Supplemental Digital Content is Available in the Text.
Key Words: HIV, oral HIV self-testing, male involvement, partner testing, antenatal care
Abstract
Background:
In Kenya, HIV testing during first antenatal care (ANC) visit is a standard practice for pregnant women. Despite a policy promoting male partner testing in ANC, few male partners accompany their partners for HIV testing. We evaluated the impact of using oral HIV self-testing on HIV couples testing among ANC clients in Kenya and their male partners.
Methods:
In a 3-arm randomized control study in eastern and central Kenya, consenting women attending the first ANC visit were randomized to receive: (1) standard-of-care and a standard information card; (2) an improved card stating the importance of male HIV testing; and (3) 2 oral HIV self-test kits and HIV testing information. Women completed a baseline and endline questionnaire, and consenting male partners were surveyed 3 months after enrolling female ANC clients. The primary outcome was HIV couples testing as reported by the female partners.
Results:
We randomized 1410 women at their first ANC visit of which 1215 were successfully followed up. One thousand one hundred thirty-three male partners consented to the survey. In the self-testing study arm 3, 79.1% (334/422) of the women reported that their partner tested for HIV as part of a couple, compared with 27% (110/406) and 35.1% (136/387) in study arm 1 and study arm 2, respectively. More than 90% of male partners who used the oral HIV self-test kits reported that it was easy to take sample and read the test results.
Conclusions:
The study demonstrates that the ANC platform offers a unique opportunity to increase HIV couples testing among men using self-testing through distribution by their female partners.
INTRODUCTION
Kenya has an estimated HIV prevalence of 5.4% among adults aged 15–49 years, although the prevalence varies by geographic region.1 It is estimated that there are about 1.5 million adults living with HIV in Kenya, with more than 60,000 new HIV infections occurring annually.1 The HIV testing program in Kenya has evolved over time, and many different strategies have been developed to increase HIV testing. Since 2008, when Kenya adopted a national HIV strategy, HIV testing has been offered through client-initiated testing and counseling, provider-initiated testing and counseling, and home-based testing and counseling. The Kenya AIDS Indicator Survey (KAIS) and the Kenya Demographic and Health Survey (KDHS) show that the number of people who had ever tested for HIV increased from 36% in 20072 to 77% in 2014,3 and HIV status awareness among infected people increased from 53% in 20124 to 76% currently.5
Current HIV testing methods in Kenya have had substantial success; however, as cumulative testing rates increase, the last segment of the untested HIV-positive population becomes harder to identify. With the current shift in the HIV programming globally to focus on reaching the 90:90:90 goal6 by 2020, other innovative approaches are needed to supplement existing approaches to accelerate identification of HIV positives and increase HIV status awareness.
In 2012, the US Food and Drug Administration (FDA) authorized the use of OraQuick® In-Home HIV Test.7 In 2017, Kenya's Pharmacy and Poisons Board approved OraQuick® for use.8 HIV self-testing (HST) offers the potential for more people to know their sero-status by circumventing some barriers, including stigma, lack of privacy, long distances to health facilities, lack of client autonomy, and poor access to health facilities.9 HST is accurate with sensitivity of over 97% and specificity of over 99%.10 A systematic review conducted in 2013 of HST in both low- and high-risk populations showed that both supervised and unsupervised testing strategies were highly acceptable, preferred, and more likely to result in partner self-testing.11 In a study conducted in Malawi, the majority (98.5%) of the participants rated the self-test as very easy.12 This study explored the potential of using HIV self-test kit to increase male partner testing in antenatal care (ANC) setting.
Partner testing is very important during the first ANC visit, as there are high rates of HIV acquisition among pregnant women as shown in studies from Kenya, Botswana, and Uganda.13 In a recent study conducted in Ethiopia, of 802 women, only 27% had attended ANC with their partner.14 In another study conducted in Kenya, female ANC clients were asked to come with their male partners in the subsequent visit, and only 16% of the women did so.15 Although pregnancy is a critical window for the prevention of mother-to-child transmission, many men hesitate to come to the ANC clinic for HIV testing. Therefore, self-testing may present an opportunity to increase partner testing during pregnancy avoiding the complex issues preventing men from attending ANC clinic. A limitation of this strategy is that it places a burden on the pregnant women to approach their male partners with the self-testing kits.
METHODS
Study Design
This was a 3-arm, individually randomized controlled study. The details of the interventions provided in each arm are described further below. The study was conducted between August 2015 and February 2016.
Ethics and Institutional Reviews
The study was approved by the Kenya Medical Research Institute, the Johns Hopkins School of Public Health, and the Medical University of South Carolina Review Boards.
Study Setting
This study took place in 14 health facilities with high-volume ANC attendance in eastern and central Kenya. The facilities have an average attendance of about 95 first ANC clients in a month and were mostly government-owned with the exception of 1 private facility owned by a church organization.
Participants
Eligible participants were pregnant women aged 18 years and older who were attending ANC for the first time in their current pregnancy. In addition, the selection criteria required that (1) the women should have had at least weekly contact with their male partners, (2) the male partners of the women were HIV-negative or of unknown status, and (3) their male partners had not tested for HIV within 3 months before enrollment. We excluded women who were concerned about the risk of violence from their male partners in the event that they hinted at the prospect of HIV testing. Male partners of women enrolled in the study were eligible to participate on consenting.
Recruitment
The health facility nurse identified women attending first ANC and referred them to the trained study nurse. The study nurse screened women for eligibility and those who agreed to participate in the study signed informed consent. The women were also informed that their male partners would be contacted to discuss HIV testing. After the consent of the female participants, the study nurse administered a structured questionnaire at baseline to collect sociodemographic and behavioral information. Informed consent was also obtained from the participants' male partners willing to participate in an interview.
Randomization
Participants were individually randomized into 1 of the 3 study arms following their informed consent. During randomization, each study arm was assigned a distinct color (yellow for study arm 1, green for study arm 2, and blue for study arm 3). Stickers with these colors equal to the number to be recruited were prepared, put in small brown opaque envelopes, sealed completely, then placed in a basket, and mixed thoroughly. Each participant was requested to randomly select an envelope from the basket indicating the arm of study to which she was assigned. After randomization, the stickers were stuck inside of the back cover of the client's personal ANC booklet for ease of identification during follow-up.
Intervention Delivery
On randomization the interventions were provided as follows:
Study arm 1 (standard-of-care): The study nurse gave the participant the standard Kenyan Ministry of Health card, inviting the male partner to come to the clinic for a discussion on the health of their family, but the card did not specifically mention HIV testing.
Study arm 2 (improved card): The study nurse gave the client an improved invitation card developed by the study team describing the benefits of HIV testing to the family and to the man's own health, as well as information on the possibility of sero-discordance.
Study arm 3 (oral HIV self-test kit and improved invitation card): The study nurse gave the client the same improved invitation card as in arm 2, plus 2 OraQuick® oral HIV self-test kits (OraSure Technologies, Bethlehem, PA), instructional material on how to use the oral self-test kit with user-friendly pictorial instructions (Supplemental Digital Content 1, http://links.lww.com/QAI/B206), and information, education, and communication materials on post-test counseling and HIV. The study nurse also offered the women skills in negotiation with their male partners on the use of the self-test kit and also instructed to inform their partners to seek confirmatory testing from any clinic of their choice should the self-test give a positive result for HIV.
The inclusion of arm 2 in the study was to evaluate the effect the provision of information on HIV would have on the HIV testing of their male partners, which was offered additionally in arm 3.
Follow-up
Three months after enrollment, participants were followed up to ascertain whether the male partner and woman tested as a couple for HIV. The study nurse contacted the women by telephone for an interview appointment to administer a structured follow-up questionnaire. In addition, the male partners that had given informed consent to the study were interviewed using a structured questionnaire. In the structured questionnaire, information was obtained from both the women and their male partners on sociodemographic characteristics, HIV testing behaviors since the baseline interview, social support, and on communication issues. In study arm 3, both the woman and her male partner were evaluated on their ability to use the oral self-test kits. The interviews for the woman and their male partners were conducted separately in a private place.
Sample Size Calculations
The sample size calculation was made by comparing study arm 1 versus arm 2, and arm 2 versus arm 3 using a 5% level of significance and power of 80%, as detailed below.
Study arm 1 versus 2 sample sizes were calculated based on an equivalence test (with 5% limit of equivalence). Previous programmatic anecdotal information from USAID-funded APHIAplus KAMILI program, partner testing at ANC was estimated at 6%, so that we assumed the same rate in study arm 1. We anticipated a small increase in HIV testing in study arm 2. However, if there was no difference between study arm 1 and 2, then 950 (475 per arm) ANC clients would be required to be 80% sure that the limits of a 2-sided 95% confidence interval would exclude a difference of more than 5% between the 2 arms.
Study arm 2 versus 3 sample sizes were calculated based on a superiority test. We assumed that study arm 3 would have an uptake of male partner HIV testing of 20%, whereas study arm 2 would reach at least 11% (the upper limit of equivalence), based on the low uptake of male partner testing in ANC settings in Kenya. Based on these assumptions, 500 (250 per arm) ANC clients would be required to have an 80% chance of detecting a difference in the partner HIV testing measure from 11% in arm 2 to 20% in arm 3 at 5% level of significance.
To achieve balance, we planned to recruit an equal number of participants in all study arms. Therefore, in each study arm, the targeted sample size was 475 female clients.
Measures
Primary Outcome
The primary outcome of the study was HIV couples testing status by any method (clinic-based testing or oral self-testing), as reported by the female partners. A critical goal of programs to prevent mother-to-child transmission (PMTCT) is to increase male partner testing, whether achieved through clinic-based, home-based, or self-testing. Moreover, in our study design, we expected that we would not be able to recruit, consent, and interview all the male partners, underscoring the importance of the woman's self-report as our primary outcome measure.
Secondary Outcome
A secondary outcome was the man's self-report of HIV couples testing to supplement the woman's self-report, and measure concordance in reporting within the couple.
Statistical Analysis
Primary analysis involved a comparison of outcome measures in study arm 3 versus both arms 1 and 2. We used a χ2 test to evaluate the unadjusted effect of study arm 3 on couples testing for HIV as reported by the woman and on the secondary outcome of couples testing for HIV as reported by the man. In separate analyses, we assessed the effect of the improved card on couples testing rates in control arm 2 versus control arm 1, in addition to using superiority tests to assess the effect of study arm 3 versus 1 and 2. We assessed the paired agreement between what women reported and what men reported using kappa statistics. We fitted a multivariable logistic regression model to adjust for sociodemographic factors that may have been imbalanced at baseline, taking into account facility clustering. Household level wealth index was computed using Rasch model based on wealth-related variables such as ownership of vehicles, bicycles, cows, type of house, etc. All analyses were conducted in R Version 3.4.3 (https://www.r-project.org/) and Stata version 12 (STATA CORP).
RESULTS
Recruitment and Follow-up
A total of 3789 women attended first ANC in 1 of the 14 facilities during the study of which 3706 (97%) were screened for eligibility, 2296 (62%) either refused or did not meet eligibility criteria, and 1410 (38%) consented to participate in the study (Fig. 1). The common reasons for exclusion were that the partner was away, woman was single, and fear of sex-based violence (51 clients).
Figure 1.
Study population: recruitment and retention.
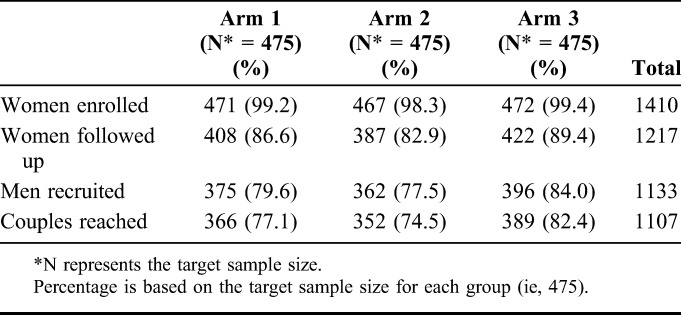
The study enrolled a total of 1410 ANC clients from which a total of 1217 (86%) were successfully followed 3 months after enrollment; the remaining 193 could not be reached. In the men's survey, the study attempted to reach all 1410 male partners of which 1133 male partners (80.4%) of the ANC clients were contacted and agreed to participate in the survey. Out of the men and women who followed up, we identified 1107 couples (Table 1). Table 1 shows that the recruitment of women was balanced between the 3 study arms; however, slightly more women (89.4%) were followed up in study arm 3 compared with arm 1 (86.6%) and arm 2 (82.9%) (P = 0.005 between arm 2 and arm 3). More men were recruited in study arm 3 compared with arm 1 and arm 2 (P = 0.02 between arm 2 and arm 3).
TABLE 1.
Number (%) of People Enrolled and Followed Up, and Couples Reached by the Study
Participant Characteristics
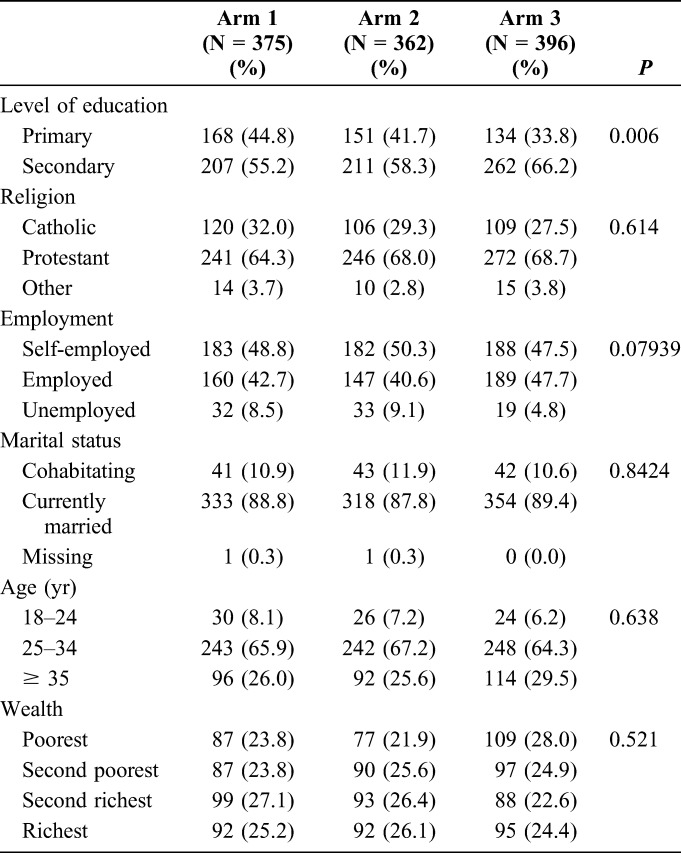
Women had relatively low level of education (overall 56% of the women had primary education or less) and high level of unemployment (over 50%), were mostly married (over 85%), and aged 25–34 years (approximately 50%) (Table 2). Most men in the study had secondary level education (>55% in each arm, 66% in study arm 3, 55% in arm 1, and 58% in arm 2) (Table 3). In addition, men in study arm 3 were slightly more likely to be employed (48%) compared with those in arm 1 and arm 2 (43% and 41%), respectively.
TABLE 2.
Characteristics of Women Enrolled Into the Study
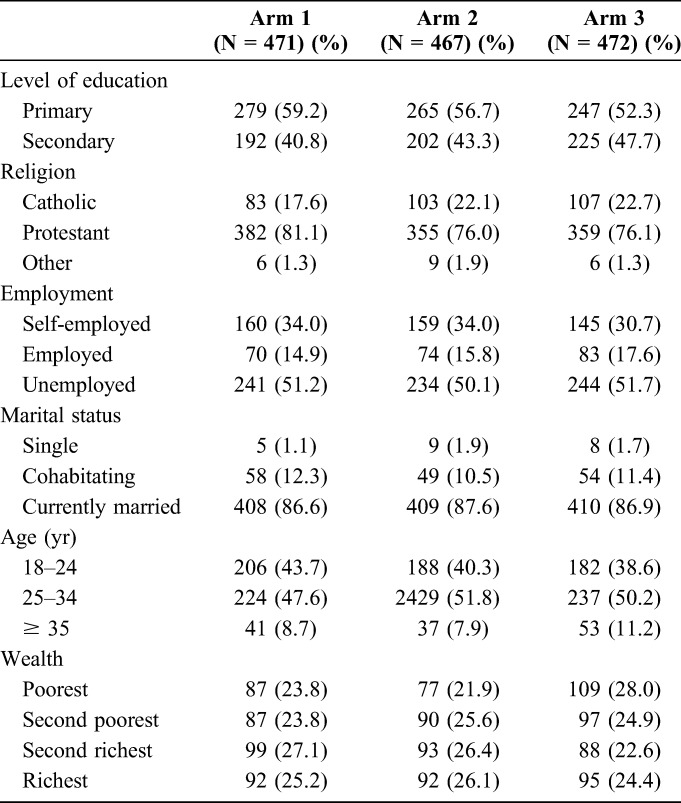
TABLE 3.
Demographic Characteristics of Men Enrolled Into the Study, n (%)
Primary Outcome: Women Reported Status of Couples Testing
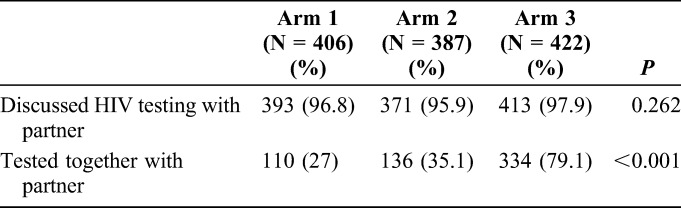
In study arm 3, 79.1% of women (334/422) reported testing together with the partner in the 3 months after the ANC visit compared with only 35.1% (136/387) in arm 2 and 27% (110/406) in arm 1, producing a statistically significant difference (1-sided test P < 0.001 comparing arm 3 and arm 2, and 2-sided test P = 0.01 comparing arm 1 and arm 2) (Table 4). Among women who reported that their partners tested for HIV, 95% (360/381) of women in study arm 3 reported that their partners tested at home, whereas nearly all (>97%) women in the control arms (study arms 1 and 2) who reported that their partners tested for HIV said that the partner tested in a clinic or at the Voluntary Counseling and Testing center. In each of the 3 arms, more than 95% of the women reported discussing HIV testing with their partners.
TABLE 4.
Women's Report of HIV Discussion and Testing With Their Partners, n (%)
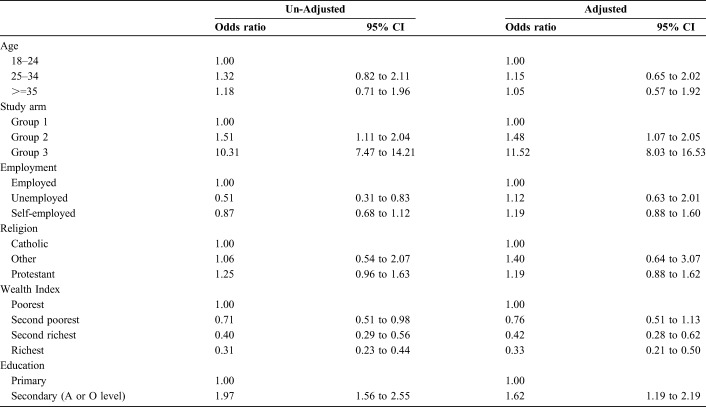
In the bivariate analysis, the study arm (intervention type), education, and wealth were strongly associated with the ANC client's report of male partner HIV testing as part of a couple (Table 5). The odds of couples testing was almost 11 times higher [10.8, 95% confidence interval (CI): 7.67 to 15.2] in study arm 3 compared with arm 1, while the odds of couples testing decreased with increased wealth and education. In the multivariate analysis, this association was slightly stronger, with an odds ratio (OR), comparing arm 3 with arm 1, of 11.5 (95% CI: 8.0 to 16.5).
TABLE 5.
Determinants of Uptake of HIV Testing Among Male Partners as Reported by Female Partners
Secondary Outcomes
Male Partner Self-Reported HIV Testing Status
In study arm 3 (intervention arm), 82% (322/393) of the men reported HIV testing as part of a couple, compared with 28% (106/375) in arm 1 and 37% (133/362) in arm 2 (1-sided test P < 0.001 comparing arms 3 and 2, and 2-sided test P = 0.02 comparing arms 1 and 2). Overall, in the intervention arm, a little over a quarter (28% or 76/274) of the men who reported to have self-tested also reported that they went to the health facility for confirmation of the test results regardless of their HIV status. The multivariable analyses showed that study arm, education, and wealth were strongly related to the male partner reporting of HIV testing as a couple. The odds of self-reported HIV testing between arm 1 and arm 2 were 10.8 (95% CI: 7.67 to 15.20), and after adjusting for other factors, the odds increased to 11.5 (95% CI: 8.03 to 16.53).
Agreement of Male and Female Partners on Self-Report of HIV Testing
Agreement between male partner self-report of HIV couples testing and women's report of HIV couples testing was strong (Cohen's kappa was 0.91 in arm 1, 0.82 in arm 2, 0.85 in arm 3, and P < 0.001 in each arm).
Usability of the kit
Among those who used the self-test kits in study arm 3, more than 80% of the participants (84% of women and 81% of men) reported that it was very easy to understand the instructions on how to conduct an oral HIV self-test, obtain the sample for the test (84% for women and 80% for men), and read the test results (92% for women and 90% for men).
DISCUSSION
In this study, female ANC clients gave oral HIV self-test kits to their male partners to test themselves in their own free time either at home or at a private location. We found remarkably high uptake of HIV testing (79.4%) in this study arm in which women were given 2 self-test kits, an improved information card on the importance of male partner testing with mention of possible sero-discordance in couples, written instructional materials, and more oral counseling compared with the other study arms that did not receive the oral HIV self-test kits (37% in control arm 2 and 28% in control arm 1). The results are similar to those of a parallel study that was undertaken in western Kenya on male partner testing among ANC and postnatal care clients, which showed 90% uptake of HIV testing among male partners who were randomized to oral HIV self-test kit.16 In addition, a formative study conducted in Uganda showed that men were willing to use HIV self-test kits when it is distributed by their female partner.17
In a follow-up survey, more than 80% of the study participants (84% of women and 81% of men) responding to the survey reported that it was easy to understand the instructions, take the swab, and read the results, despite the low education level of most of the study participants. In the study, we simplified the instructions for using the OraSure self-testing product to both a simple, easy-to-read, and pictorial format. This approach was designed to allow an average person to do step-by-step testing, as demonstrated in Supplemental Digital Content 1, http://links.lww.com/QAI/B206. These results imply that when providing HIV self-test kit, the instructions have to be simple enough for the target population.
More than a quarter (28%) of men who used the HST kit went for confirmatory testing at the health facility, a finding consistent with exploratory studies in which participants indicated that they would go for confirmatory testing if they self-test themselves for HIV.17 This finding has important implications in that providing oral HIV self-test kits to men can promote reporting of positive health-seeking behavior, which is known to be poor in men.18,19 By visiting the health facility for confirmation, men can become linked to HIV care if confirmed positive and also potentially receive other services that will generally improve their health and that of their families.
Although we did not collect data on male partners who may have tested for HIV alone, rather than as a couple, we found in this study that an overwhelming majority of women in all 3 study arms reported that they spoke with their partners about HIV, and nearly all the couples in study arm 3 tested together. This underscores the barriers removed by self-testing (eg, inconvenience and lack of privacy). Data from the KAIS found that in 83.6% of HIV-infected Kenyans living as married or cohabitating couples, neither partner knew their HIV status.20 Therefore, couples using oral HST together promote increased awareness of partner's HIV status, which is an important pillar in HIV prevention.
Although this study was designed to demonstrate the concept of how to increase male partner testing in ANC setting, the study identified less than 10 HIV-positive male partners, most likely because of the low HIV prevalence of 2.5% in eastern and central Kenya. In light of this, it is important to evaluate different strategies through which HST can contribute to identification of HIV-positive persons (the first 90 in 90:90:90), especially in this era where targeted testing is being encouraged.
In the view of the results of studies conducted in Kenya, the Ministry of Health has developed and launched the national HST guidelines, which recommends that the self-test should be used as an initial screening test. Clients testing positive with oral HIV self-test are advised to follow-up with a confirmatory test at a health facility with the possibility of linkage to care, treatment, and support services.
Limitations
The study had a couple of limitations. First, we relied on the self-report from the woman and the man for the HIV testing status, and results may exhibit self-report bias. However, the results showed strong agreement between the man and woman's response. Second, men in the study were not individually randomized into the study directly but were randomized through their female partner leading to some imbalances in the study arms in basic demographic characteristics. However, adjusted analysis showed that these imbalances did not impact distributing oral HIV self-test kits on HIV testing. Finally, provision of self-test kits to men may have triggered enhanced HIV awareness and men may have preferred to opt for clinic-based testing than do the self-test; however, this was not verified.
CONCLUSIONS
Provision of oral HIV self-tests, with a convenient, private distribution method through female partners, may be a way to reach male partners who do not accompany ANC clients for testing at the facilities. Knowledge of HIV status after the use of the oral HIV self-tests can potentially inform preventive behaviors that would reduce mother-to-child transmission of HIV.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all the study participants, research nurses, and health facility staff for participating in the study.
Footnotes
Supported by grant (TW2.02.08) from international initiative for impact evaluation (3ie) which has soon ended.
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.UNAIDS. Country Factsheets: Kenya. 2016. Available at: http://www.unaids.org/en/regionscountries/countries/kenya. Accessed June 20, 2018. [Google Scholar]
- 2.NASCOP. Kenya AIDS Indicator Survey 2007 Data Sheet. 2007. Available at: https://assets.prb.org/pdf09/kaiskenyadatasheet.pdf. Accessed June 20, 2018. [Google Scholar]
- 3.Kenya National Bureau of Statistics. Kenya 2014 Demographic and Health Survey. 2014. Available at: https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf. Accessed June 20, 2018. [Google Scholar]
- 4.National AIDS Control Council. KAIS 2012 Final Report. 2012. Available at: http://nacc.or.ke/kais-2012-final-report/. Accessed June 20, 2018. [Google Scholar]
- 5.Indravudh PP, Choko AT, Corbett EL. Scaling up HIV self-testing in sub-Saharan Africa: a review of technology, policy and evidence. Curr Opin Infect Dis. 2018;31:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. 90-90-90: An Ambitious Treatment Target to Help End the AIDS Epidemic. 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. Accessed June 5, 2018. [Google Scholar]
- 7.United States Food and Drug Administration. Information regarding the OraQuick in-home HIV test. Available at: https://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/ucm311895.htm. Accessed February 27, 2017.
- 8.UNAIDS. Kenya launches Self-Test Kits and PrEP. 2017. Available at: http://www.unaids.org/en/resources/presscentre/featurestories/2017/may/20170505_kenya. Accessed June 5, 2018. [Google Scholar]
- 9.Ganguli I, Bassett IV, Dong KL, et al. Home testing for HIV infection in resource-limited settings. Curr HIV/AIDS Rep. 2009;6:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens DR, Caroline Vrana BJ, Raviv Dlin BE, et al. A global review of HIV self-testing: themes and implications. AIDS Behav. 2018;22:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pant Pai N, Sharma J, Shivkumar S, et al. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med. 2013;10:e1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choko AT, Desmond N, Webb EL, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med. 2011;8:e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake AL, Wagner A, Richardson B, et al. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amano A, Musa A. Male involvement in PMTCT and associated factors among men whom their wives had ANC visit 12 months prior to the study in Gondar town, North west Ethiopia, December, 2014. Pan Afr Med J. 2016;24:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz DA, Kiarie JN, John-Stewart GC, et al. Male perspectives on incorporating men into antenatal HIV counseling and testing. PLoS One. 2009;4:e7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masters SH, Agot K, Obonyo B, et al. Promoting partner testing and couples testing through secondary distribution of HIV self-tests: a randomized clinical trial. PLoS Med. 2016;13:e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matovu JK, Buregyeya E, Arinaitwe J, et al. '… if you bring the kit home, you [can] get time and test together with your partner': pregnant women and male partners' perceptions regarding female partner-delivered HIV self-testing in Uganda—a qualitative study. Int J STD AIDS. 2017;28:1341–1347. [DOI] [PubMed] [Google Scholar]
- 18.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49:616–623. [DOI] [PubMed] [Google Scholar]
- 19.Pearson S, Makadzange P. Help-seeking behaviour for sexual-health concerns: a qualitative study of men in Zimbabwe. Cult Health Sex. 2008;10:361–376. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser R, Bunnell R, Hightower A, et al. Factors associated with HIV infection in married or cohabitating couples in Kenya: results from a nationally representative study. PLoS One. 2011;6:e17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.