Supplemental Digital Content is available in the text.
Keywords: biomarkers, emergency medicine, mortality, risk management, vital signs
Abstract
Objectives:
Soluble urokinase plasminogen activator receptor is a prognostic biomarker associated with critical illness, disease progression, and risk of mortality. We aimed to evaluate whether soluble urokinase plasminogen activator receptor adds prognostic value to a vital sign-based score for clinical monitoring of patient risk (National Early Warning Score) in acute medical patients.
Design:
Registry-based observational cohort study of consecutively admitted acute medical patients.
Setting:
The Acute Medical Unit, Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark.
Patients:
Acute medical patients admitted between November 18, 2013, and September 30, 2015.
Interventions:
None.
Measurements and Main Results:
Of 17,312 included patients, admission National Early Warning Score was available for 16,244 (93.8%). During follow-up, 587 patients (3.4%) died in-hospital, 859 (5.0%) within 30 days, and 1,367 (7.9%) within 90 days. High soluble urokinase plasminogen activator receptor was significantly associated with in-hospital-, 30-day-, and 90-day mortality within all National Early Warning Score groups, in particular in patients with a low National Early Warning Score; for 30-day mortality, mortality rate ratios ranged from 3.45 (95% CI, 2.91–4.10) for patients with National Early Warning Score 0–1, to 1.86 (95% CI, 1.47–2.34) for patients with National Early Warning Score greater than or equal to 9 for every doubling in soluble urokinase plasminogen activator receptor (log2-transformed). Combining National Early Warning Score, age, and sex with soluble urokinase plasminogen activator receptor improved prediction of in-hospital-, 30-day-, and 90-day mortality, increasing the area under the curve (95% CI) for 30-day mortality from 0.86 (0.85–0.87) to 0.90 (0.89–0.91), p value of less than 0.0001, with a negative predictive value of 99.0%.
Conclusions:
The addition of soluble urokinase plasminogen activator receptor to National Early Warning Score significantly improved risk prediction of both low- and high-risk acute medical patients. Patients with low National Early Warning Score but elevated soluble urokinase plasminogen activator receptor had mortality risks comparable to that of patients with higher National Early Warning Score.
Risk evaluation in hospital wards and emergency departments (EDs) is critical in order to detect early signs of clinical deterioration and enable a timely and appropriate clinical intervention. The National Early Warning Score (NEWS) and similar vital sign-based track-and-trigger systems are commonly used to monitor patients’ clinical progress and perform in-hospital risk assessment of acutely and critically ill patients (1, 2). Use of track-and-trigger systems has been found to reduce unexpected in-hospital mortality (3).
The NEWS was primarily developed and evaluated for the prediction of acute mortality, that is, 24 hours in-hospital mortality (1). However, patient risk assessment should ideally extend further. In Denmark, the admission length of stay (LOS) has shortened from 4.2 to 3.0 days during the past decade (4), and 54% of older medical patients are discharged within 24 hours directly from the EDs (5). A limitation of the currently used vital sign-based models, such as NEWS, is the failure to identify the patients with largely unaffected vital signs who still have an increased risk of deterioration in the near future. Thus, improved risk assessment is of interest in order to avoid premature discharge of high-risk patients.
Recent studies have investigated the possibility of strengthening the NEWS with various biomarkers, such as d-dimer or lactate, and this approach might improve the risk prediction (6, 7). Both d-dimer and lactate have some degree of specificity (cardiovascular disease and sepsis, respectively) and thus limited value in unspecified acute medical patients. Soluble urokinase plasminogen activator receptor (suPAR) is an inflammatory biomarker, reflecting presence, progression, and severity of disease, organ damage, and risk of mortality, in the general population and patient populations (reviewed by Desmedt et al [8–11]). suPAR is the soluble form of the membrane-bound receptor urokinase plasminogen activator receptor (uPAR) which is a central mediator of plasminogen activation and cellular processes, such as migration, adhesion, proliferation, and survival. suPAR is generated when uPAR is cleaved from the membrane of immune cells and endothelial cells during immune activation and proinflammatory conditions (12, 13). In acute medical patients, elevated suPAR is associated with chronic comorbidities, ICU admission as well as 30- and 90-day risk of readmission and mortality (10, 14).
Here, we aimed to evaluate whether suPAR can add prognostic value to NEWS and extend its prognostic value beyond 24 hours for prediction of in-hospital-, 30-day-, and 90-day mortality in a large population of acutely admitted medical patients.
MATERIALS AND METHODS
Design and Setting
This is a registry-based cohort study of consecutively admitted acute medical patients admitted to the Acute Medical Unit (AMU), Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark, between November 18, 2013, and September 30, 2015. suPAR data on a subgroup of this cohort has previously been published (10).
At admission to the AMU, each patient routinely has a standard panel of blood tests analyzed, including electrolytes, blood counts, liver function, kidney function, and markers of infection and inflammation. suPAR was added to these standard blood tests on November 18, 2013. Patients who had suPAR analyzed as part of their admission blood samples were included in this study and followed in national registries for 90 days. The outcomes were in-hospital-, 30-day-, and 90-day mortality.
Ethics Approval
All processing of personal data followed national guidelines, and the study was approved by the Danish Health and Medicines Authority (reference 3-3013-1061/2) and the Danish Data Protection Agency (reference HVH-2014–018, 02767).
Data
The index admission was identified for each patient as the first admission where suPAR was measured (10). Data on suPAR levels were extracted from the clinical laboratory information system LABKA.
Each patient’s unique civil registration (central person registry [CPR]) number was used to extract data on vital signs and NEWS from the nursing documentation system KISO (“clinical data entry schedules and overview”); diagnoses and dates of hospital admission and discharge from the National Patient Registry (NPR); and information on sex, date of birth, and vital status at the end of follow-up from the Civil Registration System.
All contacts with the secondary healthcare system are registered in the NPR. Contacts for hospital admissions less than 5 hours apart were considered coherent and coded as the same admission. If more than 5 hours had passed between two contacts, the new contact was considered a readmission.
NEWS
A track-and-trigger system based on the NEWS is routinely used upon admission to the AMU. The first NEWS registered during the index admission was used for the analyses.
The NEWS is an early warning score (EWS) based on the following vital signs and use of supplemental oxygen (1), which were recorded in KISO at admission by nurses in the AMU: respiratory rate (breaths/min) measured by manual counting of breaths, saturation (%) measured noninvasively with pulse oximetry, oxygen supplement delivered by mask or nasal cannula (L/min), heart rate (beats/min), systolic blood pressure (mm Hg), and temperature (°C) measured on the forehead. Furthermore, the level of consciousness was assessed with the AVPU score and categorized as normal (A: alert or normal sleep) or decreased consciousness (V: reacting to voice; P: reacting to pain; U: unresponsive).
Each variable is assigned a weighted score (between 0 and 3 points) depending on how much the measured value deviates from the normal level. The individual scores are summed to an aggregated NEWS.
Measured vital signs and their corresponding NEWS points were compared and checked for obvious errors, and, in case of errors, NEWS was recalculated with the corrected NEWS points to secure the accuracy of the score. If registrations of some vital signs were missing, NEWS was calculated based on the available measurements, and the missing vital signs were assigned 0 points.
Measurements
Plasma suPAR levels were analyzed as part of the standard admission blood samples at the Department of Clinical Biochemistry with the suPARnostic AUTO Flex ELISA (ViroGates A/S, Birkerød, Denmark) using an automated Siemens BEP2000 analyzer according to the manufacturer’s instructions. suPAR was measured once or bid during weekdays. Thus, the suPAR results were most often not reported in due time for clinical decisions during the acute admission.
Statistical Analysis
Continuous variables are described by median and interquartile range (IQR) and categorical variables by number (n) and percentages (%).
Six NEWS groups were created based on the recommended track-and-trigger groups used in the Capital Region of Denmark: NEWS 0–1, 2, 3–5, 6, 7–8, and greater than or equal to 9. chi-square test was used to test frequency of deaths between NEWS groups.
We performed Poisson regression analysis since it reports the results on a natural scale and is directly comparable with public available mortality data from national registries. Mortality rate ratios (MRRs) with 95% CIs were estimated by age- and sex-adjusted Poisson regression analysis for the six categorical NEWS groups, as the test for departure from trend for continuous NEWS values was statistically significant (p < 0.0001). Log-transformed length of in-hospital stay or time to death was used as offsets when calculating MRRs for in-hospital mortality or 30- and 90-day mortality, respectively. For the Poisson regression analyses, suPAR was log2-transformed. A maximum likelihood ratio test with five degrees of freedom was performed to test for an interaction between suPAR and NEWS.
The discriminative ability of NEWS and suPAR to predict outcome was analyzed with receiver operating characteristic (ROC) curve analyses. The analyses for in-hospital mortality were adjusted for LOS, and adjustments were made for age and sex for all three endpoints. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using the Youden’s index as cut-off (15).
Statistical analyses were performed with SAS Enterprise Guide 7.11 (SAS Institute, Cary, NC) and R 3.1.1 (The R Foundation for Statistical Computing, Vienna, Austria), and the plots were created with GraphPad Prism 7.02 (GraphPad Software, La Jolla, CA) and R 3.1.1.
RESULTS
During the study period, a total of 20,193 patients had routine blood tests analyzed in the AMU. Patients were excluded if suPAR was not ordered (n = 1,415), if the suPAR result was missing (n = 769), and for various other reasons (n = 697) (Fig. S1, Supplemental Digital Content 1, http://links.lww.com/CCM/E13; legend, Supplemental Digital Content 6, http://links.lww.com/CCM/E18). The final study population comprised 17,312 acute medical patients. Baseline characteristics are shown in Table 1.
TABLE 1.
Baseline Characteristics of Patients Admitted to the Acute Medical Unit
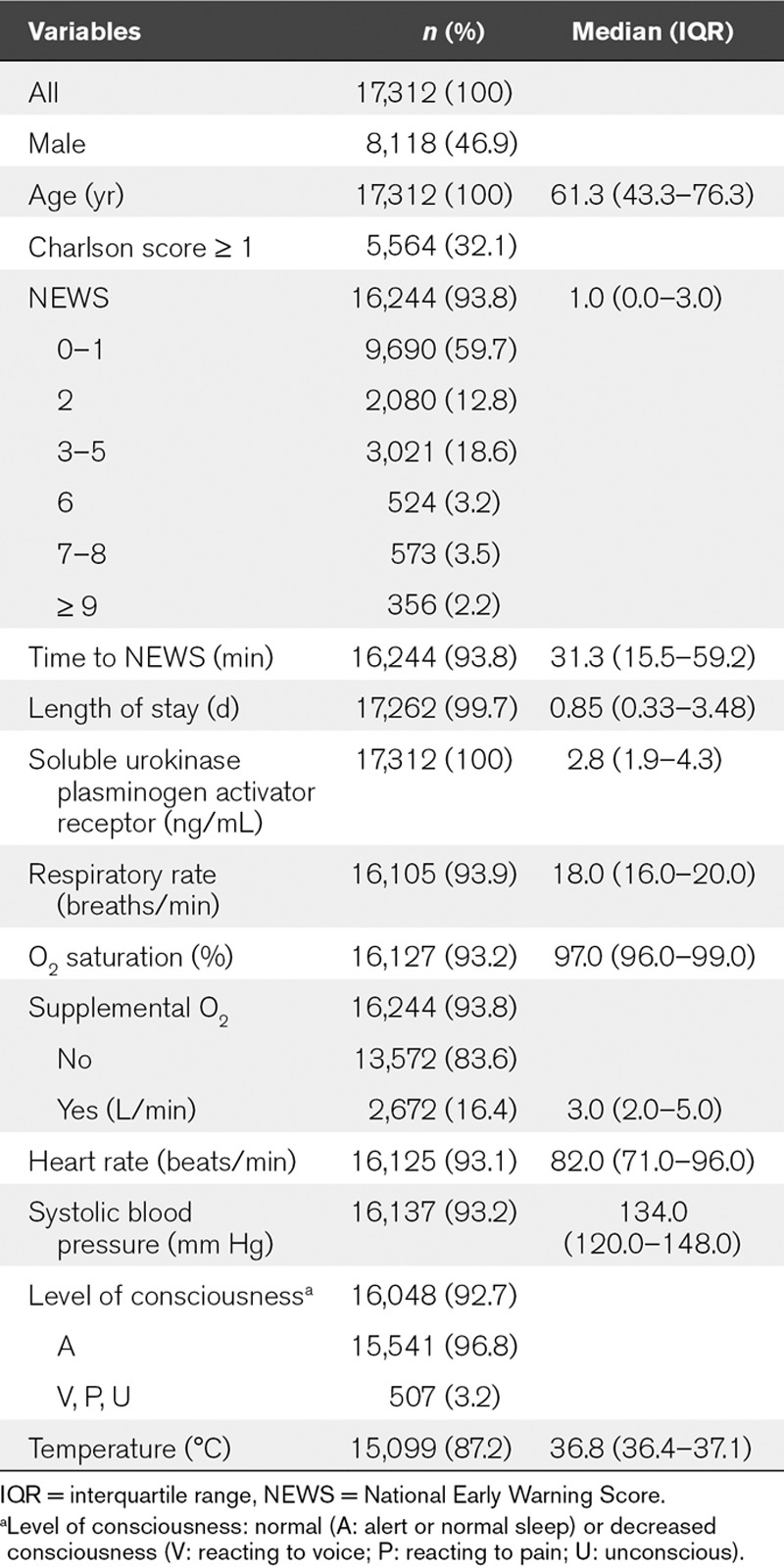
Of the final study population, 93.8% had an available NEWS (Table 1). Admission NEWS ranged from 0 to 16, and the majority of patients had a NEWS of 0–1 (Table 1).
suPAR and NEWS were significantly positively correlated (Kendall’s tau-b, 0.23; p < 0.0001) and median suPAR increased with NEWS (p < 0.0001) (Fig. S2, Supplemental Digital Content 2, http://links.lww.com/CCM/E14; legend, Supplemental Digital Content 6, http://links.lww.com/CCM/E18).
All individual NEWS variables, except for body temperature, were significantly associated with all endpoints (Table S1, Supplemental Digital Content 3, http://links.lww.com/CCM/E15). The median suPAR level at the index admission was significantly higher for patients who died compared with patients who survived (Table S1, Supplemental Digital Content 3, http://links.lww.com/CCM/E15).
The frequency of patients who died increased with NEWS for in-hospital-, 30-day-, and 90-day mortality (p < 0.0001) (Table 2). Furthermore, in Poisson regression adjusted for age and sex, MRRs for all endpoints increased with increasing NEWS group compared with patients with NEWS 0–1 (Table 2).
TABLE 2.
Mortality Rate Ratios With National Early Warning Score 0–1 as Reference or for Log2-Transformed Soluble Urokinase Plasminogen Activator Receptor Within National Early Warning Score Groups
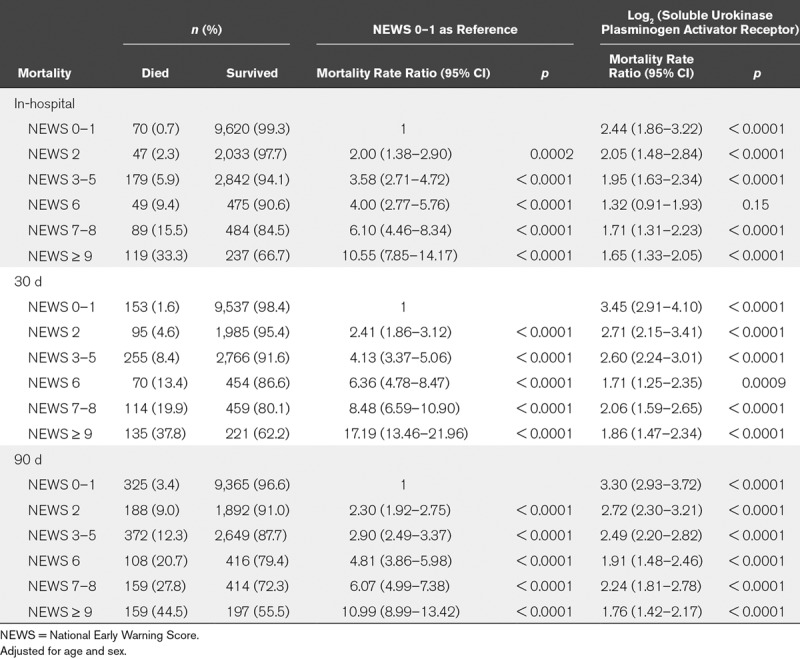
We tested the effect of suPAR on mortality within the six NEWS groups (Table 2) and tested for an interaction between NEWS and suPAR. Significant interactions were observed for 30-day mortality (χ2, 28.12; p < 0.0001) and 90-day mortality (χ2, 36.43; p < 0.0001), but not for in-hospital mortality (χ2, 8.98; p = 0.11).
suPAR was significantly associated with increased mortality rates in all NEWS groups and for all endpoints, except for in-hospital mortality for patients with a NEWS of 6 (Table 2). For each endpoint, the MRR for a doubling in suPAR was highest for patients with NEWS 0–1 (Table 2).
Compared with the rest of the population, patients with low NEWS (0–1) had the same sex distribution (p = 0.79), were younger (57.2 yr; IQR, 40.5–73.2; p < 0.0001), had lower Charlson score (0; IQR, 0–0; p < 0.0001), shorter admissions (0.64 d; IQR, 0.28–1.71; p < 0.0001), and lower mortality rates (p < 0.0001) (Table 2). But among patients with low NEWS (0–1), those with high suPAR (> 4.5 ng/mL, n = 1,513) were significantly older (75.9 yr, IQR 63.1–85.5 vs 53.5 yr, IQR 37.9–69.5), had higher Charlson score (1, IQR 0–2 vs 0, IQR 0–0), longer admissions (1.86 d, IQR 0.74–6.56 vs 0.53, IQR 0.26–1.15), and markedly higher mortality rates (in-hospital 3.30% vs 0.24%, 30-day 6.94% vs 0.59%, 90-day 14.14% vs 1.36%) compared with patients with low suPAR (≤ 4.5 ng/mL), all p value of less than 0.0001.
An increase in suPAR within one NEWS group corresponds to an increase in the mortality rate to the level of that of higher NEWS groups (Fig. 1), for example, a patient with NEWS 0–1 and a suPAR level of 4.3 ng/mL has the same risk of in-hospital mortality as a patient with NEWS 2 and a suPAR level of 2.5 ng/mL (Fig. 1A).
Figure 1.
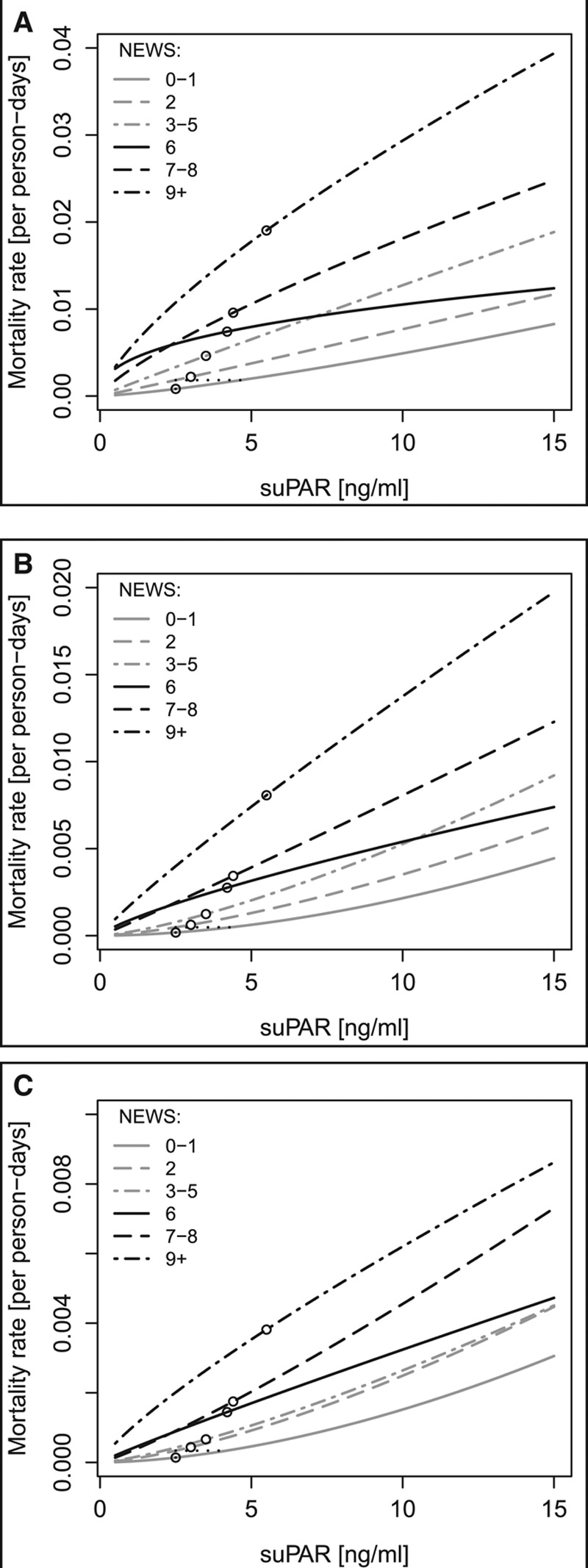
Mortality rates (per person-days) plotted against soluble urokinase plasminogen activator receptor (suPAR) level for each National Early Warning Score (NEWS) group. A, In-hospital mortality, B, 30-day mortality, and C, 90-day mortality. Circles indicate median suPAR level for the particular NEWS group. As an example, the vertical dotted lines illustrate the increased mortality rate for patients with NEWS 2 compared with NEWS 0–1 at the same suPAR level, and the horizontal dotted lines illustrate how a patient with NEWS 0–1 but higher suPAR have the same mortality rate as a patient with NEWS 2 and lower suPAR.
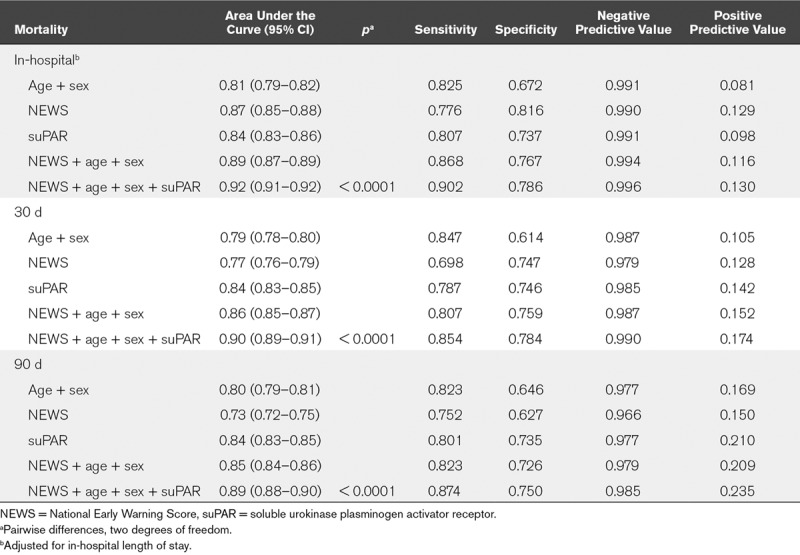
ROC curve analyses were carried out for predicting in-hospital-, 30-day-, and 90-day mortality. For all endpoints, the addition of suPAR to NEWS, sex, and age significantly improved the prediction (p < 0.0001). In particular, the PPV increased when combining suPAR, NEWS, sex, and age (Table 3).
TABLE 3.
Logistic Regression and Receiver Operating Characteristic Curve Analysis for In-Hospital-, 30-Day-, and 90-Day Mortality
Subgroup Analyses
In patients with cardiovascular disease or cancer, suPAR was similarly associated with mortality across NEWS groups, and the same tendency was found in patients with renal disease (Table S2, Supplemental Digital Content 4, http://links.lww.com/CCM/E16).
To address the predictive value of suPAR in early discharge, a subgroup analysis was carried out on patients with LOS shorter than 24 hours (n = 9,517), excluding patients with missing LOS (n = 50) and patients who died within 24 hours (n = 56). Patients discharged within 24 hours had significantly lower suPAR levels (2.3 ng/mL; IQR, 1.7–3.2) and NEWS (0; IQR, 0–2), compared with patients admitted for more than 24 hours (p < 0.0001). Among early discharged patients, patients who died had higher NEWS and suPAR compared with patients who survived (Table S3, Supplemental Digital Content 5, http://links.lww.com/CCM/E17). In Poisson regression analyses adjusted for age, sex, and NEWS, a doubling in suPAR was associated with a MRR of 3.17 (95% CI, 2.38–4.23; p < 0.0001) for 30-day mortality and 2.92 (95% CI, 2.42–3.52; p < 0.0001) for 90-day mortality.
DISCUSSION
In this study of 17,312 acutely admitted medical patients, NEWS and suPAR were both independently associated with risk of in-hospital-, 30-day-, and 90-day mortality. Combining NEWS with suPAR (and age and sex) provided a strong predictive algorithm, for in-hospital mortality as well as 30- and 90-day risk. Furthermore, the suPAR level within each of the NEWS groups provides a potent tool for reclassification of risk. Finally, we found that suPAR was predictive of outcome in patients who were discharged from the AMU after controlling for NEWS, suggesting that suPAR may aid in discharge decisions by adding information on patient risk to the NEWS.
Interestingly, the MRR for suPAR was inversely correlated to NEWS, for example, in patients with low NEWS, high suPAR carried the highest risk of mortality, even for in-hospital mortality. This finding is of major clinical relevance as suPAR improves the current identification of high-risk patients among those with unaffected vital signs.
Most likely, a high NEWS triggers an urgent and focused clinical response to stabilize vital signs and reduce patient risk. Reacting on a high NEWS often means indirectly acting on high suPAR—as suPAR and NEWS are correlated. In contrast, in patients with normal vital signs and high suPAR, risk may not be clinically recognized and fewer actions taken. Using suPAR in combination with NEWS could potentially lead to clinical interventions among patients otherwise not considered urgent or at risk, thereby ultimately saving lives. Finally, the high NPV found in this study may aid in hospital discharge decisions, and as an unspecific biomarker, the NPV may be of most clinical relevance in clinical decision-making. Among patients with LOS shorter than 24 hours, suPAR levels were generally low. However, some patients that were early discharged died within 30 days, and these patients did have high suPAR levels; it can be speculated that knowledge of suPAR could improve discharge decisions, but since we did not have information on cause of death, we were unable to investigate whether these patients actually died of the same pathology as they presented for their initial visit, or if they may have had some unrecognized, underlying disease that could maybe have been reflected in their suPAR level.
The Discriminative Ability of NEWS
In general medical emergency patients, NEWS has been found to have the best discriminative ability for death within 24 hours (area under the curve [AUC], 0.89; 95% CI, 0.89–0.90), or a combined endpoint of cardiac arrest, unanticipated ICU admission, and 24 hours mortality (AUC, 0.87; 95% CI, 0.87–0.88) compared with 33 other EWS systems (2). In our study, the AUC for NEWS for in-hospital mortality was 0.87 (95% CI, 0.85–0.88) and this was significantly improved by adding age, sex, and suPAR (0.92; 95% CI, 0.91–0.92).
NEWS was developed for the prediction of acute mortality, and we found it to be a strong marker of in-hospital mortality but less predictive for 30-day- and 90-day mortality. However, combining NEWS with a long-term prognostic biomarker such as suPAR, which has a high 30-day- and 90-day predictive value, improved prediction of mortality up to 3 months after admission, thus enhancing the usefulness of NEWS.
A number of studies have taken a similar approach and combined NEWS with biomarkers, such as lactate, d-dimer, or routine blood tests, that is, albumin, sodium, WBC count, urea, creatinine, hemoglobin, and potassium (6, 7, 16–18), but with lower discriminative ability for in-hospital mortality (16–18) compared with our study. One study of 4,624 ED patients combining NEWS and lactate levels found an AUC for 2-day mortality of 0.96 (0.94–0.98) and 0.87 (0.85–0.90) for in-hospital mortality, which was better than for NEWS alone. However, no data were reported beyond 7 days (6).
Added Prognostic Value of suPAR to NEWS
suPAR has previously been found to be associated with 30-day- and 90-day mortality after adjustment for vital signs EWS (19), but the present study is, to the best of our knowledge, the first to investigate the effect of suPAR within different NEWS groups. Combining age and sex, NEWS, and suPAR gave very high NPVs and thus a good ability to correctly identify patients with low risk, which may aid in decisions of discharge and lower the number of unnecessary admissions. The PPVs were low but significantly improved by the addition of suPAR. Additional blood biomarkers may improve the PPV further.
suPAR reflects the level of chronic inflammation in patients as well as healthy persons and is a broadly applicable marker providing information on development, presence, and severity of disease (8, 10). Similarly, suPAR has been shown to be a prognostic marker associated with disease severity, mortality, and other adverse outcomes in hospitalized patients, for example, ED patients with suspected infection (20), patients with ST-elevation myocardial infarction undergoing primary percutaneous intervention (21), bacteraemia (22), acute pancreatitis (23), acute chest pain (24), or systemic inflammatory response syndrome (25). The advantage of routinely measured biochemical tests—like suPAR at our hospital—is that these tests comprise objective measures that can be added to the similarly objective NEWS. In biological terms, suPAR is a very stable marker with respect to pre- and postsampling procedures (fasting conditions, exercise, time of day, sample handling, etc) which adds to the clinical utility (26). suPAR is therefore a broad unspecific and stable marker of patient risk that could biochemically strengthen the NEWS to improve risk assessment of patients. Automating NEWS and suPAR results must occur in order to implement a timely track-and-trigger system.
Unanswered Questions and Future Research
Further studies are needed to determine whether daily measuring of suPAR would refine the prognostic capabilities of NEWS.
Furthermore, the high NPV should be tested in a prospective interventional study, to evaluate whether patient discharge based on low NEWS and low suPAR is safe and does not increase readmission and mortality rates.
Limitations
The study has limitations. This was a single-center study including acute medical patients admitted through the AMU, and not including patients primarily admitted for surgical interventions or acute pediatric-, obstetric-, and gastroenterological patients who are admitted through specialized departments. Replications are needed in other patient populations. We excluded patients with missing suPAR results; a considerable proportion of these are probably patients with very short in-hospital stays and lack of routine blood tests. Thus, our data are limited to patients who have admission blood samples analyzed in the AMU. Measuring and registration of the NEWS variables were associated with some uncertainty; for example, measuring body temperature on the forehead (27) and respiratory rate by manual counting of breaths, in addition to manual data entry with a risk of typing errors. Another limitation concerns missing registrations of vital signs, which were assigned 0 points in the dataset, based on the rationale that a clinical response would not have been triggered. Finally, we lack information on smoking, which is a major confounder of suPAR in the general population (28), and on mobility status which has been suggested to add significantly to the NEWS (29).
In addition to study limitations, a major limitation for the clinical implementation of suPAR has been the long analysis time. Faster determination of suPAR, for example, using turbidimetric methods rather than ELISA, is needed to increase the clinical value of suPAR in acute care and to implement a timely track-and-trigger system combining information on NEWS and suPAR.
CONCLUSIONS
In this study, NEWS and suPAR were both strongly associated with risk of in-hospital-, 30-day-, and 90-day mortality in acute medical patients. The suPAR level increased with increasing NEWS, and high suPAR independently predicted risk of mortality across NEWS scores thereby adding prognostic value to the NEWS. In particular, suPAR was a stronger predictor of mortality in patients without clinical signs of risk of deterioration; patients with low NEWS but high suPAR levels had mortality risks comparable to that of patients with higher NEWS scores.
Supplementary Material
Footnotes
*See also p. 2050.
The study was performed at the Copenhagen University Hospital Amager and Hvidovre, Hvidovre, Denmark.
The funder had no role in the design of the study and collection, analysis, and interpretation of data and in writing the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Rasmussen and Haupt have received funding for travel from ViroGates A/S, Denmark, the company that produces the suPARnostic assays; she is supported by a grant from the Lundbeck Foundation (grant number R180-2014–3360). Mr. Ladelund received funding from Novo Nordisk. Dr. Eugen-Olsen is a co-founder, shareholder, and Chief Scientific Officer (currently) of ViroGates A/S. Drs. Eugen-Olsen and Andersen are named inventors on patents on soluble urokinase plasminogen activator receptor as a prognostic biomarker; the patents are owned by Copenhagen University Hospital Amager and Hvidovre, Denmark, and licensed to ViroGates A/S. Dr. Ellekilde disclosed that she does not have any potential conflicts of interest.
REFERENCES
- 1.Royal College of Physicians: National Early Warning Score (NEWS) - Standardising the assessment of acute-illness severity in the NHS. Report of a working party. 2012London, RCP. [Google Scholar]
- 2.Smith GB, Prytherch DR, Meredith P, et al. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation 2013; 84:465–470. [DOI] [PubMed] [Google Scholar]
- 3.Bunkenborg G, Samuelson K, Poulsen I, et al. Lower incidence of unexpected in-hospital death after interprofessional implementation of a bedside track-and-trigger system. Resuscitation 2014; 85:424–430. [DOI] [PubMed] [Google Scholar]
- 4.Statistics Denmark: Available at: http://www.statistikbanken.dk/10651. Accessed September 14, 2018
- 5.Juul-Larsen HG, Petersen J, Sivertsen DM, et al. Prevalence and overlap of Disease Management Program diseases in older hospitalized patients. Eur J Ageing 2017; 14:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo S, Yoon J, Lee JB, et al. Predictive value of the National Early Warning Score-lactate for mortality and the need for critical care among general emergency department patients. J Crit Care 2016; 36:60–68. [DOI] [PubMed] [Google Scholar]
- 7.Nickel CH, Kellett J, Cooksley T, et al. Combined use of the National Early Warning Score and D-dimer levels to predict 30-day and 365-day mortality in medical patients. Resuscitation 2016; 106:49–52. [DOI] [PubMed] [Google Scholar]
- 8.Desmedt S, Desmedt V, Delanghe JR, et al. The intriguing role of soluble urokinase receptor in inflammatory diseases. Crit Rev Clin Lab Sci 2017; 54:117–133. [DOI] [PubMed] [Google Scholar]
- 9.Eugen-Olsen J, Andersen O, Linneberg A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med 2010; 268:296–308. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen LJ, Ladelund S, Haupt TH, et al. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: A strong marker of disease presence and severity, readmission and mortality. A retrospective cohort study. Emerg Med J 2016; 33:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayek SS, Sever S, Ko YA, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med 2015; 373:1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blasi F, Carmeliet P. uPAR: A versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002; 3:932–943. [DOI] [PubMed] [Google Scholar]
- 13.Dekkers PE, ten Hove T, te Velde AA, et al. Upregulation of monocyte urokinase plasminogen activator receptor during human endotoxemia. Infect Immun 2000; 68:2156–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haupt TH, Petersen J, Ellekilde G, et al. Plasma suPAR levels are associated with mortality, admission time, and Charlson Comorbidity Index in the acutely admitted medical patient: A prospective observational study. Crit Care 2012; 16:R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–35. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed MA, Rudge G, Watson D, et al. Index blood tests and national early warning scores within 24 hours of emergency admission can predict the risk of in-hospital mortality: A model development and validation study. PLoS One 2013; 8:e64340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis SW, Kovacs C, Badriyah T, et al. Development and validation of a decision tree early warning score based on routine laboratory test results for the discrimination of hospital mortality in emergency medical admissions. Resuscitation 2013; 84:1494–1499. [DOI] [PubMed] [Google Scholar]
- 18.Abbott TEF, Torrance HDT, Cron N, et al. A single-centre cohort study of National Early Warning Score (NEWS) and near patient testing in acute medical admissions. Eur J Intern Med 2016; 35:78–82. [DOI] [PubMed] [Google Scholar]
- 19.Nayak RK, Allingstrup M, Phanareth K, et al. suPAR as a biomarker for risk of readmission and mortality in the acute medical setting. 2015; 62:A5146. [PubMed] [Google Scholar]
- 20.Uusitalo-Seppälä R, Huttunen R, Tarkka M, et al. Soluble urokinase-type plasminogen activator receptor in patients with suspected infection in the emergency room: A prospective cohort study. J Intern Med 2012; 272:247–256. [DOI] [PubMed] [Google Scholar]
- 21.Lyngbæk S, Marott JL, Møller DV, et al. Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Am J Cardiol 2012; 110:1756–1763. [DOI] [PubMed] [Google Scholar]
- 22.Huttunen R, Syrjänen J, Vuento R, et al. Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: A prospective cohort study. J Intern Med 2011; 270:32–40. [DOI] [PubMed] [Google Scholar]
- 23.Lipinski M, Rydzewska-Rosolowska A, Rydzewski A, et al. Soluble urokinase-type plasminogen activator receptor (suPAR) in patients with acute pancreatitis (AP) - Progress in prediction of AP severity. Pancreatology 2017; 17:24–29. [DOI] [PubMed] [Google Scholar]
- 24.Lyngbæk S, Andersson C, Marott JL, et al. Soluble urokinase plasminogen activator receptor for risk prediction in patients admitted with acute chest pain. Clin Chem 2013; 59:1621–1629. [DOI] [PubMed] [Google Scholar]
- 25.Raggam RB, Wagner J, Prüller F, et al. Soluble urokinase plasminogen activator receptor predicts mortality in patients with systemic inflammatory response syndrome. J Intern Med 2014; 276:651–658. [DOI] [PubMed] [Google Scholar]
- 26.Andersen O, Eugen-Olsen J, Kofoed K, et al. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol 2008; 80:209–216. [DOI] [PubMed] [Google Scholar]
- 27.Geijer H, Udumyan R, Lohse G, et al. Temperature measurements with a temporal scanner: Systematic review and meta-analysis. BMJ Open 2016; 6:e009509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haupt TH, Kallemose T, Ladelund S, et al. Risk factors associated with serum levels of the inflammatory biomarker soluble urokinase plasminogen activator receptor in a general population. Biomark Insights 2014; 9:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brabrand M, Kellett J. Mobility measures should be added to the National Early Warning Score (NEWS). Resuscitation 2014; 85:e151. [DOI] [PubMed] [Google Scholar]


