 which regulates the onset of cap-dependent protein translation (Crino, 2011; Meikle et al., 2007). mTORC1 activity is robustly elevated in TSC-associated lesions that contain homozygous null cells, and in the perituberal cortex (Ruppe et al., 2014). Similarly, mTORC1 is hyperactive in Tsc1 or Tsc2 null conditional mouse models, which often manifest TSC-like symptoms including seizures (reviewed by (Feliciano et al., 2013)). mTORC1 upregulation is not readily apparent in the brain of Tsc1 or Tsc2 heterozygous animals, which do not exhibit seizures or apparent neuroanatomical defects but manifest learning deficits (Ehninger et al., 2008; Goorden et al., 2007; Sato et al., 2012); however, these defects can be rescued by the mTORC1 inhibitor rapamycin, suggesting that a modest dysregulation of this kinase may underlie cognitive dysfunction. Evidence of mTORC1 hyperactivity has also been reported in the synaptic fraction of the Tsc1 heterozygous mouse brain (Bartley et al., 2014). Together with the observations that some types of heterozygous neurons exhibit subtle alterations in axon targeting, dendrite arborization and synaptic structure (Nie et al., 2010; Tavazoie et al., 2005; Zhang et al., 2016) these data implicate mTORC1 signaling in the cellular and behavioral defects associated with Tsc1 or Tsc2 heterozygosity.
In recent years, human pluripotent stem cells have become a widely used alternative models for neurological diseases as they can be directed to produce differentiated neurons or glia in vitro (Marchetto et al., 2011; Tiscornia et al., 2011; Yu et al., 2013). Modeling TSC, genome-engineered TSC2 heterozygous and homozygous human embryonic stem cell (hESC) lines have been established and used to generate neural progenitor cells (NPCs) as well as differentiated neurons and glia (Costa et al., 2016; Grabole et al., 2016). These studies first demonstrated abnormal neuronal maturation, altered synaptic activity, aberrant glia differentiation and neuroinflammation, which were particularly evident in TSC2 null cultures. An adult cell-derived induced pluripotent stem cell (iPSC) line carrying a heterozygous TSC2 mutation was also recently generated from one TSC patient, and used to identify proliferation defects in NPCs and morphological abnormalities in differentiated neurons (Li et al., 2017). Finally, heterozygous TSC patient-derived iPSC lines as well as isogenic null and control lines were established and used to generate NPCs and cerebellar Purkinje cells (Sundberg et al., 2018). This study further reported abnormal neuronal differentiation and synaptic activity, particularly affecting null cells.
In order to investigate possible developmental abnormalities of heterozygous cells in the TSC brain we established two sets of patient- and unaffected control-derived iPSCs, and further differentiated these into neural progenitor cells (NPCs) and neurons in vitro. Using this model, we identify novel cellular and molecular phenotypes in TSC2 haploinsufficient NPCs. In addition to previously identified dysregulation of mTORC1 activity we found that patient-derived progenitor cells are transiently delayed in their ability to differentiate into neurons and exhibit a profound suppression of AKT activity that is mediated by a negative feedback mechanism. Together, these findings suggest that TSC heterozygosity produces abnormal phenotypes in NPCs that potentially impact the developing brain.
2. Methods
2.1. Human Subjects and iPSC Generation
Four human subjects were recruited for this study at the Comprehensive Epilepsy Center, NYU Medical School after obtaining informed written consent from the subjects or their parents. The subject group includes two clinically diagnosed TSC patients who carry de novo heterozygous mutations in the TSC2 gene that are predicted to cause loss of function, and two unaffected controls consisting of one gender and age-matched sibling, and one age-matched individual (Table 1). Mutations sites are based on human TSC2 mRNA variant 1 sequence (GenBank NM_000548.3). Peripheral blood samples from these subjects were collected and processed at RUCDR Infinite Biologics (Piscataway, NJ) where CD4+ hematopoietic progenitor cells were isolated and transduced with Sendai viruses expressing reprogramming factors to generate iPSCs according to an established protocol (Loh et al., 2010). Multiple iPSC clones were derived from each individual, and clones were subjected to a standard set of quality control services including assays for microbiological contamination and pluripotency as defined by the expression of markers by immunofluorescence and FACS analysis. This study was conducted as described in protocols approved by the Institutional Review Board (IRB) at NYU and Rutgers University.
Table 1.
Human subject information.
| Subject | Age | Gender | TSC2 genotype | Mutation site | TSC features | |
|---|---|---|---|---|---|---|
| Same family | TSC #1 | 32 | male | 4bp deletion causing frame-shift | Nucleotides 4650-4653 (ACAA) | Refractory epilepsy, tubers, low IQ |
| CTR #5 | 30 | male | Normal | none | None | |
| Different family | TSC #6 | 18 | male | Point mutation causing premature stop | Nucleotide 2427 (C->T) | Controlled epilepsy, SEGAs, low IQ |
| CTR #8 | 19 | female | Normal | none | none |
2.2. NPC Induction
iPSC colonies were maintained in mTESR hPSC media (STEMCELL Tech) and were passaged onto Matrigel coated six well plates. To generate NPC lines the iPSC culture medium was replaced with a PSC Neural Induction Medium (NIM) consisting of Neurobasal medium and Neural Induction Supplement according to the manufacturer’s instructions (Gibco™, Life Technologies). The NIM was changed every day until day seven. Newly generated NPCs were detached using Accutase (STEMCELL Tech), passed through a 100 μm strainer, plated in Matrigel coated cell culture flasks, and expanded in Neural Expansion Medium (NEM) consisting of equal parts Neurobasal and Advanced DMEM/F-12 medium (Gibco™, Life Technologies). NPC cultures were maintained in NEM and the medium was changed every 2–3 days. Cells were passaged to new Matrigel coated flasks (Cell Star) after reaching ~90% confluence. During passaging NPCs were seeded at 2 × 107 cells per flask. NPCs were counted for seeding by manual counting with a hemocytometer. At least three NPC lines per subject were generated and used in this study.
2.3. Neuronal Differentiation
Expanded NPC lines were allowed to reach ~90% confluence in Cell Star 500 mL cell culture flasks and then detached with Accutase. Dissociated NPCs were seeded at 1 × 106 cells per well in a 24 well cell culture plate coated with 10 μg/mL laminin and poly-D-lysine. NPCs were induced to differentiate by replacing the NEM with a neural differentiation medium (NDM) containing Neurobasal, B-27 supplement without vitamin A, GlutaMAX, nonessential amino acids, 20 μg/mL BDNF, and 20 μg/mL GDNF. Cultures were differentiated for 7 to 21 days with NDM refreshed every 2–3 days.
2.4. Sanger Sequencing
NPCs generated from control and affected TSC patients were seeded at a density of 5 × 106 per well in 6 well cell culture plates. NPCs were allowed to reach ~70% confluence before lysis and RNA extraction (ThermoFisher). cDNA was then generated from RNA samples and the sequences surrounding each known mutation sites in the patients were amplified in each subject via PCR. Oligos for TSC2: Set 1 – 5’-GGAACCTGGTGCCTCACTTG-3’ (forward); Set 1 – 5’-GCTGCCACAGGGAGCTTAG-3’ (reverse); Set 2 – 5’-CACAGGCATTCAGGGACTTG-3’ (forward); Set 2 – 5’-TGAGCTTCACCACCAGAAC-3’ (reverse). Primers from set 1 were used to amplify sequences from subject lines CTR #5 and TSC #1, surrounding the patient mutation. Primers from set 2 were used to amplify sequences from subject lines CTR #8 and TSC #6, surrounding the patient mutation. PCR products were then purified and sequenced via Sanger Sequencing (Genewiz).
2.5. Immunofluorescence Staining
NPCs were seeded at 5 × 105 or 1 × 106 per well either on glass coverslips or directly onto 24 well cell culture plates coated with either Matrigel or laminin/poly-D-lysine (as appropriate for either progenitor or neuronal culture). NPCs were cultured for 48 hours whereas neurons were differentiated for 1–3 weeks before staining. The medium was then removed, and cells were washed twice with cold PBS and fixed with 4% paraformaldehyde. Fixed cells were washed twice with PBS before permeabilization with 0.1% Triton X-100, and then washed three times in PBS before blocking in 10% normal goat serum. Cells were incubated overnight at 4°C with appropriate primary antibodies (Suppl. Table 1) in a buffer containing 0.1% Triton X-100 and 10% normal goat serum. Afterwards cells were washed three times in PBS, incubated with an appropriate secondary antibody solution for one hour, and washed again three times in PBS before mounting coverslips on glass slides with Vectashield (Vector Laboratories) mounting medium. Cells fixed directly onto plates were counterstained with either RedDot2 (Biotium) or Hoechst nuclear stains (ThermoFisher). Images were captured using an Olympus microscope equipped with a Yokogawa CSU-10 spinning disk confocal head and/or inverted epifluorescence microscope. Images were corrected for brightness and contrast using ImageJ analysis software. Neuronal soma size was measured in a blind fashion from DIV14 βIII TUB-stained cultures established by differentiating 3 independent NPC lines per subject using ImageJ analysis software.
2.6. High Content Analysis of Microscopy Images
To determine the percentage of neuronal differentiation at different time points HuC/D+ cells stained using the previously described immunofluorescence protocol were loaded into an INCell Analyzer 6000 (GE Healthcare) for high-content analysis of microscopy images. For each experiment multiple (3–4) image fields/well were selected at random from 3–4 wells/subject (9–12 fields/subject/time point), and analyzed using Workstation software (GE Healthcare). Some images were manually excluded from the analysis because either cells were out of focus or captured fields were empty. n=3 independent experiments were conducted each at DIV14 and DIV21 using 3 independently generated NPC lines per subject. For DIV7 n=4 independent experiments were conducted using 3 independently generated NPC lines per subject and 1 replicate. Each experimental data point was based on a large number of cells analyzed per subject (48,000–134,000 total nuclei). The percentage of HuC/D+ cells over total DAPI-stained nuclei obtained from all independent experiments at each time point were pooled and statistically analyzed to determine the effect of the genotype on neuronal differentiation in each patient-control subject matched set.
2.7. Cell Death Assay
Cell death was assayed by seeding 5 × 105 NPCs on Matrigel coated 24 well plates. After 48 hours the medium was removed, and cells were incubated with Hoechst and Sytox (Invitrogen) nuclear stains for thirty minutes. Cells were washed twice quickly with PBS, imaged live using the INCell Analyzer 6000 and subjected to high content analysis as described above. To calculate the percentage of dead cells the number of Sytox stained cells was divided by the number of the total cell population labeled by Hoechst nuclear staining.
2.8. BrdU Labeling
NPC lines from each subject were incubated with 5-bromo-2-deoxyuridine (BrdU) for 4 h on DIV2 and 10. Cells were incubated in 2N hydrochloric acid for 30 min at 37°C, followed by neutralization with 100 mM sodium borate pH 8.5 for 10 min at room temperature. Cells were then fixed and immunofluorescence staining was performed as indicated above for other antigens.
2.9. Whole Transcriptome Analysis
Two independent NPC lines from each of the related subjects (TSC #1 and CTR #5; Table 1) were harvested for RNA extraction. Total cellular RNA was prepared using a Zymo Direct-Zol kit and delivered to RUCDR Infinite Biologics (Piscataway, NJ) for sequencing. Libraries were prepared with Illumina Tru-Seq kits and run as paired-end, 75nt/end on Illumina NextSeq. RNA-Seq reads were pseudoaligned to hashed k-mers built from the UCSC Reference Sequence library of mRNAs using Kallisto (Bray et al., 2016). Estimated counts were imported into the Sleuth package (Pimentel et al., 2017) in R/Bioconductor (Gentleman et al., 2004), where differential expression was assessed using a Wald test. Gene ontology analysis utilized the DAVID website (Huang da et al., 2009). RNA-Seq data are available from the NIH GEO archive (accession GSE111584).
2.10. Western Blotting
Cells were seeded onto six well cell culture plates coated with Matrigel at a density of 5 × 106 and allowed to expand 48 hours before lysing with RIPA cell lysis buffer containing phosphatase and protease inhibitors. Lysates were cleared via centrifugation at 12,000 x g for 10 minutes and the supernatant was collected. Soluble protein samples were combined in a 1:1 solution with loading buffer containing SDS and loaded onto Tris-Glycine gels for electrophoresis. Proteins were then transferred onto nitrocellulose membranes and blocked in 3% nonfat dry milk for one hour before incubation with appropriate primary antibodies (Suppl. Table 1). After primary incubation membranes were washed 3 times with Tris buffer saline including 0.1% tween (TBST) and incubated for one hour in an HRP-conjugated secondary antibody solution, then washed a further 3 times in TBST. Membranes were coated with ECL Western Blotting Substrate for chemiluminescence (ThermoFisher) and images of protein bands were obtained using radiographic film. Image scanning and band densitometry was performed using AlphaImager band analysis software. Western blot plots were obtained by pooling the results from n=3–5 independent experiments using 3 independently generated NPC lines per subject.
2.11. Small Molecule Treatment
For drug treatments NPCs were plated in either 24 well or 6 well cell culture plates at a density of 1 × 106 or 5 × 106, respectively. Cells were expanded for 48 hours then treated with NEM containing either 0.1% DMSO (Sigma), 5 μM RAD001 (Selleckchem), 1 μM MK2206 (Selleckchem), or 10 μM LY294002 (Selleckchem). After 24 or 48 hours samples in 6 well plates were collected for Western blotting. Cells plated for immunofluorescence in 24 well dishes were differentiated by changing the medium to NDM containing DMSO, RAD001, MK2206 or LY294002. Cells were cultured for 7 days before fixation and imaging via high content analysis of microscopy images as described previously.
2.12. Statistics
In this study 3–4 independently generated NPC lines per subject were used in each set of experiments. Sample size (n) was determined by the number of independent experiments conducted using at least 3 different lines per subject and, where indicated, additional replicate experiments using the same NPC lines cultured at a different time and after a different number of passages. Comparisons between TSC patient and control groups were conducted pairwise between one sibling-matched pair (CTR #5 and TSC #1) and non-sibling pair (CTR #8 and TSC #6). All statistical analysis was conducted using GraphPad Prism7 software. For proliferation (KI67 and BrdU staining) experiments at multiple time points significance was determined using ordinary or repeated measure two-way ANOVA with Sidak’s multiple comparisons tests. For cell death (Sytox labeling) and soma size measurements statistical significance between genotypes was determined using unpaired nonparametric t tests (Mann-Whitney). To compare neuronal differentiation (% HuC/D+ cells) in CTR and TSC sets of samples at different time points statistical significance was determined using multiple unpaired t tests corrected using the Holm-Sidak method and α= 0.05. To determine the genotype effect in Western blot experiments statistical significance was determined using either unpaired t tests or one-sample t tests, depending on the data structure. When multiple independent samples from each TSC and the corresponding CTR subject were analyzed simultaneously on the same gel we used nonparametric unpaired t tests (Mann-Whitney) to compare the means of the two groups. When independent samples were analyzed in technical triplicates and run at different times on different gels we normalized the data to the mean of the CTR value (set at 1). In this case comparison was made using one-sample t tests. For drug treatment experiments that were performed at different times with different drugs we normalized the mean percentage of HuC/D+ cells in the CTR+DMSO sample (internal control set at 1) and expressed values from other groups as fold change. Significance was determined via one-way ANOVA with Dunnett’s post-hoc analysis. For gene expression analysis we compared results obtained from 2 independent NPC lines/subject in the #1/5 pair. Transcripts having a False Discovery Rate (FDR; p value adjusted by the Benjamini-Hochberg method) of 1% or less and a beta value estimating at least a two-fold change were selected for analysis.
3. Results
3.1. Generation of NPCs from TSC patient and control subjects
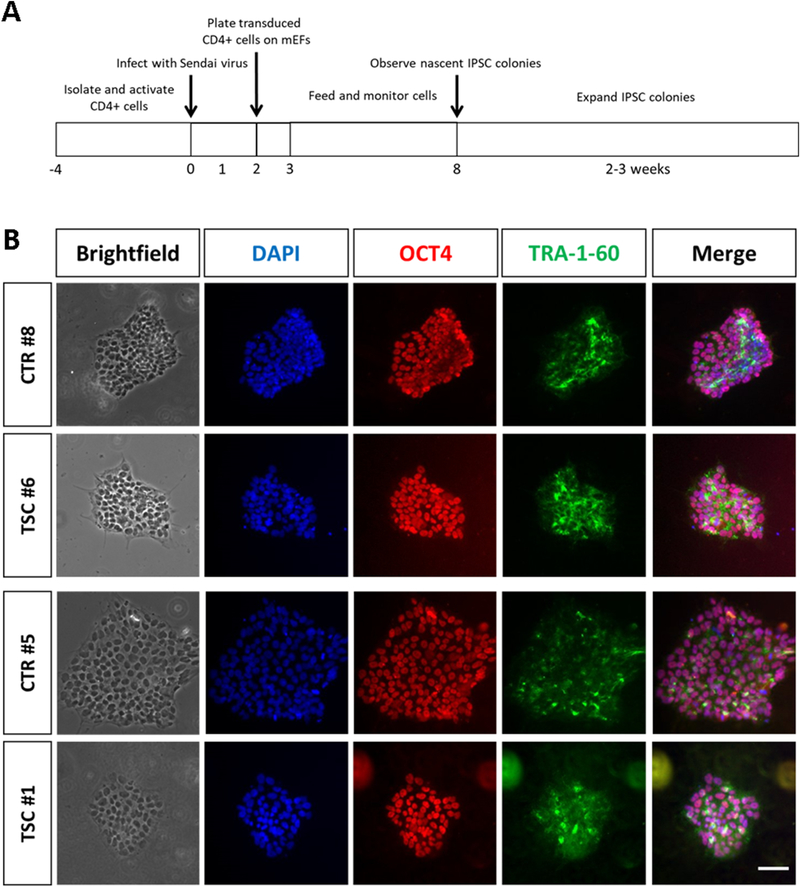
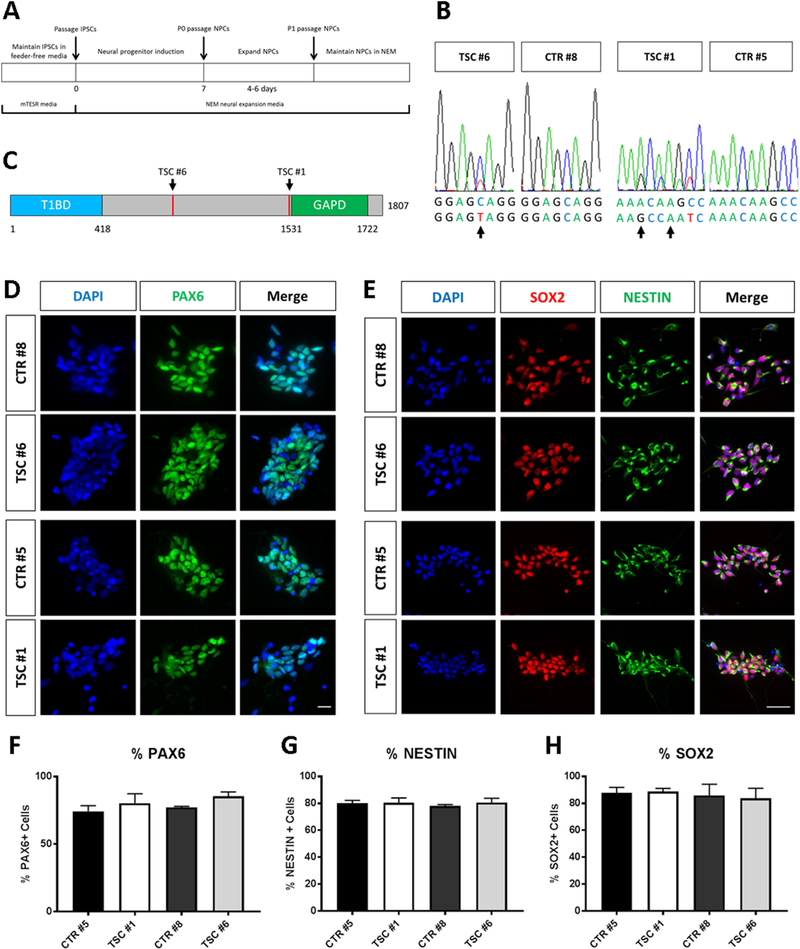
To study TSC2 heterozygous phenotypes, we developed an in vitro model of TSC by generating iPSCs from two individuals with de novo TSC2 mutations who had epilepsy, low IQ and neuroanatomical defects, and two genetically normal controls (Table 1). Patient TSC #1, sibling of unaffected CTR #5, carries a heterozygous deletion in the TSC2 gene (hg38 chr16:2,085,001–2,085,004, del ACAA) predicting a frameshift beginning at Asn1515, that results in termination after 1573 amino acids, according to the reference isoform (NM_000548). TSC #6 has a single base change (chr16:2,074,224; rs45517233), causing premature termination of TSC2 mRNA translation (Gln794->Stop). iPSCs were generated from peripheral blood cells and reprogrammed using Sendai viruses expressing the Yamanaka cocktail of transcription factors (Figure 1A). Newly generated patient- and control-derived iPSC colonies expressed the factors OCT4 and TRA-1–60 confirming their pluripotent state (Figure 1B). We next generated lineage restricted NPCs from iPSC cultures using commercial reagents and protocols, as previously described (Yan et al., 2013). After seven days of differentiation in neural expansion medium, NPCs were isolated (Figure 2A). This protocol was repeated multiple times to obtain at least 3 independently-generated NPC lines per subject. Direct sequencing of cDNA generated from NPC lines confirmed that patient-derived cells carried the expected heterozygous TSC2 mutations consisting of a frame-shift in TSC #1 and a nonsense mutation in TSC #6 (Figure 2B). Both mutations occur before the TSC2 GAP domain and are predicted to result in either a deficit in the levels of functional protein or a reduction in protein expression due to nonsense-mediated RNA decay (Figure 2C). Immunofluorescence staining confirmed the expression of NESTIN, SOX2 and PAX6 markers in both patient and control NPCs, consistent with a neural progenitor identity (Figure 2D, E). The percentage of NPCs expressing these markers was not affected by the genotype (Figure 2F-H).
Fig. 1.
Generation and characterization of subject-derived iPSCs. (A) Workflow depicting reprogramming and generation of iPSC clones from CD4+ hematopoietic progenitor cells. mEF= mouse embryonic fibroblasts. (B) Representative immunofluorescence images of successfully reprogrammed iPSC colonies obtained from TSC patients and controls. iPSC colonies stained positive for the pluripotent markers OCT4 and TRA-1–60. Nuclei are counterstained with DAPI. Scale bar = 40μm.
Fig. 2.
Generation and characterization of NPCs. (A) Workflow for generating lineage restricted NPCs from subject-derived iPSCs. (B) Sanger targeted sequencing confirms the heterozygous genotype of TSC patient NPC lines. Arrows point to double peaks in patient cells showing the presence of a point mutation resulting in a nonsense mutation in the TSC2 gene of subject TSC #6, and a four base pair deletion resulting in frameshift in subject TSC #1. (C) Schematic representation of the TSC2 protein indicating the approximate position of each patient mutation. (D, E) Immunofluorescence images of induced NPCs. NPC lines show expression of neural stem cell markers PAX6, SOX2 and NESTIN. (F-H) Quantification of the percentage of cells expressing each NPC marker relative to the total number of cells (DAPI-labeled nuclei). Data obtained from n=3 lines per subjects are plotted as mean values ± SEM. Scale bars = 20μm (D) and 50μm (E).
3.2. Characterization of NPC proliferation and viability
Since TSC signaling and mTORC1 activity are known to be important for cell growth and survival (Gan et al., 2008; Hartman et al., 2013; Lee et al., 2003; Ozcan et al., 2008; Wu et al., 2009) we sought to determine the effect of TSC2 haploinsufficiency on NPC proliferation and viability. Here we utilized high-content analysis of microscopy images in order to quantify a large number of cells from multiple technical replicate samples and multiple independent experiments in an unbiased manner. NPC proliferation was assayed by labeling cells in the active phases of the cell cycle with an antibody against KI67, and the total cell number was determined using a nuclear stain (Figure 3A, D). The percentage of KI67+ proliferating cells over the course of 2 or 10 days in vitro (DIV) was similar between patient and control progenitor lines in both subject sets analyzed (Figure 3B, E). To confirm these findings, we also labeled proliferating cells by BrdU incorporation at DIV2 and 10, and measured their percentages (Figure 3C, F). These data further demonstrate that TSC patient cells proliferate at a normal rate. To assay NPC viability we measured the percentage of Sytox-stained dying cells in similar 48-hour culture experiments (Figure 3G). The data indicate that there is no significant increase in cell death in TSC cell lines, although both patient-derived cultures exhibited a trend towards increased cell death (Figure 3H).
Fig. 3.
Analysis of growth and survival in patient and control NPC cultures. (A) Representative confocal images of NPC cultures from subjects #5 and #1 stained for the proliferative marker KI67 or BrdU to label proliferating cells at DIV 2 or 10. Nuclei are counterstained with RedDot2 or Hoechst. (B, C) Automated high content analysis of randomized image fields of cultures labeled with KI67 (B) or BrdU (C) antibodies. Ordinary two-way ANOVA with Sidak’s multiple comparison test, p>0.05. (n = 6 independent cultures at DIV2 and n= 3 independent cultures at DIV10 from 3 different NPC lines/subject). (D) Confocal images of NPC cultures from subjects #8 and #6 labeled with KI67 or BrdU antibodies and counterstained with Hoechst. (E, F) High content analysis of images obtained from cultures labeled on DIV2 and DIV10 (n = 4 independent cultures at each time point from 3 NPC lines/subject) with KI67 (E) or BrdU (F) antibodies. Repeated measures two-way ANOVA with Sidak’s multiple comparison test, p>0.05. The data show no difference in proliferation between TSC and control samples. (G) Representative confocal images of NPC cultures stained for Sytox to visualize dead cells and counterstained with Hoechst. (H) Automated high content analysis of Sytox staining shows no significant difference between each TSC group and its respective control (CTR #5 and TSC #1: n = 5 independent cultures from three NPC lines/individual; CTR #8 and TSC #6: n = 4 independent cultures from three NPC lines/individual). Significance was determined via unpaired nonparametric t tests (Mann-Whitney). Plots represent mean values ± SEM. Scale bar = 100μm.
3.3. Signaling abnormalities in TSC neural progenitors
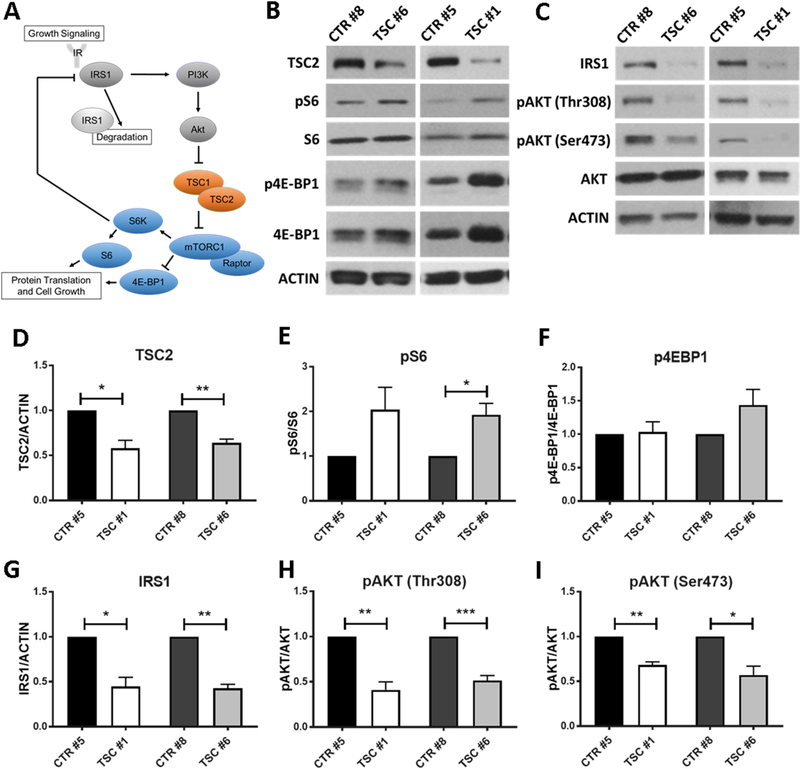
To investigate potential molecular defects in heterozygous cells derived from TSC patients we analyzed TSC2 protein expression levels and signal transduction pathways likely to be affected by TSC2 mutations (Figure 4A). Protein lysates from 3 independent NPC lines per subject were analyzed by Western blotting using an antibody against the C terminus of TSC2. The levels of this protein were significantly reduced by approximately 50% in both patients compared to control cells (Figure 4B, D). These data demonstrate that both patient mutations result in TSC2 haploinsufficiency. The TSC1/TSC2 complex is a key regulator of mTORC1 activity in response to growth factor signaling, and mTORC1 upregulation has been observed in numerous homozygous animal or cellular models of TSC (Goto et al., 2011; Huang and Manning, 2008; Inoki et al., 2003; Magri et al., 2011; Meikle et al., 2007). mTORC1 kinase activity is readily inferred by the levels of phosphorylation of downstream targets such as the ribosomal protein S6 and the translation factor 4E-BP1 (Figure 4A). TSC2 haploinsufficiency in patient progenitor cells affected mTORC1 activity, resulting in an increase in S6 phosphorylation relative to total S6 protein levels (Figure 4B and E). The phosphorylation of 4E-BP1 was not significantly affected when levels of phospho-4E-BP1 were normalized to the total protein (Figure 4B and F). However, relative levels of total 4E-BP1 appeared to be elevated in TSC cells derived from one patient, potentially confounding the interpretation of these results. Overall, these data suggest that TSC2 heterozygosity in NPCs increases mTORC1 activity, albeit to a modest degree.
Fig. 4.
Altered PI3K/AKT/mTORC1 signaling in TSC NPCs. (A) Schematic representation of the PI3K/Akt/mTORC1 signaling pathway. Growth factors and insulin receptors activate the IRS1-mediated PI3K/Akt pathway, which inhibits TSC2 and promotes mTORC1-dependent protein translation and cell growth via S6K1 and 4E-BP1 phosphorylation. Activated S6K1 elicits a negative feedback that leads to the phosphorylation and degradation of IRS1. (B) Western blotting for TSC2 and phosphorylated proteins downstream of mTORC1: ribosomal protein S6 and 4E-BP1. (C) Western blotting for upstream regulators of TSC2 activity including phosphorylated Akt and total IRS1. (D-I) Quantification of Western blot data showed significantly decrease levels of TSC2 in both patients, significantly increased phosphorylation of S6 in one TSC patient (*p<0.05) and a trending increase in the second TSC patient (p=0.13, n=4 cultures from three independently generated NPC lines). Levels of phospho-4E-BP1 relative to total protein were not significantly altered in either patient samples. Total levels of IRS1 were significantly reduced in both TSC patients (*p<0.05, **p<0.01, n=4 cultures from three independently generated NPC lines). Levels of phospho-Akt were significantly reduced in TSC NPCs at two phosphorylation sites: Thr308 (**p<0.01, ***p<0.001, n=5 cultures from three NPC lines) and Ser473 (**p<0.01, *p<0.05, n=4 independent cultures from three NPC lines). Significance was determined via one-sample t-test. Plots represent mean values ± SEM.
Upstream regulation of TSC function occurs by PI3K/Akt signaling in response to mitogenic stimuli via insulin or growth factor receptors and the insulin receptor substrate IRS1, an adapter protein that mediates PI3K activation (Figure 4A). In the presence of pro-growth stimuli, the PI3K/Akt pathway acts to phosphorylate and inhibit TSC2, resulting in the activation of mTORC1 and the increase in protein translation and cell growth (Huang and Manning, 2009; Manning and Cantley, 2003). A negative feedback loop involving S6K has been shown to suppress PI3K/Akt signaling via inhibition and degradation of IRS1 (Pederson et al., 2001; Shah and Hunter, 2006; Shah et al., 2004). Consistent with the observed activation of mTORC1 we found that IRS1 levels are dramatically reduced in both patient NPC lines (Figure 4C, G). Reduced Akt phosphorylation levels have been shown previously in homozygous models of TSC where mTORC1 activity, and thus S6K, is robustly elevated (Jaeschke et al., 2002; Meikle et al., 2008; Zhang et al., 2003). Consistent with these findings we observed that TSC2 heterozygous NPCs exhibited significantly decreased levels of phosphorylated AKT protein (Figure 4C, H and I). AKT in TSC2 heterozygous cell lines showed reduced phosphorylation at the Ser473 and Thr308 sites, both of which are required for AKT activation. The Thr308 site of AKT is phosphorylated by PI3K via 3-Phosphoinositide-dependent kinase 1 (PDK1), and reduced levels of phosphorylation at that site are indicative of reduced PI3K activity. The Ser473 site, on the other hand, is known to be phosphorylated by the mTOR-containing complex mTORC2, although the regulation of this kinase complex activity is not well understood. Together, our findings suggest that the S6K-mediated negative feedback triggered by mTORC1 upregulation is responsible for the downregulation of IRS1 and the reduced PI3K/AKT activity observed in patient TSC2 cells.
3.4. TSC NPCs exhibit neuronal differentiation defects
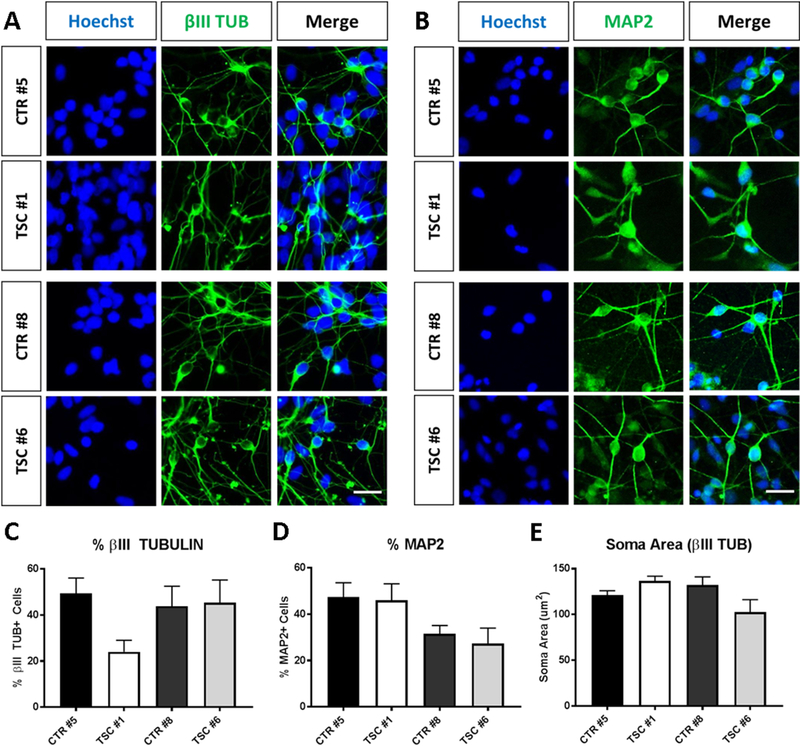
Having identified molecular abnormalities in patient-derived NPCs, next we set out to determine whether these cells are impaired in their ability to undergo neuronal differentiation. Multiple control and TSC NPC lines from both subject sets were induced to differentiate over a time course of 1–3 weeks. To identify the cell bodies of differentiated neurons we immunostained cultures with antibodies against HuC/D, an RNA-binding protein that is specifically expressed in differentiated neurons. This antibody clearly detects neuronal nuclei and cell bodies, and co-labels cells that express the classic neuronal marker MAP2 (Figure 5A and Supplemental Figure 1). We then conducted high-content analysis of microscopy images and measured neuronal differentiation by calculating the percentage of HuC/D+ cells over the total number of cells counterstained with a nuclear stain in both sets of patient and control cultures. Multiple randomized fields were captured for each time point of differentiation and the data obtained from multiple experiments using 3 independent NPC lines per subject were pooled. We found that the percentage of neurons increased through the course of differentiation in both patient and control cell lines (Figure 5B, C). However, the percentage of neurons was significantly reduced in both patient cultures compared to controls at DIV7. This effect was transient and was largely abolished at later time points in the subject set #8/6 (Figure 5B), whereas in subject set #5/1 TSC values appeared lower than control at DIV14 and DIV21, although this difference was not statistically significant at these later time points (Figure 5C). These data suggest that TSC2 heterozygous mutations cause a delay in neuronal differentiation. To further investigate this possibility, we performed Western blot analysis of early neuronal marker protein expression in DIV7 neuronal cultures. Levels of Doublecortin (DCX) and βIII TUBULIN protein were significantly reduced in both patient cultures compared to controls (Figure 5D-F), confirming that patient cultures contained fewer differentiated neurons at this time point. Using confocal imaging of DIV14 immunostained neuronal cultures we also analyzed cells derived from both sets of TSC and control NPCs. Many cells in these cultures expressed the classic neuronal markers βIII TUBULIN (TUB) and MAP2, and exhibited a typical neuronal morphology in both genotypes (Figure 6A, B). Detailed analysis of the percentage of βIII TUB+ and MAP2+ neurons in cultures obtained from 3 independent lines per subject revealed no significant difference between genotypes (Figure 6C, D), although the percentage of βIII TUB+ appeared somewhat reduced in patient TSC #1. Finally, we measured the soma size of βIII TUB -stained neurons and found that it was not altered in patient-derived neurons (Figure 6E). These findings indicate that TSC2 haploinsufficiency, unlike the homozygous condition, does not result in neuronal hypertrophy.
Fig. 5.
Delayed neuronal differentiation of TSC NPCs. (A) Representative confocal images of DIV7, 14, and 21 neuronal cultures generated from TSC and control NPC lines. An increasing proportion of the cells express the neuron-specific markers HuC/D and MAP2 over the time course of differentiation. Nuclei are counterstained with DAPI. Scale bar = 100μm. (B, C) Automated high content analysis of randomized image fields shows a steadily increasing percentage of HuC/D+ cells during the time course of differentiation for TSC and control cultures in both subject sets. However, at DIV7 TSC cultures showed a significantly lower percentage of HuC/D+ cells than control in both subject sets (*p<0.05, n = 4 independent differentiation experiments). The percentage of HuC/D+ neurons in TSC cultures was not significantly altered at DIV14 or DIV21 in either subject set (n = 3–4 independent experiments). Significance at each time point was determined via multiple unpaired t tests corrected using the Holm-Sidak method and α= 0.05. (D) Western blotting of DIV7 cultures show reduced levels of early neuronal markers DCX and βIII-TUB in patient samples from both subject sets. (E, F) Quantification of Western blot data normalized to internal controls. The decrease in DCX and βIII-TUB levels were significant in both patient groups compared to controls (**p<0.01, *p<0.05, n=5 experiments for CTR #8 and TSC #6, n = 4 for CTR #5 and TSC #1). Significance was determined via unpaired nonparametric t tests (Mann-Whitney). Plots represent mean values ± SEM.
Fig. 6.
Analysis of NPC-derived differentiated neuronal cultures. (A, B) Representative confocal images of DIV14 differentiated neuronal cultures showing expression of the βIII TUBULIN (TUB) (A) and MAP2 (B) neuronal markers (B). Nuclei are counterstained with Hoechst. Scale bar = 40μm. (C, D) Quantification of the percentage of cells expressing each neuronal marker show no difference between genotypes. Data obtained from n=3 experiments with independent lines per subjects. Significance was determined via unpaired nonparametric t tests (Mann-Whitney). (E) Analysis of the soma size of βIII TUB+ cells demonstrate no difference between genotypes. Data obtained from n=3 independent lines per subjects. Significance was determined via unpaired nonparametric t tests (Mann-Whitney). Plots represent mean values ± SEM.
Finally, we performed RNA-Seq analysis of two independent NPC lines derived from one patient (TSC #1) and his sibling control (CTR #5), identifying 513 transcripts as differentially expressed (Supplemental Figure 2 and Supplemental Table 3). Gene ontology (GO) analysis of transcripts that are depleted in TSC enriched two biological processes relating to neuron migration and development (Supplemental Table 2), supporting the view that neuronal differentiation is impaired by TSC2 heterozygous mutations. RNA-Seq reads aligning with the TSC2 gene also exhibited evidence of allelic imbalance, since a synonymous SNP (rs34012042) in TSC #1 was heterozygous but unequal (28% C, 72% T). This supports the prediction that the frameshifted mRNA may be destabilized.
3.5. AKT inhibition mimics the differentiation delay of TSC patient cells
To identify signaling alterations that may underlie the neuronal differentiation delay phenotype in TSC2 heterozygous cells we utilized a pharmacological approach focusing on PI3K, AKT and mTORC1. First, we used the rapamycin-analog RAD001 to suppress mTORC1 activity. NPC lines from one individual with TSC and significantly elevated mTORC1 activity (#6) and control (#8) were treated with 5μM RAD001 or DMSO vehicle control for 48 hours. Consistent with previous Western blot experiments (Figure 4), we found that DMSO-treated NPCs derived from the TSC2 deficient individual expressed significantly higher levels of phospho-S6 (Figure 7A, B), and reduced levels of phospho-AKT (Thr308) compared to DMSO-treated control cells (Figure 7A, C). RAD001 treatment successfully abolished elevated phospho-S6 levels in patient cultures, and also reduced phospho-S6 levels in control cells, but did not significantly affect phospho-AKT levels in either subject group (Figure 7A-C). Second, we used the AKT inhibitor MK2206 and the PI3K inhibitor LY294002 to reduce AKT activity in control cells from both unaffected subjects. NPCs from control lines were treated for 24 hours with 1μM MK2206, 10μM LY294002 or DMSO vehicle control. Western blot analysis revealed that both drugs significantly reduced phospho-AKT (Thr308) levels in both subjects compared to DMSO-treated samples (Figure 7D-F), and also reduced levels of downstream phospho-S6, as expected (Figure 7D, E).
Fig. 7.
PI3K and AKT inhibition, but not mTORC1, affects neuronal differentiation in NPCs. (A) Western blot analysis of AKT (Thr308) and S6 phosphorylation in control and TSC NPCs treated with 5 μM RAD001 or DMSO for 48 hours. (B, C) Quantification of Western blot data for the RAD001 treatment. Phospho-S6 (pS6) levels were significantly higher in TSC-DMSO compared to CTR-DMSO samples, but were strongly reduced by RAD001 treatment (B), whereas significantly lower levels of phospho-AKT (pAKT) were unaffected (C) (*p<0.05, ***p<0.001, ****p<0.0001, n=6 DMSO-treated cultures, n=3 RAD001-treated cultures). Significance was determined via one-way ANOVA with Tukey’s post-hoc analysis. (D, E) Western blot analysis of AKT (Thr308) and S6 phosphorylation in control NPCs treated with 1 μM MK2206, 10μM LY294002 or DMSO for 24 hours. (F) Quantification of the pAKT data. Both subject groups show significantly attenuated pAKT levels in MK2206-treated as well as LY294002-treated cultures compared to DMSO controls (*p<0.05, **p<0.01, ***p<0.001, n=3 cultures of 3 independent cell lines per subject). Significance was determined via one-way ANOVA with Dunnett’s multiple comparisons tests. (G) Representative confocal images of CTR #8 and TSC #6 cultures treated with DMSO, 5μM RAD001, 1μM MK2206 or 10μM LY294002, and differentiated for 7 DIV. Cultures were immunostained for the pan-neuronal marker HuC/D and counterstained with Hoechst. Scale bar = 50μm. (H, I) Automated high content analysis of treated culture images from both subject sets. The fraction of HuC/D+ cells was calculated from 3–6 experiments using 3 independent lines per subject, normalized to the internal control in each set (CTR+DMSO), and expressed as fold change. Significance was determined via one-way ANOVA with Dunnett’s multiple comparisons tests. (H) The fraction of HuC/D+ cells was significantly reduced in TSC #6+DMSO compared to CTR #8+DMSO cultures, and treatment with RAD001 did not rescue the defect. The fraction of HuC/D+ cells was similarly reduced in CTR #8+MK2206 and CTR #8+LY294002 cultures, but was not altered in CTR #8+RAD001 cultures. **p<0.01, ***p<0.001, n=4 independent cultures (RAD001 treatment), n=3 (MK2206 and LY294002 treatments), n=6 (DMSO). (I) The fraction of HuC/D+ cells was significantly reduced in MK2206- and LY294002-treated cultures compared to DMSO-treated CTR #5 cultures. *p<0.05, **p<0.01, n=3 independent experiment per treatment. Plots represent mean values ± SEM.
To test the role of mTORC1 activity in neuronal differentiation we incubated patient and control NPCs with 5μM RAD001 or DMSO, whereas to probe AKT activity we incubated control cells with 1μM MK2206 or 10μM LY294002. NPCs were induced to differentiate into neurons in the continuous presence of these inhibitors. At DIV7, neuronal cultures were immunostained with HuC/D antibodies, and the extent of neuronal differentiation was determined in each subject group by measuring the fraction of HuC/D+ cells in a large sample set by high content analysis of microscopy images (Figure 7G and Supplementary Figure S3). The data obtained from 4 independent experiments confirmed that DMSO-treated TSC cultures produce significantly fewer neurons than DMSO-treated controls at DIV7 (Figure 7H), as previously noted for untreated cultures (Figure 5). However, the RAD001 treatment failed to correct the neuronal differentiation defect in patient cells, and did not alter the differentiation of control cells (Figure 7H), despite its effectiveness in suppressing mTORC1 activity (Figure 7A, B). On the other hand, both MK2206 and LY294002 treatments significantly reduced the fraction of HuC/D+ cells in control cultures derived from both unaffected individuals, mimicking the phenotype of TSC2 haploinsufficient cell lines (Figure 7H, I). Together, these data suggest that PI3K/AKT signaling deficits in TSC2 heterozygous neural progenitors, rather than mTORC1 hyperactivity directly, may underlie changes in the process of neural differentiation.
4. Discussion
In this study, we have used a human iPSC model system to produce lineage restricted neural progenitors and neurons in vitro in order to examine the effects of TSC2 haploinsufficiency on neuronal development. Our findings demonstrate that human heterozygous TSC2 NPCs are abnormal despite lacking the hypertrophy often observed in LOH cells or lesion tissue, and that their most salient abnormality is their inability to promptly differentiate into neurons in response to appropriate growth factors. At the molecular level, this cellular phenotype likely results from the suppression of PI3K/AKT signaling activity; this in turn results from a negative feedback mechanism that is triggered by a modest upregulation of mTORC1 activity. Our data suggest that TSC2 heterozygosity in progenitor cells may be sufficient to alter brain development and function in TSC patients. These findings may explain why surgical excision of TSC lesions does not restore cognitive and behavioral function. However, our data do not dispute the notion that lesions are deleterious for brain activity. Furthermore, it is possible that microscopic lesions or undetected, dispersed homozygous null cells with impaired functionality may contribute to TSC symptomology (Marcotte et al., 2012; Sosunov et al., 2015).
The molecular signaling phenotype in TSC2 haploinsufficient neural progenitors is consistent with defects previously reported in homozygous TSC models. TSC2 heterozygous NPCs exhibited increased mTORC1 activation and decreased PI3K/AKT activation. mTORC1 activation was modest compared to that often reported in homozygous null TSC1 or TSC2 models, as expected for a haploinsufficient phenotype. Nevertheless, this activation was sufficient to elicit a classic negative feedback pathway that leads to the loss of IRS1 expression and the suppression of PI3K and AKT signaling activity. This deregulation of upstream signaling molecules was consistent and robust, and it was not accompanied by increased cell growth or proliferation, which typically correlate with high mTORC1 activity in TSC1 or TSC2 null cells. In fact, we observed normal soma size in neurons, and no significant effect on NPC proliferation. These latter findings are at odds with previous reports that described increased proliferation in TSC patient-derived NPCs. One study reported not only increased cell count and BrdU incorporation, but also increased neuronal soma size in TSC2 heterozygous cells (Li et al., 2017). These data were obtained comparing cells derived from only one TSC patient to cells obtained from unrelated unaffected controls. Another study also reported increased KI67 labeling in TSC2 heterozygous cells, although these cells, unlike ours, were cerebellar progenitors (Sundberg et al., 2018). The reason for the discrepancies are presently unclear, but they likely reflect technical differences in cell type, induction protocols or time points of analysis. Consistent with this latest study (Sundberg et al., 2018), we did not detect a significant reduction of TSC2 haploinsufficient NPC cell viability in our short-term culture system, although a modest increase in cell death was noted in both patient-derived cells. Increased cell death occurs in some models of TSC or mTOR pathway activation (Ozcan et al., 2008; Wu et al., 2009), and it was also observed in TSC2 deficient reprogrammed human stem cells (Armstrong et al., 2017). Further studies are required to determine whether the long-term survival of patient NPCs or neurons is affected, possibly as a result of reduced AKT activity, which is well known to promote survival in many systems (Brunet et al., 1999; Manning and Toker, 2017; Pap and Cooper, 1998).
One of the main findings of this study is that the progression of lineage restricted neural progenitors differentiating into neurons was perturbed in TSC2 heterozygous cell lines. This finding was very similar in both our patient-derived lines and is consistent with previous research utilizing genome-edited human iPSC lines which showed transiently reduced propensity of heterozygous and homozygous TSC2 lines to undergo neuronal differentiation (Costa et al., 2016). This was supported by RNA-Seq analyses, which identified a set of gene transcripts that were depleted in TSC NPCs and associated with functional groupings consistent with a diminished or delayed neuronal differentiation. We further showed that the differentiation delay of TSC2 heterozygous cells can be mimicked by reducing AKT activity in control cells, strongly implicating this kinase in the differentiation process. The delay in neuronal differentiation could have far reaching effects on brain development, particularly the alteration of cortical development. The formation of cortical layers and functional circuitries depend on the appropriate spatial and temporal regulation of neural progenitor proliferation, migration and differentiation (Dehay and Kennedy, 2007; Kwan et al., 2012; Noctor et al., 2004). Subtle alterations to these processes could disrupt the organization and connectivity of neurons in the cortex or subcortex. Indeed, TSC1 and TSC2 deficiencies and increased mTOR activity can cause neuronal migration defects and cortical malformations in animal models (Carson et al., 2012; Hanai et al., 2017; Zhou et al., 2011). Abnormalities in neuronal morphology, branching and connection, as well as synaptic activity have also been observed in TSC2 haploinsufficient human neurons (Costa et al., 2016; Li et al., 2017; Sundberg et al., 2018). Moreover, axonal projection, synaptic and behavioral abnormalities have been reported in heterozygous mouse models (Ehninger et al., 2008; Nie et al., 2010; Tavazoie et al., 2005). Together with the neurodevelopmental abnormality reported here, these defects could contribute to neurological deficits observed in TSC, including cognitive and behavioral abnormalities.
Despite the detectable increase of mTORC1 activity in our patient-derived NPCs, and the many documented beneficial effects of rapamycin analogs on TSC mouse models or patients (Carson et al., 2012; Crowell et al., 2015; Franz et al., 2006; Meikle et al., 2008; Zeng et al., 2008), we found that RAD001, a widely used rapamycin analog, while effectively suppressed mTORC1 activity in TSC2 haploinsufficient neural progenitors did not rescue the observed neuronal differentiation defect. However, the inhibition of PI3K and its downstream target AKT in control lines mimicked the patient phenotype. These data suggest that AKT signaling may play a more prominent role in TSC neuropathology than previously thought. Indeed, previous studies indicate that AKT activity is critical for progression of neuronal differentiation, including inhibitory neuronal differentiation (Lopez-Carballo et al., 2002; Oishi et al., 2009; Otaegi et al., 2006; Vojtek et al., 2003; Yuan et al., 2015; Zhang et al., 2014). AKT appears to play a permissive role in cell fate determination, but this role may be highly context-dependent as AKT has also been shown to maintain pluripotency and proliferation (Fishwick et al., 2010; Watanabe et al., 2006). Additionally, upstream components of the AKT pathway responding to growth stimuli have been shown to be required for differentiation, and insulin signaling specifically appears to play a role in regulating neuronal differentiation (Brooker et al., 2000; Nieto-Estevez et al., 2016; Vicario-Abejon et al., 2003). Our observation that IRS1 is strongly downregulated in patient NPCs suggests that neurodevelopmental defects in TSC may also result from the suppression of key growth signaling factors such as IGF or other IRS1-dependent stimuli. Together, these data show that PI3K/AKT activity is necessary for neuronal differentiation and fate determination, and its downregulation may be a mechanism underlying neurodevelopmental abnormalities in TSC. It is interesting to note that we detected a significant decrease in AKT phosphorylation in patient cells not only at Thr308 (a PI3K/PDK-dependent site), but also at Ser473, a target of mTORC2, a different mTOR-containing kinase complex that could play a role in neuronal differentiation. This finding suggests that mTORC2 activity may be downregulated in TSC NPCs, a possibility that was not addressed here but deserves further investigation.
One limitation of the present study is that the data were obtained from only two sets of patient and control individuals; one set (#5 and #1) consists of a well-matched set of siblings of the same gender and similar age, whereas the other (#8 and #6) consists of two individuals of similar age, but different gender and from different families. Also, no isogenic control lines were used. Despite these limitations, the fact that we observed similar cellular and molecular defects in both patient-derived cells suggest that the phenotypes are due to heterozygous TSC2 mutations and not to genetic background or cell line variability. However, further research is needed to fully elucidate the consequences of TSC2 heterozygosity, the role of PI3K/AKT signaling in brain development and neuronal differentiation, and the role of these kinases in the TSC pathophysiology.
5. Conclusions
In summary, the present study demonstrates that NPCs derived from TSC patients carrying heterozygous TSC2 mutations exhibit a neuronal differentiation delay that results from alterations in the PI3K/AKT/mTORC1 signaling pathway. Specifically, we demonstrated that a modest upregulation of mTORC1 activity downstream of TSC2 triggers a downregulation in IRS1 expression and PI3K/AKT activity through a negative feedback mechanism, and that these molecular defects likely impair neuronal differentiation, but not affect cell proliferation or growth. These findings provide new insights into the mechanisms of disease and point to alternative targets for intervention in the treatment of TSC.
Supplementary Material
HIGHLIGHTS:
Induced Pluripotent Stem Cells, Neural Progenitor Cell lines and neuronal cultures were established from Tuberous Sclerosis Complex patients and unaffected controls
Patient-derived Neural Progenitor Cells differentiated into neurons more slowly than controls
PI3K/AKT signaling and IRS1 expression were strongly decreased in patient-derived Neural Progenitor Cells
PI3K and AKT inhibition in control cells mimicked the differentiation delay noted in patient Neural Progenitor Cells
Acknowledgements
We are grateful to Jay Tischfield, Michael Sheldon and Jennifer Moore for their support and assistance in generating iPSC and NPC lines. This work was funded in part by the National Institutes of Health (NINDS grant R21 NS089441), the Tuberous Sclerosis Alliance (TSA, grant #332884), and pilot grants from the Human Genetics Institute of New Jersey Stem Cell Program to G.D., and Finding A Cure for Epilepsy and Seizures (FACES) to O.D.. A.Z. performed most experiments and wrote the manuscript draft, V.D.P and A.A. performed some experiments, R.P.H. analyzed the RNA-Seq data, O.D. recruited human subjects, G.D. supervised the project and finalized the manuscript for publication.
Abbreviations:
- TSC
Tuberous Sclerosis Complex
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- LOH
loss of heterozygosity
- iPSCs
induced pluripotent stem cells
- NPCs
neural progenitor cells
- PI3K
phosphoinositide 3-kinase
- SENs
subependymal nodules
- SEGAs
subependymal giant cell astrocytomas
- GAP
GTPase activating protein
- NEM
neural expansion medium
- DMEM
Dulbecco’s modified Eagle’s medium
- NDM
neural differentiation medium
- BDNF
Brain-derived neurotrophic factor
- GDNF
Glia-derived neurotrophic factor
- PBS
phosphate buffered saline
- TBST
Tris buffer saline with 0.1% tween
- HRP
horseradish peroxidase
- ECL
enhanced chemiluminescence
- NES
Nestin; SOX2, SRY-box 2
- PAX6
Paired box 6; TUB, βIII TUBULIN
- MAP2
microtubule-associated protein 2
- DCX
Doublecortin
- RNA-Seq
RNA sequencing
- GO
Gene ontology
Footnotes
Competing interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong LC, Westlake G, Snow JP, Cawthon B, Armour E, Bowman AB, Ess KC, 2017. Heterozygous loss of TSC2 alters p53 signaling and human stem cell reprogramming. Hum Mol Genet 26, 4629–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley CM, O’Keefe RA, Bordey A, 2014. FMRP S499 is phosphorylated independent of mTORC1-S6K1 activity. PLoS One 9, e96956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L, 2016. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34, 525–527. [DOI] [PubMed] [Google Scholar]
- Brooker GJ, Kalloniatis M, Russo VC, Murphy M, Werther GA, Bartlett PF, 2000. Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J Neurosci Res 59, 332–341. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME, 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868. [DOI] [PubMed] [Google Scholar]
- Carson RP, Van Nielen DL, Winzenburger PA, Ess KC, 2012. Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol Dis 45, 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V, Aigner S, Vukcevic M, Sauter E, Behr K, Ebeling M, Dunkley T, Friedlein A, Zoffmann S, Meyer CA, Knoflach F, Lugert S, Patsch C, Fjeldskaar F, Chicha-Gaudimier L, Kiialainen A, Piraino P, Bedoucha M, Graf M, Jessberger S, Ghosh A, Bischofberger J, Jagasia R, 2016mTORC1 Inhibition Corrects Neurodevelopmental and Synaptic Alterations in a Human Stem Cell Model of Tuberous Sclerosis. Cell Rep 15, 86–95. [DOI] [PubMed] [Google Scholar]
- Crino PB, 2011. mTOR: A pathogenic signaling pathway in developmental brain malformations. Trends Mol Med 17, 734–742. [DOI] [PubMed] [Google Scholar]
- Crowell B, Lee GH, Nikolaeva I, Dal Pozzo V, D’Arcangelo G, 2015. Complex Neurological Phenotype in Mutant Mice Lacking Tsc2 in Excitatory Neurons of the Developing Forebrain. eNeuro 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries PJ, Whittemore VH, Leclezio L, Byars AW, Dunn D, Ess KC, Hook D, King BH, Sahin M, Jansen A, 2015. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr Neurol 52, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay C, Kennedy H, 2007. Cell-cycle control and cortical development. Nat Rev Neurosci 8, 438–450. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ, 2008. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med 14, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano DM, Lin TV, Hartman NW, Bartley CM, Kubera C, Hsieh L, Lafourcade C, O’Keefe RA, Bordey A, 2013. A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits. Int J Dev Neurosci 31, 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishwick KJ, Li RA, Halley P, Deng P, Storey KG, 2010. Initiation of neuronal differentiation requires PI3-kinase/TOR signalling in the vertebrate neural tube. Dev Biol 338, 215–225. [DOI] [PubMed] [Google Scholar]
- Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dinopoulos A, Thomas G, Crone KR, 2006. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol 59, 490–498. [DOI] [PubMed] [Google Scholar]
- Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, Chin L, Williams DA, Kwiatkowski DJ, DePinho RA, 2008. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A 105, 19384–19389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J, 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y, 2007. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol 62, 648–655. [DOI] [PubMed] [Google Scholar]
- Goto J, Talos DM, Klein P, Qin W, Chekaluk YI, Anderl S, Malinowska IA, Di Nardo A, Bronson RT, Chan JA, Vinters HV, Kernie SG, Jensen FE, Sahin M, Kwiatkowski DJ, 2011. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci U S A 108, E1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabole N, Zhang JD, Aigner S, Ruderisch N, Costa V, Weber FC, Theron M, Berntenis N, Spleiss O, Ebeling M, Yeo GW, Jagasia R, Kiialainen A, 2016. Genomic analysis of the molecular neuropathology of tuberous sclerosis using a human stem cell model. Genome Med 8, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai S, Sukigara S, Dai H, Owa T, Horike SI, Otsuki T, Saito T, Nakagawa E, Ikegaya N, Kaido T, Sato N, Takahashi A, Sugai K, Saito Y, Sasaki M, Hoshino M, Goto YI, Koizumi S, Itoh M, 2017. Pathologic Active mTOR Mutation in Brain Malformation with Intractable Epilepsy Leads to Cell-Autonomous Migration Delay. Am J Pathol 187, 1177–1185. [DOI] [PubMed] [Google Scholar]
- Hartman NW, Lin TV, Zhang L, Paquelet GE, Feliciano DM, Bordey A, 2013. mTORC1 targets the translational repressor 4E-BP2, but not S6 kinase 1/2, to regulate neural stem cell self-renewal in vivo. Cell Rep 5, 433–444. [DOI] [PubMed] [Google Scholar]
- Henske EP, Wessner LL, Golden J, Scheithauer BW, Vortmeyer AO, Zhuang Z, Klein-Szanto AJ, Kwiatkowski DJ, Yeung RS, 1997. Loss of tuberin in both subependymal giant cell astrocytomas and angiomyolipomas supports a two-hit model for the pathogenesis of tuberous sclerosis tumors. Am J Pathol 151, 1639–1647. [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA, 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4, 44–57. [DOI] [PubMed] [Google Scholar]
- Huang J, Manning BD, 2008. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 412, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Manning BD, 2009. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans 37, 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A, Shepherd C, 1993. A prevalence study of autism in tuberous sclerosis. J Autism Dev Disord 23, 323–339. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL, 2003. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17, 1829–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke A, Hartkamp J, Saitoh M, Roworth W, Nobukuni T, Hodges A, Sampson J, Thomas G, Lamb R, 2002. Tuberous sclerosis complex tumor suppressor-mediated S6 kinase inhibition by phosphatidylinositide-3-OH kinase is mTOR independent. J Cell Biol 159, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Guan KL, 2013. Nutrient signaling to mTOR and cell growth. Trends Biochem Sci 38, 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenerson HL, Aicher LD, True LD, Yeung RS, 2002. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res 62, 5645–5650. [PubMed] [Google Scholar]
- Khwaja OS, Sahin M, 2011. Translational research: Rett syndrome and tuberous sclerosis complex. Curr Opin Pediatr 23, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Sestan N, Anton ES, 2012. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development 139, 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Maldonado M, Baybis M, Walsh CA, Scheithauer B, Yeung R, Parent J, Weiner HL, Crino PB, 2003. Markers of cellular proliferation are expressed in cortical tubers. Ann Neurol 53, 668–673. [DOI] [PubMed] [Google Scholar]
- Lewis JC, Thomas HV, Murphy KC, Sampson JR, 2004. Genotype and psychological phenotype in tuberous sclerosis. J Med Genet 41, 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cao J, Chen M, Li J, Sun Y, Zhang Y, Zhu Y, Wang L, Zhang C, 2017. Abnormal Neural Progenitor Cells Differentiated from Induced Pluripotent Stem Cells Partially Mimicked Development of TSC2 Neurological Abnormalities. Stem Cell Reports 8, 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, Urbach A, Heffner GC, Grskovic M, Vigneault F, Lensch MW, Park IH, Agarwal S, Church GM, Collins JJ, Irion S, Daley GQ, 2010. Reprogramming of T cells from human peripheral blood. Cell Stem Cell 7, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Carballo G, Moreno L, Masia S, Perez P, Barettino D, 2002. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem 277, 25297–25304. [DOI] [PubMed] [Google Scholar]
- Magri L, Cambiaghi M, Cominelli M, Alfaro-Cervello C, Cursi M, Pala M, Bulfone A, Garcia-Verdugo JM, Leocani L, Minicucci F, Poliani PL, Galli R, 2011. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell 9, 447–462. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC, 2003. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans 31, 573–578. [DOI] [PubMed] [Google Scholar]
- Manning BD, Toker A, 2017. AKT/PKB Signaling: Navigating the Network. Cell 169, 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Brennand KJ, Boyer LF, Gage FH, 2011. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet 20, R109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte L, Aronica E, Baybis M, Crino PB, 2012. Cytoarchitectural alterations are widespread in cerebral cortex in tuberous sclerosis complex. Acta Neuropathol 123, 685–693. [DOI] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ, 2008. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci 28, 5422–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ, 2007. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci 27, 5546–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie D, Di Nardo A, Han JM, Baharanyi H, Kramvis I, Huynh T, Dabora S, Codeluppi S, Pandolfi PP, Pasquale EB, Sahin M, 2010. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci 13, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Estevez V, Defterali C, Vicario-Abejon C, 2016. IGF-I: A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain. Front Neurosci 10, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR, 2004. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7, 136–144. [DOI] [PubMed] [Google Scholar]
- Northrup H, Koenig MK, Pearson DA, Au KS, 1993. Tuberous Sclerosis Complex. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (Eds.), GeneReviews((R)), Seattle (WA). [Google Scholar]
- Oishi K, Watatani K, Itoh Y, Okano H, Guillemot F, Nakajima K, Gotoh Y, 2009. Selective induction of neocortical GABAergic neurons by the PDK1-Akt pathway through activation of Mash1. Proc Natl Acad Sci U S A 106, 13064–13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne JP, Fryer A, Webb D, 1991. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci 615, 125–127. [DOI] [PubMed] [Google Scholar]
- Otaegi G, Yusta-Boyo MJ, Vergano-Vera E, Mendez-Gomez HR, Carrera AC, Abad JL, Gonzalez M, de la Rosa EJ, Vicario-Abejon C, de Pablo F, 2006. Modulation of the PI 3-kinase-Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci 119, 2739–2748. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS, 2008. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29, 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap M, Cooper GM, 1998. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 273, 19929–19932. [DOI] [PubMed] [Google Scholar]
- Pederson TM, Kramer DL, Rondinone CM, 2001. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes 50, 24–31. [DOI] [PubMed] [Google Scholar]
- Pimentel H, Bray NL, Puente S, Melsted P, Pachter L, 2017. Differential analysis of RNA-seq incorporating quantification uncertainty. Nature methods 14, 687–690. [DOI] [PubMed] [Google Scholar]
- Qin W, Chan JA, Vinters HV, Mathern GW, Franz DN, Taillon BE, Bouffard P, Kwiatkowski DJ, 2010. Analysis of TSC cortical tubers by deep sequencing of TSC1, TSC2 and KRAS demonstrates that small second-hit mutations in these genes are rare events. Brain Pathol 20, 1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppe V, Dilsiz P, Reiss CS, Carlson C, Devinsky O, Zagzag D, Weiner HL, Talos DM, 2014. Developmental brain abnormalities in tuberous sclerosis complex: a comparative tissue analysis of cortical tubers and perituberal cortex. Epilepsia 55, 539–550. [DOI] [PubMed] [Google Scholar]
- Sahin M, Henske EP, Manning BD, Ess KC, Bissler JJ, Klann E, Kwiatkowski DJ, Roberds SL,Silva AJ, Hillaire-Clarke CS, Young LR, Zervas M, Mamounas LA, Tuberous Sclerosis Complex Working Group to Update the Research, P., 2016. Advances and Future Directions for Tuberous Sclerosis Complex Research: Recommendations From the 2015 Strategic Planning Conference. Pediatr Neurol 60, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Kasai S, Kobayashi T, Takamatsu Y, Hino O, Ikeda K, Mizuguchi M, 2012. Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat Commun 3, 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidenhelm DK, Gutmann DH, 2004. Mouse models of tuberous sclerosis complex. J Child Neurol 19, 726–733. [DOI] [PubMed] [Google Scholar]
- Sepp T, Yates JR, Green AJ, 1996. Loss of heterozygosity in tuberous sclerosis hamartomas. J Med Genet 33, 962–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah OJ, Hunter T, 2006. Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol Cell Biol 26, 6425–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T, 2004. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol 14, 1650–1656. [DOI] [PubMed] [Google Scholar]
- Smalley SL, Tanguay PE, Smith M, Gutierrez G, 1992. Autism and tuberous sclerosis. J Autism Dev Disord 22, 339–355. [DOI] [PubMed] [Google Scholar]
- Sosunov AA, McGovern RA, Mikell CB, Wu X, Coughlin DG, Crino PB, Weiner HL, Ghatan S, Goldman JE, McKhann GM 2nd, 2015. Epileptogenic but MRI-normal perituberal tissue in Tuberous Sclerosis Complex contains tuber-specific abnormalities. Acta Neuropathol Commun 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg M, Tochitsky I, Buchholz DE, Winden K, Kujala V, Kapur K, Cataltepe D, Turner D, Han MJ, Woolf CJ, Hatten ME, Sahin M, 2018. Purkinje cells derived from TSC patients display hypoexcitability and synaptic deficits associated with reduced FMRP levels and reversed by rapamycin. Mol Psychiatry. doi: 10.1038/s41380-018-0018-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL, 2005. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci 8, 1727–1734. [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Vivas EL, Izpisua Belmonte JC, 2011. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nat Med 17, 1570–1576. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Yusta-Boyo MJ, Fernandez-Moreno C, de Pablo F, 2003. Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia. J Neurosci 23, 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek AB, Taylor J, DeRuiter SL, Yu JY, Figueroa C, Kwok RP, Turner DL, 2003. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol 23, 4417–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T, 2006. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 25, 2697–2707. [DOI] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Huang Q, Ong CN, Shen HM, 2009. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy 5, 824–834. [DOI] [PubMed] [Google Scholar]
- Yan Y, Shin S, Jha BS, Liu Q, Sheng J, Li F, Zhan M, Davis J, Bharti K, Zeng X, Rao M, Malik N, Vemuri MC, 2013. Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells. Stem Cells Transl Med 2, 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Marchetto MC, Gage FH, 2013. Therapeutic translation of iPSCs for treating neurological disease. Cell Stem Cell 12, 678–688. [DOI] [PubMed] [Google Scholar]
- Yuan H, Chen R, Wu L, Chen Q, Hu A, Zhang T, Wang Z, Zhu X, 2015. The regulatory mechanism of neurogenesis by IGF-1 in adult mice. Mol Neurobiol 51, 512–522. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M, 2008. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol 63, 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ, 2003. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest 112, 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Feliciano DM, Huang T, Zhang S, Bordey A, 2016. Hypoxia-inducible factor-1a contributes to dendritic overgrowth in tuberous sclerosis. Neurosci Lett 612, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Cheng X, Guo Y, Sun X, Chen G, Li H, Li P, Lu X, Tian M, Qin J, Zhou H, Jin G, 2014. IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3K/Akt pathway. PLoS One 9, e113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shrikhande G, Xu J, McKay RM, Burns DK, Johnson JE, Parada LF, 2011. Tsc1 mutant neural stem/progenitor cells exhibit migration deficits and give rise to subependymal lesions in the lateral ventricle. Genes Dev 25, 1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.