Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, cholecalciferol, esophagectomy, vitamin D
Abstract
Objectives:
Observational studies suggest an association between vitamin D deficiency and adverse outcomes of critical illness and identify it as a potential risk factor for the development of lung injury. To determine whether preoperative administration of oral high-dose cholecalciferol ameliorates early acute lung injury postoperatively in adults undergoing elective esophagectomy.
Design:
A double-blind, randomized, placebo-controlled trial.
Setting:
Three large U.K. university hospitals.
Patients:
Seventy-nine adult patients undergoing elective esophagectomy were randomized.
Interventions:
A single oral preoperative (3–14 d) dose of 7.5 mg (300,000 IU; 15 mL) cholecalciferol or matched placebo.
Measurements and Main Results:
Primary outcome was change in extravascular lung water index at the end of esophagectomy. Secondary outcomes included Pao2:Fio2 ratio, development of lung injury, ventilator and organ-failure free days, 28 and 90 day survival, safety of cholecalciferol supplementation, plasma vitamin D status (25(OH)D, 1,25(OH)2D, and vitamin D-binding protein), pulmonary vascular permeability index, and extravascular lung water index day 1 postoperatively. An exploratory study measured biomarkers of alveolar-capillary inflammation and injury. Forty patients were randomized to cholecalciferol and 39 to placebo. There was no significant change in extravascular lung water index at the end of the operation between treatment groups (placebo median 1.0 [interquartile range, 0.4–1.8] vs cholecalciferol median 0.4 mL/kg [interquartile range, 0.4–1.2 mL/kg]; p = 0.059). Median pulmonary vascular permeability index values were significantly lower in the cholecalciferol treatment group (placebo 0.4 [interquartile range, 0–0.7] vs cholecalciferol 0.1 [interquartile range, –0.15 to –0.35]; p = 0.027). Cholecalciferol treatment effectively increased 25(OH)D concentrations, but surgery resulted in a decrease in 25(OH)D concentrations at day 3 in both arms. There was no difference in clinical outcomes.
Conclusions:
High-dose preoperative treatment with oral cholecalciferol was effective at increasing 25(OH)D concentrations and reduced changes in postoperative pulmonary vascular permeability index, but not extravascular lung water index.
Prevention of the acute respiratory distress syndrome (ARDS) has become a focus of research in recent years, with the development of prediction scores (1), prevention trials (2, 3) and a growing evidence of other potential biological therapies to prevent ARDS (4).
ARDS is common after esophagectomy with studies reporting a frequency between 13% and 30% (2, 5–7). During open surgery, access to the esophagus is attained by deflation of one lung and maintenance of one-lung ventilation (OLV) exposing the ventilated lung to volutrauma, hyperoxia, and barotrauma (8, 9). Concurrently the deflated lung is exposed to ischemia-reperfusion injury (10). The pathophysiologic changes of lung injury post esophagectomy are similar but less exaggerated than in ARDS patients (11–13). This high-risk population has been validated for assessing potential therapies to assess biological efficacy and prevent ARDS (2).
Vitamin D (VD) deficiency is common (14). VD has important functions besides bone, and calcium homeostasis and its immunomodulatory actions may play a role in the pathogenesis of ARDS (15). Studies report a high prevalence of VD deficiency (VDD) in the critically ill (16) with an association with increased rates of infection (17, 18) acute respiratory failure (19), acute kidney failure (20), sepsis (18, 21), and mortality in some (22), but not all studies (23, 24).
We have previously reported severe VDD in a cohort of ARDS patients (25). VDD (plasma 25(OH)D < 50 nmol/L) was ubiquitous in ARDS patients and present in the vast majority of patients at risk of developing ARDS following esophagectomy. Experimental studies suggest a protective effect of VD in the lung. In a murine model of intratracheal lipopolysaccharide challenge, dietary-induced VDD resulted in exaggerated alveolar inflammation, epithelial damage, and hypoxia which were abrogated by cholecalciferol treatment (21). In vitro, 25(OH)D has trophic effects on primary human alveolar type II cells affecting over 600 genes (25). We conducted a phase 2 randomized, placebo-controlled trial to test our hypothesis that high-dose cholecalciferol treatment preoperatively reduces markers of alveolar epithelial lung injury seen post esophagectomy, a high-risk population of developing ARDS. Results of these studies have been previously reported in the form of an abstract (26).
MATERIALS AND METHODS
Study Design
A randomized, double-blind placebo-controlled trial, recruited patients from October 3, 2012, to January 26, 2015, at three hospitals in the United Kingdom. Ethics approval was obtained from South Birmingham Research Ethics Committee (REC 12/WM/0092). Trial registration identification ISRCTN27673620 and EudraCT 2012-000332-25. The study protocol has been previously published (27). Detailed methods can be found in the online supplemental material (Supplemental Digital Content 1, http://links.lww.com/CCM/D967).
Eligibility Criteria
Patients undergoing a planned thoracic esophagectomy and 18 years old or older if male, aged 55 or more than 2 years since menopause if female and were able to give written informed consent. Exclusion criteria detailed in the online supplemental material (Supplemental Digital Content 1, http://links.lww.com/CCM/D967).
Drug Randomization and Masking
The trial drug manufacturer, Novalabs (Leicester, United Kingdom), produced computer-generated randomization sequence using a block size of 10 with equal allocation between active and placebo groups. Pharmacy, research staff, clinical teams, and patients were masked to randomization and treatment allocation.
Drug Administration
Participants received either a single dose of drug (oral cholecalciferol oily solution Vigantol (Novalabs), 300,000 IU [7.5 mg; 15 mL]) or matched placebo (Miglyol 812 oil, the vehicle for cholecalciferol in Vigantol) 3–14 days prior to planned esophagectomy.
Primary Outcome
Primary outcome was change in extravascular lung water index (EVLWI) measured by Pulse Contour Continuous Cardiac Output (PiCCO2) thermodilution catheter (Pulsion Medical Systems, Feldkirchen, Germany) at the end of the esophagectomy (measured within 1 hr postoperatively). PiCCO2 EVLWI has been shown to be a marker for developing lung injury (28) and mortality in ARDS (29, 30) and has been used as the primary outcome in clinical trials in ARDS (31, 32) as well as post thoracotomy (33).
Secondary Outcomes
Prespecified trial secondary outcomes were Pao2:Fio2 ratio, development of lung injury in the first 28 days as defined by the Berlin Criteria, ventilator and organ failure free days, 28 and 90 day survival and safety (e.g., hypercalcemia), and tolerability of cholecalciferol supplementation. Plasma VD status (25(OH)D, 1,25(OH)2D, and vitamin D binding protein DBP) predrug dose, preoperatively, postoperatively, and day 3 as well as EVLWI day 1 postoperatively (measured at 9 am on day 1). PiCCO2 derived pulmonary vascular permeability index (PVPI) was added as an outcome measure prior to the conduct of the study as recent data suggest it was a better measure of alveolar-capillary permeability to differentiate nonhydrostatic edema than EVLWI (34).
Exploratory Outcomes
Details are available in the online supplemental material (Supplemental Digital Content 1, http://links.lww.com/CCM/D967).
Lung Water Measurements
EVLWI and PVPI were measured by thermodilution (PiCCO2) as previously described (35, 36). Specified time points for measurement were immediately preoperatively, within 1 hour postoperatively and day 1 postoperatively (8–9 am). Detailed methods are provided in the online supplemental material (Supplemental Digital Content 1, http://links.lww.com/CCM/D967) and published trial protocol (27).
Perioperative Care
Patients underwent a two-stage transthoracic esophagectomy which included a laparoscopic abdominal stage followed by an open thoracotomy or minimally invasive technique with thoracoscopy. All approaches required OLV. Anesthesia was performed dependent on the anesthetists preferred practice. Teams were briefed to adopt a lower tidal volume and fluid-conservative hemodynamic management approach.
Sample Size
The trial was powered to detect a change of 20% in EVLWI with a power of 80% requiring 34 patients (68 in total) in each arm to reach the primary endpoint (two-tailed p = 0.05). An additional six patients were added to allow for dropouts, such as open/close cases, unexpected deaths, and other difficulties with data collection. Detailed information about the sample size estimate is provided in the trial protocol (27).
Statistical Analysis
Data were analyzed using Graphpad PRISM 6 software package (Graphpad, San Diego, CA). All analysis was based on Intention to treat. Continuous data were assessed for normality using the Shapiro-Wilks test and the appropriate parametric or nonparametric test applied. Statistical significance was predefined as two-tailed p value of less than 0.05. To assess the significance of differences between two sets of continuous data the unpaired t test was used for parametric data, whereas the Mann-Whitney U test was used for nonparametric data, except paired data which were assessed using paired t test (parametric) or Wilcoxon signed rank test (nonparametric). Categorical data were assessed using Fisher exact test and chi-square test for larger samples.
RESULTS
Enrollment and Patient Characteristics
A total of 79 patients were enrolled in the study (Consolidated Standard of Reporting Trials) (Fig. 1). Eleven patients (14%) did not receive OLV, or it was not possible to measure the primary endpoint (EVLWI at the end of surgery). Thirty-five patients in the placebo arm and 33 in the cholecalciferol arm (n = 68; 86%) went on to complete esophagectomy and primary outcome measurement. All patients (n = 76; 96%) that proceeded to surgery were included in the analysis of efficacy and safety of cholecalciferol supplementation.
Figure 1.
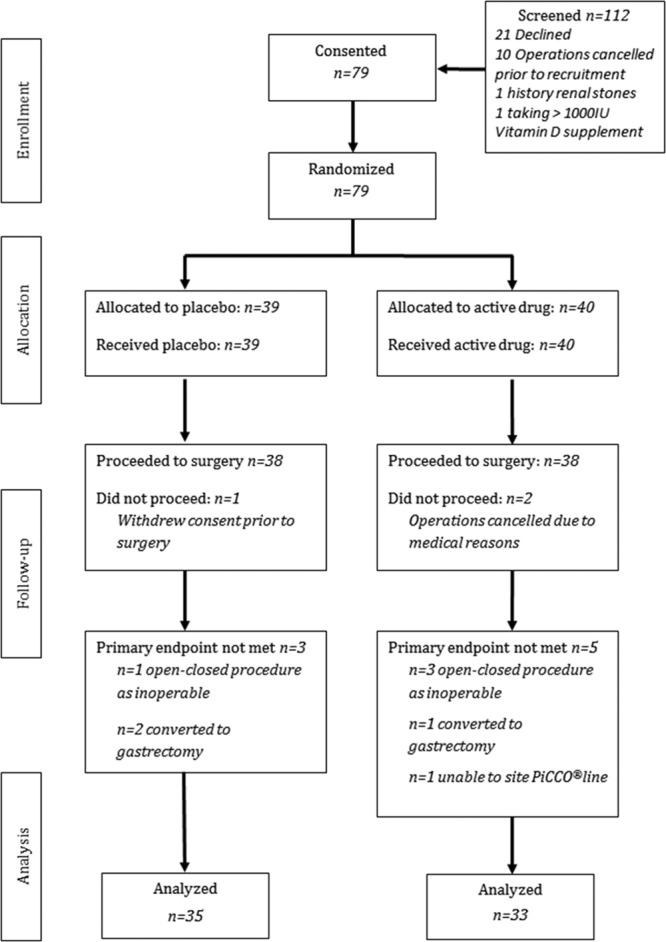
Patient Consolidated Standard of Reporting Trials flow diagram. IU = international unit, PiCCO = Pulse Contour Continuous Cardiac Output.
Patient baseline characteristics were well matched (Table 1). All procedures were performed for malignancy, and the predominant cell type was adenocarcinoma. Groups were well matched with respect to operative and anesthetic interventions (Table 2).
TABLE 1.
Patient Baseline Characteristics

TABLE 2.
Anesthetic and Operative Characteristics
Primary Outcome
There was no difference in the a priori primary outcome of change in EVLWI measured within 1 hour postoperatively placebo median +1.0 (interquartile range [IQR], 0.4–1.8) versus cholecalciferol median +0.4 mL/kg (IQR, –0.4 to –1.2 mL/kg); p value equals to 0.059 (Fig. 2A). There was no difference in absolute EVLWI between placebo and cholecalciferol arms postoperatively or postoperative day 1 time points (Table E1, Supplemental Digital Content 1, http://links.lww.com/CCM/D967). In a within-group analysis, EVLWI rose significantly preoperatively to postoperatively only in the placebo group (Fig. E1, Supplemental Digital Content 1, http://links.lww.com/CCM/D967).
Figure 2.

A, Scatter plot of fold change in extravascular lung water index (EVLWI). B, Scatter plot of fold change in pulmonary vascular permeability index (PVPI). Fold changes shown from pre- to postoperative and preoperative to day 1; black lines and squares dots placebo (n = 35); gray lines and triangle dots cholecalciferol treatment (n = 33); and data presented as medians and interquartile ranges. p values represent Mann-Whitney U test.
Secondary Outcomes
Effects on Perioperative Changes in PVPI. The change in PVPI in placebo patients was median 0.4 (IQR, 0–0.7) versus cholecalciferol median 0.1 (IQR, –0.15 to –0.35); p value equals to 0.027 (Fig. 2B). There was no difference seen in absolute values of PVPI preoperatively, postoperatively, and at day 1 between the groups (Table E2, Supplemental Digital Content 1, http://links.lww.com/CCM/D967). In a within-group analysis PVPI significantly increased pre- to postoperatively in patients who received placebo, but not VD (Fig. E2, Supplemental Digital Content 1, http://links.lww.com/CCM/D967).
Safety of Cholecalciferol Supplementation. Trial medication was well tolerated with no episodes of hypercalcemia and no reported serious adverse events related to the trial medication (detailed in the online supplemental material and Table E3, Supplemental Digital Content 1, http://links.lww.com/CCM/D967).
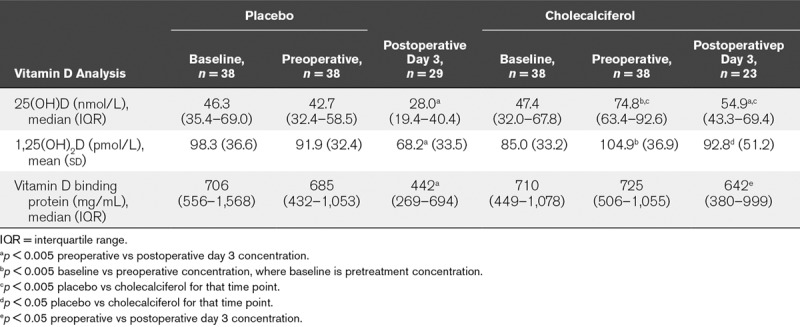
Efficacy of Cholecalciferol Supplementation Upon VD Status Perioperatively. There was no difference between groups in baseline 25(OH)D, 1,25(OH)2D, or DBP levels (Table 1). A single bolus dose of 300,000 IU of cholecalciferol resulted in significant increases in plasma 25(OH)D concentration (Table 3).
TABLE 3.
Perioperative Change in Vitamin D Status
On the day of operation 58% of patients who received placebo were deficient (25(OH)D concentrations < 50 nmol/L) compared with 5% of patients who had received the cholecalciferol single bolus dose (χ2 [1, n = 76] =22.36; p < 0.0001).
1,25(OH)2D concentrations increased significantly in patients randomized to receive cholecalciferol (Table 3) although this did not result in significantly different 1,25(OH)2D levels between groups. There was no significant change in plasma DBP concentrations.
Following the surgical insult concentrations of 25(OH)D and DBP decreased in both groups, but there were persistently higher 25(OH)D concentrations in the cholecalciferol treated arm day 3 postoperatively (Table 3). This meant that in the placebo group VDD was present in 58% on the day of operation and 90% by day 3. For the cholecalciferol supplemented patients, 5% were deficient at the time of operation increasing to 49% by day 3 postoperatively, demonstrating that critical illness induces VDD in this patient group for the first time.
Clinical Outcomes. There was no significant difference seen in Pao2:Fio2 ratio between the groups. There was no difference in ARDS rates between placebo and cholecalciferol treatment arms (placebo 4 [11%] of 35 compared with cholecalciferol 4 [12%] of 33; odds ratio, 0.94; 95% CI, 0.21–4.09). There was no difference seen in ventilator-free and organ failure free days or survival (28 or 90 d) (detailed in the online supplemental material, Supplemental Digital Content 1, http://links.lww.com/CCM/D967; and Table E4, Supplemental Digital Content 1, http://links.lww.com/CCM/D967). No patients developed renal or cardiac failure in the first 7 days.
Exploratory Outcomes. Details can be viewed in Table E5 (Supplemental Digital Content 1, http://links.lww.com/CCM/D967).
Exploratory Post Hoc Analysis of EVLWI and PVPI Measurements. Only 58 % of placebo-treated and 5% of cholecalciferol treated patients were VDD on the day of the operation. Therefore a post hoc exploratory-pooled analysis of all patients irrespective of treatment arm was performed to determine if there is a threshold effect above which the beneficial effects of 25(OH)D on EVLWI and PVPI are seen. VD sufficient patients (> 50 nmol/L) on the day of operation had significantly lower increases in EVLWI and PVPI compared with those who were deficient irrespective of treatment allocation (Fig. E3, Supplemental Digital Content 1, http://links.lww.com/CCM/D967).
DISCUSSION
In this phase 2 biological efficacy study, we sought to determine if high-dose cholecalciferol replacement attenuates the increase in alveolar-capillary permeability and development of pulmonary edema after esophagectomy. The main finding was that 300,000 IU of oral cholecalciferol treatment pre-esophagectomy did not reduce the change in EVLWI postoperatively but did reduce the change in PVPI.
Vitamin D3, or cholecalciferol, is mainly formed in the skin after exposure to sunlight (ultraviolet B). Synthesized or dietary VD is hydroxylated in the liver by CYP27A1, CYP2R1, and CYP3A4 to 25-hydroxyvitamin D3 [25(OH)D] the major circulating form of VD which is widely accepted as the key measure of VD status (37). In humans, 25(OH)D is bound to its binding protein (DBP) and requires hydroxylation to the active 1,25-dihydroxyvitamin D3 [1,25(OH)2D] by the mitochondrial enzyme CYP27B1 (37) in order to activate the VD receptor (VDR) which mediates the biological actions of 1,25(OH)2D. Both CYP27B1 and VDR are expressed by cells of the innate and adaptive immune system as well as alveolar epithelial cells (38, 39).
Our hypothesis was based upon experimental data: alveolar epithelial cells possess the ability to convert circulating 25(OH)D to active 1,25(OH)2D and activate VDR responsive genes (39), suggesting organ-specific effects. Physiologically relevant doses of 25(OH)D stimulate wound repair, cellular proliferation, and reduce soluble Fas ligand induced cell death of human type 2 alveolar epithelial cells in vitro (25), suggesting that cholecalciferol has a direct protective role on the alveolar epithelium. There may also be a protective mechanism on the pulmonary endothelium as 1,25(OH)2D has been shown to decrease expression of intercellular adhesion molecule-1 and prevent neutrophil adhesion, migration and therefore potentially the initiation of lung injury (40). Furthermore, observational studies have found that VDD is common in patients with sepsis and ARDS and an increased risk of developing ARDS in patients undergoing esophagectomy (21, 25).
The changes in PVPI and EVLWI, we observed in this study were smaller than the observational studies led us to expect. This may reflect a change in baseline VD status in the population studied, which were not as deficient as the patient cohort that this study was powered on—median 25(OH)D 25.5 nmol/L (25) compared with 43.2 nmol/L in the current study, suggesting that the beneficial effects of replacement may be greater in those with more severe VDD. Additionally, improved surgical technique and anesthetic management may also explain the smaller postoperative increases in EVLWI and PVPI between the two studies that were 5 years apart.
High-dose cholecalciferol successfully increased 25(OH)D concentrations above sufficiency (50 nmol/L) in 94.7% of cases leading to a corresponding and sustained rise in 1,25(OH)2D level perioperatively. However, following surgical trauma induced by esophagectomy, both arms had significant falls in 25(OH)D day 3 postoperatively demonstrating that critical illness can rapidly induce a state of 25(OH)D deficiency. The placebo group also saw similar falls in circulating 1,25(OH)2D perioperatively but not in the patients who received cholecalciferol, indicating that patients in the placebo group have insufficient circulating 25(OH)D to maintain plasma concentrations of 1,25(OH)2D in the perioperative period.
It is unclear how critical illness promotes this rapid fall, but our data suggest that perioperative falls in circulating DBP, the major carrier protein produced by the liver may be important. Although this could be accounted for by hemodilution (41), the cause of loss of binding proteins in acute inflammation is still unknown and could also be related to interstitial extravasation from increased vascular permeability following inflammatory responses and decreased hepatic synthesis (42). In addition, enhanced induction of activation to 1,25(OH)2D or catabolic pathways such as the formation of 24-hydroxylated or three epimer forms of VD that have recently been identified may also play a role (43).
There is also evidence that VD may be a negative acute-phase reactant postelective knee arthroscopy (44) and in acute pancreatitis in which it recovers without supplementation (45). We believe this is an important area for future research as it is critical to inform dosing regimens for further studies into rapid VD replacement in critically ill patients. Patients may require repeat dosing rather than the large single doses so far investigated and serum 25(OH)D may be an unreliable marker of VD status after an acute inflammatory insult (46).
Importantly high-dose cholecalciferol therapy was well tolerated with no frequency of hypercalcemia. The frequency of ARDS in this cohort was 11.7%. Cholecalciferol treatment did not prevent the development of ARDS, and there was no difference in other clinical outcomes, although our study was not powered to detect differences in clinical outcomes.
This study has limitations. The changes observed in PVPI and EVLWI were much lower than previously observed possibly due to the current cohort being very much less VD deficient than previously with significant numbers of patients in the control arm not being VD deficient. The lower than expected PiCCO changes and low frequency of ARDS may have led to a type II error with no difference seen in the primary outcome. The timing of the dose was not uniform, but this did not impact the level of sufficiency in the VD group on the day of operation. Our primary outcome was a measure of potential in vivo efficacy of VD on the lung barrier, but measurements of PiCCO EVLWI have limitations of under- and over-estimation due to positive end-expiratory pressure, changes in pulmonary vascular occlusion, heterogeneous lung injury, and operator-dependency (30). Finally, recent studies suggest that higher doses than we used and alternative routes of administration may be more efficacious in critical illness (47) and sepsis (48), but none have investigated effects on lung injury. Our results also support the hypothesis of benefit in the more deficient cohort and the use of a follow-up maintenance dose which we did not use. Clearly, the optimum dose of VD, best route of administration, complex metabolic pathways, and multiple isoforms that influence its bioactivity need to be established in sepsis, ARDS, and critical illness before large-scale trials are undertaken.
CONCLUSIONS
A single preoperative dose of cholecalciferol did not reduce EVLWI in the human esophagectomy model of lung injury. There was some effect on PVPI. Surgical insult results in a precipitous decrease in VD concentrations, which is an important consideration for future trial design to address clinical efficacy of VD therapy in ARDS and critical illness. It is clear that the ability to rapidly identify critically ill patients who are deficient in VD is a necessary precursor to trial enrollment of VD replacement in critically ill patients.
ACKNOWLEDGMENTS
We thank all the research, preoperative nursing, anesthetic, and surgical teams at University Hospitals Birmingham NHS Foundation Trust, Heart of England NHS Foundation Trust, and University Hospital Coventry and Warwickshire NHS Trust. In particular, Dr. Simon Smart, Dr. Jeremy Marwick, Mr. John Whiting, Mr. Ewen Griffiths, and Professor Derek Alderson from the University Hospitals Birmingham NHS Foundation Trust; Mr. Raj Nijjar, Mr. Martin Richardson; and Dr. Ruth McKenzie from the Heart of England NHS Foundation Trust. We are extremely grateful to Dr. Keith Couper and Teresa Melody from the Academic Department of Anesthesia, Critical Care, Resuscitation and Pain Department, Heart of England NHS Foundation Trust for helping to facilitate patient recruitment and trial delivery, and Dr. Anita Pye for providing overall trial support and coordination. We are grateful to Mr. Rajnikant Mehta for his independent senior statistical review of the data analysis and interpretation.
Supplementary Material
Footnotes
*See also p. 2064.
Drs. Parekh and Dancer are joint first authors.
Clinical trial registered with the International Standard Randomized Controlled Trial Registry (ISRCTN27673620) and European Union database of Randomized Controlled Trials (EudraCT 2012-000332-25).
Drs. Parekh, Fraser, Gao, Martineau, Perkins, and Thickett designed the study. Drs. Cooper, Tan, and Tucker provided expert advice and aided recruitment. Drs. Parekh, Dancer, Howells, and Mahida recruited patients. Drs. Parekh, Dancer, Scott, D’Souza, and Tang undertook biomarker assays and analyzed the data. Drs. Parekh, Dancer, and Thickett wrote the first draft of the article. All authors contributed to revising the draft critically for important intellectual content and have agreed to the final submitted version.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Parekh was funded by the Medical Research Council (MRC) (MR/J011266/1); Drs. Dancer and Thickett by the MRC (G1100196/1) trust; and Dr. Scott by the MRC (MR/L002736/1). Drs. Gao and Perkins are National Institute for Health Research Senior Investigators. Drs. Parekh, Dancer, Mahida, Tang, and Thickett received support for article research from Research Councils UK. Drs. Dancer, Tang, and Thickett’s institutions received funding from the MRC. Dr. Howells institution received funding from GlaxoSmithKline. Dr. Perkins disclosed off-label product use of vitamin D. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Gajic O, Dabbagh O, Park PK, et al. ; U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS): Early identification of patients at risk of acute lung injury: Evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 2011; 183:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins GD, Gates S, Park D, et al. ; BALTI-Prevention Collaborators: The beta agonist lung injury trial prevention. A randomized controlled trial. Am J Respir Crit Care Med 2014; 189:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kor DJ, Carter RE, Park PK, et al. ; US Critical Illness and Injury Trials Group: Lung Injury Prevention with Aspirin Study Group (USCIITG: LIPS-A): Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: The LIPS-a randomized clinical trial. JAMA 2016; 315:2406–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beitler JR, Schoenfeld DA, Thompson BT. Preventing ARDS: Progress, promise, and pitfalls. Chest 2014; 146:1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul DJ, Jamieson GG, Watson DI, et al. Perioperative risk analysis for acute respiratory distress syndrome after elective oesophagectomy. ANZ J Surg 2011; 81:700–706. [DOI] [PubMed] [Google Scholar]
- 6.Tandon S, Batchelor A, Bullock R, et al. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth 2001; 86:633–638. [DOI] [PubMed] [Google Scholar]
- 7.Park DP, Welch CA, Harrison DA, et al. Outcomes following oesophagectomy in patients with oesophageal cancer: A secondary analysis of the ICNARC Case Mix Programme Database. Crit Care 2009; 13(Suppl 2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagano A, Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci 2003; 1010:405–416. [DOI] [PubMed] [Google Scholar]
- 9.Carnesecchi S, Deffert C, Pagano A, et al. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med 2009; 180:972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari RS, Andrade CF. Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev 2015; 2015:590987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baudouin SV. Lung injury after thoracotomy. Br J Anaesth 2003; 91:132–142. [DOI] [PubMed] [Google Scholar]
- 12.Rocker GM, Wiseman MS, Pearson D, et al. Neutrophil degranulation and increased pulmonary capillary permeability following oesophagectomy: A model of early lung injury in man. Br J Surg 1988; 75:883–886. [DOI] [PubMed] [Google Scholar]
- 13.Sato N, Koeda K, Kimura Y, et al. Cytokine profile of serum and bronchoalveolar lavage fluids following thoracic esophageal cancer surgery. Eur Surg Res 2001; 33:279–284. [DOI] [PubMed] [Google Scholar]
- 14.van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab 2011; 25:671–680. [DOI] [PubMed] [Google Scholar]
- 15.Parekh D, Thickett DR, Turner AM. Vitamin D deficiency and acute lung injury. Inflamm Allergy Drug Targets 2013; 12:253–261. [DOI] [PubMed] [Google Scholar]
- 16.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med 2009; 360:1912–1914. [DOI] [PubMed] [Google Scholar]
- 17.Quraishi SA, Litonjua AA, Moromizato T, et al. Association between prehospital vitamin D status and hospital-acquired bloodstream infections. Am J Clin Nutr 2013; 98:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moromizato T, Litonjua AA, Braun AB, et al. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med 2014; 42:97–107. [DOI] [PubMed] [Google Scholar]
- 19.Thickett DR, Moromizato T, Litonjua AA, et al. Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: A retrospective cohort study. BMJ Open Respir Res 2015; 2:e000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun AB, Litonjua AA, Moromizato T, et al. Association of low serum 25-hydroxyvitamin D levels and acute kidney injury in the critically ill. Crit Care Med 2012; 40:3170–3179. [DOI] [PubMed] [Google Scholar]
- 21.Parekh D, Patel JM, Scott A, et al. Vitamin D deficiency in human and murine sepsis. Crit Care Med 2017; 45:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun AB, Gibbons FK, Litonjua AA, et al. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality*. Crit Care Med 2012; 40:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cecchi A, Bonizzoli M, Douar S, et al. Vitamin D deficiency in septic patients at ICU admission is not a mortality predictor. Minerva Anestesiol 2011; 77:1184–1189. [PubMed] [Google Scholar]
- 24.Lucidarme O, Messai E, Mazzoni T, et al. Incidence and risk factors of vitamin D deficiency in critically ill patients: Results from a prospective observational study. Intensive Care Med 2010; 36:1609–1611. [DOI] [PubMed] [Google Scholar]
- 25.Dancer RC, Parekh D, Lax S, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015; 70:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dancer R, Parekh D, Scott A, et al. T2 Vitamin D supplementation reduces perioperative systemic and alveolar inflammation in patients undergoing oesophagectomy: Results of the Vindaloo Trial. Thorax 2015; 70(Suppl 3):A1 [Google Scholar]
- 27.Parekh D, Dancer RC, Lax S, et al. Vitamin D to prevent acute lung injury following oesophagectomy (VINDALOO): Study protocol for a randomised placebo controlled trial. Trials 2013; 14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kor DJ, Warner DO, Carter RE, et al. Extravascular lung water and pulmonary vascular permeability index as markers predictive of postoperative acute respiratory distress syndrome: A prospective cohort investigation. Crit Care Med 2015; 43:665–673. [DOI] [PubMed] [Google Scholar]
- 29.Jozwiak M, Silva S, Persichini R, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome*. Crit Care Med 2013; 41:472–480. [DOI] [PubMed] [Google Scholar]
- 30.Jozwiak M, Teboul JL, Monnet X. Extravascular lung water in critical care: Recent advances and clinical applications. Ann Intensive Care 2015; 5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins GD, McAuley DF, Thickett DR, et al. The beta-agonist lung injury trial (BALTI): A randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 2006; 173:281–287. [DOI] [PubMed] [Google Scholar]
- 32.Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011; 183:620–626. [DOI] [PubMed] [Google Scholar]
- 33.Licker M, Tschopp JM, Robert J, et al. Aerosolized salbutamol accelerates the resolution of pulmonary edema after lung resection. Chest 2008; 133:845–852. [DOI] [PubMed] [Google Scholar]
- 34.Kushimoto S, Taira Y, Kitazawa Y, et al. ; PiCCO Pulmonary Edema Study Group: The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: A prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit Care 2012; 16:R232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkins GD, Gao F, Thickett DR. In vivo and in vitro effects of salbutamol on alveolar epithelial repair in acute lung injury. Thorax 2008; 63:215–220. [DOI] [PubMed] [Google Scholar]
- 36.Craig TR, Duffy MJ, Shyamsundar M, et al. Extravascular lung water indexed to predicted body weight is a novel predictor of intensive care unit mortality in patients with acute lung injury. Crit Care Med 2010; 38:114–120. [DOI] [PubMed] [Google Scholar]
- 37.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281. [DOI] [PubMed] [Google Scholar]
- 38.Adams JS, Hewison M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 2008; 4:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J Immunol 2008; 181:7090–7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen SF. 1 alpha, 25-dihydroxyvitamin D3 decreased ICAM-1 and ELAM-1 expressions on pulmonary microvascular endothelial cells and neutrophil motivation. J Steroid Biochem Mol Biol 1995; 52:67–70. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan A, Ochola J, Mundy J, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care 2010; 14:R216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quraishi SA, Camargo CA., Jr Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care 2012; 15:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkinson C, Taylor AE, Hassan-Smith ZK, et al. High throughput LC-MS/MS method for the simultaneous analysis of multiple vitamin D analytes in serum. J Chromatogr B Analyt Technol Biomed Life Sci 2016; 1014:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr 2011; 93:1006–1011. [DOI] [PubMed] [Google Scholar]
- 45.Bang UC, Novovic S, Andersen AM, et al. Variations in serum 25-hydroxyvitamin D during acute pancreatitis: An exploratory longitudinal study. Endocr Res 2011; 36:135–141. [DOI] [PubMed] [Google Scholar]
- 46.Yousefzadeh P, Shapses SA, Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. Int J Endocrinol 2014; 2014:981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amrein K, Schnedl C, Holl A, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA 2014; 312:1520–1530. [DOI] [PubMed] [Google Scholar]
- 48.Quraishi SA, De Pascale G, Needleman JS, et al. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit Care Med 2015; 43:1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]


