Supplemental Digital Content is available in the text.
Keywords: astrocyte, astrogliosis, endoplasmic reticulum stress, old astrocyte specifically induced substance, spinal cord injury
Abstract
To investigate the relationship between endoplasmic reticulum (ER) stress mediated by old astrocyte specifically induced substance (OASIS) and astrogliosis in spinal cord injury (SCI). SCI models were established using adult male mice deficient for OASIS and C57BL/6 (wild-type mice) mice. After SCI, recovery and astrogliosis were examined in the mice at specific time points using functional and histological methods. After SCI, functional recovery was better in the OASIS-deficient mice than in the wild-type mice. OASIS deletion did not inhibit astrocyte migration but reduced the excessive accumulation of N-cadherin-expressing reactive astrocytes that formed the glial scar around the injury site. In addition, OASIS deletion increased the number of serotonin-positive axons in spinal cord regions caudal to the injury site. These findings suggested that the OASIS-mediated ER stress response inhibits the repair of the injured spinal cord by promoting the development of N-cadherin-expressing reactive astrocytes that form glial scars following injury. OASIS deletion inhibited the development of N-cadherin-positive reactive astrocytes that form glial scars and promoted axon growth and functional recovery after SCI. These results suggest that the ER stress response mediated by OASIS could be a new target in the treatment of SCI.
Introduction
The endoplasmic reticulum (ER) is the entrance site for proteins destined for the secretory pathway or extracellular environment 1. Various pathophysiological conditions, such as ER-calcium depletion, oxidative stress, hypoglycemia, altered glycosylation, mutated protein expression, and hypoxia, interfere with the correct folding of proteins and increase the accumulation of unfolded or misfolded proteins in the ER 1,2. This condition is termed ER stress, and excessive and/or long-term stress results in apoptotic cell death 1,3. To manage the accumulation of unfolded proteins, the ER ubiquitously expresses three major transducers, protein kinase R-like ER kinase, inositol-requiring 1, and activating transcription factor 6, which sense unfolded proteins in the ER lumen. They provoke an unfolded protein response (UPR), which leads to the production of ER-resident chaperones and attenuates global protein translation and ER-associated degradation, to remove misfolded or unfolded proteins. Another ER stress transducer is the old astrocyte specifically induced substance (OASIS), which is expressed in specific cells, including astrocytes and osteoblasts 2,4,5. The activation of OASIS by mild ER stress has been reported to accelerate the differentiation of neural precursor cells into astrocytes in the fetal brain 6. In this study, we examined the function of OASIS in astrogliosis following spinal cord injury (SCI). SCI is a severe condition that occurs throughout the world with an annual incidence of 15–40 cases per million 7. SCI consists of two stages. After mechanical injury owing to an external force, the injured area enlarges owing to secondary damage, including the increase in inflammation, hypoxia, ischemia, and demyelination, and disruption of the blood–brain barrier 8. Previous treatments have included the administration of methylprednisolone, but none were sufficiently clinically effective 9. Previous studies 10,11 have focused on reactive astrogliosis, which is a pathological hallmark of SCI and is a defense mechanism that minimizes and repairs the initial damage but eventually leads to inhibitory effects through glial scar formation 12. Controlling astrogliosis is one of the most important steps in the successful treatment of SCI. The function of the ER stress response has been reported to be related to apoptosis, but no studies have revealed the relationship between ER stress and astrogliosis in SCI. Here, we show that the OASIS-mediated ER stress response positively regulates reactive astrogliosis, which results in glial scar formation following SCI.
Materials and methods
The experiments described in this study were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All protocols were approved by the ethics committee of Hiroshima University.
Old astrocyte specifically induced substance-deficient mice
C57BL/6 and OASIS−/− mice were used in this study. The OASIS−/− mice were supplied from the Department of Biochemistry of the Graduate School of Biomedical and Health Sciences of Hiroshima University 13. They were bred and maintained at the animal facility of Hiroshima University.
Spinal cord injury model
Adult male mice (10 weeks old) that were deficient for OASIS [OASIS knockout (KO) mice] and C57BL/6 mice [wild-type (WT) mice] were anesthetized by the sustained inhalation of 2.0% isoflurane and air (2.0 l/min). After a T10 thoracic laminectomy, the spinal cord was contused with an Infinite Horizon Impactor (70 kdyn; Precision Systems and Instrumentation, LLC, Lexington, Kentucky, USA) 14.
Assessment of motor function
After SCI, the recovery of hindlimb motor function was assessed by locomotor ratings made using the Basso Mouse Scale (BMS) on days 1, 3, 5, 7, 14, and 42 15. The BMS scores of all mice were 9 (maximum score) before SCI and 0 on day 1 after SCI.
Tissue preparation
The mice were deeply anesthetized by the sustained inhalation of 2.0% isoflurane and air (2.0 l/min) and then transcardially perfused with PBS and then 4% paraformaldehyde in 0.1 M PBS. Their spinal cords were resected in 10-mm-long sections with the contused areas centered and then fixed in 4% paraformaldehyde for 24 h at 4°C. Subsequently, the spinal cords were immersed in 15% sucrose for 24 h, followed by 30% sucrose for 3 days for cryoprotection, and then submerged in compound and stored in a freezer at −80°C.
Immunohistochemistry
Ten-micron-thick sagittal sections were cut on a cryostat and then carefully mounted on saline-coated glass slides, air dried, and treated with serum-free protein blocker (Agilent Technologies Denmark ApS, Glostrup, Denmark) for 60 min at room temperature. The sections were then stained with primary antibodies. The primary antibodies used in this study were as follows: goat anti-glial fibrillary acidic protein (GFAP, 1 : 200; Abcam plc, Cambridge, UK), rat anti-F4/80 (1 : 200; BD Biosciences, San Jose, California, USA), mouse anti-nestin (1 : 100; BD Biosciences), rabbit anti-N-cadherin (1 : 400; Abcam plc), goat anti-serotonin (5-HT; 1 : 600; Abcam plc), rabbit anti-cleaved caspase-3 (1 : 400; Cell Signaling Technology Inc., Danvers, Massachusetts, USA), and rat anti-myelin basic protein (MBP, 1 : 400; Abcam plc). The secondary antibodies were Alexa Fluor 568-conjugated donkey anti-goat IgG (1 : 500), Alexa Fluor 594-conjugated donkey anti-rat IgG (1 : 500), Alexa Fluor 568-conjugated donkey anti-mouse IgG (1 : 500), Alexa Fluor 594-conjugated donkey anti-rabbit IgG (1 : 500), Alexa Fluor 488-conjugated donkey anti-goat IgG (1 : 500), Alexa Fluor 488-conjugated goat anti-rabbit IgG (1 : 400), and Alexa Fluor 594-conjugated goat anti-rat IgG (1 : 400; all from Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) solution was applied for 5 min for nuclear staining. All spinal cord sections were examined using a fluorescent microscope (BZ-9000; Keyence Corporation, Osaka, Japan).
Quantitative assessment of N-cadherin, axons, and apoptotic oligodendrocytes
The N-cadherin- and 5-HT-positive areas were measured using ImageJ software (National Institutes of Health, Maryland, USA), as previously described 16. The areas of the 5-HT-positive axons were measured at two different sites (caudal region adjacent to the epicenter and 500 µm caudal to the epicenter) 17. The number of apoptotic oligodendrocytes was calculated at two different regions in the white matter caudal to the injury site 18. Cells labeled for MBP, cleaved caspase-3, and DAPI were counted by investigators who were blind to the groups in five sections each from five mice from each group. The labeled sections were examined using the BZ-9000 microscope.
Statistical analysis
All measured values other than the BMS scores were expressed as mean±SD. The data were analyzed using the Statistical Package for Social Sciences (SPSS, IBM Japan, Tokyo, Japan). Unpaired Student’s t-tests were used to compare the two groups. The BMS scores were expressed as mean±standard error and statistically analyzed using two-way repeated-measures analysis of variance for group and time. P values less than 0.05 were considered statistically significant.
Results
Motor function recovery
All WT and KO mice had BMS scores of 9 (maximum score) before the SCI, and their scores were reduced to ∼1, 1 day after SCI. The scores of both the WT and KO mice gradually improved over time. Significant effects for group were identified using repeated-measures analysis of variance (P<0.0001 for group, time, and group×time interaction). The scores of the KO mice were significantly higher than those of the WT group on and after day 7 after SCI (Fig. 1). These findings indicated that OASIS deletion improved the functional recovery of mice after SCI.
Fig. 1.
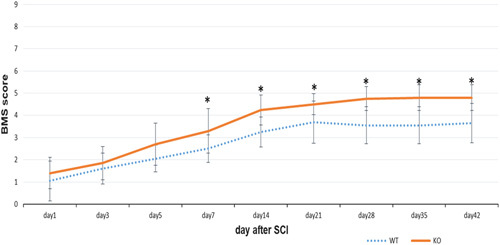
The Basso Mouse Scale (BMS) scores made after spinal cord injury (SCI). Hindlimb motor function was more improved in knockout (KO) mice on and after day 7 (*P<0.05, Student’s t-test). WT, wild type.
Generation and migration of reactive astrocytes following spinal cord injury
The astrocyte precursor cells expressing GFAP and nestin were mainly observed around the central canal of the spinal cord at almost the same intensity in the WT and KO mice on day 7 after SCI (Supplementary Fig., Supplemental digital content 1, http://links.lww.com/WNR/A491 which demonstrates GFAP- and nestin-positive astrocytes). On day 14 after SCI, accumulation of F4/80-positive macrophages in the central part of the injury site, with surrounding GFAP-positive astrocytes, was observed (Fig. 2). No significant differences were found for the F4/80-negative and GFAP-negative areas between the WT and KO mice. These findings suggested that OASIS deletion did not affect the generation and migration of reactive astrocytes following SCI.
Fig. 2.

The formation of astrogliosis on day 14 after spinal cord injury (SCI; scale bar=100 µm). (a) Digital images of astrocytes immunostained for glial fibrillary acidic protein (GFAP; green) and trichrome stained for 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; blue) and anti-F4/80 (red). Astrogliosis is observed in knockout (KO) mice, but the intensity is lower than that in wild-type (WT) mice. (b) Area showing positive immunoreactivity to F4/80 and negative immunoreactivity to GFAP. No differences were observed in the F4/80-positive and GFAP-negative areas between WT and KO mice.
N-cadherin-positive astrocytes
N-cadherin-positive astrocytes were located in the region adjacent to the GFAP-negative central part of the injury site in both WT and KO mice (Fig. 3). However, the N-cadherin-positive area in the KO mice was significantly smaller than that in the WT mice. These findings showed that OASIS deletion decreased the development of N-cadherin-expressing reactive astrocytes.
Fig. 3.
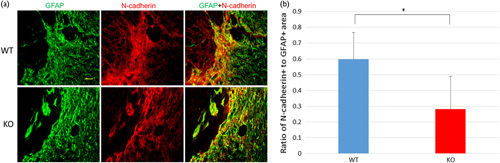
Digital images of sections stained for glial fibrillary acidic protein (GFAP; green) and N-cadherin (red) on day 42 after spinal cord injury (SCI; scale bar=40 µm). (a) The expression of GFAP and N-cadherin is observed in both groups, but the width of the N-cadherin-positive area overlapping the GFAP-positive area is smaller in the knockout (KO) mice than in the wild-type (WT) mice. (b) The ratio of N-cadherin to GFAP staining at the injured site is significantly smaller in the WT mice than in the KO mice (*P<0.05, Student’s t-test).
Axon growth
To assess the contribution of descending nerve fibers to motor function improvement, immunohistochemistry for 5-HT (marker of descending serotonergic axons) was performed (Supplementary Fig., Supplemental digital content 2, http://links.lww.com/WNR/A492, which shows spinal cord sections on day 42 after SCI). The 5-HT-positive areas were measured in two lesions at the injury site (caudal region adjacent to the epicenter) and 500 µm distal to the lesion. In both lesions, the area of the 5-HT-positive axons in the KO mice was significantly greater than that in the WT mice. These findings suggested that OASIS deletion enhanced axon growth after SCI.
Apoptotic oligodendrocytes
Immunohistochemistry for cleaved caspase-3 (apoptotic marker), MBP (oligodendrocyte marker), and DAPI was performed on day 7 after SCI (Fig. 4). The numbers of apoptotic oligodendrocytes (trichrome-stained cells) were counted in two regions of the white matter caudal to the injury site. The numbers in WT mice were significantly higher than those in KO mice.
Fig. 4.
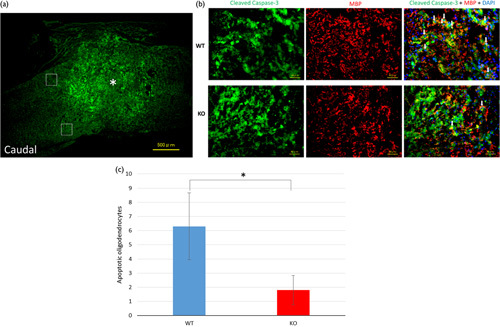
Spinal cord sections from day 7 after spinal cord injury (SCI) immunostained for cleaved caspase-3, myelin basic protein (MBP), and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). (a) The asterisk shows the epicenter of the injury site (scale bar=500 µm). The white squares indicate the two regions in the white matter. (b) Enlarged images of the regions (scale bar=40 µm) are shown. These digital images show apoptotic cells immunostained for cleaved caspase-3 (green) and oligodendrocytes stained for myelin basic protein (MBP; red) and DAPI (blue). The white arrows show trichrome-stained apoptotic oligodendrocytes. (c) The cells co-labeled with cleaved caspase-3 and MBP were defined as apoptotic oligodendrocytes. The number of apoptotic oligodendrocytes immunostained for cleaved caspase-3, MBP, and DAPI at the injury site. The number of trichrome-stained cells was significantly higher in the wild-type (WT) mice than in the knockout (KO) mice (*P<0.05, Student’s t-test).
Discussion
Our findings demonstrated that OASIS deletion did not affect the generation and migration of reactive astrocytes but rather inhibited the development of N-cadherin-positive reactive astrocytes around the injury site and enhanced axon growth after SCI.
In this study, we examined astrogliosis following SCI from the aspect of ER stress. The UPR in the ER is a homeostatic response that occurs under ER stress conditions 19. The ER stress signals are detected by sensors that are located in the membrane and that transmit new signals to deal with the stress. Of the two types of sensors, one is ubiquitous, and the other is expressed in specific cells. We focused on the sensor specifically expressed in astrocytes, OASIS, and examined its contribution to astrogliosis. This study is the first report of the relationship of OASIS to astrogliosis after SCI.
Astrogliosis following SCI has two contrasting characteristics. One is beneficial and prevents the spinal cord from expanding owing to inflammation and reconstructs the blood–spinal barrier 12. The suppression of astrogliosis has been reported to enlarge the damaged lesion, which hampers recovery from the injury 10. In contrast, astrogliosis also forms a morphological barrier called a glial scar that disturbs axonal regeneration and leads to poor motor function recovery.
The UPR mediated by OASIS has previously been reported to promote astrocyte differentiation in the fetal brain 6. However, OASIS deletion did not affect the generation of astrocyte precursor cells expressing nestin and GFAP following SCI in the present study. In addition, OASIS deletion did not decrease the proliferation and migration of reactive astrocytes. These findings suggested that OASIS might not contribute to the differentiation of neural precursor cells in SCI. Therefore, OASIS deletion might not inhibit the beneficial effects of astrogliosis in the acute and subacute phases of SCI.
In contrast, these findings revealed that OASIS deletion decreased N-cadherin-positive reactive astrocytes around the injury site and promoted axon growth and functional recovery after SCI. N-cadherin has been reported to play a prominent role in the injury response of astrocytes, including the formation and maintenance of the glial scar 20. A previous study has shown that the astrocyte-specific KO of N-cadherin results in impairments in astrogliosis in response to brain injury 21. Furthermore, another previous study has reported that N-cadherin downregulation in reactive astrocytes prevents glial scar formation and promotes axonal regeneration and functional recovery after SCI 22. These findings suggested that the ER stress response mediated by OASIS positively regulates the development of N-cadherin-positive scar-forming astrocytes that inhibit axon growth and functional recovery after SCI.
In this study, we also evaluated apoptotic oligodendrocytes at the injury site. Fewer apoptotic oligodendrocytes were observed in OASIS KO mice. Although the detailed mechanisms are unknown, we assumed that the beneficial effects of astrogliosis play a role in protecting oligodendrocytes from apoptosis. Therefore, the ER stress response mediated by OASIS could promote the apoptosis of oligodendrocytes that inhibit improvements in functional recovery after SCI.
Although studies on SCI have been performed for decades, we still do not have a definitive cure for SCI, which sometimes has tragic outcomes. We believe that a better understanding of the relationship between OASIS and astrogliosis will provide new options for the treatment of SCI. Therefore, additional studies are needed to elucidate the detailed mechanisms and roles of OASIS.
Conclusion
The deletion of OASIS inhibited the development of N-cadherin-positive reactive astrocytes that form glial scars and promoted axon growth and functional recovery after SCI. Thus, the ER stress response mediated by OASIS could be a new target for the treatment of SCI.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.
Acknowledgements
This study was supported by a MEXT/JSPS KAKENHI Grant-in-Aid for Scientific Research (C) awarded to N.K. (grant no. 17K10931).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest 2002; 110:1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito A. Physiological functions of endoplasmic reticulum stress transducer OASIS in central nervous system. Anat Sci Int 2014; 89:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000; 403:98–103. [DOI] [PubMed] [Google Scholar]
- 4.Honma Y, Kanazawa K, Mori T, Tanno Y, Tojo M, Kiyosawa H, et al. Identification of a novel gene, OASIS, which encodes for a putative CREB/ATF family transcription factor in the long-term cultured astrocytes and gliotic tissue. Brain Res Mol Brain Res 1999; 69:93–103. [DOI] [PubMed] [Google Scholar]
- 5.Kondo S, Saito A, Asada R, Kanemoto S, Imaizumi K. Physiological unfolded protein response regulated by OASIS family members, transmembrane bZIP transcription factors. IUBMB Life 2011; 63:233–239. [DOI] [PubMed] [Google Scholar]
- 6.Saito A, Kanemoto S, Kawasaki N, Asada R, Iwamoto H, Oki M, et al. Unfolded protein response, activated by OASIS family transcription factors, promotes astrocyte differentiation. Nat Commun 2012; 3:967. [DOI] [PubMed] [Google Scholar]
- 7.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001; 26:S2–S12. [DOI] [PubMed] [Google Scholar]
- 8.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars) 2011; 71:281–299. [DOI] [PubMed] [Google Scholar]
- 9.Evaniew N, Belley-Côté EP, Fallah N, Noonan VK, Rivers CS, Dvorak MF. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a systematic review and meta-analysis. J Neurotrauma 2016; 33:468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 2006; 12:829–834. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004; 24:2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi-Abdolrezaee S, Billakanti R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol 2012; 46:251–264. [DOI] [PubMed] [Google Scholar]
- 13.Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol 2009; 11:1205–1211. [DOI] [PubMed] [Google Scholar]
- 14.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 2003; 20:179–193. [DOI] [PubMed] [Google Scholar]
- 15.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006; 23:635–659. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med 2006; 12:1380–1389. [DOI] [PubMed] [Google Scholar]
- 17.Kamei N, Kwon SM, Alev C, Nakanishi K, Yamada K, Masuda H, et al. Ex-vivo expanded human blood-derived CD133+ cells promote repair of injured spinal cord. J Neurol Sci 2013; 328:41–50. [DOI] [PubMed] [Google Scholar]
- 18.Magnuson DS, Lovett R, Coffee C, Gray R, Han Y, Zhang YP, et al. Functional consequences of lumbar spinal cord contusion injuries in the adult rat. J Neurotrauma 2005; 22:529–543. [DOI] [PubMed] [Google Scholar]
- 19.Mori K. Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem 2009; 146:743–750. [DOI] [PubMed] [Google Scholar]
- 20.Vázquez-Chona F, Geisert EE., Jr N-cadherin at the glial scar in the rat. Brain Res 1999; 838:45–50. [DOI] [PubMed] [Google Scholar]
- 21.Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc Natl Acad Sci USA 2013; 110:11612–11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med 2017; 23:818–828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.neuroreport.com.


