Abstract
This study aims to determine whether a semi-quantitative assessment of inflammatory response in tumor and stroma on routine hematoxylin and eosin-stained (H&E) slides can predict survival in patients with epithelioid malignant pleural mesothelioma (MPM). H&E sections of 175 epithelioid MPM specimens from a single institution (1989–2009) were reviewed. Patients who received neoadjuvant chemotherapy were excluded from analysis. Each tumor was histologically assessed for acute and chronic inflammatory response both within the tumor and the stromal component. Inflammatory response was graded: low (none to mild infiltrate) or high (moderate to severe infiltrate). Log-rank test and Cox proportional hazards regression were used to investigate the association between the degree of inflammation (acute/tumor, acute/stroma, chronic/tumor, and chronic/stroma) and overall survival (OS). Patients with high chronic inflammatory response in stroma (n = 59) had improved survival compared to low (n = 116) (median OS = 19.4 vs. 15.0 months, P = 0.01). This prognostic stratification remained significant in stage III patients (median OS = 16.0 vs. 9.3 months, P = 0.03). In multivariate analysis, chronic inflammation in stroma was an independent predictor of survival (HR = 0.659, 95% CI 0.464–0.937, P = 0.02). While high degree of chronic inflammatory cell infiltration in the stromal component was associated with improved overall survival, degree of other inflammatory responses did not show significant correlation with OS. Our study for the first time investigates inflammatory response in tumor and stroma and not only suggests the prognostic value of inflammatory response in epithelioid MPM but also provides rationale for investigation of immunotherapy to benefit epithelioid MPM patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1073-8) contains supplementary material, which is available to authorized users.
Keywords: Epithelioid mesothelioma, Tumor stroma, Immune response, Prognosis
Introduction
The host inflammatory response is a critical component in tumor progression as well as potential marker of prognosis in solid tumors [1–3]. In colorectal cancer, tumor-infiltrating immune cells were shown to be a stronger predictor of survival than the conventional histopathological methods [4]. These studies have not only underscored the prognostic value of inflammatory response in cancer patients but also emphasized the importance of three essential parameters in systematically assessing the tumor-associated inflammatory response: type, density, and location (tumor and stroma) of inflammatory cells.
Malignant pleural mesothelioma (MPM) is a rare, yet aggressive, disease with a median survival of 9–12 months [5]. Currently accepted prognosticators include histology and the staging system developed by the International Mesothelioma Interest Group [6]. Few studies have investigated the prognostic value of tumor-infiltrating immune cells in MPM [7–11] and suggested a correlation between lymphoid infiltration and prolonged overall survival [7–9]. However, due to the rarity and heterogeneity of MPM, these studies involving small series of patients investigate heterogeneous pathology (epithelioid, biphasic, and sarcomatoid) and patients undergoing different treatment modalities. More importantly, no study to date has investigated the immune cell infiltration with regard to their location—tumor and the tumor-associated stroma.
We herein report a comprehensive pathologic analysis of inflammatory response in the largest series to date MPM cohort of epithelioid histology, the most prevalent subtype of MPM. The aims of this study were (a) to systematically assess the type (acute versus chronic), density, and location (tumor versus stroma) of tumor-associated inflammation and (b) to determine whether assessment of such inflammation on routine hematoxylin and eosin-stained (H&E) slides can independently predict prognosis in MPM patients, thus providing an inexpensive marker that can be obtained within the routine assessment by the pathologists. To minimize confounding influences on prognosis, we focused our investigation only on patients with the epithelioid histology who had not received any neoadjuvant therapy. As recent report has demonstrated the prognostic value of inflammatory marker in the peripheral blood of MPM patients undergoing chemotherapy [12], we also sought to investigate the prognostic value of peripheral blood neutrophil to lymphocyte ratio (NLR) in our chemo-naïve cohort.
Materials and methods
Patients and pathologic samples
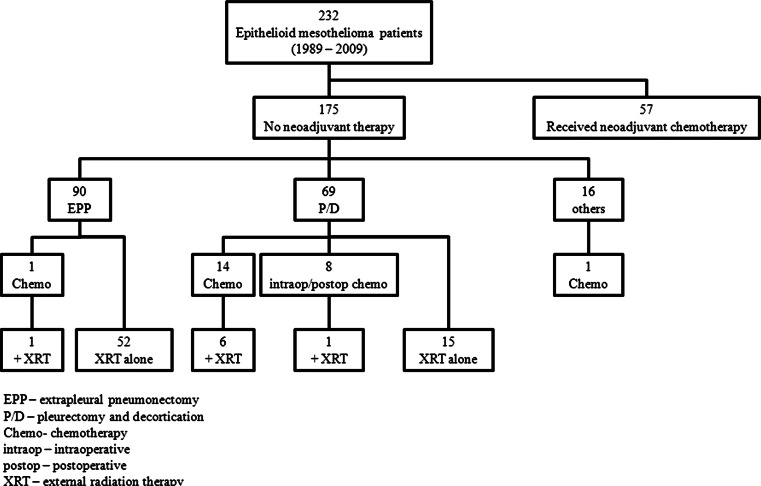
Information on 305 patients with pathologic diagnosis of MPM between 1989 and 2009 at Memorial Sloan-Kettering Cancer Center was obtained from the Thoracic Surgery mesothelioma database and the Department of Pathology database after internal review board approval. Review of pathological reports and confirmation by two pathologists (K.K. and W.D.T) yielded 232 epithelioid, 47 biphasic, and 26 sarcomatoid MPM. Patients with the epithelioid histology whose tumor slides were available were included in the study. Lymphohistiocytoid histology was not included. Patients who had received neoadjuvant chemotherapy (n = 57) were excluded. The study cohort consisted of 175 patients, and the treatment history of this cohort is shown in Fig. 1.
Fig. 1.
Patient disposition by treatment. Breakdown of patients by treatment they received. Main study cohort consisted of 175 patients who did not receive neoadjuvant chemotherapy
Pathologic diagnosis was based on standard histologic, histochemical, and immunohistochemical criteria [13, 14]. As a positive immunohistochemical marker of MPM, we used calretinin, WT-1, cytokeratin 5/6, and D2-40. As negative markers, we used carcinoembryonic antigen, CD15, B72.3, BerEP4, and thyroid transcription facor-1. In cases before positive mesothelial markers were available, negative markers were used for making the diagnosis of MPM. Staging was based on the sixth edition of the American Joint Commission on Cancer Staging Manual [15].
Variables recorded in the database included age, gender, laterality, pathologic stage, surgical procedure, and multimodality therapy. Laboratory values examined as potential prognostic factors included baseline white blood cell count (WBC), lymphocyte, and neutrophil. Peripheral blood neutrophil to lymphocyte ratio (NLR) was defined as the absolute neutrophil count divided by the absolute lymphocyte count. NLR of ≥5 was considered high as per previous report [12]. All patients were followed until date of death or date of last follow-up.
Evaluation of inflammatory response
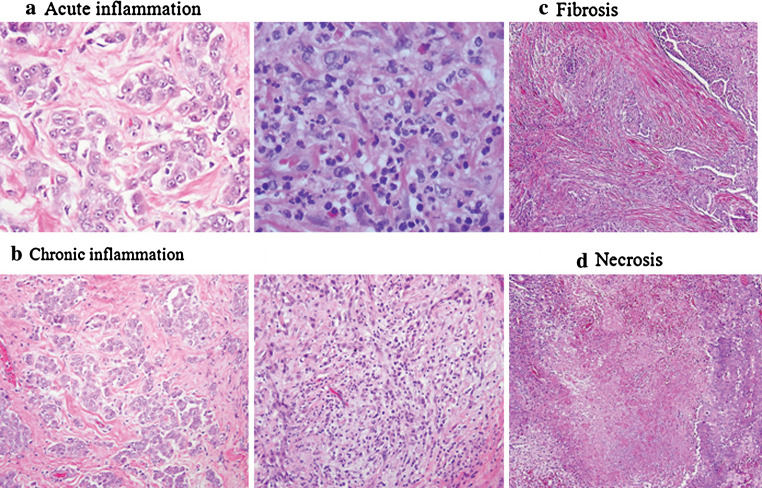
For each tumor, all available H&E slides, which included a median of 9 slides per patient (range: 1–43), were independently reviewed by two pathologists (K.K. and W.D.T). One slide with the most inflammatory response was selected for further review. Each slide was then assessed for two types of inflammatory response—(1) acute response representing neutrophils (Fig. 2a) and (2) chronic representing lymphocytes and plasma cells (Fig. 2b)—in both the intra-tumoral and stromal component of the tumor. Intra-tumoral component included tumor nests containing tumor cells and intra-tumoral stroma. Stromal component was considered as cells adjacent to the tumor nest. Areas near foreign body granulomas were excluded to prevent assessment of foreign body reaction. We semi-quantitatively evaluated the area of tumor or stroma with inflammatory cells as a percentage of the total area assessed in ten fields at a magnification of 400 (0.237 mm2). Using these percentages, each inflammatory response was scored on a 0–3 scale as follows: for acute inflammatory response, 0 for 0%, 1 for 0–1%, 2 for 1–5%, 3 for >5% and for chronic response, 0 for 0%, 1 for 0–10%, 2 for 10–50%, and 3 for >50%. Each specimen was also assessed for the presence of two inflammation-related features—fibrosis and necrosis (Fig. 2c, d), which were estimated in 10% increments in relation to the entire tumor tissue.
Fig. 2.
Assessed pathologic features in epithelioid MPM (hematoxylin and eosin stain; original magnification, a × 400, b, c, d × 200). a Acute inflammatory response: near absence of acute inflammatory cells on left, representing a score 1 and abundance on right, representing a score 3. b Chronic inflammatory response: near absence of chronic inflammatory cells on left, representing a score 1 and abundance on right, representing a score 3. c MPM tumor cells surrounded by an abundance of fibrotic stroma. d MPM tumor cells with a large area of necrotic center
Statistical analysis
Associations between clinicopathologic variables and each inflammatory response were analyzed using Pearson chi-square test or Fisher exact test. Kaplan–Meier method was used to estimate the probability of survival as a function of time, and differences in the survival of patient subgroups were compared by using the log-rank test. Multivariate analyses were performed using the Cox proportional hazard regression model to study the effects of different variables on survival. All P values were based on two-tailed statistical analysis, and a P value <0.05 was considered to indicate statistical significance. All analysis was performed using SAS statistical software (version 8.02; SAS Institute Inc, Cary, NC).
Results
Demographics
Clinical and pathologic profile of the analysis cohort is outlined in Table 1. There were 175 patients with a median age 64 years (range: 29–83), 73% of them were men. MPM involved the left chest in 45% of the patients and the right in 55%. Nine (5%) patients were stage I, 40 (23%) were stage II, 103 (59%) were stage III, and 23 (13%) were stage IV. By procedure, 51% underwent extrapleural pneumonectomy (EPP), 39% pleurectomy/decortication and the remaining 9% had other procedures, including biopsies, exploratory thoracotomies, palliative pleurectomies, and video-assisted thoracoscopic surgeries.
Table 1.
Demographics
| Variables | Number (%) | P value* |
|---|---|---|
| All patients | 175 | |
| Age | ||
| ≤ 65 | 96 (55%) | 0.14 |
| > 65 | 79 (45%) | |
| Gender | ||
| Female | 48 (27%) | 0.27 |
| Male | 127 (73%) | |
| Asbestos | ||
| Yes | 77 (44%) | 0.52 |
| No | 43 (25%) | |
| Unknown | 55 (31%) | |
| Smoking | ||
| Yes | 95 (54%) | 0.42 |
| No | 36 (21%) | |
| Unknown | 44 (25%) | |
| Laterality | ||
| Left | 79 (45%) | 0.05† |
| Right | 96 (55%) | |
| Stage | ||
| I–II | 49 (28%) | 0.003† |
| III–IV | 126 (72%) | |
| Procedure | ||
| EPP | 90 (51%) | |
| P/D | 69 (39%) | |
| Others | 16 (9%) | |
| Lymphatic invasion | ||
| Absent | 92 (53%) | |
| Present | 83 (47%) | 0.01† |
| Vascular invasion | ||
| Absent | 130 (74%) | |
| Present | 45 (26%) | 0.06† |
| Fibrosis | ||
| Absent | 123 (70%) | |
| Present | 52 (30%) | 0.25 |
| Necrosis | ||
| Absent | 140 (80%) | |
| Present | 35 (20%) | 0.25 |
EPP extrapleural pneumonectomy, P/D pleurectomy/decortication
* P values from univariate analyses in predicting overall survival
† Factors included in multivariate analysis
With a median follow-up time of 15 months, median overall survival (OS) was 16.0 months (95% CI: 13.5–19 months), with a 2-year OS of 32% and 5-year OS of 11%. On univariate analyses, advanced stage (stage III–IV) (P = 0.003) and lymphatic invasion (P = 0.01) were associated with shorter OS, while right-sided disease (P = 0.05) and vascular invasion approached statistical significance (P = 0.06) (Table 1).
Associations between inflammatory responses and survival
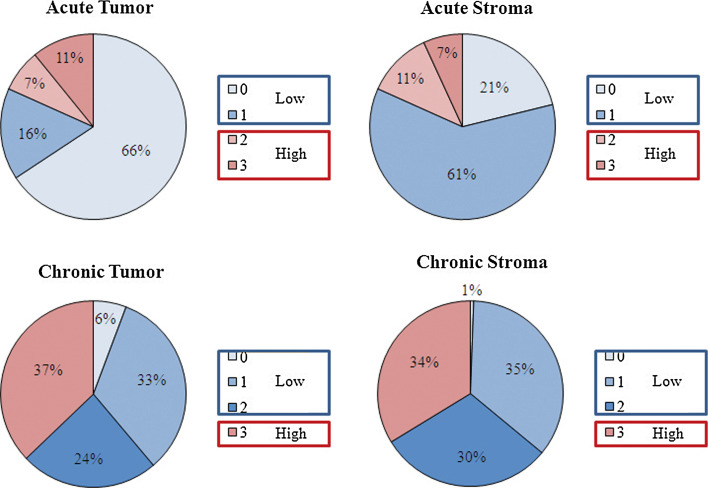
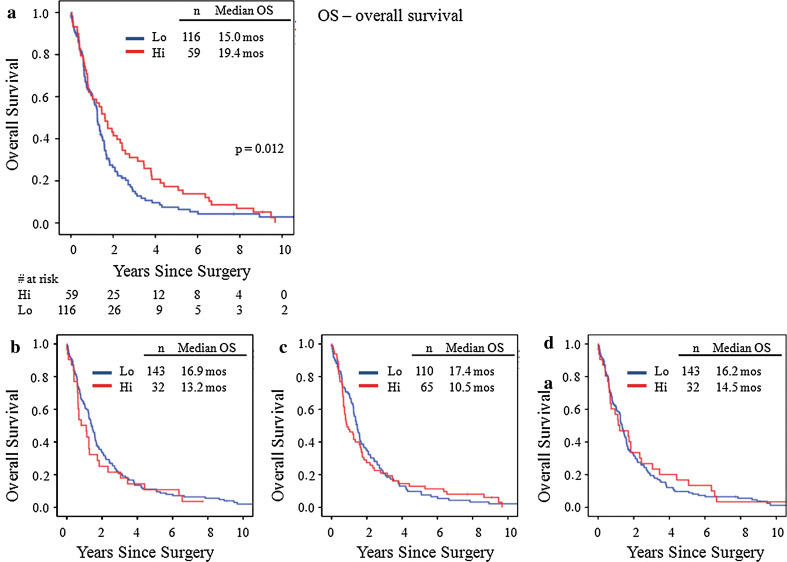
The distribution of the study cohort by the degree of inflammation in each type/location is shown in Fig. 3, and these scores were dichotomized for statistical analysis. Acute response was very sparse and was grouped as low (absent) for scores of 0 and 1 and high for scores of 2 and 3. For chronic response, 0, 1, and 2 were grouped as low and 3 as high. Survival curves by type/location of inflammatory response are shown in Fig. 4. Of the four type/location combinations, chronic response in stroma was a significant predictor of survival. Patients with high chronic response in stroma experienced a longer overall survival (n = 59, 19.4 months) compared to those with low response (n = 116, 15.0 months), P = 0.01 (Fig. 4a). This association remained significant in 103 stage III patients, the largest cohort in the series. Stage III patients with high chronic response in stroma experienced longer survival (n = 42, 16.0 months) compared to low (n = 61, 9.3 months), P = 0.03. There was no significant association between the other three type/location combinations and survival. A multivariate analysis confirmed that chronic inflammation in stroma is independently associated with prolonged survival after controlling for stage, laterality, and lymphatic/vascular invasions (HR = 0.66, 95% CI 0.46–0.94, P = 0.02, Table 2).
Fig. 3.
Distribution by type and location of inflammation. Distribution of each inflammatory response by the degree of infiltration on a scale of 0–3. For acute response, scores of 0 and 1 were grouped as low and 3 and 4 grouped as high. For chronic response, scores of 0, a, and 2 were grouped as low and 3 as high
Fig. 4.
Overall survival by type & location of inflammatory response. a Overall survival by chronic inflammatory response in stroma. Patients with high response had a median survival of 19.4 months compared to 15 months for low, P = 0.012. b Overall survival by acute inflammatory response in tumor. Patients with high response had a median survival of 13.2 months compared to 16.9 months for low, P = 0.57. c Overall survival by acute inflammatory response in stroma. Patients with high response had a median survival of 10.5 months compared to 17.4 months for low, P = 0.90. d Overall survival by chronic inflammatory response in tumor. Patients with high response had a median survival of 14.5 months compared to 16.2 months for low, P = 0.51
Table 2.
Multivariate analysis in predicting overall survival
| Variables | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Stromal inflammation | 0.659 | 0.464–0.937 | 0.02 |
| Stage (III & IV vs. I & II) | 1.72 | 1.16–2.55 | 0.007 |
| Laterality (right vs. left) | 1.43 | 1.01–2.01 | 0.04 |
| Lymphatic invasion | 1.39 | 0.95–2.03 | 0.09 |
| Vascular invasion | 1.50 | 0.97–2.34 | 0.07 |
When similar analysis was performed on the cohort including the 57 patients who had received neoadjuvant therapy (n = 232), a trend was observed for the protective association of chronic inflammation in the stroma—patients with high response (n = 82) experienced a median survival of 17.4 months compared to 15.0 months for low response (n = 150), P = 0.06.
Associations between inflammatory responses and clinicopathologic variables
We next investigated the association of each inflammatory response with other clinical variables. High stromal chronic response was marginally associated with advanced stage (P = 0.05). In addition, women (P = 0.08) and patients without asbestos exposure (P = 0.08) tended to have high stromal chronic response more often, although this association did not reach significance.
We also investigated the relationship of each inflammatory response to other pathologic features—lymphatic/vascular invasions, fibrosis, and necrosis. Vascular invasion was associated with high response of both acute and chronic inflammation in tumor (P = 0.01 for acute, P = 0.02 for chronic). The distribution by fibrosis and necrosis is shown in Supplementary Fig. 1. Based on the distribution, each feature was grouped as present or absent using a cut-off value of 50% for fibrosis and 10% for necrosis. Interestingly, fibrosis was associated with low level of three inflammatory responses—acute and chronic in tumor and chronic in stroma (P = 0.005, 0.001, and 0.05, respectively). As expected, necrosis was associated with high acute inflammation in both tumor and stroma (P < 0.001 for tumor, P = 0.02 for stroma), as well as chronic response in tumor (P = 0.006). In an analysis of 57 patients who received neoadjuvant chemotherapy, presence of necrosis was a predictor of shorter OS (P = 0.034, data not shown), although this significance was not observed in the study cohort of chemo-naïve patients. No association was observed between lymphatic invasion and any of the inflammatory responses.
Peripheral blood neutrophil to lymphocyte ratio (NLR)
As peripheral blood NLR has recently been shown to be prognostic in MPM patients undergoing systemic chemotherapy [12], we sought to investigate whether these observations can be applied to our cohort of patients both with and without neoadjuvant chemotherapy. For the cohort that received neoadjuvant chemotherapy, NLR was available for 44 patients as laboratory values could not be obtained for patients before 1998. Of these 44, 35 belonged in the low group (NLR < 5) and 9 in the high group. The high NLR group experienced a shorter median OS of 5.4 months compared to 17.9 months observed in the low NLR group, but overall, a significant association between the two groups was not noted (P = 0.26), perhaps due to a small number of patients.
For the cohort that did not receive neoadjuvant chemotherapy, NLR was available for 76 patients. Again, a much shorter median OS was observed in the high NLR group (n = 22, 10.2 months) compared to the low NLR group (n = 54, 18.9 months), although this did not reach statistical significance (P = 0.34).
Discussion
Prognostic value of tumor-infiltrating immune cells has been well investigated in many solid malignancies [1–3, 16], and recent reports emphasize the importance of analyzing such data specifically in relation to tumor and tumor stroma [4]. The tumor-associated stroma has been implicated to play an important role as a regulator of tumor progression/regression [17, 18], yet it has not been explored in studies investigating inflammatory response in MPM. While several small studies in MPM have shown a correlation between lymphoid infiltration and prolonged overall survival [7–9], we sought to improve upon these studies from two perspectives. First, to minimize confounding effects of histology on our results, we focused on a large series of only the epithelioid MPM, the most common histology. Secondly, to our knowledge, this is the first study in MPM to assess the inflammatory response in two compartments of the tumor microenvironment—tumor and stroma.
Our main finding was that increased chronic inflammation in the stroma is an independent predictor of prolonged survival in epithelioid MPM patients. This may indicate the protective role of immune cells in MPM and highlights the importance of studying the stromal component of the tumor. In ovarian and colorectal cancers, intra-tumoral inflammatory cells have been shown to be prognostic [1, 2], while in non-small cell lung cancer and more recently breast cancer, presence of inflammatory cells in the stroma has shown prognostic significance [3, 16, 19]. Our finding of the chronic inflammatory cells in the stroma is intriguing and provides support to investigate the tumor stroma and the type of inflammatory cells in the stroma for better understanding the tumor immunology of MPM.
The significance of our findings is two fold. First, this information obtained from an assessment on routine H&E slides provides a simple, additional prognostic factor that can be obtained during routine pathologic diagnosis. While epithelioid MPM has relatively better prognosis compared to biphasic or sarcomatoid MPM, there is a lack of prognostic markers to further stratify clinical outcome within the epithelioid histologic subtype, the most common subtype representing 70% of MPM patients. To confirm that adjuvant therapies did not have a confounding effect on our observations, we further performed subanalysis for the 90 patients who underwent extrapleural pneumonectomy (EPP) and subsequent adjuvant therapies. Among these, 53 patients (59%) received adjuvant external radiation therapy; however, no difference in the prognostic value of stromal chronic inflammation was observed between patients with and those without adjuvant therapies (data not shown).
Second, our finding forms a rationale for investigating immuno or immunomodulating therapy that promotes lymphocyte infiltration in this disease, which is resistant to currently available therapeutic options. As has been shown by Hegmans et al. [20], the tumor microenvironment of MPM is rich in immunosuppressive cytokine and cells. Therapies designed to modulate such environment to promote immune cell infiltration may result in beneficial effects. One such approach was demonstrated in a recent preclinical work by Fridlender et al., who has shown that blockade of chemokine CCL2 augments CD8 + T cell response [21].
In contrast to what we observed in the stroma, inflammation within the tumor nest showed an association with vascular invasion—a negative predictor of survival. This emphasizes the polarity and complexity of tumor-associated inflammation, as it can be both tumor-promoting and tumor-suppressing [22]. One limitation of our study is that the assessment of inflammatory response on H&E slides does not provide specific phenotyping of the cells. Chronic inflammatory cells represent lymphocytes that consist of CD3 + T cells and CD20 + B cells. T cells can be further characterized by specific markers such as CD4, CD8, CD45RO, and FoxP3. While two studies have found high level of CD8 + T cells to be associated with better prognosis in MPM, neither study emphasized the location factor, and both studies are relatively small [8, 9]. As we have shown what seems to be an opposing effect of the same inflammatory response depending on the location, the findings of CD8 + T cells need further exploration with specific focus on location. Shigematsu et al. [23] also found that tumor-infiltrating B cells recognize mesothelioma-associated antigens in murine models. The clinical significance of B cells has not been investigated in human MPM tumors. Since both T and B cells [24] have polarized functions within the tumor microenvironment [25], future studies will focus on further dissecting the immune microenvironment of MPM with particular attention to the specific type of cells.
Finally, we investigated the prognostic value of peripheral blood NLR in our cohort. The association of elevated NLR and poor prognosis has been demonstrated in many other malignancies [26–28] and was also recently shown in MPM patients undergoing systemic therapy. We did not observe a significant association—median survival was 10.1 and 18.9 months for high and low NLR, respectively, in our study, perhaps due to small number of patients with NLR values. Of note, our study cohort consisted of patients receiving surgery as the main treatment with no neoadjuvant chemotherapy, while Kao et al. investigated patients undergoing systemic therapy as the sole treatment. In our subset analysis of patients who had received neoadjuvant chemotherapy and surgical resection, median OS was 5.4 and 17.9 months for high and low NLR, respectively.
In conclusion, we have demonstrated that high chronic inflammatory response in the tumor stroma is an independent predictor of prolonged survival in epithelioid MPM patients. Our finding (1) provides a prognostic factor that can be obtained from examination of routine H&E slides, (2) emphasizes the importance of evaluating two often-ignored, yet important features of the tumor: tumor-infiltrating immune cells as well as the stromal component, and (3) provides rationale to investigate immune-based approach as a potential therapy in this challenging disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Joe Dycoco for his help with the mesothelioma database within the Division of Thoracic Surgery. This work was supported in part by Mesothelioma Applied Research Foundation (MARF) Grant in memory of Lance S. Ruble, Stony Wold-Herbert Fund, New York State Empire Clinical Research Investigator Program (ECRIP), American Association for Thoracic Surgery (AATS)—Third Edward D. Churchill Research Scholarship, IASLC—International Association for the Study of Lung Cancer Young Investigator Award, National Lung Cancer Partnership/LUNGevity Foundation Research Grant, William H. Goodwin and Alice Goodwin and the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center.
Conflict of interest
All authors affirm that we have no actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations.
References
- 1.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc P-H, Trajanoski Z, Fridman W-H, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 3.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund L-T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 4.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc P-H, Trajanoski Z, Fridman W-H, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 5.Robinson BWS, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 6.Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, DeCamp MM, Jr, Swanson SJ, Bueno R, Lukanich JM, Baldini EH, Mentzer SJ. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results of 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–65. doi: 10.1016/S0022-5223(99)70469-1. [DOI] [PubMed] [Google Scholar]
- 7.Leigh RA, Webster I. Lymphocytic infiltration of pleural mesothelioma and its significance for survival. S Afr Med J. 1982;61:1007–1009. [PubMed] [Google Scholar]
- 8.Anraku M, Cunningham KS, Yun Z, Tsao M-S, Zhang L, Keshavjee S, Johnston MR, de Perrot M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2008;135:823–829. doi: 10.1016/j.jtcvs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Yamada N, Oizumi S, Kikuchi E, Shinagawa N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T, Torigoe T, Nishimura M. CD8 + tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother. 2010;59(10):1543–1549. doi: 10.1007/s00262-010-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alì G, Boldrini L, Lucchi M, Mussi A, Corsi V, Fontanini G. Tryptase mast cells in malignant pleural mesothelioma as an independent favorable prognostic factor. J Thorac Oncol. 2009;4:348–354. doi: 10.1097/JTO.0b013e3181989ddb. [DOI] [PubMed] [Google Scholar]
- 11.Mudhar HS, Wallace WAH. No relationship between tumour infiltrating lymphocytes and overall survival is seen in malignant mesothelioma of the pleura. Eur J Surg Oncol (EJSO) 2002;28:564–565. doi: 10.1053/ejso.2002.1294. [DOI] [PubMed] [Google Scholar]
- 12.Kao SCH, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, Clarke SJ. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–5813. doi: 10.1158/1078-0432.CCR-10-2245. [DOI] [PubMed] [Google Scholar]
- 13.Travis W, Colby T, Corrin B, Shimosato Y, Brambilla E. World health organization international histological classification of tumours: histological subtyping of lung and pleural tumours. 3. Berlin: Springer; 1999. [Google Scholar]
- 14.Travis W, Brambilla E, Muller-Hermelink H, Harris C. Pathology and genetics. Tumours of the lung, pleura, thymus, and heart. Lyon: IARC Press; 2004. [Google Scholar]
- 15.Greene F, Page D, Fleming I, Fritz A, Balch C, Haller D, Morrow M. AJCC cancer staging manual. New York: Springer; 2002. [Google Scholar]
- 16.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8 + T cells and CD4 + T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol Mech Dis. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 18.Blankenstein T. The role of tumor stroma in the interaction between tumor and immune system. Curr Opin Immunol. 2005;17:180–186. doi: 10.1016/j.coi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud SMA, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AHS, Ellis IO, Green AR. Tumor-infiltrating CD8 + lymphocytes predict clinical outcome in breast cancer. J Clinical Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 20.Hegmans JPJJ, Hemmes A, Hammad H, Boon L, Hoogsteden HC, Lambrecht BN. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur Respir J. 2006;27:1086–1095. doi: 10.1183/09031936.06.00135305. [DOI] [PubMed] [Google Scholar]
- 21.Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, Crisanti MC, Wang L-CS, Heitjan D, Snyder LA, Albelda SM. CCL2 blockade augments cancer immunotherapy. Cancer Res. 2010;70:109–118. doi: 10.1158/0008-5472.CAN-09-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 23.Shigematsu Y, Hanagiri T, Kuroda K, Baba T, Mizukami M, Ichiki Y, Yasuda M, Takenoyama M, Sugio K, Yasumoto K. Malignant mesothelioma-associated antigens recognized by tumor-infiltrating B cells and the clinical significance of the antibody titers. Cancer Sci. 2009;100:1326–1334. doi: 10.1111/j.1349-7006.2009.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouaziz J-D, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 25.Johansson M, DeNardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An X, Ding P-R, Li Y-H, Wang F-H, Shi Y-X, Wang Z-Q, He Y-J, Xu R-H, Jiang W-Q. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 27.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 28.Sarraf K, Belcher E, Raevsky E, Nicholson A, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.