Abstract
The 2012 neuropathological criteria for the diagnosis of Alzheimer disease (AD) summarize the extent of AD neuropathological change with an ABC score, which is a composite of the Thal stage of amyloid deposition (A), the Braak stage of neurofibrillary tangles (NFTs) (B), and the CERAD neuritic plaque score (C). NFTs and neuritic plaques are well-established contributors to cognitive impairment, but whether the Thal amyloid stage independently predicts antemortem cognition remains unknown. We used the National Alzheimer’s Coordinating Center autopsy data set to build adjacent-categories logit regression models with CDR-SOB and Mini-Mental State Examination (MMSE) scores as cognitive outcome variables. Increasing CERAD scores were independently associated with higher CDR-SOB scores, whereas increasing Braak NFT stages predicted both higher CDR-SOB and lower MMSE scores. Increasing Thal amyloid stages were not significantly independently associated with either outcome measure. Increasing ABC scores predicted higher CDR-SOB and lower MMSE scores. These results raise the possibility that Thal amyloid stages do not substantially contribute to predicting antemortem cognition compared to CERAD neuritic plaque scores and Braak NFT stages, and suggest that the diffuse amyloid deposits participating in the assignment of Thal amyloid stages are neutral with respect to clinically detectable cognitive and functional changes.
Keywords: ABC score, Alzheimer disease, Amyloid plaques, National Alzheimer’s Coordinating Center, Neuritic plaques, Neurofibrillary tangles, Thal stages.
INTRODUCTION
Although the definite diagnosis of Alzheimer disease (AD) continues to require the demonstration of the 2 pathological hallmarks of the disease, namely amyloid plaques and neurofibrillary tangles (NFTs), neuropathological criteria for the diagnosis of AD have recently been updated under the auspices of the National Institute on Aging (NIA) and the Alzheimer’s Association (AA) ( 1 , 2 ). The main difference of the 2012 NIA-AA guidelines with respect to the prior set of criteria, the 1997 NIA–Reagan Institute criteria ( 3 ), is the use of descriptive scores to grade the extent of AD neuropathological changes regardless of the presence of cognitive impairment; that is, without making any assumption about the cognitive status of the person prior to death. Thus, the 2012 NIA-AA guidelines acknowledge the evidence that the AD pathological process is a continuum that begins decades before the onset of cognitive symptoms and that certain individuals remain asymptomatic despite abundant plaques and NFTs.
According to the 2012 NIA-AA guidelines, the extent of AD neuropathological change is summarized by an ABC score, which is a composite of 3 scores: Thal phases of amyloid deposition (A) ( 4 ), Braak stage of NFTs (B) ( 5 , 6 ), and Consortium to Establish a Registry for Alzheimer Disease (CERAD) score of neuritic amyloid plaques (C) ( 7 ). These guidelines also differ from the previous in that they incorporate another amyloid grading system: Thal amyloid staging. Using anti-β-amyloid (Aβ) immunohistochemistry in multiple cortical and subcortical regions from a large number of autopsy subjects, Thal et al distinguished up to 5 phases of amyloid deposition that follow a top-down anatomical progression: phase 0 or no amyloid; phase 1 or isocortical; phase 2 or limbic; phase 3 or basal ganglia; phase 4 or basal forebrain and midbrain; and phase 5 or pons/medulla oblongata and cerebellum ( 4 ).
The NIA-AA-sponsored panel of experts suggested the use of 2 amyloid measures in the ABC score (CERAD and Thal) because it was uncertain which marker of amyloid deposition would be most informative for various studies, including correlations between pathological and clinical measures. Moreover, it seemed likely that the 2 measures would be complementary. First, the 2 scores measure different types of amyloid deposits, the Thal phases refer to any amyloid deposits, both diffuse and dense-core plaques, because they are based on anti-Aβ immunohistochemistry, whereas the CERAD score refers exclusively to neuritic (usually dense-core) plaques. Second, the CERAD score reflects the abundance (not the spatial-temporal distribution) of neuritic plaques (none, sparse, moderate, and frequent) in 3 areas of the isocortex (frontal, temporal, and parietal), whereas the Thal stages reflect the spatial-temporal distribution (not the abundance) of amyloid deposition throughout the encephalon.
It is well established that both NFTs and neuritic plaques correlate with the extent of cognitive decline ( 8–10 ). In 2012, however, no large systematically collected data set including both clinical and Thal staging data was available. With the accumulation of such data since the 2012 revision of the AD neuropathological diagnostic criteria, we can now address the question of whether Thal stage provides additional predictive value with regard to the patient’s antemortem cognitive status. We took advantage of the National Alzheimer’s Coordinating Center (NACC) Neuropathology Data Set to investigate the contribution of Thal amyloid phases to antemortem cognitive impairment as measured by the Clinical Dementia Rating Sum of Boxes (CDR-SOB) and the Mini-Mental State Examination (MMSE).
MATERIALS AND METHODS
Inclusion and Exclusion Criteria
Subjects were participants in a longitudinal cohort study of aging in any of the past and present 34 NIA-funded Alzheimer Disease Centers (ADCs). This multicenter study is ongoing since 2005 and has been described in detail elsewhere ( 11–13 ). Briefly, subjects underwent a baseline visit and annual follow-up visits in which the Uniform Data Set forms were completed, including a minimum of subject demographics and standard motor, behavioral, functional, and neuropsychological assessments. Subjects or the next of kin were offered an autopsy after death and, if accepted, a uniform neuropathological assessment was carried out at the ADC Neuropathology Core. In January 2014, this neuropathological assessment was updated to include for the first time the Thal amyloid staging, among other recent advances and progress in the classification of neurodegenerative diseases (Neuropathology Data Set form version 10). Thus, the present study sample was selected from the NACC autopsy cohort between January 2014 and May 2015 (June 2015 data freeze).
Subjects were eligible if they met the following inclusion criteria: 1) at least 1 Uniform Data Set evaluation available (CDR-SOB with or without MMSE), 2) Thal amyloid stage available, and 3) final clinical evaluation within 2 years of death. To minimize statistical noise, subjects with a primary neuropathological diagnosis other than AD neuropathological changes, such as frontotemporal lobar degeneration (FTLD-tau, FTLD-TDP43, FTLD-FUS, etc.), amyotrophic lateral sclerosis and other motor neuron diseases, tangle-only dementia, argyrophilic grain disease, multiple system atrophy, prion disease, Huntington disease and other trinucleotide diseases, Down syndrome, multiple sclerosis and other demyelinating diseases, traumatic brain injury and chronic traumatic encephalopathy, abscess and encephalitides, primary neoplasm and metastases, among others, were excluded from the analyses. The presence of incidental Lewy bodies and cerebrovascular disease were allowed to reflect the frequent coexistence between these pathologies and AD neuropathological changes observed in population-based studies ( 8 , 9 , 14–16 ).
Data Collection
Demographic and clinical data used in this study included sex, years of education, age at death, age at onset of cognitive symptoms, duration of clinical disease (age at death-age at onset), APOE genotype, most recent CDR-SOB score, and most recent MMSE score. The CDR-SOB is a cognitive and functional measure with values ranging from 0 to 18, and with higher values indicating worse cognitive/functional status ( 17 ). The MMSE is a global cognitive measure with values ranging from 0 to 30, and with lower values indicating worse cognition ( 18 ).
Neuropathological variables included Braak staging of NFTs (0 or none; I-II: entorhinal; III-IV or limbic, and V-VI or isocortical) ( 5 , 6 ); Thal staging of amyloid deposition (here categorized following NIA-AA guidelines as 0 or no amyloid, 1/2 or isocortical/allocortical, 3 or basal ganglia, and 4/5 or brainstem/cerebellum) ( 4 ); CERAD scoring of neuritic plaques (none, sparse, moderate, or frequent) ( 7 ); presence of incidental Lewy bodies in any region, and the extent of vascular pathology, specifically cerebral amyloid angiopathy (CAA), small and large vessel disease, and hippocampal sclerosis. Current NACC neuropathology guidelines recommend the use of a semiquantitative grading system of the overall severity (rather than an individual vessel) to assess arteriosclerosis, atherosclerosis, and CAA (none, mild, moderate, or severe). Arteriosclerosis refers to the hyalinosis of the media and adventitia (arteriolosclerosis) of small parenchymal and/or leptomeningeal vessels, whereas atherosclerosis refers to the presence of intimal and medial fibro-fatty atheromatous plaques in large arteries at the base of the brain (ie, circle of Willis). Hippocampal sclerosis was defined as the presence of selective neuronal loss and gliosis (“sclerosis”) limited to CA1 and subiculum, with variable additional involvement of endplate, CA2, entorhinal cortex, and amygdala.
Statistical Analyses
The association of demographic and neuropathological variables with ordinal categories of CDR-SOB or MMSE was examined with adjacent-categories logit models ( 19 ), with potentially different effects for different adjacent categories of CDR-SOB or MMSE. The proportional odds assumption of each demographic and neuropathological variable was checked using likelihood ratio tests of nested models. Details of the model selection process can be found in the supplementary methods section of our previous publication ( 10 ). The VGAM package in R software was used to fit the adjacent-categories logit models ( 20 ). Adjacent-categories logit regression models that examined the ability of the ABC score to predict cognitive performance prior to death used subjects with “not AD” as reference and were adjusted for demographic variables only ( Table 2 , model 1) or for both demographic variables and concurrent pathologies that are routinely assessed by the neuropathologists (Lewy bodies, CAA, small and large vessel disease, and hippocampal sclerosis) ( Table 2 , model 2). The other models were constructed in a stepwise fashion ( Tables 3 and 4 ): The simpler model (model 1) only included demographic variables (sex, age at death, and education). Next, the AD pathologic hallmarks; that is, CERAD scores of neuritic plaques and Braak stages of NFTs, were added to the model (model 2). In a third step, the Thal amyloid stages were added to the model (model 3). In the last step, concurrent pathologies were added to the model (model 4 or full model).
TABLE 2.
Value of the ABC Score to Predict CDR-SOB and MMSE
|
CDR-SOB
|
MMSE
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ABC score (“not AD” as reference) |
Model 1
|
Model 2
|
Model 1
|
Model 2
|
||||
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Low AD neuropathological change | 0.93 (0.57, 1.50) | 0.757 | 0.77 (0.45, 1.31) | 0.336 | 2.46 (0.57, 10.59) | 0.227 | 1.46 (0.34, 6.30) | 0.614 |
| Intermediate AD neuropathological change | 1.60 (1.01, 2.54) | 0.046 | 1.39 (0.82, 2.34) | 0.221 | 6.28 (1.49, 26.51) | 0.012 | 3.67 (0.87, 15.46) | 0.076 |
| High AD neuropathological change | 2.77 (1.72, 4.45) | <0.001 | 2.34 (1.35, 4.08) | 0.003 | 11.45 (2.71, 48.39) | 0.001 | 7.91 (1.86, 33.65) | 0.005 |
Analyses in model 1 are adjusted by sex, age at death, and education; analyses in model 2 are adjusted by sex, age at death, education, and concurrent pathologies. The interpretation of the effect of the ABC score on CDR-SOB and MMSE is similar to the interpretation of the effect of CERAD neuritic plaque score described in the footnote of Tables 3 and 4 .
ABC, amyloid, Braak, CERAD scores; AD, Alzheimer disease; CDR-SOB, Clinical Dementia Rating Sum of Boxes; CERAD, Consortium to Establish a Registry for Alzheimer Disease; CI, confidence interval; MMSE, Mini-Mental State Examination; OR, odds ratio.
TABLE 3.
Summary of Results of the Adjacent-Categories Logit Regression Model With CDR-SOB Intervals as Outcome Variable
|
Model 1 (only demographics)
|
Model 2 (Model 1+ CERAD neuritic plaque score + Braak NFT stage)
|
Model 3 (Model 2+Thal amyloid stage)
|
Model 4 (Model 3+concurrent pathologies)
|
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sex (female as reference) | 0.92 (0.77, 1.11) | 0.403 | 1.01 (0.79, 1.28) | 0.965 | 0.99 (0.78, 1.27) | 0.957 | 0.98 (0.77, 1.25) | 0.861 |
| Age at death (in 5-years unit) | 0.89 (0.85, 0.93) | <0.001 | 0.96 (0.89 1.02) | 0.198 | 0.95 (0.88, 1.02) | 0.144 | 0.95 (0.88, 1.03) | 0.226 |
| Education (in 4-years unit) | 0.99 (0.89, 1.11) | 0.902 | 1.10 (0.95, 1.27) | 0.220 | 1.09 (0.94, 1.26) | 0.258 | 1.13 (0.95, 1.34) | 0.154 |
| CERAD neuritic plaque score (none/sparse as reference) | ||||||||
| Moderate | 1.63 (1.12, 2.38) | 0.011 | 1.58 (1.04, 2.38) | 0.030 | 1.73 (1.08, 2.76) | 0.022 | ||
| Frequent | 2.43 (4.53, 3.84) | <0.001 | 2.26 (1.39, 3.68) | 0.001 | 2.67 (1.54, 4.63) | <0.001 | ||
| Braak NFT stage (none/I/II as reference) | ||||||||
| Stage III/IV | 1.09 (0.74, 1.61) | 0.648 | 1.13 (0.76, 1.67) | 0.545 | 1.09 (0.71, 1.68) | 0.680 | ||
| Stage V/VI | 1.65 (1.08, 2.54) | 0.022 | 1.55 (1.00, 2.42) | 0.052 | 1.49 (0.89, 2.49) | 0.129 | ||
| Thal amyloid stage (0 as reference) | ||||||||
| Stage 1/2 | 0.74 (0.41, 1.35) | 0.331 | 0.66 (0.35, 1.28) | 0.219 | ||||
| Stage 3 | 0.86 (0.49, 1.51) | 0.597 | 0.71 (0.37, 1.37) | 0.312 | ||||
| Stage 4/5 | 1.05 (0.61, 1.81) | 0.859 | 0.91 (0.50, 1.67) | 0.765 | ||||
| Cerebral amyloid angiopathy (none as reference) | ||||||||
| Mild | 0.85 (0.60, 1.20) | 0.349 | ||||||
| Moderate | 1.18 (0.78, 1.78) | 0.437 | ||||||
| Severe | 0.89 (0.57, 1.40) | 0.621 | ||||||
| Lewy bodies (present vs absent) | 1.08 (0.83, 1.42) | 0.561 | ||||||
| Arteriosclerosis (none as reference) | ||||||||
| Mild | 0.95 (0.63, 1.42) | 0.789 | ||||||
| Moderate | 1.18 (0.80, 1.76) | 0.405 | ||||||
| Severe | 1.38 (0.85 2.24) | 0.197 | ||||||
| Hippocampal sclerosis (present vs absent) | 1.99 (1.09, 3.64) | 0.025 | ||||||
Six ordinal levels of the response variable CDR-SOB (0.0, [0.5, 3], [3.5, 6.0], [6.5, 12.0], [12.5, 17.5], and 18.0) are represented by k = 0, 1, 2, 3, 4, 5. Therefore, it follows that the higher the k, the worse the cognitive status. Following are some examples of interpretation based on model 4. Effect of education: holding other factors constant, the odds ratio of having CDR-SOB at a certain category (k = 1, 2, 3, 4, 5) versus the adjacent lower category (k-1) associated with every 4-year increase in education is 1.13, with a 95% CI (0.95, 1.34). Effect of CERAD neuritic plaque score: holding other factors constant, the odds of having CDR-SOB at a certain category (k = 1, 2, 3, 4, 5) versus the adjacent lower category (k − 1) for subjects with moderate neuritic plaques are 1.73 times the odds for subjects with sparse or no neuritic plaques, with a 95% CI (1.08, 2.76). Similarly, holding other factors constant, the odds of having CDR-SOB at a certain category (k = 1, 2, 3, 4, 5) versus the adjacent lower category (k − 1) for subjects with frequent neuritic plaques are 2.67 times the odds for subjects with sparse or no neuritic plaques, with a 95% CI (1.54, 4.63).
CDR-SOB, Clinical Dementia Rating Sum of Boxes; CERAD, Consortium to Establish a Registry for Alzheimer Disease; NFT, neurofibrillary tangle; OR, odds ratio.
TABLE 4.
Summary of Results of the Adjacent-Categories Logit Regression Model With MMSE Intervals as Outcome Variable
|
Model 1 (only demographics)
|
Model 2 (Model 1+ CERAD neuritic plaque score + Braak NFT stage)
|
Model 3 (Model 2+Thal amyloid stage)
|
Model 4 (Model 3+concurrent pathologies)
|
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sex (female as reference) | 1.01 (0.79, 1.28) | 0.957 | 1.20 (0.88, 1.63) | 0.256 | 1.21 (0.89, 1.66) | 0.223 | 1.42 (0.99, 2.04) | 0.055 |
| Age at death (in 5-years unit) | 0.87 (0.82, 0.93) | <0.001 | 0.92 (0.84 1.00) | 0.058 | 0.92 (0.84, 1.00) | 0.061 | 0.95 (0.86, 1.05) | 0.325 |
| Education (in 4-years unit) | 0.91 (0.79, 1.05) | 0.203 | 0.91 (0.75, 1.10) | 0.327 | 0.90 (0.74, 1.09) | 0.290 | 0.90 (0.72, 1.13) | 0.355 |
| CERAD neuritic plaque score (none/sparse as reference) | ||||||||
| Moderate | 1.59 (0.81, 3.12) | 0.181 | 1.30 (0.64, 2.67) | 0.466 | 1.24 (0.56, 2.76) | 0.597 | ||
| Frequent | 2.00 (0.97, 4.09) | 0.059 | 1.72 (0.80, 3.67) | 0.162 | 1.71 (0.73, 4.00) | 0.215 | ||
| Braak NFT stage (none/I/II as reference) | ||||||||
| Stage III/IV | 1.32 (0.65, 2.65) | 0.439 | 1.26 (0.62, 2.56) | 0.531 | 1.30 (0.59, 2.87) | 0.522 | ||
| Stage V/VI | 3.40 (1.62, 7.16) | 0.001 | 3.58 (1.64, 7.85) | 0.001 | 4.64 (1.78, 12.08) | 0.002 | ||
| Thal amyloid stage (0 as reference) | ||||||||
| Stage 1/2 | 2.25 (0.47, 10.79) | 0.310 | 1.71 (0.36, 8.06) | 0.500 | ||||
| Stage 3 | 3.39 (0.74, 15.44) | 0.115 | 1.94 (0.41, 9.23) | 0.403 | ||||
| Stage 4/5 | 2.51 (0.55, 11.48) | 0.234 | 1.47 (0.32, 6.84) | 0.624 | ||||
| Cerebral amyloid angiopathy (none as reference) | ||||||||
| Mild | 1.10 (0.73, 1.68) | 0.642 | ||||||
| Moderate | 1.36 (0.82, 2.26) | 0.231 | ||||||
| Severe | 1.19 (0.69, 2.06) | 0.525 | ||||||
| Lewy bodies (present vs absent) | 1.64 (1.13, 2.39) | 0.010 | ||||||
| Arteriosclerosis (none as reference) | ||||||||
| Mild | 0.82 (0.48, 1.42) | 0.487 | ||||||
| Moderate | 0.83 (0.50, 1.40) | 0.495 | ||||||
| Severe | 1.10 (0.59 2.06) | 0.763 | ||||||
| Hippocampal sclerosis present vs absent) | 2.24 (0.95, 5.29) | 0.067 | ||||||
Five ordinal levels of the response variable MMSE ([27, 30], [21, 26], [15, 20], [10, 14], [0, 9]) are represented by k = 0, 1, 2, 3, 4. Therefore, it follows that the higher the k, the worse the cognitive status. Following are some examples of interpretation based on model 4. Effect of education: holding other factors constant, the odds ratio of having MMSE at a certain category (k = 1, 2, 3, 4) versus the adjacent lower category (k-1) associated with every 4-year increase in education is 0.90, with a 95% CI (0.72, 1.13). Effect of CERAD neuritic plaque score: holding other factors constant, the odds of having MMSE at a certain category (k = 1, 2, 3, 4) versus the adjacent lower category (k-1) for subjects with moderate neuritic plaques are 1.24 times the odds for subjects with sparse or no neuritic plaques, with a 95% CI (0.56, 2.76). Similarly, holding other factors constant, the odds of having MMSE at a certain category (k = 1, 2, 3, 4) versus the adjacent lower category (k-1) for subjects with frequent neuritic plaques are 1.71 times the odds for subjects with sparse or no neuritic plaques, with a 95% CI (0.73, 4.00).
CERAD, Consortium to Establish a Registry for Alzheimer Disease; NFT, neurofibrillary tangle; MMSE, Mini-Mental State Examination; OR, odds ratio.
Following previous studies ( 10 ), years of education was treated as a continuous variable in the adjacent-categories logit models but was categorized in 4-year intervals–roughly corresponding to the education stages of high school, undergraduate college, and post-college education–to express the odds ratio for education level. Similarly, although the age at death was treated as a continuous variable, we used 5-year intervals to express the odds ratio.
CDR-SOB was categorized in intervals roughly corresponding to subjective complaints and mild cognitive impairment (CDR-SOB 0.5 to 3.0), mild dementia (CDR-SOB 3.5 to 6.0), moderate dementia (CDR-SOB 6.5 to 12.0), severe dementia (CDR-SOB 13.0 to 17.0), and end-stage dementia (CDR-SOB 18.0). The MMSE was categorized in intervals inspired by the UK National Institute of Health and Care Excellence (NICE) guidelines on the management of dementia ( 21 ): MMSE 27 to 30 (mild cognitive impairment), MMSE 21 to 26 (mild dementia), MMSE 15 to 20 (moderate dementia), MMSE 10 to 14 (moderately severe dementia), and MMSE <10 (severe dementia). As expected, CDR-SOB and MMSE scores showed a strong negative correlation (rho= –0.755, p < 0.0001, Spearman rank correlation test). When modeling the association of demographic and neuropathologic variables with ordinal categories of MMSE using adjacent categories models, we reversed the MMSE categories and used its highest interval (27 to 30) as the reference category, so that the odds ratios for MMSE would have the same direction as those for CDR-SOB in Tables 2, 3, and 4 .
RESULTS
Description of the Sample
Figure 1 depicts the selection procedure based on inclusion and exclusion criteria. The NACC autopsy cohort from January 2014 to May 2015 consists of a total of 523 subjects of which 192 subjects were eligible for this study. The MMSE was not available in 29 of these subjects: 21 due to a “behavioral/cognitive problem” (usually subjects too impaired to obtain a MMSE), 5 due to a “physical problem” (hearing or visual loss), and 3 due to “other problems.” Therefore, analyses with the MMSE as outcome variable included 163 subjects.
FIGURE 1.

Flow-chart of the selection process of study subjects. Note that the June 2015 data freeze includes data until May 31 st . UDS = Uniform Data Set as approved by the National Alzheimer's Coordinating Center (NACC).
Table 1 shows the demographic and clinical characteristics of the study subjects split by Thal amyloid stage. Supplementary Table 1 shows the demographic and clinical characteristics of the study subjects split by CDR-SOB and MMSE intervals.
TABLE 1.
Demographic and Clinical Characteristics of Study Subjects Classified by Thal Amyloid Stages
|
Thal amyloid stages
|
Total (column %) | ||||
|---|---|---|---|---|---|
| 0 | 1/2 | 3 | 4/5 | ||
| Number (row %) | 13 (6.8) | 17 (8.9) | 22 (11.5) | 140 (72.9) | 192 (100.0) |
| Sex | |||||
| n male (row %) | 8 (7.6) | 9 (8.6) | 12 (11.4) | 76 (72.4) | 105 (54.7) |
| n female (row %) | 5 (5.8) | 8 (9.2) | 10 (11.5) | 64 (73.6) | 87 (45.3) |
| Education (y) | 16.0 ± 2.7 | 15.3 ± 2.5 | 16.0 ± 3.1 | 15.3 ± 3.5 | 15.4 ± 3.3 |
| Age at death (y) | 89.4 ± 8.4 | 84.0 ± 9.9 | 85.1 ± 7.9 | 79.6 ± 10.5 | 81.3 ± 10.4 |
| Age at onset (y) | |||||
| n (row %) | 9 (5.4) | 12 (7.2) | 16 (9.6) | 130 (77.8) | 167 (100.0) |
| Mean ± SD | 81.4 ± 11.3 | 76.1 ± 11.1 | 79.5 ± 10.9 | 69.0 ± 10.7 | 71.2 ± 11.5 |
| Symptom duration (y) | |||||
| n (row %) | 9 (5.4) | 12 (7.2) | 16 (9.6) | 130 (77.8) | 167 (100.0) |
| Mean ± SD | 8.0 ± 4.0 | 7.6 ± 2.6 | 7.4 ± 5.7 | 9.8 ± 4.5 | 9.3 ± 4.5 |
| Time from last evaluation to death (mo) | 7.1 ± 4.0 | 11.1 ± 7.7 | 8.7 ± 5.0 | 8.7 ± 5.9 | 8.8 ± 5.9 |
| APOE genotype, n (row %) | |||||
| ϵ2/ϵ3 | 2 (16.7) | 1 (8.3) | 3 (25.0) | 6 (50.0) | 12 (7.8) |
| ϵ2/ϵ4 | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (66.7) | 3 (2.0) |
| ϵ3/ϵ3 | 7 (9.3) | 11 (14.7) | 11 (14.7) | 46 (61.3) | 75 (49.0) |
| ϵ3/ϵ4 | 0 (0.0) | 3 (6.0) | 4 (8.0) | 43 (86.0) | 50 (32.7) |
| ϵ4/ϵ4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 (100.0) | 13 (8.5) |
| Missing/unknown/not assessed | 4 (10.3) | 2 (5.1) | 3 (7.7) | 30 (76.9) | |
Education, age at death and onset, symptom duration, and time from last evaluation to death are expressed as mean ± SD. Numbers in parentheses represent row percentages unless otherwise indicated. Some values for age at onset and symptom duration were missing; therefore, numbers of subjects with available data are reported.
y, years; mo, months; n, number.
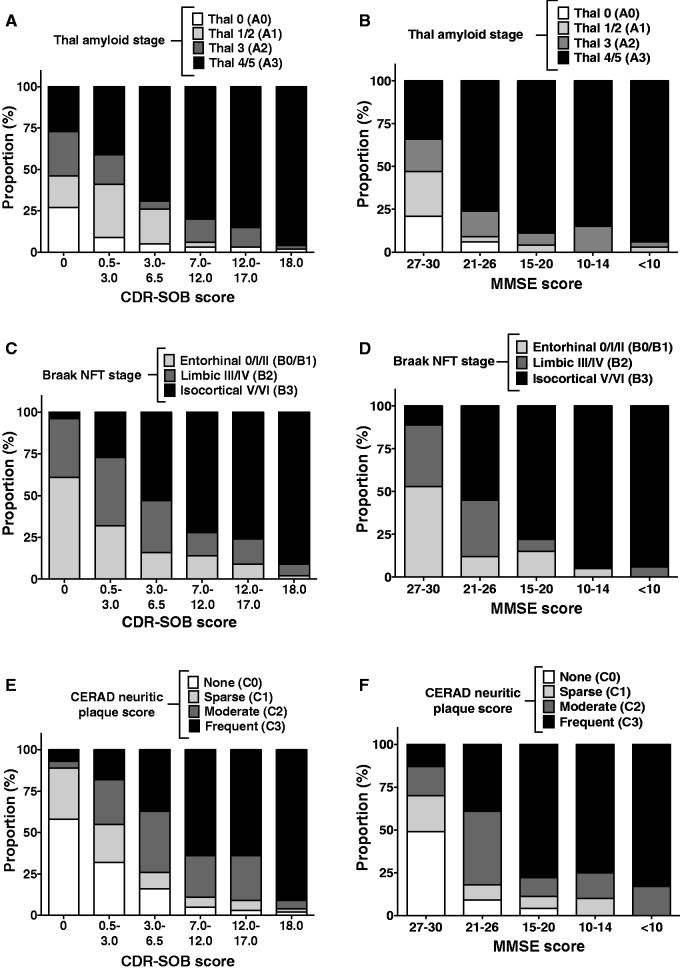
Supplemental Tables 2 and 3 describe the severity of each of the AD neuropathological changes (Thal amyloid stage, CERAD score of neuritic plaques, and Braak NFT stage) split by CDR-SOB and MMSE categories, respectively. Bar graphs in Figure 2 show graphically that the severity of each of these AD neuropathological changes appears to correlate with the CDR-SOB score in a more continuous fashion compared to the correlations with the MMSE score, which appear to be more binary relative to high MMSE score intervals versus all the other intervals.
FIGURE 2.
Correlations between AD neuropathological change and cognitive outcomes. Bar graphs illustrate the distribution of the severity of each AD neuropathological change (Thal amyloid stage [ A, B ], Braak NFT stage [ C, D ], and CERAD neuritic plaque score [ E, F ]) across CDR-SOB ( A, C, E ) or MMSE ( B, D, F ) score intervals.
Bar graphs in Figure 3 show the cross-correlations between the scores of AD neuropathological changes. The Thal amyloid staging and CERAD scoring of neuritic plaques have good correlation; ie, most subjects with early Thal stages (1/2 or A1 using the NIA-AA nomenclature) exhibit none or sparse neuritic plaques (CERAD C0 or C1), and most subjects with advanced Thal stages (4/5 or A3 by NIA-AA criteria) also have frequent neuritic plaques (CERAD C3) ( Fig. 3A ). The agreement between both amyloid measures was estimated using a weighted kappa with Fleiss-Cohen κ coefficient weights ( 19 ) and comparing Thal 0 versus CERAD none, Thal 1/2 versus CERAD sparse, Thal 3 versus CERAD moderate, and Thal 4/5 versus CERAD frequent. The resultant κ coefficient of 0.69 (95% CI: 0.60–0.79) indicates a moderate agreement. The CERAD neuritic plaque score also shows a good correlation with the Braak NFT stage because increasing densities of neuritic plaques are associated with higher Braak NFT stages ( Fig. 3B ). In sharp contrast, the correlation between Thal amyloid stages and Braak NFT stages does not appear to be as strong ( Fig. 3C ), mainly because both subjects with no amyloid (Thal 0 or A0) and those with isocortical amyloid deposition with or without limbic involvement (Thal 1/2 or A1) have early (0/I/II or B1) or intermediate (III/IV or B2) Braak NFT stages in similar proportions.
FIGURE 3.

Cross-correlations between AD neuropathological changes. Bar graphs illustrate the correlation between Thal amyloid stage and CERAD neuritic plaque score ( A ), between Braak NFT stage and CERAD neuritic plaque score ( B ), and between Thal amyloid stage and Braak NFT stage ( C ).
Last, Supplemental Tables 4 and 5 depict the presence and severity of each of the incidental comorbid pathologies (CAA, arteriosclerosis/small vessel ischemic disease, atherosclerosis of the circle of Willis, hippocampal sclerosis, and Lewy bodies) split by CDR-SOB and MMSE intervals, respectively.
The ABC Score Predicts Cognitive Performance Prior to Death
The ABC score combines the Thal amyloid stage, the CERAD score of neuritic plaques, and the Braak NFT stage to distinguish 4 categories: not AD, and low, intermediate, and high AD neuropathological change. According to the NIA-AA guidelines, “intermediate” or “high AD neuropathological change” is considered sufficient explanation for dementia; thus, individuals with cognitive impairment at the time of death and these levels of AD neuropathological change should be reported with a final diagnosis of AD. By contrast, “low AD neuropathological change” is not considered an adequate explanation for cognitive impairment or dementia, and other comorbid pathologies are usually found in subjects in this scenario.
Compared to “not AD,” both intermediate and high AD neuropathological change predicted a higher CDR-SOB and a lower MMSE scores ( Table 2 , model 1). These significant associations were “dose-dependent”; that is, the odds ratios were greater for high than for intermediate AD neuropathological change, although the confidence intervals were quite large for the MMSE. The addition of concurrent incidental pathologies to the model lowered the predictive value of the ABC score; thus, the intermediate AD neuropathological change was no longer significantly associated with either CDR-SOB or MMSE score ( Table 2 , model 2) when compared to “not AD.” This result is consistent with the idea that the strength of association of AD-related pathological changes with cognition weakens in the presence of other causes of brain damage.
Neuritic Plaques and NFTs, but Not Thal Amyloid Stages, Independently Associate With Cognition
We next investigated the contribution of demographics and neuropathological measures to cognitive impairment with special focus on Thal amyloid stages. Of the demographic variables, only age at death had a significant association with the outcome measures. An older age at death was negatively associated with cognitive impairment prior to death, as assessed by both CDR-SOB and MMSE ( Tables 3 and 4 , model 1). However, this effect lost statistical significance after adding the hallmark AD neuropathological changes (neuritic plaques and NFTs) to the analyses (model 2), and was further attenuated by the addition of Thal amyloid stages (model 3) and, particularly, comorbid pathologies (model 4).
Compared to none or sparse neuritic plaques, the presence of moderate and frequent neuritic plaques was significantly associated with a higher CDR-SOB ( Table 3 , model 2), although no significant association was observed between CERAD score of neuritic plaques and MMSE after controlling for demographic variables ( Table 4 , model 2). Compared to a Braak NFT stage 0/I/II, Braak NFT V/VI stage was significantly associated with both a higher CDR-SOB ( Table 3 , model 2) and a lower MMSE ( Table 4 , model 2). It should be noted that we reversed the MMSE categories and used its highest interval (27 to 30) as the reference category in the modeling so that the odds ratios for MMSE would have the same direction as those for CDR-SOB in Tables 2, 3, and 4 .
Adding the Thal amyloid stage as a covariate to these analyses did not change the magnitude or direction of any of the above associations (model 3 in Tables 3 and 4 ). Compared to no amyloid deposition (Thal stage 0), increasing Thal amyloid stages were not significantly associated with any of the outcome measures, CDR-SOB or MMSE, after adjusting for demographic variables, CERAD score of neuritic plaques, and Braak NFT stage. For the association between Thal stages and CDR-SOB, the odds ratios ranged between 0.74 and 1.05 with confidence intervals centered near 1, suggesting a null association. However, for the association between Thal stages and MMSE, the odds ratios ranged between 2.25 and 3.39 and had wide confidence intervals with large upper limits, suggesting the existence of a signal that might be weakened due to insufficient statistical power. We estimated a power of only ∼18% to detect an odds ratio of 2.65 for a Thal stage >0 versus 0 relative to low versus high MMSE score.
Effect of Concurrent Pathologies on Cognition
We next investigated the effects of concurrent incidental pathologies commonly found in the aging brain on the outcome measures ( Tables 3 and 4 , model 4). The addition of concurrent incidental pathologies to the analyses did not substantially alter the non-significant associations between Thal amyloid stages and CDR-SOB or MMSE described above. On the other hand, the statistically significant associations between moderate/frequent neuritic plaques and CDR-SOB and between Braak NFT V/VI stage and MMSE remained significant. However, the association between Braak NFT V/VI stage and CDR-SOB lost its statistical significance with the addition of comorbid pathologies ( Table 3 , model 4).
Of note, the presence of hippocampal sclerosis was independently associated with worse cognitive impairment prior to death as assessed by CDR-SOB, but its association with MMSE did not reach statistical significance. Moreover, the presence of incidental Lewy bodies was independently associated with a lower MMSE, but not with a higher CDR-SOB. Last, neither the severity of CAA, nor that of arteriosclerosis/small vessel ischemic disease and atherosclerosis of the circle of Willis, was significantly associated with either outcome measure in this selected autopsy cohort.
DISCUSSION
Our results raise the possibility that the Thal amyloid stages have little contribution to the prediction of cognitive performance prior to death compared to the CERAD score of neuritic plaques and the Braak NFT stage. Indeed, increasing Thal amyloid stages were neither significantly associated with CDR-SOB or MMSE, nor significantly modified the odds ratios of the associations between CERAD score of neuritic plaques and Braak NFT stage and CDR-SOB or MMSE ( Tables 3 and 4 , model 3), after controlling for the demographic variables sex, age at death, and education. We did observe a non-significant signal of its association with MMSE score (but not with CDR-SOB), suggesting that our study may be underpowered to detect an effect of the Thal amyloid stages on the MMSE score. Last, although the ABC score, which comprises Thal amyloid stage, CERAD score of neuritic plaques, and Braak NFT stage, proved to be a good predictor of cognitive performance prior to death, our analyses suggest that this association was mainly driven by the two classic AD neuropathological hallmarks, neuritic plaques and NFTs.
In a large single-center brain bank, Murray et al recently found that Braak NFT stage but not Thal amyloid stage correlates with the MMSE score obtained within 3 years from death ( 22 ), an observation that is in agreement with our present findings. However, because their multiple linear regression models did not include the CERAD neuritic plaque score, they could not address the question of the added value of the Thal amyloid staging over the CERAD score. A limitation of our analysis is the number of missing MMSE values in our sample (29 out of 192 subjects). Although we were not able to ascertain this in every case, we hypothesize that these missing values correspond to the most impaired subjects based on the codes for why missing and their high CDR-SOB scores. We reasoned that if this interpretation is correct and if these MMSE values had been available, they would have constituted another category of MMSE (lower than those observed) and would not have had much impact on our model estimates given the flexibility of the model. By contrast, if these missing MMSE values are actually distributed among the observed MMSE categories and if the neuropathology of these individuals is different from that of those with observed MMSE, then the results could be different from what we report here.
That Thal amyloid stage and CERAD neuritic plaque score are not equivalent measures of amyloid deposition is supported by the modest concordance between both indices, estimated as a κ = 0.60 in a recent small (n = 82) autopsy sample from a single center brain bank ( 23 ), and as a κ = 0.69 in our present larger multicenter study. Further in vivo evidence for a (at least to some extent) different amyloid pathological substrate underlying these 2 scores comes from recent studies correlating the antemortem uptake of fibrillar amyloid PET radiotracers with the postmortem Thal stage and showing that amyloid PET scans only turn positive at advanced Thal stages (Thal stage ≥2 for [ 11 C]-PiB ( 22 ) and Thal stage ≥4 for [ 18 F]-flutemetamol [ 24 ]).
Because Thal stage scores the spatial distribution of Aβ-immunoreactive plaques across the encephalon without discerning dense-core from diffuse deposits or neuritic from non-neuritic plaques, it is tempting to point to the diffuse amyloid deposits encompassed by the Thal staging scheme to explain the observed lack of significant association between the Thal amyloid stages and the severity of cognitive impairment. On the other hand, because both neuritic and diffuse amyloid deposits coexist in the cortex in AD, our analysis of CERAD and Thal stages does not completely distinguish whether amyloid-related toxicity in the cortex is partially resultant from either type of amyloid deposit. However, our overall analysis suggests that diffuse amyloid per se is not strongly associated with cognitive impairments. Multiple lines of evidence support the idea that diffuse amyloid deposits are associated with less neural system damage and do not contribute to the Alzheimer dementia and behavioral syndrome. First, numerous postmortem clinicopathological studies have shown that many cognitively intact elderly subjects exhibit some amount of diffuse Aβ deposits in the cerebral cortex ( 25–29 ). Also, multiple postmortem histopathological studies have established that diffuse plaques are not neurotoxic, as judged by the negligible or minimal surrounding synaptic loss ( 30 , 31 ), dystrophic neurites ( 32–34 ), activated microglia ( 35–40 ), and reactive astrocytes ( 36 , 41 ).
Of note, the strength of the associations between AD neuropathological changes and cognitive outcomes was attenuated by statistical adjustment for common pathologies incidentally found in the brain of elderly people (model 2 in Table 2 , and model 4 in Tables 3 and 4 ). In a previous study in a similar but larger cohort representing the AD clinicopathological continuum, we reported an independent contribution of neuritic plaques and NFTs to cognitive impairment as assessed with CDR-SOB score within 2 years before death, even after adjusting for these comorbid pathologies ( 10 ). In that study, we also found a protective effect of education against cognitive decline, whereas moderate and severe CAA, severe small vessel disease (arteriosclerosis), and presence of hippocampal sclerosis but not presence of Lewy bodies, were all independently associated with a more severe antemortem cognitive decline ( 10 ). Because the original autopsy cohort from which the study sample was selected (the NACC autopsy cohort), the eligibility criteria (except for the requirement of an available Thal amyloid stage in this study), and the statistical methods used in both studies are analogous, differences between the studies are most likely attributable to sample size (835 in previous study and 192 in present study).
In summary, our analyses in the NACC research cohort raise the possibility that the Thal amyloid staging system does not substantially add predictive value regarding cognitive performance prior to death to the classic measures of NFTs and neuritic plaques. The ABC score does associate significantly with cognitive measures, but this association is driven primarily by both CERAD score of neuritic plaques and Braak NFT stage. These findings suggest that the diffuse amyloid deposits that participate in the assignment of Thal amyloid stages are neutral with respect to clinically detectable changes in cognition and function. Future studies should investigate the correlations between Thal amyloid stages and more sensitive neuropsychological tests targeting specific cognitive domains.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to patients and relatives involved in research in the Alzheimer Disease Centers across the United States.
REFERENCES
- 1. Hyman BT, Phelps CH, Beach TG , et al. . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease . Alzheimers Dement J Alzheimers Assoc 2012. ; 8 : 1 – 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Montine TJ, Phelps CH, Beach TG , et al. . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach . Acta Neuropathol 2012. ; 123 : 1 – 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease . The National Institute on Aging, and Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of alzheimer’s disease . Neurobiol Aging 1997. ; 18 : S1 – 2 [PubMed] [Google Scholar]
- 4. Thal DR, Rüb U, Orantes M , et al. . Phases of a beta-deposition in the human brain and its relevance for the development of AD . Neurology 2002. ; 58 : 1791 – 800 [DOI] [PubMed] [Google Scholar]
- 5. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes . Acta Neuropathol 1991. ; 82 : 239 – 59 [DOI] [PubMed] [Google Scholar]
- 6. Braak H, Alafuzoff I, Arzberger T , et al. . Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry . Acta Neuropathol 2006. ; 112 : 389 – 404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mirra SS, Heyman A, McKeel D , et al. . The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease . Neurology 1991. ; 41 : 479 – 86 [DOI] [PubMed] [Google Scholar]
- 8. Matthews FE, Brayne C, Lowe J , et al. . Epidemiological pathology of dementia: Attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study . PLoS Med 2009. ; 6 : e1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neuropathology Group . Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) . Lancet 2001. ; 357 : 169 – 75 [DOI] [PubMed] [Google Scholar]
- 10. Serrano-Pozo A, Qiang J, Monsell SE , et al. . Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center . J Neuropathol Exp Neurol 2013. ; 72 : 1182 – 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beekly DL, Ramos EM, Lee WW , et al. . The National Alzheimer’s Coordinating Center (NACC) database: The Uniform Data Set . Alzheimer Dis Assoc Disord 2007. ; 21 : 249 – 58 [DOI] [PubMed] [Google Scholar]
- 12. Morris JC, Weintraub S, Chui HC , et al. . The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers . Alzheimer Dis Assoc Disord 2006. ; 20 : 210 – 6 [DOI] [PubMed] [Google Scholar]
- 13. Weintraub S, Salmon D, Mercaldo N , et al. . The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The neuropsychologic test battery . Alzheimer Dis Assoc Disord 2009. ; 23 : 91 – 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dolan H, Crain B, Troncoso J , et al. . Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort . Ann Neurol 2010. ; 68 : 231 – 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schneider JA, Arvanitakis Z, Bang W , et al. . Mixed brain pathologies account for most dementia cases in community-dwelling older persons . Neurology 2007. ; 69 : 2197 – 204 [DOI] [PubMed] [Google Scholar]
- 16. Sonnen JA, Larson EB, Crane PK , et al. . Pathological correlates of dementia in a longitudinal, population-based sample of aging . Ann Neurol 2007. ; 62 : 406 – 13 [DOI] [PubMed] [Google Scholar]
- 17. Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules . Neurology 1993. ; 43 : 2412 – 4 [DOI] [PubMed] [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician . J Psychiatr Res 1975. ; 12 : 189 – 98 [DOI] [PubMed] [Google Scholar]
- 19. Agresti A, Agresti A. Categorical Data Analysis [Internet] . Hoboken, NJ, USA: : John Wiley & Sons, Inc; . 2002 [Google Scholar]
- 20. Yee TM. The VGAM package for categorical data analysis . J Stat Softw 2010. ; 32 : 1 – 34 [Google Scholar]
- 21. National Institute for Health and Care Excellence (NICE). Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease [Internet]. 2011. . Available at: http://www.nice.org.uk/guidance/ta217/resources/donepezil-galantamine-rivastigmine-and-memantine-for-the-treatment-of-alzheimers-disease-82600254699973 . Access date: 11/15/2015
- 22. Murray ME, Lowe VJ, Graff-Radford NR , et al. . Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum . Brain 2015. ; 138 : 1370 – 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boluda S, Toledo JB, Irwin DJ , et al. . A comparison of Aβ amyloid pathology staging systems and correlation with clinical diagnosis . Acta Neuropathol 2014. ; 128 : 543 – 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thal DR, Beach TG, Zanette M , et al. . [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer’s disease: Specific detection of advanced phases of amyloid-β pathology . Alzheimers Dement 2015. ; 11 : 975 – 85 [DOI] [PubMed] [Google Scholar]
- 25. Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease . Neurology 1992. ; 42 : 1681 – 8 [DOI] [PubMed] [Google Scholar]
- 26. Knopman DS, Parisi JE, Salviati A , et al. . Neuropathology of cognitively normal elderly . J Neuropathol Exp Neurol 2003. ; 62 : 1087 – 95 [DOI] [PubMed] [Google Scholar]
- 27. Price JL, McKeel DW, Jr, Buckles VD , et al. . Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease . Neurobiol Aging 2009. ; 30 : 1026 – 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmitt FA, Davis DG, Wekstein DR , et al. . “Preclinical” AD revisited: Neuropathology of cognitively normal older adults . Neurology 2000. ; 55 : 370 – 6 [DOI] [PubMed] [Google Scholar]
- 29. Delaère P, Duyckaerts C, He Y , et al. . Subtypes and differential laminar distributions of beta A4 deposits in Alzheimer’s disease: Relationship with the intellectual status of 26 cases . Acta Neuropathol 1991. ; 81 : 328 – 35 [DOI] [PubMed] [Google Scholar]
- 30. Masliah E, Mallory M, Hansen L , et al. . Quantitative synaptic alterations in the human neocortex during normal aging . Neurology 1993. ; 43 : 192 – 7 [DOI] [PubMed] [Google Scholar]
- 31. Masliah E, Terry RD, Mallory M , et al. . Diffuse plaques do not accentuate synapse loss in Alzheimer’s disease . Am J Pathol 1990. ; 137 : 1293 – 7 [PMC free article] [PubMed] [Google Scholar]
- 32. Joachim CL, Morris JH, Selkoe DJ. Diffuse senile plaques occur commonly in the cerebellum in Alzheimer’s disease . Am J Pathol 1989. ; 135 : 309 – 19 [PMC free article] [PubMed] [Google Scholar]
- 33. Suenaga T, Hirano A, Llena JF , et al. . Modified Bielschowsky and immunocytochemical studies on cerebellar plaques in Alzheimer’s disease . J Neuropathol Exp Neurol 1990. ; 49 : 31 – 40 [DOI] [PubMed] [Google Scholar]
- 34. Wisniewski HM, Sadowski M, Jakubowska-Sadowska K , et al. . Diffuse, lake-like amyloid-beta deposits in the parvopyramidal layer of the presubiculum in Alzheimer disease . J Neuropathol Exp Neurol 1998. ; 57 : 674 – 83 [DOI] [PubMed] [Google Scholar]
- 35. Grundke-Iqbal I, Fleming J, Tung YC , et al. . Ferritin is a component of the neuritic (senile) plaque in Alzheimer dementia . Acta Neuropathol 1990. ; 81 : 105 – 10 [DOI] [PubMed] [Google Scholar]
- 36. Itagaki S, McGeer PL, Akiyama H , et al. . Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease . J Neuroimmunol 1989. ; 24 : 173 – 82 [DOI] [PubMed] [Google Scholar]
- 37. Mattiace LA, Davies P, Dickson DW. Detection of HLA-DR on microglia in the human brain is a function of both clinical and technical factors . Am J Pathol 1990. ; 136 : 1101 – 14 [PMC free article] [PubMed] [Google Scholar]
- 38. Mattiace LA, Davies P, Yen SH , et al. . Microglia in cerebellar plaques in Alzheimer’s disease . Acta Neuropathol 1990. ; 80 : 493 – 8 [DOI] [PubMed] [Google Scholar]
- 39. Ohgami T, Kitamoto T, Shin RW , et al. . Increased senile plaques without microglia in Alzheimer’s disease . Acta Neuropathol 1991. ; 81 : 242 – 7 [DOI] [PubMed] [Google Scholar]
- 40. Sasaki A, Yamaguchi H, Ogawa A , et al. . Microglial activation in early stages of amyloid beta protein deposition . Acta Neuropathol 1997. ; 94 : 316 – 22 [DOI] [PubMed] [Google Scholar]
- 41. Mrak RE, Sheng JG, Griffin WS. Correlation of astrocytic S100 beta expression with dystrophic neurites in amyloid plaques of Alzheimer’s disease . J Neuropathol Exp Neurol 1996. ; 55 : 273 – 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.