Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, airway pressure release ventilation, mechanical ventilation, protocol, respiratory failure, volume control
Abstract
Objectives:
Low tidal volume (= tidal volume ≤ 6 mL/kg, predicted body weight) ventilation using volume control benefits patients with acute respiratory distress syndrome. Airway pressure release ventilation is an alternative to low tidal volume-volume control ventilation, but the release breaths generated are variable and can exceed tidal volume breaths of low tidal volume-volume control. We evaluate the application of a low tidal volume-compatible airway pressure release ventilation protocol that manages release volumes on both clinical and feasibility endpoints.
Design:
We designed a prospective randomized trial in patients with acute hypoxemic respiratory failure. We randomized patients to low tidal volume-volume control, low tidal volume-airway pressure release ventilation, and traditional airway pressure release ventilation with a planned enrollment of 246 patients. The study was stopped early because of low enrollment and inability to consistently achieve tidal volumes less than 6.5 mL/kg in the low tidal volume-airway pressure release ventilation arm. Although the primary clinical study endpoint was Pao2/Fio2 on study day 3, we highlight the feasibility outcomes related to tidal volumes in both arms.
Setting:
Four Intermountain Healthcare tertiary ICUs.
Patients:
Adult ICU patients with hypoxemic respiratory failure anticipated to require prolonged mechanical ventilation.
Interventions:
Low tidal volume-volume control, airway pressure release ventilation, and low tidal volume-airway pressure release ventilation.
Measurements and Main Results:
We observed wide variability and higher tidal (release for airway pressure release ventilation) volumes in both airway pressure release ventilation (8.6 mL/kg; 95% CI, 7.8–9.6) and low tidal volume-airway pressure release ventilation (8.0; 95% CI, 7.3–8.9) than volume control (6.8; 95% CI, 6.2–7.5; p = 0.005) with no difference between airway pressure release ventilation and low tidal volume-airway pressure release ventilation (p = 0.58). Recognizing the limitations of small sample size, we observed no difference in 52 patients in day 3 Pao2/ Fio2 (p = 0.92). We also observed no significant difference between arms in sedation, vasoactive medications, or occurrence of pneumothorax.
Conclusions:
Airway pressure release ventilation resulted in release volumes often exceeding 12 mL/kg despite a protocol designed to target low tidal volume ventilation. Current airway pressure release ventilation protocols are unable to achieve consistent and reproducible delivery of low tidal volume ventilation goals. A large-scale efficacy trial of low tidal volume-airway pressure release ventilation is not feasible at this time in the absence of an explicit, generalizable, and reproducible low tidal volume-airway pressure release ventilation protocol.
Mechanical ventilation is a life-saving therapy in patients with acute hypoxemic respiratory failure and acute respiratory distress syndrome (ARDS) although it may induce ventilator-induced lung injury (VILI) (1–7). The highest quality of evidence demonstrates that a volume control (VC) ventilation strategy using low tidal volume (LTV) of 6 mL/kg of predicted body weight (PBW) with plateau pressure (Pplat) less than 30 cm H2O reduces mortality in patients with ARDS compared with a target tidal volume (VT) equal to 12 mL/kg PBW and Pplat less than 50 cm H2O (1). VTs even 1 or 2 mL/kg above LTV in patients with ARDS are associated with increased mortality (8). Many clinicians advocate for LTV ventilation for treatment of ARDS, with some arguing benefit in patients without ARDS (9–12).
Airway pressure release ventilation (APRV) is a pressure-limited mode of mechanical ventilation observed to improve oxygenation (13–17).
APRV is proposed as an alternative to VC-LTV because it attempts to minimize time spent at a low positive end-expiratory pressure (PEEP, or Plow) and increase mean airway pressure (Paw) by maximizing time spent at a high PEEP, or Phigh, but it does not target release volumes (14, 18, 19). Early studies of APRV demonstrate improvements in patient’s oxygenation with lower peak inspiratory pressures (PIPs) and higher Paws compared to patients treated with conventional ventilation (20–23). Observational studies also suggest an improved hemodynamic profile and enhanced patient comfort (both related to spontaneous breathing) resulting in reduced sedation requirements with APRV (24–27). PEEP optimization may improve oxygenation but occurs at the expense of higher measured tidal or release volumes that could increase VILI (28, 29). Therefore, we created an exportable APRV-LTV protocol that adapted the Habashi (14) protocol to employ an LTV ventilation strategy that targets average release volumes less than 6.5 mL/kg.
We proposed APRV-LTV would confer the benefits of APRV and limit the risk of VILI (14, 18). An ideal APRV-LTV protocol is reproducible and generalizable, enhances patient comfort, and confers advantages to hemodynamics and clinically relevant endpoints. We defined feasibility as successful implementation of an APRV-LTV protocol that consistently achieved VT less than or equal to 6.5 mL/kg with high clinician compliance. Before conducting a large multicenter efficacy trial comparing mortality between APRV-LTV and VC-LTV, we assessed the feasibility of an APRV-LTV protocol compared with traditional APRV and VC in a randomized, three-arm design among patients with acute hypoxemic respiratory failure.
METHODS
We performed a randomized controlled feasibility trial of VC-LTV versus APRV-LTV versus APRV in patients with respiratory failure admitted to one of four ICUs located in three Intermountain Healthcare hospitals over 49 months with first enrollment in September 2011 after trial registration with clinical.trials.gov (NCT01339533). The Central Region Intermountain Institutional Review Board (IRB) approved the protocol (IRB number: 1015758) with oversight from an independent data safety and monitoring board (DSMB). We employed three explicit mechanical ventilation paper protocols in patients with respiratory failure less than 24 hours and anticipated to require the ventilator for greater than 24 hours. Patients were assigned via computer-generated permuted 3–6 block randomization with sealed, opaque, and sequentially numbered envelopes into one of three arms: 1) conventional VC-LTV ventilation, 2) APRV-LTV, and 3) traditional APRV (14) (Supplement A, Supplemental Digital Content 1, http://links.lww.com/CCM/E39; Supplement B, Supplemental Digital Content 2, http://links.lww.com/CCM/E40; and Supplement C, Supplemental Digital Content 3, http://links.lww.com/CCM/E41). An identical continuous positive airway pressure protocol with a trigger Fio2 less than or equal to 0.60 and a PEEP or Phigh less than or equal to 10 comprised the weaning phase, which is found in Supplement B, along with daily compliance (Supplement D, Supplemental Digital Content 4, http://links.lww.com/CCM/E42) and power analysis (Supplement E, Supplemental Digital Content 5, http://links.lww.com/CCM/E43) for planned enrollment of the initial study aimed to justify a larger multicenter efficacy trial. The study was terminated early to maximize enrollment in Prevention and Early Treatment of Acute Lung Injury Network competing trials and after a planned 50 patient data review by the DSMB revealed significant variability in VTs in the APRV-LTV protocol.
Protocols
We operationalized the three protocols with decision tree logic for adhering to each arm’s stated purpose (Supplement A, Supplemental Digital Content 1, http://links.lww.com/CCM/E39; Supplement B, Supplemental Digital Content 2, http://links.lww.com/CCM/E40; and Supplement C, Supplemental Digital Content 3, http://links.lww.com/CCM/E41). We used the Draeger EvitaXL ventilator (Draeger, Houston, TX) for all protocols. Each protocol was written in paper format with cell branching logic as a preliminary step for computerization and exportability. The VC-LTV titrates PEEP and Fio2 based on the ARDS Network (ARDSNet) recommended Fio2 and PEEP tables modified for high altitude (1, 30). The oxygenation and ventilation tables were used by our institution in ARDSNet trials. The VC-LTV protocol sets a target VT of 6.0 mL/kg but with auto-flow can result in measured VT greater than 6.0 mL/kg. Release volumes and VTs are measured as exhale volumes at the exhalation valve of the ventilator. Respiratory therapists interact with the patient and the ventilator at least every 2 hours, often more frequently, and record the average values over several breaths in the electronic ventilator record. APRV-LTV titrates Plow and/or Phigh to target release volumes equal to 6.0 mL/kg. Traditional APRV followed previously published protocols (14) (Supplement C, Supplemental Digital Content 3, http://links.lww.com/CCM/E41). APRV-LTV was developed by two respiratory therapists and three pulmonary and critical care physicians and involved testing in an artificial lung simulation laboratory with quality improvement project iterative refinement of the protocol during clinical care (Supplement B, Supplemental Digital Content 2, http://links.lww.com/CCM/E40). All participating clinicians stated equipoise regarding the study.
After randomization, the respiratory therapist set the ventilator parameters per the specific arm protocol directions. APRV settings were determined by setting Phigh 3 above the Paw reading for the patient before randomization. Traditional APRV settings followed the Habashi (14) protocol to set Plow equal to 0 with timehigh and Plow titrated per patient response. APRV-LTV settings were determined by setting Phigh 3 above the Paw reading for the patient before randomization. Plow was set at a minimum of 5 and titrated with Phigh to target a release volume equals to 6 mL/kg. Work of breathing for each patient was observed and adjusted for P.01 as described in the protocol (Supplement B, Supplemental Digital Content 2, http://links.lww.com/CCM/E40; and Supplement C, Supplemental Digital Content 3, http://links.lww.com/CCM/E41).
Data Collection
As part of standard clinical practice, respiratory therapists measure and electronically record ventilator parameters every 2 hours, including Paw, PIP, VT, exhaled volume, and Pplat when indicated. We report average daily ventilator parameters. VT (mL/kg PBW) was defined as the measured exhaled volume for the VC-LTV and the measured exhaled release volume each APRV arm. We also collected the exhaled volume and rate of spontaneous breaths in the APRV arms. Phigh and PIP were treated as interchangeable for APRV, and Pplat is not measured. Lowest mean arterial pressure (MAP) and average daily central venous pressure (CVP) were also recorded. The medication dosing data were reported for baseline day as randomization time to 6:00 am the following day, and then 6:00 am to 6:00 am for each subsequent study day. Dosage is summarized only for days when medications were administered. Total dosing for vasoactive medications was calculated as the sum of the norepinephrine equivalent (NEE) doses for each vasoactive medication (31).
Subjects
We screened all patients greater than or equal to 18 years old with hypoxemic respiratory failure daily (Fig. 1). Informed consent was obtained by qualified surrogate. Randomization blocks were stratified as either ARDS or hypoxemic respiratory failure. If patients met the criteria for ARDS (Pao2/Fio2 ratio, chest radiograph infiltrates, absence of clinical evidence of left atrial hypertension) (1), they were stratified to the ARDS group. Otherwise patients were stratified as hypoxemic respiratory failure. Patients were randomized within 24 hours of the initiation of invasive mechanical ventilatory support.
Figure 1.
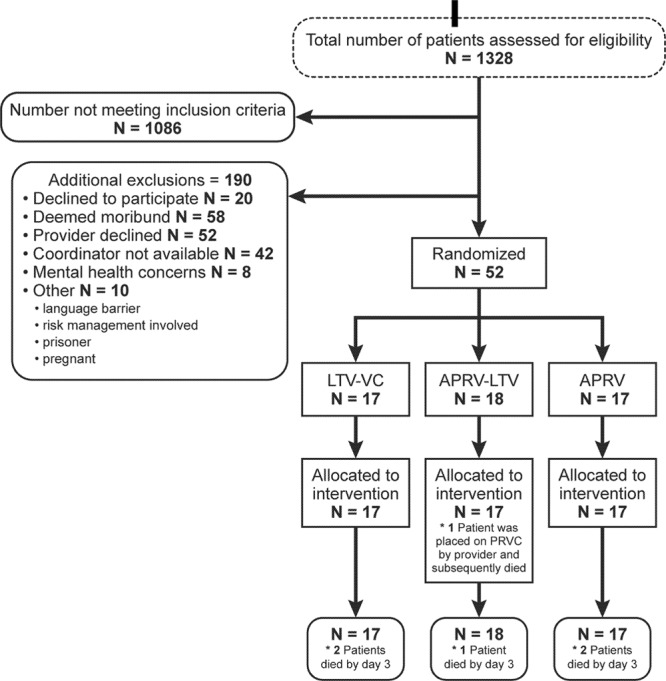
Consort diagram. *Primary analysis was Pao2/Fio2 ratio on study day 3. APRV = airway pressure release ventilation, LTV = low tidal volume, PRVC = pressure-regulated volume control, VC = volume control.
We excluded patients with known pregnancy or a primary diagnosis of acute coronary syndrome, major cardiac dysrhythmia, severe chronic respiratory disease, or severe traumatic head injury. We also excluded patients receiving chronic mechanical ventilation, neuromuscular disease, moribund patients, or patients enrolled in another interventional trial.
Statistical Analysis
The primary outcome was Pao2/Fio2 at day 3. Secondary outcomes included hospital mortality, ICU and hospital length of stay (LOS), ventilator-free days (VFDs), sedation medication received, vasoactive medication received, reintubation, and barotrauma. We used the Kruskal-Wallis test for three-way comparison of the continuous outcomes and Fisher exact test for the binary outcomes. We also performed a linear regression analysis adjusted for severity of illness (using Acute Physiology and Chronic Health Evaluation II) for clinical outcomes. We compared daily administration of vasoactive medications, sedatives, benzodiazepines, opioids, atypical antipsychotics, and paralytics for each protocol. We also compared total daily dosages of continuous medications including weighted mean NEE, dexmedetomidine, and propofol. As we collected the ventilator parameters, medication information, and compliance daily for each patient, we used generalized linear mixed-effects models with the appropriate link function to estimate the mean and 95% CI for each parameter of interest. The model included a random intercept for patient, and ventilator protocol was the only fixed effect included. An F test for protocol effect was conducted for each model, followed by Tukey adjusted pairwise comparisons when necessary.
RESULTS
Patients
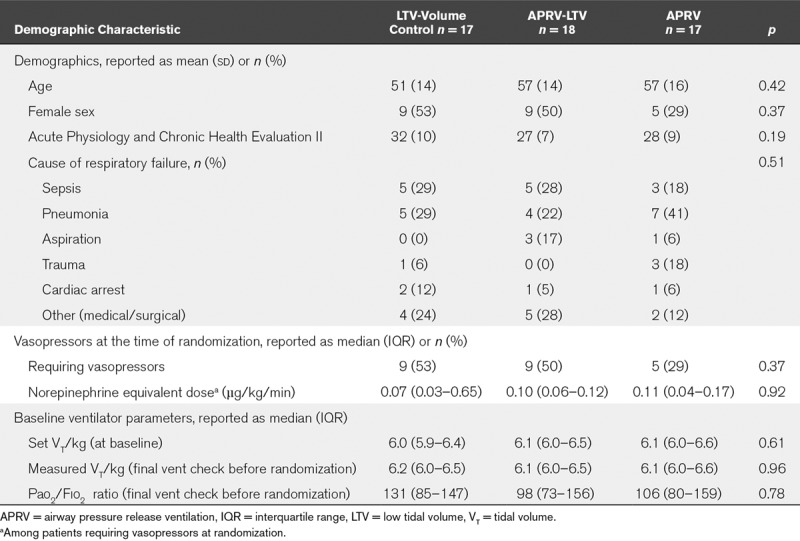
We enrolled a total of 52 patients in four participating ICUs with n equal to 17, 18, and 17 in the VC-LTV, APRV-LTV, and APRV arms, respectively (Fig. 1). In the mixed surgical, trauma, and medical study ICUs, the majority of patients who were screened for eligibility did not meet the inclusion criteria (Fig. 1). There was no difference in Pao2/Fio2 ratio between the groups before randomization or at the time of protocol initiation (VC-LTV = 131, APRV-LTV = 98, APRV = 106; p = 0.78) (Table 1). Baseline demographics did not differ significantly across the three arms (Table 1).
TABLE 1.
Demographic and Baseline Information
Clinical Outcomes
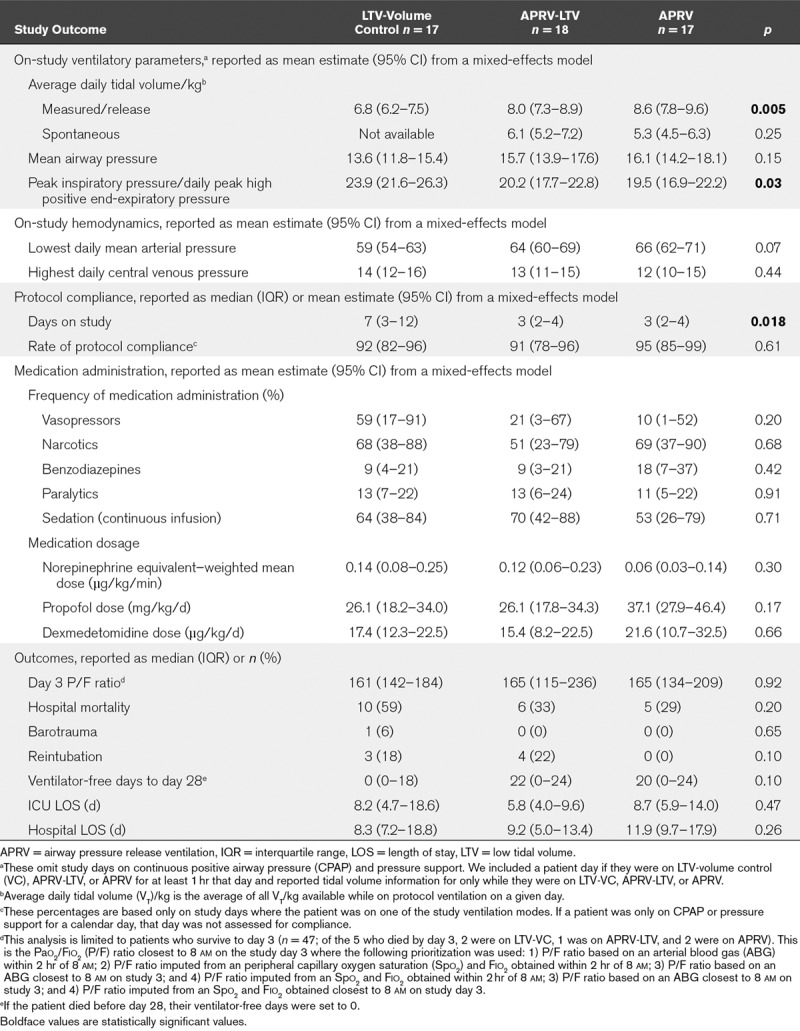
In-hospital mortality for the cohort was 40% (21/52) with no significant difference among the arms (p = 0.20). We found no difference in day 3 Pao2/Fio2 ratio, barotrauma, ICU LOS, hospital LOS, VFDs, or reintubation rate among the arms (Table 2); this persisted after linear regression adjusted for severity of illness.
TABLE 2.
On-Study Ventilatory Parameters, Protocol Compliance, and Outcomes
Ventilator Parameters
Figure 2 depicts the average daily measured VT by patient for each protocol arm. Thirty-five percent of APRV and 17% of APRV-LTV patients had at least one study day with average measured release volume greater than 12 mL/kg (Fig. 2). Patients in the VC-LTV arm had significantly lower average daily VTs with less variability than those in either APRV arm (VC = 6.8 mL/kg [6.2–7.5 mL/kg], APRV-LTV = 8.0 mL/kg [7.3–8.9 mL/kg], APRV = 8.6 mL/kg [7.8–9.6 mL/kg]; p = 0.005).
Figure 2.
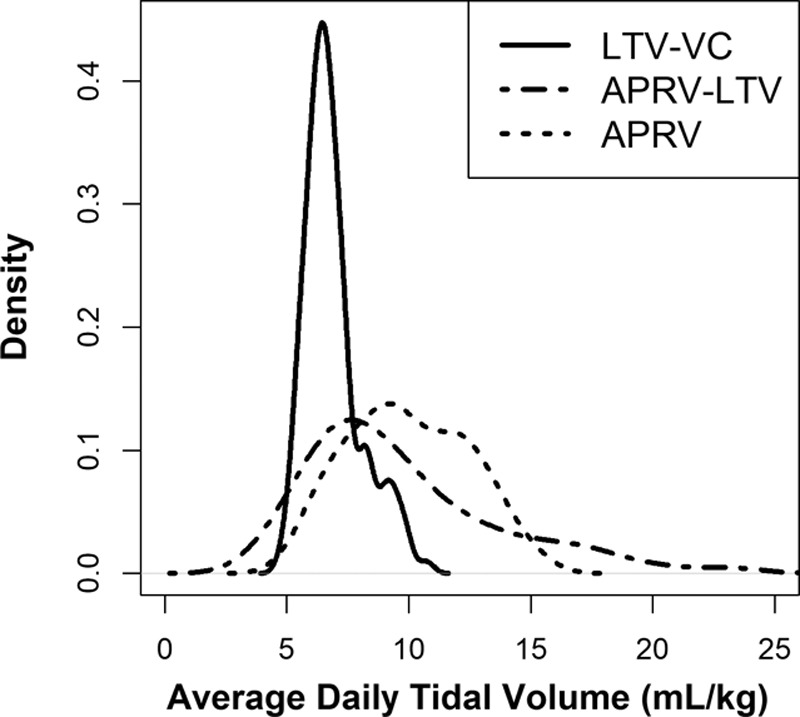
Density plot of the distribution of average daily tidal volumes by protocol. APRV = airway pressure release ventilation, LTV = low tidal volume, VC = volume control.
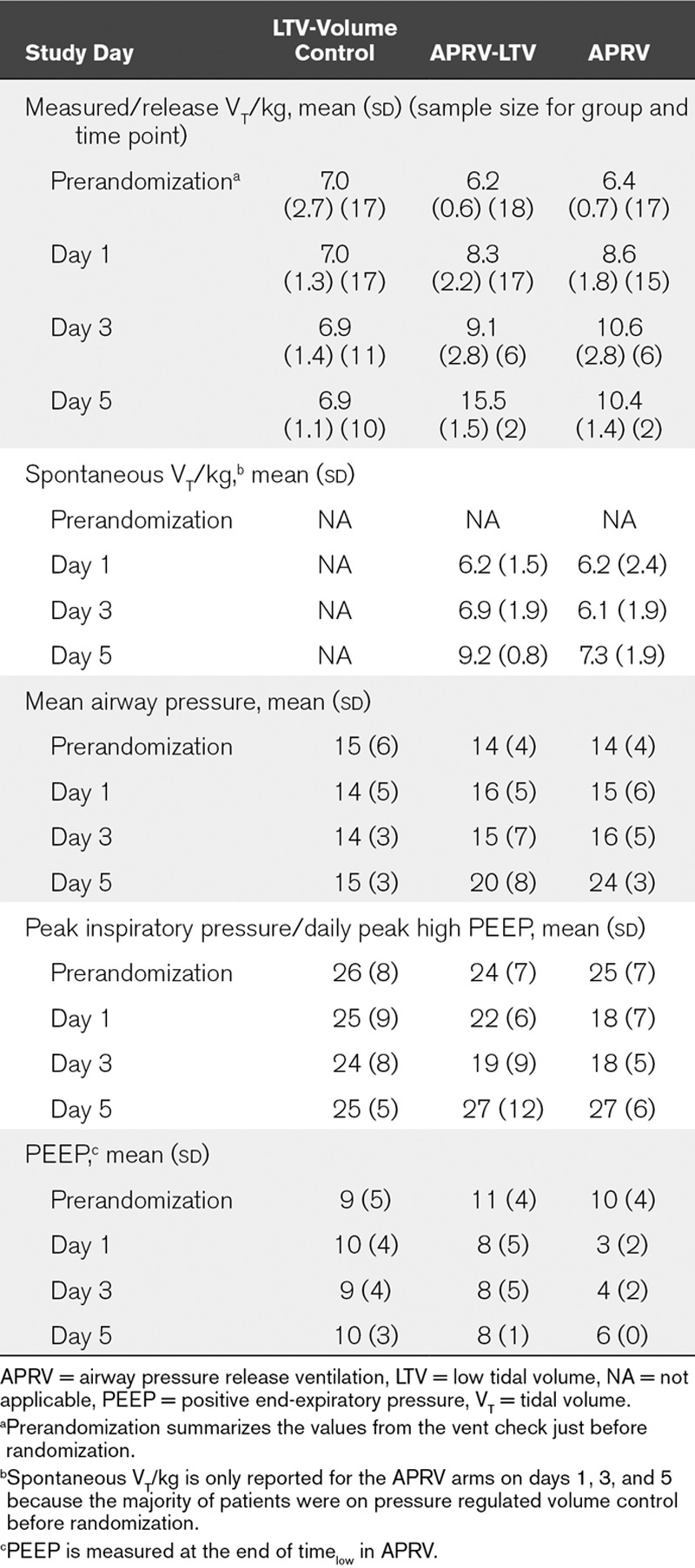
We found no significant difference between Paw (VC-LTV = 13.6, APRV-LTV = 15.7, APRV = 16.1; p = 0.15) between arms, but higher PIP in the conventional arm (VC-LTV = 23.9, APRV-LTV = 20.2, APRV = 19.5; p = 0.03) (Table 2). The lowest daily MAP and highest daily CVP were also similar. Average daily compliance for each protocol was similar (p = 0.61) and ranged between 91% and 95% (Table 2). Mean and sds of ventilator parameters through study day 5 is presented in Table 3.
TABLE 3.
Ventilator Parameters Across the Study Period
Medication Administration
The proportion of patient days on which patients received vasoactive medication was 59% in the VC-LTV arm compared with 10% and 21% in the APRV arms (p = 0.20). We observed no significant difference in the rate of benzodiazepine receipt among the arms (VC-LTV = 9%, APRV-LTV = 8%, APRV = 18%; p = 0.42). Similarly, there was no statistical difference in the total dosage of dexmedetomidine (VC-LTV = 17.4 mg/kg/d, APRV-LTV = 15.4 mg/kg/d, APRV = 21.6 mg/kg/d; p = 0.66) or propofol medication (VC = 26.1 μg/kg/d, APRV-LTV = 26.1 μg/kg/d, APRV = 37.1 μg/kg/d; p = 0.17). The rate of administration of paralytics, opioids, and typical or atypical antipsychotics was also similar (Table 2).
DISCUSSION
This is the first multicenter randomized controlled trial (RCT) of an LTV ventilation APRV protocol tracking release volumes in patients with hypoxemic respiratory failure. We observed no difference in oxygenation on day 3 of mechanical ventilation between VC-LTV and APRV, although the study was underpowered. Similarly, we observed no difference in sedation requirements, VFDs, or hospital mortality among the protocols. We importantly demonstrate that in four ICUs with years of ventilator protocol experience, neither the APRV-LTV nor the standard APRV protocol was associated with adherence to a LTV (6 mL/kg) ventilation target; that both APRV protocols resulted in variable release volumes with many greater than 12 mL/kg. Despite high daily compliance, our APRV-LTV protocol was unable to achieve LTV targets. Given the evidence in support of LTV ventilation, we propose that in the absence of a protocol that demonstrates successful achievement of LTV with APRV, the use of APRV should be limited to controlled clinical trials (8, 28).
The mortality benefits of APRV remain unproven. APRV appears to improve oxygenation or Pao2/Fio2 ratio in patients with hypoxemic respiratory failure (5, 16, 32–42). Although clinicians often titrate mechanical ventilation to optimize oxygenation, LTV is what confers mortality benefit, not improvement in oxygenation (1). Furthermore, clinicians titrate APRV with different application, making comparisons of published prospective studies difficult (18, 39, 42–44), and making a reproducible APRV protocol challenging. The single-center study by Zhou et al (42) favors APRV over LTV but may be contingent on the individual titration of APRV for each patient. Animal data and one observational study suggest that early application of APRV may reduce the occurrence of ARDS (33, 39, 40, 45–48). However, higher quality evidence indicates that LTV ventilation may be a better strategy more consistently applied for ARDS prevention (7, 8, 10–12, 49–51). Our study results do not suggest that APRV will improve patient comfort or reduce mortality and are supported by Lalgudi Ganesan et al (52) who found no difference in sedation and increased mortality with APRV. They suggest that a higher spontaneous-to-mandatory breath ratio observed in the APRV group could portend harm (52). Because of increased release volumes observed with APRV in our study, APRV may be inferior to VC-LTV ventilation. Although the harms of large release volumes in humans are yet unproven, animal studies demonstrate greater alveolar microstrain and decreased dynamic alveolar homogeneity with larger release volumes (47, 53). We assert that until implementation of APRV successfully adheres to LTV ventilation or can be tested in a reproducible manner across several centers, APRV compares unfavorably with LTV ventilation for prevention or treatment of ARDS (8, 11, 52). We express concern that although a multicenter study of APRV is justified, it may not be feasible using current protocols.
Prospective evaluations of the efficacy of APRV in patients are limited (13, 26, 54, 55). Our study is the second RCT comparing APRV to VC-LTV ventilation in a mixed population with acute hypoxemic respiratory failure, and the first prospective trial of an explicit APRV-LTV protocol. Maxwell et al (54) found no difference in clinical outcomes between APRV and VC-LTV in trauma patients. The single-center study by Zhou et al (42) reported an increase in VFDs and reduced sedation requirements with traditional APRV. The findings by Zhou et al (42) may be linked to key design elements as sedation and VFDs are linked; respiratory therapists manipulated APRV settings per subject and changed sedation medication to achieve spontaneous breath goals that may have introduced treatment bias (42). The individualized treatment by Zhou et al (42) of each APRV patient also raises concerns about the feasibility of reproducing the trial in other centers. Our study used explicit reproducible ventilator protocols (Supplement B, Supplemental Digital Content 2, http://links.lww.com/CCM/E40) that were managed by the respiratory therapists and titration of sedation was managed independent of the ventilator by the bedside nurses. Our observations do not support previously noted reduced sedation requirements with APRV (25, 26).
Spontaneous breathing during APRV occurs any time during the ventilator cycle and is recorded as tidal breathing. The two-way valve on the Draeger EvitaXL allows for the patient to inhale or exhale during Plow, Phigh, or during the release phase. The majority of time during the ventilator cycle in APRV is spent at Phigh; therefore, tidal breathing occurs largely at Phigh and generates pressures above Phigh. Many APRV protocols add tube compensation to the spontaneous efforts which enhances tidal efforts similar to pressure support. Release breaths serve to augment ventilation and are titrated (28, 56, 57). A tidal breath occurring at the end of Phigh allows the patient to augment the release breath with exhalation of their tidal breath. Similarly, spontaneous inspiration during the ascending phase of the release breath allows the patient to inhale additional volume above that required to reach Phigh. Duration of the release breath is short, meant to prevent alveolar collapse and is determined by the time spent at Plow (14, 58). Release volume is quickly returned, is rarely tracked, yet represents the volume required to reestablish Phigh. Alveoli can collapse very quickly, and the main determinant of how much collapse occurs is the volume that leaves the lungs during Plow. We submit that the release breath, not the spontaneous efforts in APRV, represents the volume change experienced in the alveoli. Neither Zhou et al (42) nor Maxwell et al (54) report measured release volumes. Our findings highlight the variability in measured release and spontaneous breaths during APRV ventilation (Fig. 2 and Table 2).
Inconsistent VTs with corresponding increases in transalveolar pressure may confer risk of VILI during spontaneous efforts in mechanically ventilated patients and such variability is common in APRV (38, 52, 57, 59–62). Increased driving pressure and transalveolar pressures may be more directly related to VILI (60, 61). In our findings, 35% of APRV and 17% of APRV-LTV patients had at least one study day where the average measured release volume was greater than 12 mL/kg (Fig. 2) which supports concerns about potential harms (28, 61, 63). The Paw in APRV is often considered to be similar to the intrinsic PEEP required by the patient to achieve the critical opening pressure on the hysteresis curve but often it approaches Phigh (28, 64, 65). In our study, both APRV protocols demonstrated numerically higher Paw compared with VC-LTV. Spontaneous breaths at higher Paw may result in even higher transalveolar pressures with unknown risks (28, 37, 61, 63, 64). Additional clinical studies measuring transalveolar pressure during release breaths and spontaneous efforts on APRV are warranted.
This study has several limitations. Clinicians were not blinded to the treatment arm. Although our study is comparable in size to other APRV trials (52, 54), we did not reach our target enrollment. Our study is thus underpowered, and negative findings of this trial may represent type 2 statistical error. The study was originally designed to evaluate oxygenation but was stopped prematurely due to inability to achieve LTV in APRV. Our patient enrollment was also smaller than enrollment in the trial by Zhou et al (42). However, our study cohort and mortality rate (consistent with severe to moderate ARDS) are representative of the population that currently triggers the clinical use of APRV (66). Our study protocol did not include an explicit sedation, pain management, or vasoactive medication protocol. Titration of sedation and pain control were performed by bedside clinicians using standard goal-directed delirium (Confusion Assessment Method for the ICU) and sedations assessments (Richmond Agitation-Sedation Scale). Similarly, vasoactive medications were titrated to goal MAP determined by the clinical team. It is possible that patients in the APRV arms were inappropriately perceived as more agitated or uncomfortable. Our data, however, are likely to mimic clinical application of sedation management with score-targeted sedation guidelines. Our findings may be specific to our protocol. It is unclear why titration of Plow and Phigh did not result in targeted release breath values or if a different APRV-LTV protocol can achieve an average VT less than 6.5 mL/kg. Although daily compliance was high, we used a paper protocol without assessment of compliance to each individual instruction. It is possible that an electronic APRV protocol would have tracked compliance more accurately and identified areas where the APRV-LTV protocol could have been changed to better achieve LTV targets.
CONCLUSIONS
An APRV-LTV protocol was implemented with adequate protocol compliance and resulted in highly variable release volumes that do not consistently achieve an LTV target (< 6.5 mL/kg). Average daily release volumes often exceeded 12 mL/kg. Our APRV-LTV protocol designed to limit release volumes was not feasible. Current APRV protocols are unable to achieve consistent and reproducible delivery of LTV ventilation goals. A large- scale efficacy trial of APRV-LTV is not feasible in the absence of a reproducible and easily generalizable APRV-LTV protocol.
ACKNOWLEDGMENT
We thank Terry Clemmer from the Division of Pulmonary and Critical Care Medicine, LDS Hospital, Salt Lake City, UT, for his contributions to this article.
Supplementary Material
Footnotes
Dr. Hirshberg, Ms. Carpenter, and Drs. Dean, Orme, and Grissom helped with conception. Dr. Hirshberg, Ms. Peterson, Ms. Carpenter, and Ms. Wilson helped with data acquisition. Drs. Hirshberg and Lanspa, Ms. Peterson, Ms. Carpenter, and Ms. Wilson helped with data analysis. Drs. Hirshberg, Lanspa, Brown, Orme, and Grissom helped with writing the article. All authors helped with revising the article for important intellectual content and approval of final copy.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by grants from Intermountain Research Medical Foundation.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Brower RG, Matthay MA, Morris A, et al. ; Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 2.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998; 338:347–354. [DOI] [PubMed] [Google Scholar]
- 3.Chacko B, Peter JV, Tharyan P, et al. Pressure-controlled versus volume-controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev 2015; 1:Cd008807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillette MA, Hess DR. Ventilator-induced lung injury and the evolution of lung-protective strategies in acute respiratory distress syndrome. Respir Care 2001; 46:130–148. [PubMed] [Google Scholar]
- 5.Mehaffey JH, Charles EJ, Sharma AK, et al. Airway pressure release ventilation during ex vivo lung perfusion attenuates injury. J Thorac Cardiovasc Surg 2017; 153:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham T, Brochard LJ, Slutsky AS. Mechanical ventilation: State of the art. Mayo Clin Proc 2017; 92:1382–1400. [DOI] [PubMed] [Google Scholar]
- 7.Sahetya SK, Mancebo J, Brower RG. Fifty years of research in ARDS. Vt selection in acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 196:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needham DM, Yang T, Dinglas VD, et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med 2015; 191:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: A preventive randomized controlled trial. Crit Care 2010; 14:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Futier E, Constantin JM, Paugam-Burtz C, et al. ; IMPROVE Study Group: A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013; 369:428–437. [DOI] [PubMed] [Google Scholar]
- 11.Neto AS, Simonis FD, Barbas CS, et al. ; PROtective Ventilation Network Investigators: Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: A systematic review and individual patient data analysis. Crit Care Med 2015; 43:2155–2163. [DOI] [PubMed] [Google Scholar]
- 12.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: A meta-analysis. JAMA 2012; 308:1651–1659. [DOI] [PubMed] [Google Scholar]
- 13.Varpula T, Pettilä V, Nieminen H, et al. Airway pressure release ventilation and prone positioning in severe acute respiratory distress syndrome. Acta Anaesthesiol Scand 2001; 45:340–344. [DOI] [PubMed] [Google Scholar]
- 14.Habashi NM. Other approaches to open-lung ventilation: Airway pressure release ventilation. Crit Care Med 2005; 33:S228–S240. [DOI] [PubMed] [Google Scholar]
- 15.Sundar KM, Thaut P, Nielsen DB, et al. Clinical course of ICU patients with severe pandemic 2009 influenza A (H1N1) pneumonia: Single center experience with proning and pressure release ventilation. J Intensive Care Med 2012; 27:184–190. [DOI] [PubMed] [Google Scholar]
- 16.Ferdowsali K, Modock J. Airway pressure release ventilation: Improving oxygenation: Indications, rationale, and adverse events associated with airway pressure release ventilation in patients with acute respiratory distress syndrome for advance practice nurses. Dimens Crit Care Nurs 2013; 32:222–228. [DOI] [PubMed] [Google Scholar]
- 17.Young D, Lamb SE, Shah S, et al. ; OSCAR Study Group: High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013; 368:806–813. [DOI] [PubMed] [Google Scholar]
- 18.Jain SV, Kollisch-Singule M, Sadowitz B, et al. The 30-year evolution of airway pressure release ventilation (APRV). Intensive Care Med Exp 2016; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieman GF, Satalin J, Andrews P, et al. Personalizing mechanical ventilation according to physiologic parameters to stabilize alveoli and minimize ventilator induced lung injury (VILI). Intensive Care Med Exp 2017; 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang AA, Steinfeld A, Gropper C, et al. Demand-flow airway pressure release ventilation as a partial ventilatory support mode: Comparison with synchronized intermittent mandatory ventilation and pressure support ventilation. Crit Care Med 1994; 22:1431–1437. [DOI] [PubMed] [Google Scholar]
- 21.Räsänen J, Cane RD, Downs JB, et al. Airway pressure release ventilation during acute lung injury: A prospective multicenter trial. Crit Care Med 1991; 19:1234–1241. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Tanigawa K, Ota K, et al. Practical use of airway pressure release ventilation for severe ARDS–a preliminary report in comparison with a conventional ventilatory support. Hiroshima J Med Sci 2009; 58:83–88. [PubMed] [Google Scholar]
- 23.González M, Arroliga AC, Frutos-Vivar F, et al. Airway pressure release ventilation versus assist-control ventilation: A comparative propensity score and international cohort study. Intensive Care Med 2010; 36:817–827. [DOI] [PubMed] [Google Scholar]
- 24.Fan E, Khatri P, Mendez-Tellez PA. Review of a large clinical series: Sedation and analgesia usage with airway pressure release and assist-control ventilation for acute lung injury. J Intensive Care Med 2008; 23:376–383. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care 2001; 5:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putensen C, Zech S, Wrigge H, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 2001; 164:43–49. [DOI] [PubMed] [Google Scholar]
- 27.Sydow M, Burchardi H, Ephraim E, et al. Long-term effects of two different ventilatory modes on oxygenation in acute lung injury. Comparison of airway pressure release ventilation and volume-controlled inverse ratio ventilation. Am J Respir Crit Care Med 1994; 149:1550–1556. [DOI] [PubMed] [Google Scholar]
- 28.Myers TR, MacIntyre NR. Respiratory controversies in the critical care setting. Does airway pressure release ventilation offer important new advantages in mechanical ventilator support? Respir Care 2007; 52:452–458.; discussion 458–460 [PubMed] [Google Scholar]
- 29.Shanholtz C, Brower R. Should inverse ratio ventilation be used in adult respiratory distress syndrome? Am J Respir Crit Care Med 1994; 149:1354–1358. [DOI] [PubMed] [Google Scholar]
- 30.Brower RG, Lanken PN, MacIntyre N, et al. ; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network: Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336. [DOI] [PubMed] [Google Scholar]
- 31.Brown SM, Lanspa MJ, Jones JP, et al. Survival after shock requiring high-dose vasopressor therapy. Chest 2013; 143:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies SW, Leonard KL, Falls RK, Jr, et al. Lung protective ventilation (ARDSNet) versus airway pressure release ventilation: Ventilatory management in a combined model of acute lung and brain injury. J Trauma Acute Care Surg 2015; 78:240–249.; discussion 249–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emr B, Gatto LA, Roy S, et al. Airway pressure release ventilation prevents ventilator-induced lung injury in normal lungs. JAMA Surg 2013; 148:1005–1012. [DOI] [PubMed] [Google Scholar]
- 34.Kollisch-Singule M, Emr B, Jain SV, et al. The effects of airway pressure release ventilation on respiratory mechanics in extrapulmonary lung injury. Intensive Care Med Exp 2015; 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kollisch-Singule M, Jain S, Andrews P, et al. Effect of airway pressure release ventilation on dynamic alveolar heterogeneity. JAMA Surg 2016; 151:64–72. [DOI] [PubMed] [Google Scholar]
- 36.Mireles-Cabodevila E, Kacmarek RM. Should airway pressure release ventilation be the primary mode in ARDS? Respir Care 2016; 61:761–773. [DOI] [PubMed] [Google Scholar]
- 37.Morimoto Y, Sugimoto T, Arase H, et al. Successful management using airway pressure release ventilation for severe postoperative pulmonary edema. Int J Surg Case Rep 2016; 27:93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richard JC, Lyazidi A, Akoumianaki E, et al. Potentially harmful effects of inspiratory synchronization during pressure preset ventilation. Intensive Care Med 2013; 39:2003–2010. [DOI] [PubMed] [Google Scholar]
- 39.Roy SK, Emr B, Sadowitz B, et al. Preemptive application of airway pressure release ventilation prevents development of acute respiratory distress syndrome in a rat traumatic hemorrhagic shock model. Shock 2013; 40:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song S, Tian H, Yang X, et al. The clinical effect of airway pressure release ventilation for acute lung injury/acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016; 28:15–21. [DOI] [PubMed] [Google Scholar]
- 41.Testerman GM, Breitman I, Hensley S. Airway pressure release ventilation in morbidly obese surgical patients with acute lung injury and acute respiratory distress syndrome. Am Surg 2013; 79:242–246. [PubMed] [Google Scholar]
- 42.Zhou Y, Jin X, Lv Y, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med 2017; 43:1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose L, Hawkins M. Airway pressure release ventilation and biphasic positive airway pressure: A systematic review of definitional criteria. Intensive Care Med 2008; 34:1766–1773. [DOI] [PubMed] [Google Scholar]
- 44.Facchin F, Fan E. Airway pressure release ventilation and high-frequency oscillatory ventilation: Potential strategies to treat severe hypoxemia and prevent ventilator-induced lung injury. Respir Care 2015; 60:1509–1521. [DOI] [PubMed] [Google Scholar]
- 45.Roy S, Habashi N, Sadowitz B, et al. Early airway pressure release ventilation prevents ARDS-a novel preventive approach to lung injury. Shock 2013; 39:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kollisch-Singule M, Emr B, Smith B, et al. Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. J Am Coll Surg 2014; 219:968–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kollisch-Singule M, Emr B, Smith B, et al. Mechanical breath profile of airway pressure release ventilation: The effect on alveolar recruitment and microstrain in acute lung injury. JAMA Surg 2014; 149:1138–1145. [DOI] [PubMed] [Google Scholar]
- 48.Andrews PL, Shiber JR, Jaruga-Killeen E, et al. Early application of airway pressure release ventilation may reduce mortality in high-risk trauma patients: A systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg 2013; 75:635–641. [DOI] [PubMed] [Google Scholar]
- 49.Eisner MD, Thompson T, Hudson LD, et al. ; Acute Respiratory Distress Syndrome Network: Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 164:231–236. [DOI] [PubMed] [Google Scholar]
- 50.Kallet RH, Corral W, Silverman HJ, et al. Implementation of a low tidal volume ventilation protocol for patients with acute lung injury or acute respiratory distress syndrome. Respir Care 2001; 46:1024–1037. [PubMed] [Google Scholar]
- 51.Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med 2007; 357:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lalgudi Ganesan S, Jayashree M, Singhi SC, et al. Airway pressure release ventilation in pediatric acute respiratory distress syndrome: A randomized controlled trial. Am J Respir Crit Care Med. 2018 Apr 11 doi: 10.1164/rccm.201705-0989OC. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Kollisch-Singule M, Jain S, Andrews P, et al. Effect of airway pressure release ventilation on dynamic alveolar heterogeneity. JAMA Surg 2016; 151:64–72. [DOI] [PubMed] [Google Scholar]
- 54.Maxwell RA, Green JM, Waldrop J, et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. J Trauma 2010; 69:501–510.; discussion 511 [DOI] [PubMed] [Google Scholar]
- 55.Varpula T, Jousela I, Niemi R, et al. Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury. Acta Anaesthesiol Scand 2003; 47:516–524. [DOI] [PubMed] [Google Scholar]
- 56.Daoud EG, Farag HL, Chatburn RL. Airway pressure release ventilation: What do we know? Respir Care 2012; 57:282–292. [DOI] [PubMed] [Google Scholar]
- 57.Muscedere JG, Mullen JB, Gan K, et al. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994; 149:1327–1334. [DOI] [PubMed] [Google Scholar]
- 58.Daoud EG. Airway pressure release ventilation. Ann Thorac Med 2007; 2:176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beitler JR, Sands SA, Loring SH, et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: The BREATHE criteria. Intensive Care Med 2016; 42:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372:747–755. [DOI] [PubMed] [Google Scholar]
- 61.Henderson WR, Chen L, Amato MBP, et al. Fifty years of research in ARDS. Respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 196:822–833. [DOI] [PubMed] [Google Scholar]
- 62.Perinel-Ragey S, Baboi L, Guérin C. Variability of tidal volume in patient-triggered mechanical ventilation in ARDS. Respir Care 2017; 62:1437–1446. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida T, Rinka H, Kaji A, et al. The impact of spontaneous ventilation on distribution of lung aeration in patients with acute respiratory distress syndrome: Airway pressure release ventilation versus pressure support ventilation. Anesth Analg 2009; 109:1892–1900. [DOI] [PubMed] [Google Scholar]
- 64.Kacmarek RM, Kirmse M, Nishimura M, et al. The effects of applied vs auto-PEEP on local lung unit pressure and volume in a four-unit lung model. Chest 1995; 108:1073–1079. [DOI] [PubMed] [Google Scholar]
- 65.Daoud EG, Chatburn RL. Comparing surrogates of oxygenation and ventilation between airway pressure release ventilation and biphasic airway pressure in a mechanical model of adult respiratory distress syndrome. Respir Investig 2014; 52:236–241. [DOI] [PubMed] [Google Scholar]
- 66.Ferguson ND, Cook DJ, Guyatt GH, et al. ; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group: High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368:795–805. [DOI] [PubMed] [Google Scholar]


