Abstract
Recent studies have indicated that the structure of the axon initial segment (AIS) of neurons is highly plastic in response to changes in neuronal activity. Whether an age-related enhancement of neuronal responses in the visual cortex is coupled with plasticity of AISs is unknown. Here, we compare the AIS length and the distribution of Nav1.6, a key Na+ ion channel in action potential (AP) initiation, along the AIS of layer II/III neurons in the primary visual cortex (V1) of young adult and aged rats, which were examined previously in a single-unit recording study. In that study, we found that V1 neurons in aged rats showed a significantly higher spontaneous activity and stronger visually evoked responses than did neurons in young rats. Our present study shows that the mean AIS length of layer II/III neurons in the V1 area of aged rats was significantly shorter than that of young adult rats. Further, the proportion of AIS with the Nav1.6 distribution was also reduced significantly in aged rats relative to young rats, as indicated by a decrease in the mean Nav1.6 immunofluorescence optical density within AISs and a specific decrease in Nav1.6 immunofluorescence optical density near the proximal region of the AIS. Our results indicate that aging results in both shortening of AISs and reduction of Nav1.6 Na+ ion channel distribution along AISs, which accompanies enhanced neuronal activity. This age-related morphological plasticity may lower the AP amplitude by reducing Na+ ion entry during AP initiation, spare ATPs consumed by Na+ ion pumps during membrane potential restoration, and thus balance the energy expenditure caused by an increased firing rate of cortical neurons during the aging process.
Keywords: aging, axon initial segment, neuronal response, primary visual cortex
Introduction
The axonal initial segment (AIS), a specialized subcellular region linking the axon to the somato-dendritic compartments, is a functional region where action potentials (APs) initiate in most neurons 1,2. Recent studies have found that the length and position of AIS showed a considerable plasticity during normal development 3, neuronal activity alterations 4, sensory deprivation 5, and brain disorders 6. This AIS plasticity is believed to work as a homeostatic mechanism in maintaining the stability of neuronal activity 4,7.
Previous electrophysiological studies have indicated that neurons in the visual cortex of aged individuals show a significantly higher spontaneous activity and stronger visually evoked responses to all stimuli than those of young adults 8–11. This age-dependent improvement in neuronal activity is likely caused by a weakened intracortical inhibition, especially GABAergic inhibition 8,10, during the aging process. The enhanced neuronal excitation results in a reduction in the signal-to-noise ratio and response selectivity to visual stimuli, and it thus impairs visual cortical signaling in the aged individuals 9. Whether an age-dependent increase in neuronal activity can trigger structural plasticity of AISs to maintain the excitation/inhibition balance in the visual cortex has not been fully confirmed, although a length change of AISs has been observed in the cerebral cortex of senescent marmoset monkeys 12.
Our recent study has shown that neurons in the V1 area of the rat show an increased excitation during senescence, as indicated by a higher spontaneous activity and stronger visually evoked response of neurons in aged rats than in young adult rats 8. Using these rats as subjects, this study further assesses age-related plasticity of AISs, with a focus on layer II/III neurons in the V1 area. We first examined the AIS length of V1 neurons in young adult and aged rats by labeling the AIS with anti-AnkyrinG, an antibody that has specific binding to an AIS scaffolding protein 2,3. In addition, we observed the distribution of Nav1.6, a key Na+ ion channel in AP initiation 13,14, along AISs of V1 neurons in rats of both age groups. We sought to determine whether age-associated plasticity of AISs occurs in mammalian species other than monkeys 12 and to confirm whether AIS plasticity accompanies neuronal activity changes in the visual cortex during aging.
Materials and methods
Experimental subjects
Subjects used in this study were six young adult Sprague-Dawley (SD) rats (male, 3 months old, body weight 200–300 g) and six aged SD rats (male, 22 months old, body weight 500–600 g). All rats were purchased from Nanjing Qing-Long-Shan Animal Breeding Farm (Jiangning District of Nanjing city, certificate no. SX1207) and were housed individually in different cages with an environmental temperature of 18–25°C, a 12-h light–dark cycle, and ad libitum food and water. All subjects, including aged rats, were healthy, with a history of age and health examination recorded by veterinarians. They were examined ophthalmoscopically before the experiment to confirm that no optical or retinal problems impaired their visual function. All animal treatments were performed in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Academic and Ethics Committee of Anhui Normal University.
Brain tissue preparation
To confirm whether the neuronal function in the V1 area of rats changed with age, we examined the response property of V1 neurons for each subject using single-unit recording techniques before harvesting the brain tissue. The recording was performed over the V1 area of the left hemisphere for 7–9 h using standard methods, as reported in our previously published papers 8,9,15. Briefly, the animal was anesthetized adequately with urethane (20%, 1.2 g/kg body weight, intraperitoneal) and were maintained in a normal healthy condition (expired pCO2 3.8%, body temperature 37.5°C, blood oxygen saturation SpO2≥94%) during recording using an artificial respirator, a heating pad, and a physiological monitoring system 8. After electrophysiological recording, each rat was deeply anaesthetized with an intraperitoneal injection of urethane (20%, 1.8 g/kg body weight) and then perfused through the heart with 0.9% saline, followed by 2% paraformaldehyde in 0.1 M PBS. Cortical tissues containing V1 on the right hemisphere were removed from the skull, post-fixed in 4% paraformaldehyde at 4°C for 1 h, and cryoprotected by sequential incubation in 20% (8 h) and 30% (overnight) sucrose until the tissue sank. The brain tissue was embedded in OCT compound (Tissue-Tek 4583, Sakura Finetek USA, Inc., California, USA) and coronal sections at a thickness of 30 µm were cut on a Leica cryostat (Leica Biosystems Inc., Buffalo Grove, Illinois, USA). Serial frozen sections were collected in order, placed in wells filled with cryoprotectant solution (ethylene glycol-based; 30% ethylene glycol, 30% sucrose, 1% PVP-40, in 0.1 M PB pH 7.4), and stored temporarily at −20°C for subsequent histological examination and immunofluorescent labeling.
Fluorescence immunohistochemistry
Immunofluorescent reactions were performed on free-floating sections following previously published methods 3. Briefly, sections were preincubated with QuickBlock Blocking Buffer (P0260; Beyotime, Shanghai, China) for 1 h. After washing in PBS (3×10 min), the sections were incubated overnight at 4°C with primary antibodies diluted in QuickBlock Primary Antibody Dilution Buffer (P0262; Beyotime). The primary antibodies used in this study were mouse anti-AnkyrinG (1 : 1000, MABN466; Merck Millipore, Shanghai, China), rabbit anti-NeuN (1 : 3000, ab177487; Abcam, Shanghai, China), and rabbit anti-Nav1.6 (1 : 1000, ASC-009; Alomone Labs, Jerusalem, Israel). After several washes in PBS, sections were incubated with the secondary antibodies in QuickBlock Secondary Antibody Dilution Buffer (P0265; Beyotime) for 2 h at room temperature. The secondary antibodies were goat anti-Rabbit IgG H&L (Alexa Fluor 488) (1 : 1000, ab150077; Abcam) and goat anti-Mouse IgG H&L (Alexa Fluor 555) (1 : 1000, ab150114; Abcam). After secondary antibody incubation and several washes in PBS, sections were mounted on clean glass slides with glycerol and sealed with nail polish. Control sections were labeled simultaneously using the same procedure as described above, with the exception that the primary antibody was substituted with QuickBlock Primary Antibody Dilution Buffer.
Confocal image acquisition and data analysis
Images were taken with a confocal laser scanning microscope (FV1000; Olympus, Tokyo, Japan) using a 20× or 60× objective. Automated sequential acquisition of multiple channels was performed. The frame size was 1024×1024 pixels. For each image, ten confocal planes were Z-stacked with a step of 0.56 µm. Stacks of images were merged into a maximum intensity projection and saved as a tiff file.
Image analysis was carried out using Image-Pro Plus 6.0 software (Media Cybernetics Inc., Rockville, USA) with standard settings 15,16. Images were preprocessed by histogram stretching and a sharpening filter to enhance the contrast. An AIS was only included in the data analysis if the AnkyrinG immunosignal identified a single entity within the image and the corresponding soma (labeled by the NeuN antibody) was clearly distinguishable. AISs and the ends overlapping into the somatic domain as well as into the distal section of the axon were smoothly traced. The traced curve was straightened automatically and the length of each AIS was output to an Excel file from Image-Pro Plus. The AIS length was converted from pixels into microns on the basis of spatial calibration. Over 700 AISs (over 100 AISs/rat) from layer II/III of the V1 area in each age group were randomly selected and measured.
To assess the age-associated change in Nav1.6 distribution along AISs, we measured the mean immunofluorescence optical density (IOD) of Nav1.6 within the AISs in each stack of images after background calibration, histogram segmentation, and area filtration using Image-Pro Plus. One hundred stacks of images from each age group were randomly selected and measured. To further examine whether the distribution of Nav1.6 channels in different regions of the AISs was modified equally by aging, we measured the mean IOD of Nav1.6 at different positions along each individual AIS. First, we used the area of interest duplicate function of Image-Pro Plus to extract the Nav1.6 fluorescence signal from each double-labeled AIS using the AnkyrinG fluorescence signal as a reference. Next, each AIS was normalized to 1.0 in length and the IOD of Nav1.6 at different positions along each AIS was measured sequentially with a step of 1/25 AIS line length using a self-written macro plugged in the Batch_Process script of Image-Pro Plus. Finally, the IOD distribution pattern of Nav1.6 along the AISs was obtained by averaging the measured values across all normalized AISs. One hundred AISs from each age group were randomly selected and measured. All mean values are expressed as mean±SD. The difference between young and aged rats was determined using the χ2-test, analysis of variance, or t-test, and P values less than 0.05 were considered statistically significant.
Results
Age-related change of axon initial segment length
We visualized AISs with an antibody that binds specifically to the AIS scaffolding protein AnkyrinG. To determine the AIS proximal position relative to the cell soma, we labeled the cell body of neurons with an antibody against NeuN, which was also used as a marker for the identification of different cortical layers. Double immunoreactive labeling showed that both AnkyrinG and NeuN fluorescence were strong in the cortical layer II/III of V1 in both young adult and aged rats (Fig. 1). It is evident that the AnkyrinG protein, an important AIS scaffolding molecule, was distributed mostly within AISs, although a few proteins were scattered in the Ranvier nodes along axons. Similar to previous studies 3,12, most neurons in the cortical layer II/III of young and aged rats showed no evident gap between the soma and the proximal end of the AIS (Fig. 1). Thus, the AIS position relative to the soma was not examined in this study, and the AIS length of layer II/III neurons in both age groups was measured quantitatively.
Fig. 1.
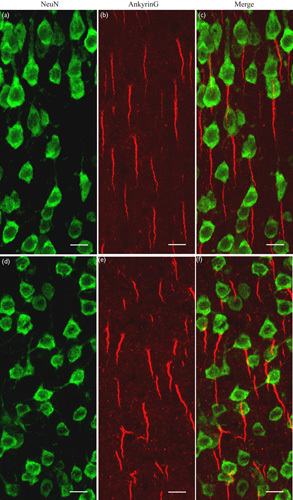
Immunofluorescent double labeling showing the cell body (green) and axon initial segment (AIS) (red) of neurons at layer II/III of the V1 cortical area from young adult (a–c) and aged (d–f) rats. (a, d) Cell bodies of neurons labeled with anti-NeuN. (b, e) AISs of neurons labeled with anti-AnkyrinG, a specific cytoskeletal protein. (c, f) The merged images of cell bodies and the corresponding AISs. Most neurons show no evident gap between the cell body and the AIS. The scale bar represents 10 μm.
Our results show that the majority of neurons in layer II/III of young rats (74.1%) had an AIS longer than 18 μm, whereas the majority of neurons in layer II/III of aged rats (69.9%) had an AIS shorter than 18 μm (Fig. 2). Statistical analysis showed that the percentage of neurons within a certain range of AIS length in layer II/III of aged rats was significantly different from that of young adult rats [χ2(11)=301.118, P<0.0001]. Further, the mean AIS length in aged rats (mean=16.9±3.1, n=704) was significantly smaller than that in young adult ones (mean=19.8±2.8, n=715) (independent-samples t-test, P<0.0001). The mean AIS length in aged rats was 14.6% smaller than that in young rats. This result indicates that aging significantly shortens the AIS length of layer II/III neurons in the V1 cortical area of the rat.
Fig. 2.
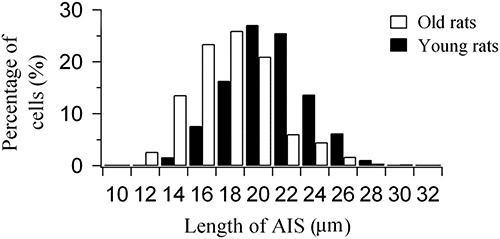
Percentage of layer II/III neurons within a certain range of axon initial segment (AIS) length in the V1 cortical area of young adult and aged rats.
Age-associated change of Nav1.6 distribution along axon initial segment
An age-related shortening of AISs may reduce the distribution of Na+ ion channels along AISs 17. To examine this possibility, we quantitatively measured the IOD of Nav1.6, a key voltage-gated Na+ ion channel in AP initiation, within AISs in layer II/III of the V1 cortical area in both age groups.
Double immunoreactive labeling showed that Nav1.6 and AnkyrinG fluorescence were evident at layer II/III of V1 in both young adult and aged rats (Fig. 3). Similar to AnkyrinG, Nav1.6 distributed mostly along AISs, although some proteins were scattered in the Ranvier nodes along axons. In contrast, the immunofluorescence of Nav1.6 in layer II/III of aged rats was weaker than that of young adult rats (Fig. 3). Statistical analysis showed that the mean Nav1.6 IOD within AISs in aged rats (mean=0.14±0.02, n=100) was significantly lower than that in young rats (mean=0.18±0.03, n=100) (independent-samples t-test, P<0.0001; Fig. 4a). To examine whether the distribution of Nav1.6 Na+ ion channels along AISs was affected equally by aging, we quantitatively measured the Nav1.6 IOD at different positions of AISs after normalization of the AIS length in both age groups (see “Confocal image acquisition and data analysis” in the Materials and methods). Statistical analysis indicated that the mean IOD of Nav1.6 varied significantly at different positions along normalized AISs within both the young and the aged group [young rats: F(24, 2500)=365.681, P<0.0001; aged rats: F(24, 2500)=130.717, P<0.0001], with a higher IOD value at the distal region of AISs and a lower IOD value at the proximal region and the distal end of AISs (Fig. 4b). The mean Nav1.6 IOD along normalized AISs of aged rats was significantly decreased compared with that of young adults [F(1, 5000)=618.144, P<0.0001], but this aging-related effect depended significantly on the region along the AIS [F(24, 5000)=46.874, P<0.0001]. Compared with young rats, the mean IOD of Nav1.6 at the most proximal region (0.04–0.16 along the AIS length from the soma) and the distal region (0.52–1.0 AIS length from the soma) of AISs in aged rats showed no significant change (independent-samples t-test, n=100, all P>0.05), whereas the mean IOD of Nav1.6 at the location of 0.20, 0.24, 0.28, 0.32, 0.36, 0.40, 0.44, and 0.48 AIS length from the soma decreased by 42.3, 57.7, 67.5, 65.2, 62.4, 56.9, 34.9, and 22.6%, respectively (independent-samples t-test, n=100, all P<0.0001; Fig. 4b). Therefore, the AIS region with a high intensity of Nav1.6 clusters was significantly shortened during senescence.
Fig. 3.
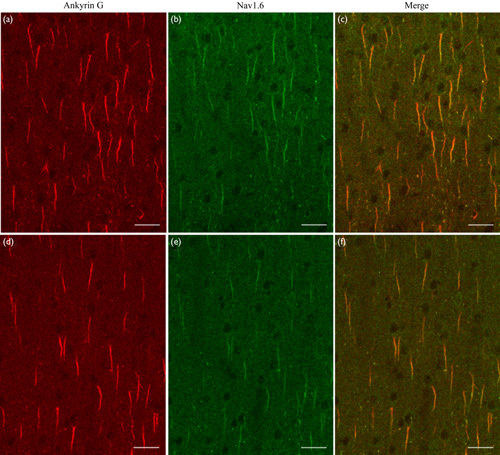
Immunofluorescent double labeling showing the axon initial segment (AIS) (red) and the distribution of Nav1.6 Na+ ion channels (green) along AISs of layer II/III neurons in the V1 cortical area of young adult (a–c) and aged (d–f) rats. (a, d) AISs of neurons labeled with anti-AnkyrinG, a specific scaffolding protein. (b, e) The distribution of Nav1.6 Na+ ion channels along AISs. (c, f) Merged images of AnkyrinG and Nav1.6 fluorescence along AISs. The scale bar represents 30 μm.
Fig. 4.
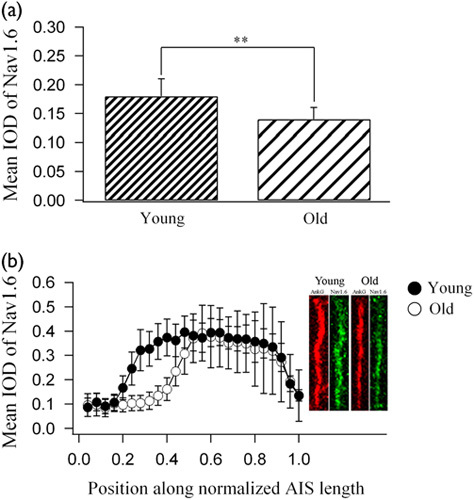
Statistics of Nav1.6 immunofluorescence optical density (IOD) within axon initial segments (AISs) of layer II/III neurons in the V1 cortical area of young adult and aged rats. (a) The mean IOD of Nav1.6 within AISs of neurons in aged and young adult rats. (b) The change in the mean Nav1.6 IOD at different positions along normalized AISs of neurons in young adult and aged rats. The point 0.0 on the x-axis denotes the soma position and the point 1.0 denotes the AIS end. The right panel within (b) represents typical samples of AIS (labeled with anti-AnkyrinG) and Nav1.6 fluorescence signals along AISs of young and aged rats. All AISs are normalized to 1.0 in length for the measurement of Nav1.6 fluorescence at different positions along the AIS.
Discussion
The AIS, which links the axon to the soma, is a subcellular structure organized by specific cytoskeletal proteins, especially AnkyrinG, and enriched with voltage-gated Na+ ion channels, and it therefore functions as the site that converts synaptic potentials into APs in neurons 17,18. A growing body of evidence indicates that AISs not only vary in structure and molecular components in a cell-specific manner 2,19 but also show considerable plasticity during neuronal activity alteration 1,5, brain disorders 6, and normal brain development 3,12. In general, plasticity of AISs represents an adaptive change that compensates for the imbalance between neuronal excitation and inhibition, and is thus beneficial for maintaining homeostasis of network activity 4,12. In addition, the AIS structure shows a change in length or position (or both) relative to the soma. For example, AISs become shorter and/or more remote from the soma when neuronal activity increases, whereas AISs become longer and/or move closer to the soma when neuronal activity is reduced 3,4,20.
It is reported widely that neuronal excitation increases during aging, as indicated by an increased stimulus-evoked response and highly enhanced spontaneous activity 8–11. On the basis of the plastic changes in the AIS structure reported previously, it is anticipated that neurons with increased excitation during aging may induce AIS shortening and/or relocation away from the soma 4,18. We examined these possibilities in this study. Unexpectedly, our results showed that most neurons in aged and young adult rats showed no evident gap between the soma and the AIS proximal end, as reported previously for the visual cortex at different developmental stages 3. Consistent with our expectation, the mean AIS length of neurons in layer II/III in the V1 area of aged rats was significantly smaller than that of young rats. This result is in agreement with a recent report on the cerebral cortex of senescent marmoset monkeys 12. Collectively, these results indicate that AIS shortening could be a plastic structural change that occurs widely in the aged mammalian cortex.
The implication of age-related AIS shortening in cortical neurons remains unclear. A simple explanation is a compensatory response to the increased neuronal excitation in the aged visual cortex 4,12 because a shortened AIS can reduce the availability of binding sites for Na+ ion channels for AP initiation, as observed in the current study, and thus decrease neuronal excitability. However, this interpretation is inconsistent with evidence shown by electrophysiological studies. Numerous investigations have indicated that neurons in the visual cortex of several species, including rats 8, show markedly enhanced excitation during the aging process, including a stronger visually evoked response and spontaneous activity, in aged individuals compared with young ones 8–10. This means that aging-related AIS plasticity does not primarily contribute to maintaining the excitation/inhibition balance in the aged cortex. Another explanation is that shortening of AISs may serve to maintain energy homeostasis in the brain during aging. Because of a higher firing rate, cortical neurons in the aged brain will need more Na+ ion pumps and ATP to return Na+ ions outside the cell during AP repolarization. However, a number of investigations have shown that the expression of Na+ ion pumps is significantly reduced during aging 21–23. Therefore, neurons in the aged brain need a new strategy to solve the Na+ ion transport problem. Several previous studies have reported that cortical neurons show a decreased AP amplitude during aging 24,25, which indicates that neurons in the aged brain may show reduced Na+ ion entry during AP initiation. In the current study, we have found that AISs of V1 neurons shorten during aging, and the Nav1.6 Na+ ion channel distribution is reduced. Taken together, an age-related reduction in AIS length and Nav1.6 Na+ ion channel distribution may cause a significant reduction in Na+ ion entry during AP initiation at the AIS, which will reduce the energy consumption by Na+ ion pumps during membrane repolarization. Therefore, this AIS morphological change may not only solve the problem of Na+ ion pump shortage but also balance the energy expenditure caused by the increased neuronal firing rate in the aged brain.
Acknowledgements
This research work was supported by the National Natural Science Foundation of China (31171082, 31771181); the Foundation of Anhui University Scientific Research Innovation Platform Team (AT161805); the Foundation of Key Laboratory for Conservation and Use of Important Biological Resources of Anhui Province, College of Life Sciences (591601); and the Foundation of the Molecular Biology and Biotechnology Institute of Anhui Normal University (20170720).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Grubb MS, Shu Y, Kuba H, Rasband MN, Wimmer VC, Bender KJ. Short- and long-term plasticity at the axon initial segment. J Neurosci 2011; 31:16049–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature 2006; 444:1069–1072. [DOI] [PubMed] [Google Scholar]
- 3.Gutzmann A, Ergul N, Grossmann R, Schultz C, Wahle P, Engelhardt M. A period of structural plasticity at the axon initial segment in developing visual cortex. Front Neuroanat 2014; 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada R, Kuba H. Structural and functional plasticity at the axon initial segment. Front Cell Neurosci 2016; 10:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature 2010; 465:1075–1078. [DOI] [PubMed] [Google Scholar]
- 6.Hsu WC, Nilsson CL, Laezza F. Role of the axonal initial segment in psychiatric disorders: function, dysfunction, and intervention. Front Psychiatry 2014; 5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen AV, Cotel F, Perrier JF. Plasticity of the axon initial segment: fast and slow processes with multiple functional roles. Neuroscientist 2017; 23:364–373. [DOI] [PubMed] [Google Scholar]
- 8.Ding Y, Zheng Y, Liu T, Chen T, Wang C, Sun Q, et al. Changes in GABAergic markers accompany degradation of neuronal function in the primary visual cortex of senescent rats. Sci Rep 2017; 7:14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiol Aging 2006; 27:155–162. [DOI] [PubMed] [Google Scholar]
- 10.Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science 2003; 300:812–815. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Xie X, Li X, Chen B, Zhou Y. Functional degradation of visual cortical cells in aged rats. Brain Res 2006; 1122:93–98. [DOI] [PubMed] [Google Scholar]
- 12.Atapour N, Rosa MGP. Age-related plasticity of the axon initial segment of cortical pyramidal cells in marmoset monkeys. Neurobiol Aging 2017; 57:95–103. [DOI] [PubMed] [Google Scholar]
- 13.Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. Nat Neurosci 2009; 12:996–1002. [DOI] [PubMed] [Google Scholar]
- 14.Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. Role of axonal Nav 1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol 2008; 100:2361–2380. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Wang Q, He F, Ding Y, Sun Q, Hua T, Xi M. Dietary restriction affects neuronal response property and GABA synthesis in the primary visual cortex. PLoS One 2016; 11:e0149004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isaacs J, Mallu S, Batchelor M. Modification of commercially available image analysis software for semi-automated qualitative analysis of axon regeneration and myelination in the rat sciatic nerve. J Neurosci Methods 2014; 233:45–49. [DOI] [PubMed] [Google Scholar]
- 17.Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. Ankyrin(G) is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 1998; 143:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuba H. Structural tuning and plasticity of the axon initial segment in auditory neurons. J Physiol 2012; 590:5571–5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci 2008; 28:14329–14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 2010; 465:1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Lores Arnaiz GR, Ordieres MGL. Brain Na(+), K(+)−ATPase activity in aging and disease. Int J Biomed Sci 2014; 10:85–102. [PMC free article] [PubMed] [Google Scholar]
- 22.Kocak H, Oner P, Oztas B. Comparison of the activities of Na(+), K(+)−ATPase in brains of rats at different ages. Gerontology 2002; 48:279–281. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka Y, Ando S. Synaptic aging as revealed by changes in membrane potential and decreased activity of Na+, K(+)−ATPase. Brain Res 1990; 506:46–52. [DOI] [PubMed] [Google Scholar]
- 24.Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex 2005; 15:409–418. [DOI] [PubMed] [Google Scholar]
- 25.Luebke JI, Chang YM. Effects of aging on the electrophysiological properties of layer 5 pyramidal cells in the monkey prefrontal cortex. Neuroscience 2007; 150:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]


