Supplemental Digital Content is available in the text.
Keywords: acute kidney injury, delirium, endothelial activation, endotoxemia, Klotho, sepsis
Abstract
Objectives:
To determine the applicability of recombinant Klotho to prevent inflammation and organ injury in sepsis in man and mice.
Design:
Prospective, clinical laboratory study using “warm” human postmortem sepsis-acute kidney injury biopsies. Laboratory study using a mouse model of endotoxemia.
Setting:
Research laboratory at a university teaching hospital.
Subjects:
Adult patients who died of sepsis in the ICU and control patients undergoing total nephrectomy secondary to renal cancer; male C57BL/6 and Klotho haploinsufficient mice.
Interventions:
Lipopolysaccharide (0.05 mg/kg) injection and kill after 4, 8, and 24 hours. Mice received recombinant Klotho (0.05 mg/kg) 30 minutes prior to lipopolysaccharide (1 mg/kg) injection. Mice treated with saline were included as controls.
Measurements and Main Results:
Quantitative reverse transcription polymerase chain reaction and immunohistochemical staining were used to quantify Klotho messenger RNA and protein expression in the kidney of sepsis-acute kidney injury patients and the kidney and brain of mice. The messenger RNA and protein expression of damage markers, inflammatory cytokine, chemokines, and endothelial adhesion molecules were also determined in mice. Renal neutrophil influx was quantified. We found significantly lower renal Klotho messenger RNA and protein levels in sepsis-acute kidney injury biopsies than in control subjects. These findings were recapitulated in the kidney and brain of lipopolysaccharide-challenged mice. Decreased Klotho expression paralleled an increase in kidney damage markers neutrophil gelatinase-associated lipocalin and kidney injury molecule-1. Administration of recombinant Klotho prior to lipopolysaccharide injection attenuated organ damage, inflammation and endothelial activation in the kidney and brain of mice. Furthermore, less neutrophils infiltrated into the kidneys of recombinant Klotho mice compared with lipopolysaccharide only treated mice.
Conclusions:
Renal Klotho expression in human sepsis-acute kidney injury and in mouse models of sepsis was significantly decreased and correlated with renal damage. Recombinant Klotho intervention diminished organ damage, inflammation, and endothelial activation in the kidney and brain of lipopolysaccharide-challenged mice. Systemic Klotho replacement may potentially be an organ-protective therapy for septic patients to halt acute, inflammatory organ injury.
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (1). With a mortality rate of around 25–40%, sepsis is a leading cause of mortality worldwide, especially in critically ill patients (2). This high mortality is both acute and long-term, as many patients die in subsequent months after recovery from sepsis (3). Furthermore, sepsis survivors often suffer from a range of long-term sequelae, including chronic kidney disease (CKD), cardiovascular disease, as well as cognitive and physical decline. A detailed understanding of sepsis-induced organ failure is still lacking, resulting in the lack of curative therapeutic options available. These options are still limited to antibiotic treatment and organ support and have remained unchanged for the last 3 decades. The development of therapeutic approaches for patients with sepsis is therefore sorely needed in order to limit and/or reverse organ injury as a result of sepsis.
Klotho is a single-pass transmembrane protein that is predominantly expressed in the kidney, parathyroid gland, and the choroid plexus in the brain (4, 5). It is proteolytically cleaved by the alpha-secretases ADAM10 and 17, to generate soluble Klotho (6–10) which is secreted into the blood, urine, or cerebrospinal fluid (CSF) (6, 11–14). Soluble Klotho functions as a humoral factor with beneficial effects including suppression of apoptosis, cellular senescence, and fibrosis, in addition to promoting autophagy and displaying antioxidative stress properties (6, 15, 16). The majority of circulating Klotho is derived from the kidney (6, 17).
Ischemic acute kidney injury (AKI) in mice was paralleled by an acute Klotho deficiency and intervention with recombinant Klotho (rKL) facilitated recovery of renal and extrarenal organ function (12, 18–20). Klotho-deficient mice display a higher mortality in experimental sepsis (21). Diminished Klotho levels proportional to a drop in glomerular filtration rate were observed in mice with CKD (12). Restoration of Klotho levels via genetic manipulation, viral-based delivery or administration of rKL protein normalized phosphate levels, improved kidney function, decreased urinary protein, and ameliorated tubulointerstitial damage in mice (19, 22, 23). Similar to AKI, acute brain injury is also commonly found in sepsis patients and presents as delirium (24, 25). Lipopolysaccharide (LPS) challenge has been shown to cause CNS inflammation, microglial activation and behavioral and cognitive dysfunction in mice (26, 27). Apart from the kidney, Klotho is also expressed in the choroid plexus (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/E2), the part of the brain responsible for producing CSF and a component of the blood-CSF barrier (28). Systemic inflammatory conditions such as sepsis, have been shown to compromise the blood-CSF barrier function in vivo, allowing leakage from the blood into the CNS via the CSF (29). In mice, Klotho deletion resulted in cognitive impairment whereas overexpression or supplementation of Klotho improved cognitive function (30, 31).
Since Klotho has multiple organ-protective effects, its replacement may serve as a therapeutic strategy for septic patients in order to limit acute organ damage and/or prevent acute-to-chronic organ dysfunction. In this study, we hypothesized that Klotho messenger RNA (mRNA) and protein levels were reduced in postmortem kidney biopsies from septic patients and in the organs of LPS-treated mice. Apart from inflammation, disrupted microvascular integrity and resulting leukocyte transmigration from the blood into underlying tissues are known to be drivers of organ failure in sepsis. We therefore additionally postulated that rKL administration prior to LPS challenge would have an organ-protective effect by attenuating inflammation and microvascular disturbances.
MATERIALS AND METHODS
Patients
Postmortem kidney biopsies were collected in patients with sepsis and renal failure as described elsewhere (32, 33). Patients 18 years old and older who died of sepsis in the ICU were included in the study. Patients with preexisting CKD, active autoimmune disorders with renal involvement, and treatment with immune-suppressive medication were excluded from this study. All patients were classified as having septic shock according to the International Sepsis Definitions (34). In addition, renal failure in all patients was classified according to the Risk, Injury, Failure, Loss of kidney function and End-stage kidney disease (RIFLE) criteria (35). In patients undergoing complete nephrectomy as a result of kidney cancer, a healthy part of tissue was isolated from the kidney cortex as far away as possible from the tumor. A dedicated renal pathologist at our hospital assessed all biopsies and considered them to be healthy. Thus, we define these biopsies in our article as healthy. Patients with previous renal function loss were excluded. Written informed consent was given by the relatives of all septic patients. These biopsies are referred to as “warm” because of the short time interval between patient death and biopsy collection. All biopsies were taken within an average of 33 minutes after death or in the operating theatre immediately after removal of the kidney in nephrectomy patients. These measures were taken in order to avoid unwanted necropsy which may have tainted our pathophysiological findings. The Medical Ethics Review Committee (METC) of the University Medical Center Groningen reviewed and waived this study (METC 2011/372).
Mice
Ten week old male C57BL/6 mice (Harlan Laboratories, Horst, The Netherlands) and 9 week old male Klotho haploinsufficient (Kl+/–) mice were housed in a specific pathogen-free facility, maintained on chow and water ad libitum and housed in temperature-controlled chambers (24°C) with a 12 hour light/dark cycle. Generation of haploinsufficient (Klotho+/–) mice has been described elsewhere (36). All experiments were performed in compliance with the animal ethics committee of the University of Groningen and Radboud University Nijmegen.
Endotoxemia Mouse Model and Klotho Intervention
C57BL/6 mice were challenged by intraperitoneal injection of LPS (0.05 mg/kg Escherichia coli, serotype 026:B6 (15,000 endotoxin U/g) and killed 4, 8, and 24 hours after LPS administration. For the Klotho intervention experiments, mice were first intraperitoneal injected with recombinant mouse Klotho (rKL) (0.05 mg/kg; 1819-KL; R & D Systems, Abington, United Kingdom) in saline before receiving intraperitoneal LPS (1 mg/kg) 30 minutes later. Vehicle-injected mice were administered the same volume of saline intraperitoneal. Mice were killed 4 hours after the LPS or vehicle administration. Prior to kill, mice were anesthetized with oxygen/isoflurane and blood was drawn via aortic puncture. Organs were harvested and either snap frozen on liquid nitrogen and stored at –80°C until further analysis, or fixed in formalin for histological analysis.
Gene Expression Analysis by Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated from mouse kidney and brain cryosections using a RNAeasy Mini Plus Kit (Qiagen, Leusden, The Netherlands), according to the manufacturer’s instructions. RNA integrity was analyzed, complementary DNA synthesized and quantitative reverse transcription polymerase chain reaction performed as described in detail (37) and briefly in the supplemental methods (Supplemental Digital Content 1, http://links.lww.com/CCM/E2).
Immunohistochemistry and Morphometric Analysis
Immunochemical staining of Klotho, VCAM-1, E-selectin, and Ly6G on formalin-fixed paraffin-embedded human kidney and mouse kidney and brain tissue is described in the supplemental methods (Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Stained tissue slides were scanned using a Hamamatsu Nanozoomer 2.0HT (Hamamatsu Photonics, Hamamatsu, Japan) at a magnification of 4×. Morphometric analysis was performed using the Aperio Imagescope positive pixel analysis v9.1 algorithm (Aperio Technologies, Vista, CA). Neutrophil infiltration was quantified by counting the number of neutrophils present in all glomeruli of the kidney sections.
Enzyme-Linked Immunosorbent Assay
Plasma neutrophil gelatinase-associated lipocalin (NGAL) and interleukin (IL)–6 and renal tissue NGAL and myeloperoxidase protein levels were determined by enzyme-linked immunosorbent assay as described in the supplemental methods (Supplemental Digital Content 1, http://links.lww.com/CCM/E2).
Blood Urea Nitrogen Determination
Blood urea nitrogen (BUN) quantification was performed using the QuantiChrom Urea Assay Kit (DIUR-100; BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions.
Statistical Analysis
To compare two means, a two-tailed unpaired Student t test was used, assuming equal variances. To compare multiple means a one-way analysis of variance followed by Bonferroni post hoc analysis was used. Statistical analyses were performed using GraphPad Prism Software v7 (GraphPad Prism Software, San Diego, CA). Differences were considered significant when p value of less than 0.05.
RESULTS
Renal Klotho Levels Are Reduced in Critically Ill Patients With Sepsis-AKI
Previous studies have reported a reduction in renal Klotho expression in mice following ischemic-AKI (6, 15, 18, 20). We hypothesized that renal Klotho expression is reduced in human sepsis-AKI patients compared with control conditions. To investigate this hypothesis we collected “warm” postmortem biopsies from patients who died of sepsis and renal failure in our ICU. Clinical and laboratory details of our patient biopsy cohorts can be found in Supplemental Tables 1 and 2 (Supplemental Digital Content 1, http://links.lww.com/CCM/E2). We found significantly lower renal Klotho mRNA levels in sepsis-AKI biopsies than in control subjects. This reduced expression paralleled increased mRNA levels of kidney damage markers NGAL and kidney injury molecule-1 (Fig. 1A). Additionally, we found a clear reduction in renal Klotho protein levels in sepsis-AKI biopsies when compared with control (Supplemental Fig. 2, A and B, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). In our study, both groups of patients were of similar age, an average of 68 (40–83) and 65 (47–79) for sepsis and control group patients respectively. We found a trend (p = 0.06) suggesting a correlation between increasing age and reduced renal Klotho levels in control individuals. However, this correlation was completely lost in patients with sepsis (Supplemental Fig. 2C, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Furthermore, the extent of Klotho mRNA and protein level reduction was not dependent on the degree/severity of renal failure as characterized in our sepsis-AKI patients using the RIFLE criteria (Fig. 1B; and Supplemental Fig. 2D, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Taken together, these findings indicate that Klotho expression is reduced in patients with sepsis-AKI, which was accompanied by an increase in kidney damage markers. Additionally, sepsis has a greater impact on renal Klotho levels than age.
Figure 1.
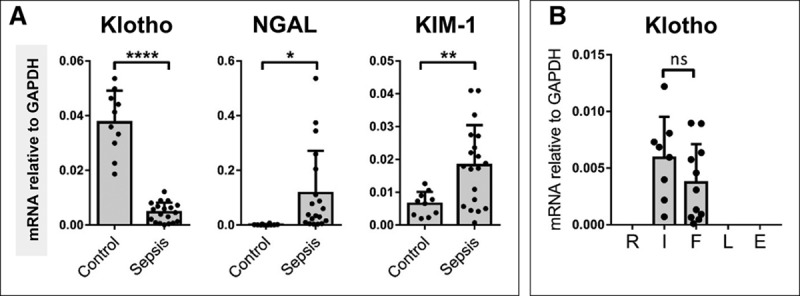
Renal Klotho levels are reduced in critically ill patients with sepsis-induced acute kidney injury (AKI) while renal damage markers are increased. A, Postmortem kidney biopsies were collected from patients with sepsis-AKI (n = 19). Kidney tissue was also obtained from control subjects (n = 10). Klotho, neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule (KIM)-1 messenger RNA (mRNA) expression were determined by quantitative reverse transcription polymerase chain reaction using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. B, Sepsis-AKI patients (n = 19) were also categorized by the extent of renal failure, Risk, Injury, Failure, Loss of kidney function and End-stage kidney disease (RIFLE) criteria. Each dot represents an individual subject, and the bars represent the mean ± sd. *p < 0.05, **p < 0.005, ****p < 0.0001. ns = not significant.
Renal Klotho mRNA and Protein Levels Are Reduced in Mice Subjected to LPS Challenge
We proceeded to investigate the kinetics of renal Klotho down-regulation using a mouse model for sepsis in which mice are killed at different time points after LPS challenge. The results reflected our observations in human sepsis-AKI, we found a significant down-regulation in renal Klotho gene expression in LPS-challenged mice compared with control mice (Fig. 2). Reduced Klotho mRNA and protein levels were already observed at 4 hours after LPS administration (Fig. 2; and Supplemental Fig. 3, Supplemental Digital Content 1, http://links.lww.com/CCM/E2), and was paralleled by an up-regulation of NGAL mRNA (Fig. 2). Increased NGAL mRNA levels were also found in kidneys of Kl+/– mice compared with wild-type (WT) mice (Supplemental Fig. 4, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Likewise, increased mRNA levels of E-selectin and intercellular adhesion molecule (ICAM)-1 as well as retinoic acid-inducible gene (RIG)-I, a receptor involved in the regulation of LPS-mediated endothelial activation (38) were observed in Kl+/– mice indicating that diminished renal Klotho levels lead to renal damage and endothelial activation (Supplemental Fig. 4, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Interestingly, Klotho mRNA levels had not recovered by 24 hours after LPS challenge (Fig. 2) despite clinical recovery of the mice.
Figure 2.
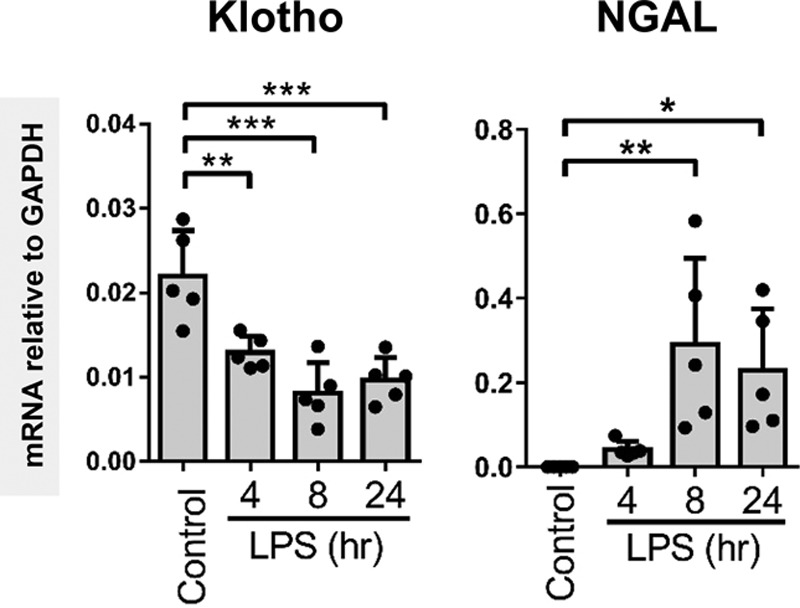
Renal Klotho messenger RNA (mRNA) and protein levels are reduced in mice subjected to lipopolysaccharide (LPS) challenge. Renal mRNA expression of Klotho and renal damage marker neutrophil gelatinase-associated lipocalin (NGAL) in control and LPS-challenged (0.05 mg/kg intraperitoneal) mice killed at the indicated time points after LPS administration. mRNA expression levels were determined by quantitative reverse transcription polymerase chain reaction using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. Each dot represents an individual mouse. Bars represent the mean ± sd. *p < 0.05, **p < 0.005, ***p < 0.001.
rKL Protein Administration Protects Against LPS-Mediated Kidney Damage and Inflammation In Vivo
In previous studies, Klotho administration was shown to ameliorate ischemic-AKI (6, 15). We hypothesized that Klotho administration prior to LPS challenge would have a renal protective effect. We administered rKL (0.05 mg/kg) to mice 30 minutes prior to LPS challenge and killed the mice 4 hours after LPS injection. We found that rKL intervention mice that received LPS had significantly lower plasma BUN and plasma NGAL levels compared with mice treated with LPS only (Supplemental Fig. 5, A and B, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Additionally, the proinflammatory cytokine IL-6 levels in plasma were decreased in rKL + LPS mice compared with LPS mice (Supplemental Fig. 5C, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Although attenuated, the levels of BUN, NGAL, and IL-6 levels did not reach basal levels in rKL pretreated mice. We found that renal NGAL mRNA and protein levels were also significantly lower in rKL + LPS mice than in mice treated with LPS only (Fig. 3; and Supplemental Fig. 5D, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Renal inflammation was significantly inhibited by rKL pretreatment, with lower IL-6, IL-8, and monocyte chemoattractant protein-1 mRNA expression levels in rKL + LPS mice compared with mice treated with LPS only (Fig. 3). We did not find any detrimental effects or rKL injection alone when examining the well-being of mice, plasma markers, and organ pathology (data not shown). These results collectively indicate that rKL administration is renoprotective in a mouse sepsis model.
Figure 3.
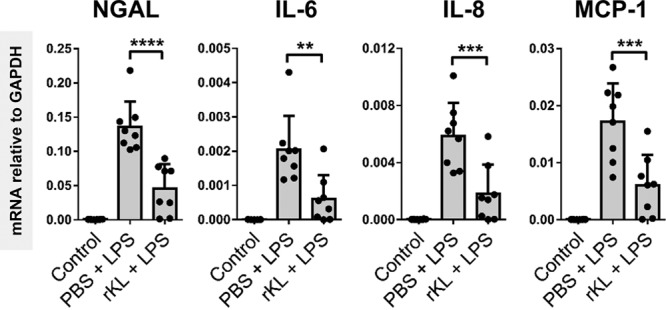
Recombinant Klotho (rKL) protein administration protects against lipopolysaccharide (LPS)-mediated kidney damage and inflammation in vivo. Renal neutrophil gelatinase-associated lipocalin (NGAL), interleukin (IL)–6, IL-8, and monocyte chemoattractant protein (MCP)-1 messenger RNA (mRNA) expression in control, LPS, and rKL + LPS mice was determined by quantitative reverse transcription polymerase chain reaction using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. Each dot represents an individual mouse. Bars represent the mean ± sd. **p < 0.005, ***p < 0.001.
rKL Protein Administration Attenuates LPS-Mediated Endothelial Activation and Associated Renal Neutrophil Infiltration
In sepsis, activated endothelial cells promote the transmigration of leukocytes from the blood into the underlying tissues through endothelial cell-leukocyte interactions that can be detrimental to organ function. We therefore proceeded to investigate whether Klotho administration was also able to attenuate LPS-induced endothelial adhesion molecule expression in WT mice. Renal E-selectin and ICAM-1 mRNA expression were significantly reduced in rKL pretreated mice compared with LPS only exposure (Fig. 4). Surprisingly, vascular cell adhesion protein (VCAM)-1 gene expression was unaltered by Klotho administration prior to LPS exposure. Semiquantification of E-selectin and VCAM-1 protein confirmed decreased E-selectin, but unaltered VCAM-1 expression in rKL pretreated mice compared with mice treated with LPS only (Supplemental Fig. 6, A and B, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Since endothelial activation plays a key role in immune cell infiltration into tissue, we investigated renal neutrophil localization. Indeed, the number of Ly6G-positive neutrophils infiltrating in the kidneys of rKL pretreated mice was significantly reduced compared with the number in LPS-only mice which was confirmed by quantification of the mean number of infiltrating neutrophils per glomerulus (Supplemental Fig. 6, C and D, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Additionally, as a result of attenuated neutrophil infiltration, reduced levels of myeloperoxidase protein were found in rKL pretreated mice compared with LPS-only treated mice (Supplemental Fig. 6E, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Taken together, our data indicate that Klotho administration attenuates endothelial adhesion molecule expression and neutrophil infiltration in the kidneys of LPS-treated mice.
Figure 4.
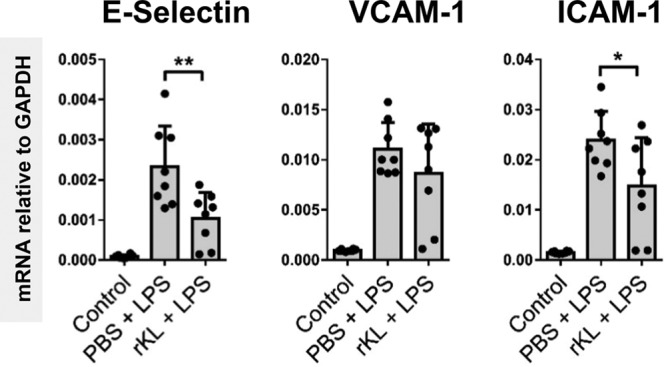
Recombinant Klotho (rKL) protein administration attenuates lipopolysaccharide (LPS)-mediated endothelial activation. Renal messenger RNA (mRNA) expression levels of E-selectin, vascular cell adhesion protein (VCAM)-1, and intercellular adhesion molecule (ICAM)-1 in control vehicle-treated mice (n = 8) or LPS (1 mg/kg) challenged mice (LPS; n = 8), or LPS (1 mg/kg) challenged mice that received recombinant Klotho (0.05 mg/kg) 30 min prior to LPS injection (rKL + LPS; n = 8). All mice were killed 4 hr after LPS or vehicle injection. Each dot represents an individual mouse. Bars represent the mean ± sd. *p < 0.05, **p < 0.005. GAPDH = glyceraldehyde 3-phosphate dehydrogenase, PBS = phosphate-buffered saline
Klotho Levels Are Reduced in the Brain of LPS-Challenged Mice, and rKL Protein Administration Diminishes LPS-Mediated Inflammatory Activation
Our initial experiments focused on the relationship between Klotho and sepsis-induced AKI. However, in sepsis, multiple organs are affected, including the brain. Similar to our findings in the kidney, we found a reduction in brain Klotho mRNA levels in LPS-challenged mice (Supplemental Fig. 1, A and B, Supplemental Digital Content 1, http://links.lww.com/CCM/E2). Based on these findings, we hypothesized that rKL intervention would attenuate brain inflammation and microvascular disturbances in endotoxemic mice. We indeed found a reduction in IL-6, IL-8, IL-1β, and tumor necrosis factor-α mRNA levels in brains of rKL pretreated, LPS-challenged mice compared with LPS-only mice (Fig. 5). Furthermore, E-selectin and ICAM-1 mRNA induction in the brain was also attenuated in rKL pretreated mice compared with LPS-only mice (Fig. 5). VCAM-1 gene expression levels did not differ between rKL + LPS and LPS-only mice, which is comparable to the results observed in the kidney. Our findings show that acute lowering of Klotho expression in response to LPS exposure is not limited to the kidney but also present in the brain. Additionally, we found that rKL administration protected against subsequent LPS-induced inflammation and endothelial adhesion molecule expression in the brain.
Figure 5.
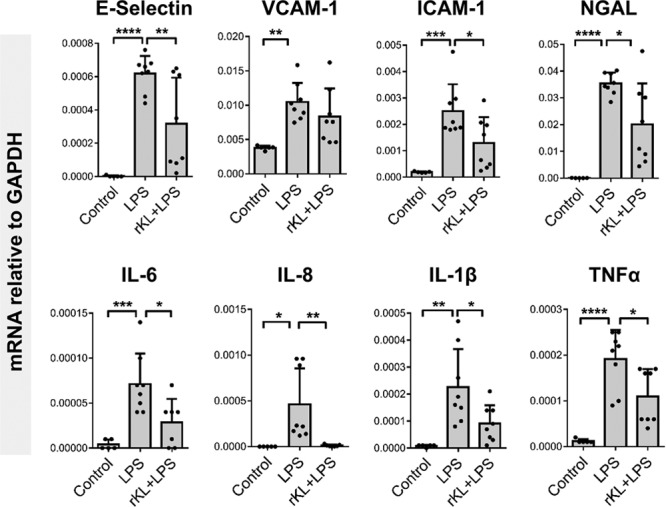
Klotho levels are reduced in the brain of endotoxemic mice and are rescued by recombinant Klotho administration prior to lipopolysaccharide (LPS) challenge. Brain messenger RNA (mRNA) expression levels of E-selectin, vascular cell adhesion protein (VCAM)-1, intercellular adhesion molecule (ICAM)-1, neutrophil gelatinase-associated lipocalin (NGAL), interleukin (IL)–6, IL-8, IL-1β, and tumor necrosis factor (TNF)–α in control vehicle-treated mice (n = 5) or LPS (1 mg/kg) challenged mice (LPS; n = 8), or LPS (1 mg/kg) challenged mice that received recombinant Klotho (0.05 mg/kg) 30 min prior to LPS injection (recombinant Klotho [rKL] + LPS; n = 8). All mice were killed 4 hr after LPS or vehicle treatment. All mRNA expression levels were determined by quantitative reverse transcription polymerase chain reaction using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. Each dot represents an individual mouse. Bars represent the mean ± sd. *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001.
DISCUSSION
The kidney is one of the most affected organs in critical illness with nearly half of critically ill patients suffering from AKI. Delirium is characterized by mental status impairments which, similar to AKI, can have lasting consequences in critically ill patients. Patients with sepsis often suffer from AKI and delirium, which was found to be an accelerator of existing cognitive decline (39–41). Hence, an intervention that can reduce acute kidney “and” brain injury as well as limit the development of chronic kidney injury and cognitive decline in sepsis survivors, would provide an important step forward for the clinical care for sepsis patients.
The exact pathophysiology of septic-AKI is still unknown, yet it is becoming increasingly clear that it is different from ischemic-AKI both in experimental models and in the clinical situation. In contrast to findings observed in ischemic-AKI, we as well as others have found little evidence of severe acute tubular necrosis in human septic-AKI postmortem studies (42–44) (Aslan et al, unpublished findings, 2018). These findings were recapitulated recently in an elegant experimental study of septic-AKI in sheep that reported that as severe septic-AKI developed, hardly any obvious renal pathologic disturbances were found. Renal blood flow and oxygen consumption were also unchanged (45). Hence, renal responses to sepsis are a decline in renal function almost certainly due to functional changes without major structural changes. Thus, the reduction of Klotho mRNA and protein levels which we found in septic-AKI patients, as well as in our LPS-challenged mice is not due to major ischemic and apoptotic damage.
We furthermore showed that the reduction in Klotho in mouse endotoxemia was not limited to the kidney but also occurred in the brain, most probably the choroid plexus, of LPS-treated mice. Lower expression levels of Klotho in LPS-treated mice remained for up to 24 hours despite clinical recovery of the mice. Recovery was defined by the absence of renal inflammation and examining the general well-being of the mice. It is as yet unclear how long it takes for renal Klotho levels to recover after LPS challenge. Using a low dose of LPS which we used in our in vivo studies, Klotho levels had recovered at 4 weeks after the initial LPS challenge (data not shown). The kinetics of Klotho down-regulation and recovery in the organs of septic patients are unknown. Interestingly, renal inflammation, thought to be a driver of Klotho down-regulation, is absent at the time of biopsy (data not shown) and therefore, the low levels of Klotho in the human sepsis-AKI biopsies were not due to persistent inflammation but rather likely due to impaired recovery after the severe inflammatory insult occurring at the initial phase of sepsis. Removal of circulating inflammatory mediators by RRT after sepsis had been initiated did not have any influence on renal Klotho levels in septic patients (data not shown).
The protective effect of rKL supports the hypothesis that Klotho is a mediator and not just a marker of renal damage. The molecular target of Klotho on cells, and a “Klotho receptor” remains to be elucidated. Klotho is however known to interact and protect the endothelium (46–49). Since microvascular integrity is disrupted, which can act as a driver of organ failure in sepsis, we hypothesized that rKL would attenuate endothelial activation in LPS-challenged mice. Diminished endothelial activation in both the kidney and brain of LPS-treated mice confirmed this hypothesis, which coincided with less renal neutrophil influx. We recently identified RIG-I as an intracellular regulator of LPS-mediated endothelial activation responses (38). Klotho was shown to inhibit senescence-associated endothelial activation and inflammation in aged cells and in mice via interaction with RIG-I (46). It is tempting to speculate that rKL attenuated endothelial activation and inflammation via RIG-I in our LPS-challenged mice.
We found peripheral injection of rKL to have protective effects on the brain but how this occurs is unknown. Previously, the uptake of labeled Klotho into different rat organs was quantified using autoradiographs. The kidney showed the highest uptake of Klotho, followed by the spleen, liver, and heart. Minimal uptake of Klotho was found in the brain (6), suggesting that rKL is unable to cross the blood brain barrier (BBB). However, the BBB is disrupted in LPS-challenged mice (50) and therefore systemic rKL may be able to cross the BBB enabling its protective effects. An alternative hypothesis is that the peripheral response of rKL mediates a protective effect on the Brain. Recently, mice injected with a fragment of Klotho, similar to its excreted form, that despite being unable to enter the brain, enhanced cognition and motor functions in mice (51). The ability of rKL to induce protective effects in multiple organs and especially the brain is of therapeutic value since this will bypass the need to deliver rKL directly into the brain. Further work is needed to elucidate exactly how peripheral rKL protects the brain in the setting of sepsis.
Recent studies in mice found that rKL administration in postischemic-AKI was found to prevent CKD progression (18, 20). Similarly, chronic administration of rKL was shown to slow down the progression of CKD and cardiac complications (20). Based on these and our own findings we speculate that rKL will also prevent the progression of acute-to-chronic organ dysfunction after sepsis recovery. However, this would have to be tested in additional sepsis mouse models and then subsequently in patients.
Sepsis survivors have a shorter life expectancy since they lose organ function, amongst others kidney and neurologic reserve, which renders them at increased risk of organ failure after recovery, and the development of chronic illnesses such as CKD, cardiovascular disease, and cognitive decline (52). These features resemble normal aging which suggest that critical illness can lead to gradual organ function decline and premature aging. Klotho is known to promote longevity, and conversely disturbances in Klotho levels or function were associated with senescent phenotypes (4, 53). Hence, Klotho depletion and impaired recovery may drive accelerated aging in sepsis survivors.
Our study does have important limitations to consider. Our in vivo LPS-challenged mouse model is limited to understanding the initial early inflammatory responses associated with sepsis. The mice recover quickly which does not leave a large window for intervention after LPS challenge. Hence, the applicability of rKL to prevent organ failure, death and chronic disease progression in sepsis would have to be tested in additional septic mouse models. Recently, while this article was under review, such experiments were performed by Chen et al (54). They found that renal klotho was reduced in cecal ligation puncture (CLP) mice, corroborating our own findings. Additionally, they found that administration of rKL 1 hour after CLP surgery reduced renal damage as determined by BUN and serum creatinine levels (54). The kinetics of acute Klotho down-regulation in septic patient organs is also unknown. To do this, patients entering the ICU with suspected infection would have to be biopsied as early as possible before organ failure occurs. However, critically ill patients are rarely biopsied because of the inherent high risk to the patient. As a result, obtaining biopsies from septic patients is not desirable with the only remaining option being to perform the biopsy post mortem in nonsurvivors. All of our patients suffered from septic shock, but an additional nephrotoxic mechanism in septic patients can never be excluded. However, all known nephrotoxic drugs were avoided or given in such a way that plasma levels could be checked. Sepsis-induced AKI was determined on clinical grounds as there is currently no diagnostic tool to define patients. Our current study focused on investigating Klotho levels septic-AKI patients. Whether this is also the case for nonseptic AKI critically ill patients is currently unknown but will be investigated in future studies. However, based on preclinical in vivo models, one might speculate that renal Klotho levels may also be reduced in other nonseptic critically ill patients. We found reduced renal Klotho levels in mice undergoing Haemorrhagic shock and resuscitation, a preclinical model for major bleeding and trauma (data not shown), but the reduction was not as severe as observed in endotoxemic or CLP mice. This suggests that the mechanism of Klotho depletion likely differs depending on whether infectious or sterile injury are the cause of AKI.
In summary, we conclude that Klotho expression in septic patients and in LPS-challenged mice is significantly decreased and negatively correlated with renal damage markers. rKL intervention diminished organ damage, inflammation, and endothelial activation in the kidney and brain of LPS-treated mice. Hence, Klotho replacement or treatments that prevent Klotho down-regulation might be a therapeutic strategy for septic patients to limit initial organ damage and the consequent progression of chronic organ dysfunction.
ACKNOWLEDGMENTS
We thank Henk Moorlag, Rianne M. Jongman, and Peter J. Zwiers for their excellent technical support.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the Department of Critical Care Research Foundation, University Medical Center Groningen.
Dr. van Meurs was supported by a Kolff grant of the Dutch Kidney Foundation (13OKJ35). The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369:840–851. [DOI] [PubMed] [Google Scholar]
- 3.Prescott HC, Osterholzer JJ, Langa KM, et al. Late mortality after sepsis: Propensity matched cohort study. BMJ 2016; 353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390:45–51. [DOI] [PubMed] [Google Scholar]
- 5.Olauson H, Mencke R, Hillebrands JL, et al. Tissue expression and source of circulating αKlotho. Bone 2017; 100:19–35. [DOI] [PubMed] [Google Scholar]
- 6.Hu MC, Shi M, Zhang J, et al. Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 2016; 27:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CD, Tung TY, Liang J, et al. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 2014; 53:5579–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloch L, Sineshchekova O, Reichenbach D, et al. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett 2009; 583:3221–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 2004; 565:143–147. [DOI] [PubMed] [Google Scholar]
- 10.Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 2007; 104:19796–19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Hwang KH, Park KS, et al. Biological role of anti-aging protein Klotho. J Lifestyle Med 2015; 5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu MC, Shi M, Zhang J, et al. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 2010; 24:3438–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bian A, Neyra JA, Zhan M, et al. Klotho, stem cells, and aging. Clin Interv Aging 2015; 10:1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuro-o M. Klotho and aging. Biochim Biophys Acta 2009; 1790:1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Shi M, Zhang J, et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 2010; 78:1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haruna Y, Kashihara N, Satoh M, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A 2007; 104:2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindberg K, Amin R, Moe OW, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 2014; 25:2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi M, Flores B, Gillings N, et al. αKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol 2016; 27:2331–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravikumar P, Li L, Ye J, et al. αKlotho deficiency in acute kidney injury contributes to lung damage. J Appl Physiol (1985) 2016; 120:723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu MC, Shi M, Gillings N, et al. Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 2017; 91:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue S, Suzuki-Utsunomiya K, Sato T, et al. Impaired innate and adaptive immunity of accelerated-aged Klotho mice in sepsis. Crit Care 2012; 16:1 [Google Scholar]
- 22.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011; 22:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu MC, Shi M, Cho HJ, et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 2015; 26:1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisani MA, Murphy TE, Van Ness PH, et al. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med 2007; 167:1629–1634. [DOI] [PubMed] [Google Scholar]
- 25.Siew ED, Fissell WH, Tripp CM, et al. Acute kidney injury as a risk factor for delirium and coma during critical illness. Am J Respir Crit Care Med 2017; 195:1597–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreuder L, Eggen BJ, Biber K, et al. Pathophysiological and behavioral effects of systemic inflammation in aged and diseased rodents with relevance to delirium: A systematic review. Brain Behav Immun 2017; 62:362–381. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham C. Systemic inflammation and delirium: Important co-factors in the progression of dementia. Biochem Soc Trans 2011; 39:945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlatou MG, Remaley AT, Gold PW. Klotho: A humeral mediator in CSF and plasma that influences longevity and susceptibility to multiple complex disorders, including depression. Transl Psychiatry 2016; 6:e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandenbroucke RE, Dejonckheere E, Van Lint P, et al. Matrix metalloprotease 8-dependent extracellular matrix cleavage at the blood-CSF barrier contributes to lethality during systemic inflammatory diseases. J Neurosci 2012; 32:9805–9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai T, Yamada K, Kim HC, et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: A role of oxidative stress. FASEB J 2003; 17:50–52. [DOI] [PubMed] [Google Scholar]
- 31.Dubal DB, Yokoyama JS, Zhu L, et al. Life extension factor klotho enhances cognition. Cell Rep 2014; 7:1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aslan A, Jongman RM, Moser J, et al. The renal angiopoietin/Tie2 system in lethal human sepsis. Crit Care 2014; 18:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aslan A, van Meurs M, Moser J, et al. Organ-specific differences in endothelial permeability-regulating molecular responses in mouse and human sepsis. Shock 2017; 48:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med 2003; 29:530–538. [DOI] [PubMed] [Google Scholar]
- 35.Bellomo R, Ronco C, Kellum V, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woudenberg-Vrenken TE, van der Eerden BC, van der Kemp AW, et al. Characterization of vitamin D-deficient klotho(-/-) mice: Do increased levels of serum 1,25(OH)2D3 cause disturbed calcium and phosphate homeostasis in klotho(-/-) mice? Nephrol Dial Transplant 2012; 27:4061–4068. [DOI] [PubMed] [Google Scholar]
- 37.Kurniati NF, Jongman RM, vom Hagen F, et al. The flow dependency of Tie2 expression in endotoxemia. Intensive Care Med 2013; 39:1262–1271. [DOI] [PubMed] [Google Scholar]
- 38.Moser J, Heeringa P, Jongman RM, et al. Intracellular RIG-I signaling regulates TLR4-independent endothelial inflammatory responses to endotoxin. J Immunol 2016; 196:4681–4691. [DOI] [PubMed] [Google Scholar]
- 39.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009; 72:1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: A population-based cohort study. Brain 2012; 135:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med 2014; 370:185–186. [DOI] [PubMed] [Google Scholar]
- 42.Lerolle N, Nochy D, Guérot E, et al. Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med 2010; 36:471–478. [DOI] [PubMed] [Google Scholar]
- 43.Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 2013; 187:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014; 41:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maiden MJ, Otto S, Brealey JK, et al. Structure and function of the kidney in septic shock. A prospective controlled experimental study. Am J Respir Crit Care Med 2016; 194:692–700. [DOI] [PubMed] [Google Scholar]
- 46.Liu F, Wu S, Ren H, et al. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 2011; 13:254–262. [DOI] [PubMed] [Google Scholar]
- 47.Nagai R, Saito Y, Ohyama Y, et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci 2000; 57:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun 1998; 248:324–329. [DOI] [PubMed] [Google Scholar]
- 49.Kusaba T, Okigaki M, Matui A, et al. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci U S A 2010; 107:19308–19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raj DD, Moser J, van der Pol SM, et al. Enhanced microglial pro-inflammatory response to lipopolysaccharide correlates with brain infiltration and blood-brain barrier dysregulation in a mouse model of telomere shortening. Aging Cell 2015; 14:1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leon J, Moreno AJ, Garay BI, et al. Peripheral elevation of a Klotho fragment enhances brain function and resilience in young, aging, and α-synuclein transgenic mice. Cell Rep 2017; 20:1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bucaloiu ID, Kirchner HL, Norfolk ER, et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012; 81:477–485. [DOI] [PubMed] [Google Scholar]
- 53.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science 2005; 309:1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Tong H, Chen Y, et al. Klotho ameliorates sepsis-induced acute kidney injury but is irrelevant to autophagy. Onco Targets Ther 2018; 11:867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]


