Supplemental Digital Content is Available in the Text.
Keywords: HIV, Pain, Systematic review, Psychosocial factors
Abstract
Chronic pain remains a prevalent and disabling problem for people living with HIV in the current antiretroviral treatment era. Psychosocial treatments may have promise for managing the impact of this pain. However, research is needed to identify psychosocial processes to target through such treatments. The current systematic review and meta-analysis examined the evidence for psychosocial factors associated with pain, disability, and quality of life in people living with HIV and persistent pain. Observational and experimental studies reporting on the association between one or more psychosocial factors and one or more pain-related variables in an adult sample of people living with HIV and pain were eligible. Two reviewers independently conducted eligibility screening, data extraction, and quality assessment. Forty-six studies were included in the review and 37 of these provided data for meta-analyses (12,493 participants). “Some” or “moderate” evidence supported an association between pain outcomes in people with HIV and the following psychosocial factors: depression, psychological distress, posttraumatic stress, drug abuse, sleep disturbance, reduced antiretroviral adherence, health care use, missed HIV clinic visits, unemployment, and protective psychological factors. Surprisingly, few studies examined protective psychological factors or social processes, such as stigma. There were few high-quality studies. These findings can inform future research and psychosocial treatment development in this area. Greater theoretical and empirical focus is needed to examine the role of protective factors and social processes on pain outcomes in this context. The review protocol was registered with PROSPERO (CRD42016036329).
1. Introduction
HIV remains a significant global health concern with 36.7 million people living with HIV worldwide.130 The availability of combined antiretroviral therapy (cART) has drastically improved life expectancy.9,93,120 In well-resourced countries, and increasingly in less well-resourced regions, the shift in HIV from a terminal illness to a chronic condition has led to a focus on disease and symptom management.59
Chronic pain is a common symptom in people with HIV. Data from one systematic review indicate that 54% to 83% of people with HIV may experience clinically meaningful persistent pain, and these estimates seem to be stable from the pre- to current-cART era.80 Neuropathic pain is a frequent complication of HIV and/or antiretroviral therapy.15 Approximately 42% to 66% of people with HIV have peripheral sensory neuropathy (HIV-SN), and around 54% to 78% of these experience neuropathic pain.84,88,129 Importantly, pain in people with HIV is associated with increased disability and reduced quality of life.27
There are few pharmacological options for managing chronic HIV-related pain. A systematic review of 14 randomized controlled trials (RCTs) of pharmacotherapy for painful HIV-SN found efficacy only for topical capsaicin, smoked cannabis, and subcutaneous nerve growth factor.85 However, nerve growth factor is not clinically available, capsaicin is not feasible in lower-resourced settings, and a subsequent review of cannabis showed no effect on neuropathic pain and concerns about long-term side effects.32 Additional negative RCTs of pregabalin, capsaicin, and amitriptyline have been published.17,24,103
In the wider literature, psychological approaches are common in chronic pain management.34 Psychological treatments, including cognitive–behavioural therapy (CBT), are associated with improved functioning and mood for chronic pain that is primarily musculoskeletal.124 However, research on psychological treatments for pain in HIV is less well developed. Only 2 RCTs have examined CBT for people with HIV and chronic pain, but interpretation of these trials is hampered by small samples118 and high dropout rates.29 An observational study of CBT for HIV-related pain showed similarly poor treatment completion.21,113 There is a clear need for improving psychological treatments for people with HIV-related pain.
Improving psychological approaches for chronic pain in HIV will require consideration of the psychosocial complexities associated with HIV. For example, stigma, mental health problems, and substance abuse may influence pain and treatment engagement in people with HIV.36,69,71,111,123,132 However, research has not systematically examined psychosocial factors associated with pain in this context. The systematic review by Parker et al. (2014), which estimated the prevalence of pain in HIV, described 5 studies reporting psychosocial factors. However, that review did not specifically include assessment of psychosocial factors in the eligibility criteria. Furthermore, 33 potentially eligible studies were excluded due to low-quality ratings,80 which limits our understanding of the range of psychosocial factors examined in this context. Therefore, we conducted a systematic review and meta-analyses to examine the associations between psychosocial factors and persistent pain in HIV. Because the aims of the review were exploratory, we did not formulate specific hypotheses about the associations between these variables.
2. Methods
The review protocol was registered with PROSPERO (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016036329).
2.1. Inclusion and exclusion criteria
2.1.1. Inclusion
(1) People with HIV aged 18 years and older.
(2) The original protocol specified the study must have a “(sub)sample with average pain duration of ≥3 months.” After piloting this criterion, a large number of studies did not define or report pain duration. We contacted authors to enquire about pain chronicity; however, these data were generally not available. Given the potentially high prevalence of chronic pain in HIV,80 we decided to include studies with (sub)samples of ambiguous pain duration, provided that “pain” vs “no pain” subgroup analyses were reported in studies for which chronic pain was not an eligibility criterion or which did not report pain duration.
(3) Data on presence of pain, pain intensity, functioning, and/or quality of life.
(4) Data on one or more psychosocial variable, representing any potentially modifiable cognitive, affective, behavioural, or interpersonal process. Adherence to antiretroviral therapy and health care use variables represent modifiable behaviour patterns. Therefore, we considered these as psychosocial variables eligible for this review.
(5) Observational (cross-sectional, case-control, or prospective) or experimental studies (RCTs) reporting between- or within-groups associations between pain and psychosocial variables in a (sub)sample with pain.
(6) Any language, from any region, from 1981 onwards (the date that HIV was identified in the literature).
(7) Studies (published and unpublished) with an available full-text. Where only abstracts or trial registration summaries were available, the authors provided unpublished data for the review. Unpublished studies are commonly included in systematic reviews, given recognition of overestimation of effects in published research.62 In addition, studies conducted in lower-resourced countries where HIV is particularly prevalent may not always proceed through to publication. Therefore, the inclusion of unpublished studies and dissertations may help overcome this disparity and allows us to consider potential contextual differences.
2.1.2. Exclusion
(1) Studies only measuring associations between unchangeable demographic factors (eg, age, ethnicity) and pain. While piloting the eligibility criteria, we found a number of studies reporting history of injecting drug use as participants' HIV risk factor. Given lack of further information about substance abuse history or current abuse, we excluded studies for which injecting drug use history was the only psychosocial factor. Likewise, we excluded studies reporting only average units of alcohol consumed, rather than alcohol abuse.
(2) Qualitative studies.
2.2. Search strategy
We searched the following databases during March 2016: Medline, EMBASE, CINAHL, PsycINFO, Cochrane, and Web of Science. We also searched ISRCTN, clinicaltrials.gov, and EU Clinical Trials Register. Reference lists of eligible studies were searched and key authors were contacted. We reran the search in August 2017. The search included terms for the target population (HIV or AIDS), outcome (chronic pain), and exposure measurement (psychosocial factors). Relevant search terms were identified from previous reviews on pain in HIV,80,124 psychosocial factors in HIV,99 and psychosocial factors in chronic pain40 (Appendix A, available at http://links.lww.com/PAIN/A643).
2.3. Data extraction
Two reviewers (W.S. and C.A.) independently screened titles/abstracts and full-texts for eligibility. The following data were extracted from eligible studies: year; design; country; sample size; demographics (ie, age, sex, and race/ethnicity); clinical factors (ie, HIV duration, use of ART, CD4+ count and viral load, and pain duration and type); assessment of pain and psychosocial variables; and, statistical analyses. In cases where both cross-sectional and prospective data reported the same (or an overlapping) cohort and variables, the prospective analyses were extracted. Data were extracted from all studies by W.S., and independently by C.A. and K.K. who each extracted data from approximately half of the studies. Disagreements regarding eligibility and data extraction were discussed to reach consensus and, where discrepancies remained, W.S. discussed these with the wider team. The reviewers were not blinded to the authorship of the studies reviewed.
2.4. Quality assessment
We assessed the methodological quality of studies using an adapted version of quality assessment tools used in previous systematic reviews of observational studies relevant to pain and HIV.3,40,80 The quality assessment tool contained items assessing: study purpose, recruitment, response rates, sample description, assessment measurements, data analysis, and confounding/matching (Appendix B, available at http://links.lww.com/PAIN/A643). Additional items assessed features specific to prospective designs. Thus, quality scores differed for cross-sectional and prospective studies. In some cases, the overall study design did not correspond to the nature of the data extracted for the purpose of this review. In addition, in some cohorts, a cross-sectional design was used to examine one psychosocial variable, whereas a prospective design was used to examine another variable using the same sample. In all cases, the quality assessment was applied to the design used for the nature of the data extracted for a given psychosocial variable. Quality assessment items were rated as “positive” (1), “negative” (0), or “unclear” (?), and total scores were computed and classified as low (<50%), medium (50%-80%), and high (>80%).3,40 W.S. completed quality assessment ratings for all studies, whereas C.A. and K.K. each independently completed the quality assessment for approximately half of the studies. The strength of evidence was assessed according to the levels outlined by Ariëns et al.3 in a systematic review of observational studies of psychosocial risk factors for neck pain: (1) Strong: consistent results in multiple high-quality prospective and/or case-control studies; (2) Moderate: consistent results in multiple prospective and/or case-control studies; (3) Some Evidence: findings in one prospective or case-control study, or consistent findings in multiple cross-sectional studies with at least one high-quality study; and (4) Inconclusive: Inconsistent findings in multiple studies or consistent findings in multiple low-quality cross-sectional studies.3
2.5. Data synthesis
Meta-analyses were conducted using Stata 15.0 where there were at least 2 studies43 with the same design and effect estimate of the association between the same pain (eg, intensity) and psychosocial variables (eg, depression). We took a broad approach to the meta-analyses,37,45 and grouped psychosocial variables on the basis that they reflected conceptually similar underlying constructs with overlapping measurement content. All analyses were conducted using random effects, given likely heterogeneity.43 Between-study heterogeneity (I2 statistic) was interpreted as low (<25%), medium (25%-50%), and high (>50%).124 For between-groups comparisons of continuous data, mean values, SDs, and sample sizes were extracted to compute the pooled standardized mean difference (SMD). For between-groups comparisons of dichotomous data, events data and sample sizes were extracted. Where events data were not reported, odds or hazard ratios, 95% confidence intervals (95% CIs), and sample sizes were extracted. To aggregate studies reporting a mixture of odds ratios (ORs) and events data, ORs were first computed from studies reporting events data and then pooled with ORs reported in other studies. Odds ratios and hazard ratios were analysed separately. Where applicable, correlation coefficients (Pearson r) were extracted with sample sizes. We transformed r to Fisher z and computed 95% CIs of z to compute the pooled estimate.18,100
Data extracted were from bivariate analyses. Multivariate data (eg, adjusted ORs) were only extracted where bivariate data were not available. We focused on bivariate analyses because many studies did not report multivariate analyses. Moreover, studies that reported multivariate models varied substantially with respect to control variables included and inconsistently used psychosocial variables as independent or dependent variables. Taken together, these differences limit meaningful interpretation of multivariate analyses across studies.
Several studies presented data on more than 2 pain/no pain groups, often with idiosyncratic group definitions, which limited our ability to compare studies. Where studies reported 3 or more groups, we collapsed these into 2 to represent groups with and without pain (eg, frequent/moderate/severe vs infrequent/mild/none), and computed effects between these. This approach facilitated more direct comparison across studies and thus enabled us to include a larger number of studies in the analyses. For studies comparing participants on the presence of neuropathy, we prioritised extracting data from these comparisons in the following order depending on the data reported: (1) painful vs nonpainful neuropathy; (2) painful neuropathy vs no neuropathy; and (3) neuropathy vs no neuropathy. Where there was more than one measure of the same variable, we extracted data for the measure with the widest usage or the longer measure to increase reliability.124 Our protocol specified that funnel plots would be inspected to assess for publication bias. However, due to the relatively small number of studies in each meta-analysis and the likelihood of high heterogeneity, inspection of funnels plots was not appropriate109 and, therefore, was not undertaken.
We conducted sensitivity analyses to examine the influence of the following study and patient characteristics on the findings: certainty of pain chronicity, pain type, immune functioning and viral suppression, ART treatment era, and health care system. With the exception of the pain chronicity analysis, these sensitivity analyses were prespecified. Given the large number of potential analyses, we restricted sensitivity analyses to the between-groups SMDs for depression because this was the analysis with the largest number of studies.
3. Results
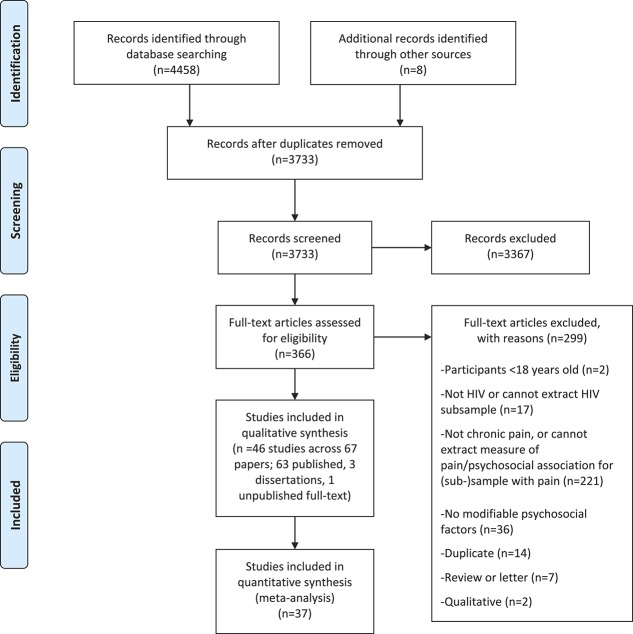
Forty-six studies were included in the review (13,480 participants) and 37 of these provided data for meta-analyses (12,493 participants; Fig. 1) . Most (83%) were conducted in the United States, with 4 studies from South Africa,79,88,121,122 and one each from the United Kingdom,84 Thailand,89 Uganda,95 and Russia.114 Participants were primarily recruited from HIV clinics or using multifaceted strategies that also included recruitment from substance abuse clinics and community outreach. One study recruited exclusively from a methadone clinic,8 whereas 2 others recruited in high-poverty areas.39,110 The samples comprised predominantly men in 41 studies, with the proportion of men in these studies ranging from 51%50 to 100%.28,104 Five studies (4 from South Africa and 1 from the United States) recruited women exclusively79,92 or predominantly (proportion of women ranging from 72% to 88%).88,121,122 The mean age ranged from 30.1 (SD = 5.2)114,115 to 51.0 (SD = 9.3) years.119 HIV duration was not consistently reported; however, of the studies providing data, duration ranged from 2.09 (SD = 1.22)102 to 16.95 years (SD = 8.70).119 Eighteen studies (39%) reported on mixed HIV/AIDS samples (reported proportion with AIDS ranged from 10% to 74%). Four studies included only participants with AIDS, one study excluded patients with AIDS, whereas 23 studies did not clearly report the proportion (if any) with AIDS. Supplemental Table 1 shows further demographic characteristics of the study samples (available at http://links.lww.com/PAIN/A643).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA75) flow diagram.
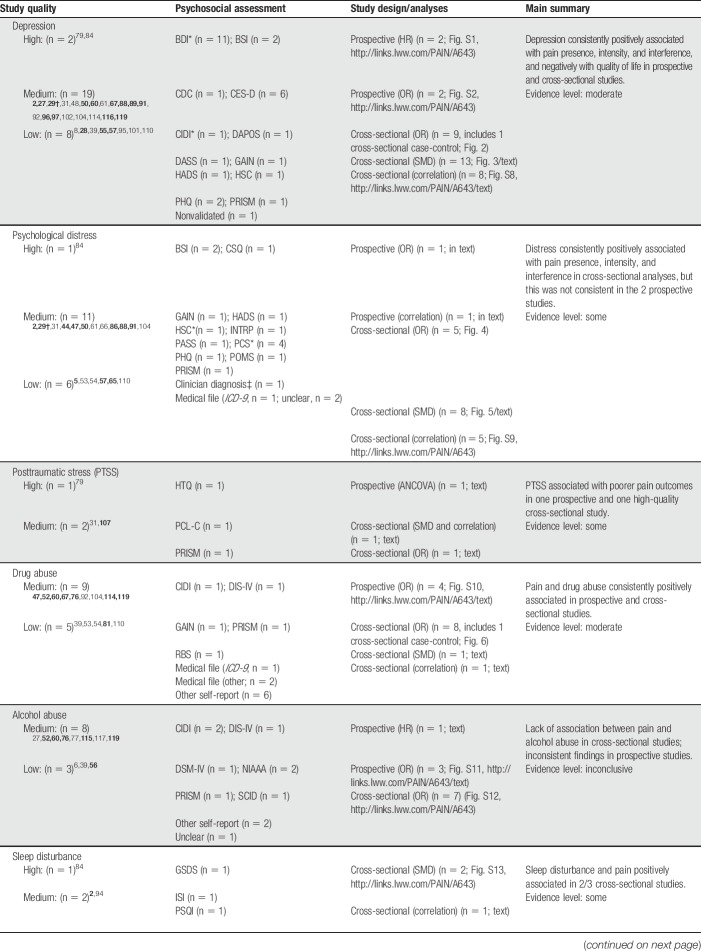
Table 1 provides a summary of study designs, quality, and evidence level for each psychosocial factor. The studies showed substantial variability in the measurement of pain and psychosocial variables. Most studies (63%) were of medium quality. Fifteen studies were of low quality, and only 2 were of high quality.79,84 The most common limitations included unclear reporting of response rates, no a priori sample size justification, and poor reporting of HIV and pain characteristics. There is no single agreed upon strategy to best address low-quality studies within meta-analyses, an issue which is compounded by the arbitrary nature of study quality scoring and cutoff points.43 This can be dealt with by only including high-quality studies, performing sensitivity analyses, or including all studies irrespective of quality and discussing risk of bias.43 Given that only 2 of 46 studies were rated as high quality, a meta-analysis of these cannot be regarded as reflecting most of the studies. Sensitivity analyses would likewise not be meaningful. Including all studies is thus the most justifiable approach for the current data. Although we have chosen to focus on data from bivariate analyses for reasons outlined in the Methods, studies that reported a multivariate model of the association between psychosocial and pain variables are shown in bold in Table 1 for ease of reference.
Table 1.
Summary of the evidence for psychosocial variables.
3.1. Depression
Depression was the most frequently assessed psychological variable, investigated in 29 studies. Two prospective studies reported hazard ratios for baseline depression predicting time to onset of symptomatic neuropathy. The pooled hazard ratio was significant and indicated that baseline depression was more severe in participants who developed symptomatic neuropathy at follow-up than those who did not: HR = 1.04 (95% CI 1.02-1.07), z = 3.23, P = 0.001 (supplemental Figure 1, available at http://links.lww.com/PAIN/A643). Heterogeneity was 0.0%. Two further prospective studies reported ORs. The pooled OR was significant and indicted that higher baseline depression symptoms were associated with greater likelihood of follow-up pain: OR = 2.26 (95% CI 1.47-3.47), z = 3.72, P < 0.001 (supplemental Figure 2, available at http://links.lww.com/PAIN/A643). Heterogeneity was medium (40.1%). Nine cross-sectional studies provided events data or ORs. The pooled OR was significant such that depression was more likely in participants with vs without pain: OR = 2.65 (95% CI 1.62-4.34), z = 3.90, P < 0.001 (Fig. 2). Heterogeneity was high (83.0%). Twelve cross-sectional studies provided data to compute SMDs (Fig. 3). The overall effect was significant and showed moderately greater depression in participants with vs without pain: SMD = 0.68 (95% CI 0.42-0.93), z = 5.22, P < 0.001. Heterogeneity was high (I2 = 89.2%). Another cross-sectional study that reported the median and interquartile range found no difference in depression between groups with (n = 125) and without pain (n = 72).88
Figure 2.
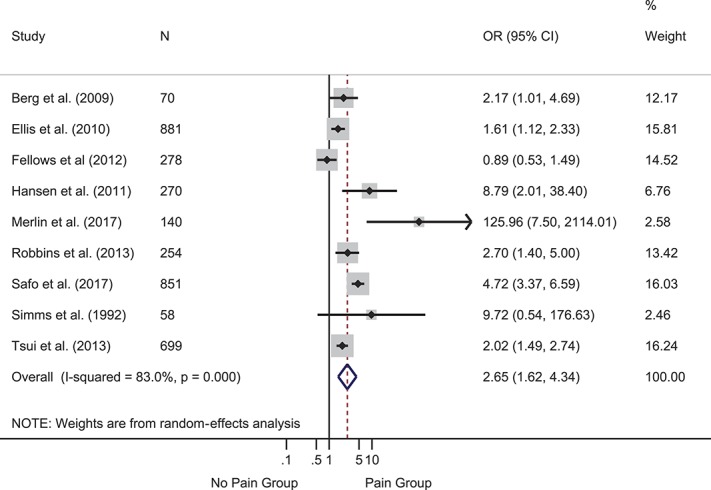
Forest plot of cross-sectional odds ratios (ORs) for depression. Depression was more likely in participants with vs without pain, as reflected in the pooled OR of >1. Gray boxes show weighting of individual studies; the red dotted line indicates the pooled effect around which effects from individual studies vary; the blue diamond shows the 95% CI around the pooled effect. CI, confidence interval.
Figure 3.
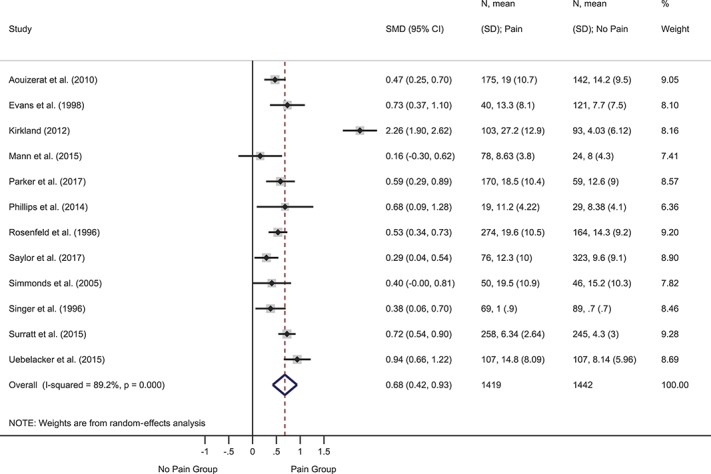
Forest plot of cross-sectional standardized mean differences (SMDs) for depression. Depression symptoms were more severe in participants with vs without pain, as indicated by a positive pooled SMD. CI, confidence interval.
Six cross-sectional studies reported correlation coefficients between depression and pain severity. The pooled correlation was small, but significant: Fisher z = 0.26 (95% CI 0.18-0.33), z = 6.77, P < 0.001. Heterogeneity was 0.0%. One additional study found a nonsignificant correlation, although the coefficient was not reported.27 Four cross-sectional studies reported correlations between depression and pain interference/disability. The pooled correlation was moderate: Fisher z = 0.48 (95% CI 0.41-0.56), z = 12.48, P < 0.001. Heterogeneity was 0.0%. Three cross-sectional studies reported correlations between depression and quality of life. The pooled correlation was large and significant: Fisher z = −0.52 (95% CI −0.75 to −0.30), z = 4.51, P < 0.001. Heterogeneity was high at 73.3% (all correlation analyses, supplemental Figure 8, available at http://links.lww.com/PAIN/A643). One final cross-sectional study (n = 120) reported a moderate correlation between pain presence and depression.55
3.2. Depression sensitivity analyses
We conducted sensitivity analyses on the SMDs for depression between pain and no pain groups (supplemental Figures 3–7, available at http://links.lww.com/PAIN/A643). We excluded data from the Kirkland study here because the SMD from this study was substantial and seemed to be driving heterogeneity in the primary meta-analysis. Excluding the Kirkland data reduced heterogeneity from I2 = 89.2% to 52.2%. Thus, 11 studies were included in sensitivity analyses.
The pooled SMD for depression was medium for studies with certain (0.61, 95% CI 0.11-1.12, z = 2.39, P = 0.02; I2 = 75.1%) and uncertain pain chronicity (0.53, 95% CI 0.41-0.64, z = 9.04, P < 0.001; I2 = 34.5%). In the analysis by pain type, the pooled SMD for depression was moderate in studies with mixed pain types (0.75, 95% CI 0.58-0.92, z = 8.51, P < 0.001; I2 = 31.7%) and for which pain type was not reported (0.52, 95% CI 0.39-0.65; z = 7.91, P < 0.001; I2 = 0.0%). By contrast, studies with neuropathic pain (0.31, 95% CI 0.11-0.52; z = 2.96, P = 0.003; I2 = 0.0%) or headache (0.38, 95% CI 0.06-0.70, z = 2.34, P = 0.02) showed small but significant differences between groups on depression.
The pooled SMD for depression was moderate for studies in which participants had less than adequate immune functioning and viral suppression (0.56, 95% CI 0.42-0.70, z = 7.77, P < 0.001; I2 = 0.0%) and for studies in which these indicators were uncertain (0.53, 95% CI 0.33-0.73, z = 5.28, P < 0.001; I2 = 69.1%). There were no studies with “adequate” functioning on these indices in this analysis. Studies from the pre-cART (0.49, 95% CI 0.32-0.66, z = 5.75, P < 0.001; I2 = 0.0%), cART (0.73, 95% CI 0.37-1.10, z = 3.91, P < 0.001), and current-cART era (0.55, 95% CI 0.38-0.72, z = 6.21, P < 0.001; I2 = 62.5%) all had moderate or near-moderate pooled SMDs. Finally, pooled SMDs for depression were similar in studies from the United States, which has a mixed health care system (0.57, 95% CI 0.43-0.72, z = 7.58, P < 0.001; I2 = 55.7%), and one study from the United Kingdom, which has universal health care (0.68, 95% CI 0.09-1.28; z = 2.25, P = 0.03). The pooled SMD of 2 studies conducted in lower- and middle-income countries was smaller, but statistically significant (0.43, 95% CI 0.14-0.72, z = 2.90, P = 0.004; I2 = 54.2%).
3.3. Psychological distress
Eighteen studies examined variables representing psychological distress, including anxiety-related constructs and the presence of “mental illness,” which generally described a combination of anxiety and depression. Five cross-sectional studies provided events data (Fig. 4). The pooled OR was significant and indicated that participants with pain were more likely to have psychological distress than those without pain: OR = 2.56 (95% CI 1.67-3.90), z = 4.34, P < 0.001. Heterogeneity was high (I2 = 68.3%). One prospective study (n = 127) found that baseline mental illness did not predict presence of pain over follow-up.54 Seven cross-sectional studies provided mean values and SDs (Fig. 5). The pooled SMD showed a large and statistically significant difference between groups such that distress was worse in participants with vs without pain (SMD = 0.85, 95% CI 0.35-1.35); z = 3.33, P = 0.001). Heterogeneity was very high (I2 = 95.4%). One further study that reported the median and interquartile range found no difference between groups.88
Figure 4.
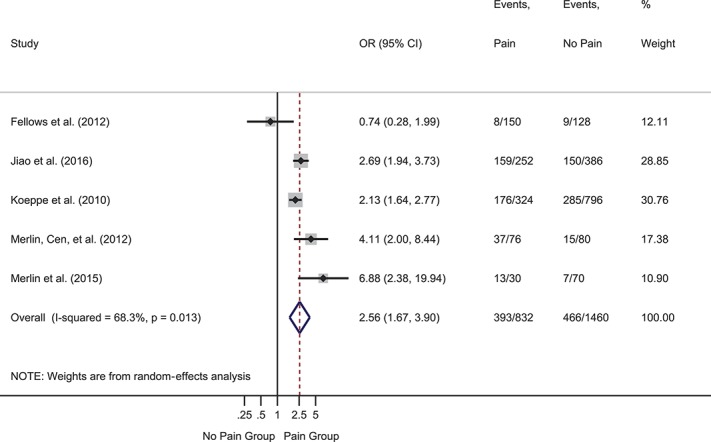
Forest plot of cross-sectional events data for psychological distress. Distress was more likely in participants with vs without pain, as reflected in the pooled odds ratio (OR) of >1. CI, confidence interval.
Figure 5.
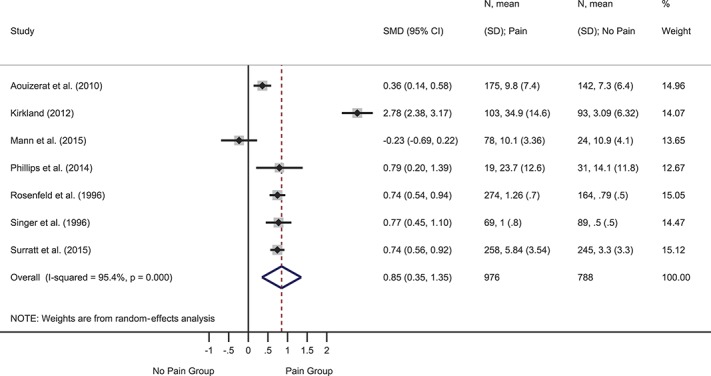
Forest plot of cross-sectional standardized mean differences (SMDs) for psychological distress. Distress was more severe in participants with vs without pain, as reflected by a positive pooled SMD. CI, confidence interval.
Four cross-sectional studies reported correlations between distress and pain severity (supplemental Figure 9, available at http://links.lww.com/PAIN/A643). The pooled correlation was moderate: Fisher z = 0.35 (95% CI 0.09-0.60), z = 2.68, P = 0.007. Heterogeneity was high (82.2%). Three cross-sectional studies reported correlations between distress and pain interference/disability. The pooled correlation was moderate: Fisher z = 0.59 (95% CI 0.24-0.93), z = 3.34, P = 0.001. Heterogeneity was high (81.2%). One prospective study (n = 45-62) found a nonsignificant correlation between change in distress and pain severity after CBT, and a significant, moderate correlation between change in distress and pain interference.44,86 Finally, one cross-sectional study reported a nonsignificant correlation between pain intensity and distress (r not reported), and a small negative correlation between distress and quality of life.88
3.4. Posttraumatic stress
Three studies investigated posttraumatic stress. These studies are reported separate from studies measuring psychological distress, given the specificity of posttraumatic stress as a variable. Different study designs and analyses precluded meta-analysis. One prospective study (n = 143) found that posttraumatic stress symptoms (PTSSs) were associated with significantly higher pain severity and interference over time in a sample with HIV and persistent pain.107 One high-quality cross-sectional study found that participants with pain (n = 170) had significantly higher PTSS than those without pain (n = 59).79 Within the pain group in this study, there was a nonsignificant correlation between PTSSs and pain severity, and small but significant correlations between PTSSs and pain interference (positive correlation) and quality of life (negative correlation).79 Posttraumatic stress disorder did not differ between groups with (n = 150) and without (n = 128) neuropathy in another cross-sectional study.31
3.5. Drug abuse
Fourteen studies examined drug abuse. We prioritised extracting opioid abuse data when multiple drug abuse categories were reported, given the relevance of opioid use in chronic pain. Two prospective studies reported ORs for pain predicting heroin use at the time of follow-up. The pooled OR indicated that participants with pain at baseline were more likely at follow-up to be using heroin: OR = 1.70 (95% CI 1.22-2.38), z = 3.13, P = 0.002 (supplemental Figure 10, available at http://links.lww.com/PAIN/A643). Heterogeneity was low (I2 = 14.0%). Conversely, another prospective study (n = 493) reported that baseline opioid use disorder history predicted new onset of neuropathic pain, OR = 2.87 (1.31-6.28), P < 0.01.60 One low-quality prospective study (n = 127) found that baseline drug abuse history did not predict the presence of pain at follow-up, 0.55 (0.25-1.21).54 These 2 studies could not be combined due to different coding of the dependent variable.
Eight cross-sectional studies reported events data (Fig. 6). The pooled OR was significant such that participants with pain were more likely to have comorbid drug abuse than those without pain: OR = 1.59 (95% CI 1.12-2.26), z = 2.58, P = 0.01. Heterogeneity was high (I2 = 69.8%), mainly attributable to one study that found the opposite effect, such that participants with symptomatic distal sensory polyneuropathy were less likely to have opioid use disorder than those with asymptomatic distal sensory polyneuropathy.76 One low-quality cross-sectional study (n = 503) found that participants with “untreated” pain had greater dependence symptoms than those with “treated” pain or without pain.110 Another low-quality cross-sectional study (n = 73) found a small positive correlation between “aberrant drug behaviours” and pain interference, but not quality of life.81
Figure 6.
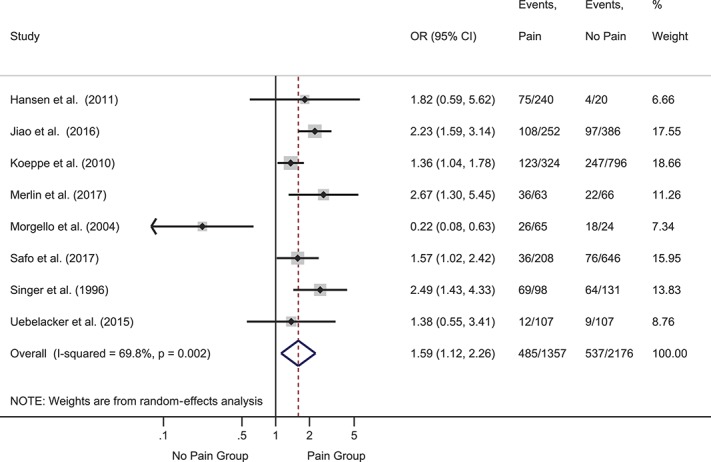
Forest plot of cross-sectional events data for drug abuse. Drug abuse was more likely in participants with vs without pain, as reflected in the pooled odds ratio (OR) of >1. CI, confidence interval.
3.6. Alcohol abuse
Eleven studies investigated alcohol abuse. Two prospective studies reported ORs for baseline pain predicting subsequent alcohol abuse. The pooled OR was not significant: OR = 0.94 (95% CI 0.39-2.26), z = 0.13, P = 0.90 (supplemental Figure 11, available at http://links.lww.com/PAIN/A643). Heterogeneity was high (84.1%). Two additional prospective studies examined baseline alcohol abuse as a predictor of developing pain/neuropathy but could not be combined due to different analyses. Both studies reported a nonsignificant association between these variables.60,77 Seven cross-sectional studies provided events data or ORs. The pooled OR was not significant: OR = 1.22 (95% CI 0.92-1.62), z = 1.36, P = 0.17 (supplemental Figure 12, available at http://links.lww.com/PAIN/A643). Heterogeneity was medium (I2 = 39.0%).
3.7. Sleep disturbance
Three studies investigated sleep disturbance. Two cross-sectional studies reported mean values and SDs. The pooled effect was significant and showed moderately greater sleep problems in participants with vs without pain: SMD = 0.66 (95% CI 0.45-0.87), z = 6.12, P < 0.001. Heterogeneity was 0.0% (supplemental Figure 13, available at http://links.lww.com/PAIN/A643). Another cross-sectional (n = 45) study reported a significant correlation between pain severity and sleep disturbance, and a nonsignificant correlation between sleep and functioning.94
3.8. Antiretroviral nonadherence
Seven studies investigated the association between pain and suboptimal ART adherence. Data were analysed separately according to whether the adherence variable was coded in the direction of nonadherence or adherence. One prospective study (n = 258) reported that severe pain at baseline predicted higher odds (OR = 1.37, 95% CI 1.02-1.85) of follow-up ART nonadherence.46 One cross-sectional study provided events data, whereas another provided an OR. The pooled OR was significant and indicated that participants with pain were more likely to report nonadherence: OR = 1.40 (95% CI 1.07-1.82), z = 2.50, P = 0.01 (supplemental Figure 14, available at http://links.lww.com/PAIN/A643). Heterogeneity was 0.00%. One cross-sectional study (n = 42) found significant positive correlations between pain severity and adherence forgetfulness and fears.57
Two cross-sectional studies reported data for the association between pain and adherence (events data or OR). The pooled OR was less than one, indicating the likelihood of adherence was lower in participants with pain, but this was not statistically significant: OR = 0.32 (95% CI 0.08-1.32), z = 1.57, P = 0.12 (supplemental Figure 15, available at http://links.lww.com/PAIN/A643). Heterogeneity was high (74.3%). Finally, one low-quality cross-sectional study (n = 377) found that pain presence was not associated with adherence in a structural equation model.72
3.9. Health care use
Six studies examined health care use. One prospective study (n = 1521) found that baseline pain predicted significantly higher odds (OR = 1.6, 95% CI 1.2-2.0) of urgent care visits.68 Two cross-sectional studies reported events data. The pooled OR was not significant: OR = 0.98 (95% CI 0.58-1.66, z = 0.07, P = 0.94) (supplemental Figure 16, available at http://links.lww.com/PAIN/A643). Heterogeneity was 0.0%. Two further cross-sectional studies reported mean values and SDs. The pooled effect was small but significant, such that participants with pain had greater health care use than those without pain: SMD = 0.36 (95% CI 0.21-0.51, z = 4.66, P < 0.001). Heterogeneity was 0.0% (supplemental Figure 17, available at http://links.lww.com/PAIN/A643). One further cross-sectional study (n = 1120) found that participants with pain and daily opioid use had more clinic visits than those with pain without daily opioid use and those without pain (SD not reported).53
3.10. Missed HIV clinic visits
Two prospective studies reported ORs for baseline presence of pain predicting missed HIV clinic visits over 1-year follow-up. The pooled OR was significant, such that those with pain at baseline had higher odds of a missed HIV clinic visit: OR = 1.42 (95% CI 1.13-1.79), z = 2.98, P = 0.003 (supplemental Figure 18, available at http://links.lww.com/PAIN/A643). Heterogeneity was 0.0%.
3.11. Unemployment
Seven cross-sectional studies provided events data or ORs for the association between unemployment and pain. The pooled OR was significant and indicated that participants with pain had higher odds of being unemployed than those without pain: OR = 2.09 (95% CI 1.59-2.76, z = 5.25, P < 0.001) (supplemental Figure 19, available at http://links.lww.com/PAIN/A643). Heterogeneity was moderate (48.6%). One further cross-sectional study (n = 229) that did not have data available for meta-analysis likewise found that participants with pain were significantly more likely to be unemployed than those without pain.79
3.12. Protective factors
Five studies examined protective psychological factors. One prospective study (n = 62) found significant small and medium correlations between change in self-reported pain acceptance during CBT and posttreatment pain severity and interference, respectively.86 One cross-sectional case-control study observed lower resilience in participants with (n = 99) vs without pain (n = 98; medium effect); however, this study found nonsignificant correlations between resilience and pain severity and interference in the pain group.121
One high-quality cross-sectional study found that participants with pain (n = 170) reported lower disease management self-efficacy than did those without pain (n = 59) (small effect).79 Within the pain group in this study, there were nonsignificant correlations between self-efficacy and pain severity and interference, and a small positive correlation between self-efficacy and quality of life.79 One low-quality cross-sectional study found that those with greater adherence self-efficacy were less likely to report pain (n = 70).8 Finally, one low-quality cross-sectional study found lower mean self-reported optimism in participants with (n = 50) vs without pain (n = 46) (small effect).101
3.13. Social factors
Four studies investigated social factors. The BEACON study (n = 377) explored social processes across 3 papers, 2 of which describe prospective data (medium quality), whereas the third reported cross-sectional data (low quality). Baseline chronic pain predicted “negative social support” (ie, overly intrusive or insensitive responses from others and a lack of support) at 12 months, controlling for baseline social support.73 Another prospective analysis showed that no chronic pain at baseline predicted greater support reciprocity at follow-up.74 Chronic pain was associated with significantly poorer ratings of patient–provider engagement in cross-sectional analyses.72
Two studies examined self-reported stigma, but could not be combined. One medium-quality cross-sectional study (n = 50) found a moderate positive correlation between stigma and pain severity.122 One low-quality cross-sectional study (n = 201) found that participants with “pain disorder” reported higher stigma scores than those without “pain disorder.”98 One medium-quality cross-sectional study found no difference in mean number or quality of self-reported social supports between participants with (n = 274) and without pain (n = 164).91
4. Discussion
This review including over 13,000 participants found “some” or “moderate” evidence supporting an association between pain outcomes and depression, psychological distress, posttraumatic stress, drug abuse, sleep disturbance, health care use, missed HIV clinic visits, ART adherence, unemployment, and protective psychological factors in people with HIV. Surprisingly few studies have examined protective psychological factors or social processes. There is a lack of high-quality research on psychosocial factors related to chronic pain in people with HIV. These findings can inform future research and treatment development in this area.
The association between depression and poorer self-reported pain outcomes in HIV is consistent with the wider pain literature.4,64 Data from prospective studies suggest depression is a risk factor for pain. However, caution is warranted in this interpretation, given the observational nature of studies. There is likely a bidirectional relationship, with shared neurobiological pathways, cognitive appraisals, and behavioural disengagement underpinning this association.4,7,14 Evidence supporting the association between pain and sleep disturbance is consistent with the wider literature that reports reciprocal associations between pain, sleep, and depression.106
There was substantial variability in the assessment of “psychological distress,” which may have contributed to the statistical heterogeneity observed. Although different measures were used to assess variables such as pain catastrophizing, pain-related fear, stress, and general anxiety, these measures overlap conceptually and in item content. The consistency of results within the psychological distress category suggests the findings are robust across different assessment methods. Several studies assessed “mental illness” based on a range of diagnoses in participants' medical file without clear diagnostic criteria. Studies exploring mental health diagnoses should use valid and reliable criteria and, ideally, semi-structured clinician-administered interviews as the gold standard.128 In light of high rates of posttraumatic stress disorder (PTSD) in HIV,99 further research is particularly needed to understand the role of PTSD in pain in this context. Alternately, rather than focusing on specific mental health diagnoses, research investigating psychosocial processes that explain the impact of a range of psychological difficulties may prove useful moving forward.42
Few studies investigated fear-avoidance model variables, such as pain catastrophizing and pain-related fear, which have dominated the musculoskeletal pain literature. Fear of movement is strongly associated with musculoskeletal pain disability.20 However, neuropathic pain is often spontaneous and not clearly provoked by movement, although it may inhibit movement. Therefore, research is needed to determine the relevance of fear-avoidance model constructs in neuropathic pain that is common in HIV.
A bidirectional association between drug abuse and pain in HIV is suggested by prospective data showing opioid abuse as both predictor60 and outcome114 of pain. In a population where there are concerns about analgesic prescribing,58 poorly managed pain may contribute to increased abuse of nonprescribed opioids, which may be exacerbated by depression.114 Alternately, prolonged opioid abuse may disrupt descending pain inhibition, exacerbating pain.49 Differing definitions of drug and alcohol abuse across studies may account for variability in effects. Future research on substance abuse in this context should use validated assessments, either screening tools or diagnostic interviews that capture key features of abuse, such as continued use despite harm.1,41,128
Adherence to ART and retention in care are psychosocial factors unique to the HIV context and are of vital importance, given their associations with mortality, morbidity, and drug resistance.112 The finding that pain was associated with reduced ART adherence and missed HIV clinic visits highlights the necessity of adequate pain management in HIV. Understanding the links between pain, ART adherence, and retention in care likely requires consideration of other psychosocial factors, such as substance abuse and depression, which may mediate or moderate this association.68,92
Findings that pain was associated with greater unemployment and health care use highlight the individual, societal, and economic costs of pain in HIV. This is consistent with the broader literature, although health care use is typically underassessed in trials of psychotherapy for chronic pain.87 Studies assessing health care use were restricted to the United States, whereas the unemployment–pain link was consistent in studies from the United States, Russia, and South Africa. The association between pain and health care use differed across studies on the basis of the type of health care assessed. Assessment of the most frequently accessed services (eg, general practitioner visits), rather than relatively infrequent events (eg, hospitalisations), may increase the interpretability of future health care data.
Surprisingly, only 5 studies assessed protective psychological factors. The lack of studies on protective factors mirrors historical trends in the general pain literature, although there has been greater focus on protective factors more recently. The focus on “maladaptive” responses to pain is problematic because such responses can be understood as a function of their short-term utility.126 Moreover, abnormal conceptualizations often fail to specify pathways through which recovery and successful functioning occur when pain is present. The psychological flexibility model, within which pain acceptance has been conceptualised, might prove useful for future research.63
A recent proposal for updating the definition of pain highlights the central role of social factors.127 However, our review identified only 4 studies exploring interpersonal variables. The lack of research on stigma in relation to pain is particularly surprising because managing stigma is key to the success of the HIV/AIDS response.108 Stigma has recently been highlighted as important for the well-being of patients with chronic pain in general.23,125 Future research is needed to determine the function of stigma in chronic pain in people with and without HIV.
The study samples included in our review varied widely in terms of the proportion of men and women, participant age, ethnicity, and duration and severity of HIV. Our sensitivity analyses support the potential applicability of findings across pain types, ART treatment eras, and health care systems. Due to poor reporting of viral loads and CD4+ counts, our analysis stratifying by these indicators is difficult to interpret. Caution is also warranted regarding the cross-cultural applicability of the findings because most studies were from the United States. One South African study with a predominantly female sample found patients with and without pain did not differ on depression or anxiety, likely due to high scores across the sample.88 Socioeconomic factors, such as poverty and gender, may thus alter the relationships between pain, functioning, and mental health.88,121 Care is needed in applying Western psychological concepts in non-Western cultures.51,82,83 Research must also determine unique cultural features that influence the experience and expression of pain in HIV.
A guiding theoretical model is needed to integrate psychosocial processes relevant to pain and HIV. Such a model should make specific predictions about the relative contributions of cognitive, affective, behavioural, and sociocultural processes in relation to specific pain outcomes. This review identified a number of closely related psychosocial constructs. Therefore, a theoretical model may benefit from identifying a key set of nonoverlapping variables.63 This may draw on prominent models within the field of pain, such as the fear-avoidance19 and psychological flexibility models,63 and those within the HIV literature that focus heavily on sociocultural perspectives to understand the impact of processes, such as stigma, on well-being.78
The current findings suggest the relevance of psychosocial treatments to manage persistent pain in HIV. To the best of our knowledge, only 3 small RCTs have evaluated CBT and mindfulness-based treatment.29,35,118 Nonrandomized trials of CBT113 and hypnosis25 have also been conducted. Further evaluation of psychosocial treatments for HIV and chronic pain is thus needed. The development of treatments that specifically target psychosocial factors identified in this review with “some” or “moderate” evidence may prove fruitful.
Several limitations warrant consideration. We used a comprehensive search strategy that included efforts to identify gray literature to limit publication bias; however, relevant studies may have been missed, given the broad nature of the search. We used an adapted quality assessment tool. Although we based this on previously validated tools, the adaptations may have limited the reliability and validity of our quality assessment. Assessment of pain was inadequate in many studies. Future research should assess information regarding pain duration, intensity, location, and type. Studies investigating chronic pain should specify eligibility criteria in line with recognized definitions: the presence of daily, clinically meaningful pain intensity and functional interference for at least 3 months.10,26,131 Given the relevance of neuropathic pain in this population, the use of well-validated screening tools of neuropathy signs and symptoms is important.16,33,129
This review identified a large number of psychosocial factors. As evidence on specific psychosocial factors develops in this area, it may be useful for a future review to use a more targeted approach to synthesize data on a smaller number of prespecified variables. We focused on bivariate analyses and dichotomized multiple between-groups analyses to facilitate comparison across studies and minimise pairwise comparisons. However, this may have limited an in-depth understanding of psychosocial factors from multivariate models and more subtle subgroup analyses. Future research examining the association between psychosocial factors and pain outcomes in HIV should consider controlling for such variables as age, sex/gender, race/ethnicity, socioeconomic status, HIV duration, current and nadir CD4+ count and current and peak viral load, current and past ART regimens, and other medical comorbidities (eg, hepatitis C, diabetes, and tuberculosis). Where multiple psychosocial variables are included, sufficient rationale for each variable should be provided and care should be taken to minimize overlap in assessment content between variables.
Despite these limitations, this is the first systematic review to specifically explore psychosocial variables associated with persistent pain in HIV. From this review, it is recommended that researchers (1) focus greater attention on protective psychological factors and social processes, such as stigma and processes to undermine stigma; (2) use higher-quality assessment tools; and (3) develop and test treatments to target key psychosocial factors to improve pain outcomes in HIV. Improving quality of life is a priority as people with HIV live longer. Adequate, whole-person pain management is vital to achieve this goal.
Conflict of interest statement
The authors have no conflict of interest to declare.
This research is an independent work supported by the National Institute for Health Research (NIHR Postdoctoral Fellowship, W. Scott, PDF-2015-08-059). L.M. McCracken is partly funded through the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health. Dr. Kemp reports grants from the European Commission (FP7 Neuropain #HEALTH F2-2013-602891) during the conduct of the study. Dr. Rice reports grants from Orion Pharma, other from Spinifex/Novartis, personal fees from Imperial College Consultants, outside the submitted work. In addition, Dr. Rice has a patent, Rice A.S.C., Vandevoorde S. and Lambert D.M Methods using N-(2-propenyl)hexadecanamide and related amides to relieve pain. WO 2005/079771 pending, and a patent, Okuse K. et al., Methods of treating pain by inhibition of vgf activity EP13702262.0/ WO2013 110945, pending.
Supplementary Material
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A643.
Supplemental video content
Video content associated with this article can be found at http://links.lww.com/PAIN/A645.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Ali R, Meena S, Eastwood B, Richards I, Marsden J. Ultra-rapid screening for substance-use disorders: the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST-Lite). Drug Alcohol Depend 2013;132:352–61. [DOI] [PubMed] [Google Scholar]
- [2].Aouizerat BE, Miaskowski CA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, Lee KA. Risk factors and symptoms associated with pain in HIV-infected adults. J Assoc Nurses AIDS Care 2010;21:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ariëns GA, Mechelen Wv, Bongers PM, Bouter LM, van der Wal G. Psychosocial risk factors for neck pain: a systematic review. Am J Ind Med 2001;39:180–93. [DOI] [PubMed] [Google Scholar]
- [4].Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity—a literature review. Arch Intern Med 2003;163:2433–45. [DOI] [PubMed] [Google Scholar]
- [5].Bakka JC. The relationships of physical functioning, psychological distress, and negative thoughts to pain in people with AIDS [doctoral thesis]. Ann Arbor: UMI Company, 1995. [Google Scholar]
- [6].Banerjee S, McCutchan JA, Ances BM, Deutsch R, Riggs PK, Way L, Ellis RJ. Hypertriglyceridemia in combination antiretroviral-treated HIV-positive individuals: potential impact on HIV sensory polyneuropathy. AIDS 2011;25:F1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95–110. [Google Scholar]
- [8].Berg KM, Cooperman NA, Newville H, Arnsten JH. Self-efficacy and depression as mediators of the relationship between pain and antiretroviral adherence. AIDS Care 2009;21:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013;339:961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. PAIN 2008;136:380–7. [DOI] [PubMed] [Google Scholar]
- [11].Breitbart W, McDonald MV, Rosenfeld B, Passik SD, Hewitt D, Thaler H, Portenoy RK. Pain in ambulatory AIDS patients. I: pain characteristics and medical correlates. PAIN 1996;68:315–21. [DOI] [PubMed] [Google Scholar]
- [12].Breitbart W, Passik S, McDonald MV, Rosenfeld B, Smith M, Kaim M, Funesti-Esch J. Patient-related barriers to pain management in ambulatory AIDS patients. PAIN 1998;76:9–16. [DOI] [PubMed] [Google Scholar]
- [13].Breitbart W, Rosenfeld B, Passik S, Kaim M, Funesti-Esch J, Stein K. A comparison of pain report and adequacy of analgesic therapy in ambulatory AIDS patients with and without a history of substance abuse. PAIN 1997;72:235–43. [DOI] [PubMed] [Google Scholar]
- [14].Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399–409. [DOI] [PubMed] [Google Scholar]
- [15].Cherry CL, Wadley AL, Kamerman PR. Painful HIV-associated sensory neuropathy. Pain Manage 2012;2:543–52. [DOI] [PubMed] [Google Scholar]
- [16].Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology 2005;65:1778–81. [DOI] [PubMed] [Google Scholar]
- [17].Clifford DB, Simpson DM, Brown S, Moyle G, Brew BJ, Conway B, Tobias JK, Vanhove GF. A randomized, double-blind, controlled study of NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. J Acquir Immune Defic Syndr 2012;59:126–33. [DOI] [PubMed] [Google Scholar]
- [18].Corey DM, Dunlap WP, Burke MJ. Averaging correlations: expected values and bias in combined Pearson rs and Fisher's z transformations. J Gen Psychol 1998;125:245–61. [Google Scholar]
- [19].Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain 2012;28:475–83. [DOI] [PubMed] [Google Scholar]
- [20].Crombez G, Vlaeyen JWS, Heuts PHTG, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. PAIN 1999;80:329–39. [DOI] [PubMed] [Google Scholar]
- [21].Cucciare MA, Sorrell JT, Trafton JA. Predicting response to cognitive-behavioral therapy in a sample of HIV-positive patients with chronic pain. J Behav Med 2009;32:340–8. [DOI] [PubMed] [Google Scholar]
- [22].Davis L, Evans S, Fishman B, Haley A, Spielman L. Predictors of attrition in HIV-positive subjects with peripheral neuropathic pain. AIDS Care 2004;16:395–402. [DOI] [PubMed] [Google Scholar]
- [23].De Ruddere L, Craig KD. Understanding stigma and chronic pain: a state of the art review. PAIN 2016;157:1607–10. [DOI] [PubMed] [Google Scholar]
- [24].Dinat N, Marinda E, Moch S, Rice AS, Kamerman PR. Randomized, double-blind, crossover trial of amitriptyline for analgesia in painful HIV-associated sensory neuropathy. PLoS One 2015;10:e0126297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dorfman D, George MC, Schnur J, Simpson DM, Davidson G, Montgomery G. Hypnosis for treatment of HIV neuropathic pain: a preliminary report. Pain Med 2013;14:1048–56. [DOI] [PubMed] [Google Scholar]
- [26].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [27].Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM. Continued high prevalence and adverse clinical impact of human immunodeficiency virus–associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol 2010;67:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Evans S, Ferrando S, Sewell M, Goggin K, Fishman B, Rabkin J. Pain and depression in HIV illness. Psychosomatics 1998;39:528–35. [DOI] [PubMed] [Google Scholar]
- [29].Evans S, Fishman B, Spielman L, Haley A. Randomized trial of cognitive behavior therapy versus supportive psychotherapy for HIV-related peripheral neuropathic pain. Psychosomatics 2003;44:44–50. [DOI] [PubMed] [Google Scholar]
- [30].Evans S, Weinberg BA, Spielman L, Fishman B. Assessing negative thoughts in response to pain among people with HIV. PAIN 2003;105:239–45. [DOI] [PubMed] [Google Scholar]
- [31].Fellows RP, Byrd DA, Elliott K, Robinson-Papp J, Mindt MR, Morgello S. Distal sensory polyneuropathy is associated with neuropsychological test performance among persons with HIV. J Int Neuropsychol Soc 2012;18:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T. Neuropathic pain: an updated grading system for research and clinical practice. PAIN 2016;157:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581. [DOI] [PubMed] [Google Scholar]
- [35].George MC, Wongmek A, Kaku M, Nmashie A, Robinson-Papp J. A mixed-methods pilot study of mindfulness-based stress reduction for HIV-associated chronic pain. Behav Med 2017;43:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Golin C, Isasi F, Bontempi JB, Eng E. Secret pills: HIV-positive patients' experiences taking antiretroviral therapy in North Carolina. AIDS Educ Prev 2002;14:318–29. [DOI] [PubMed] [Google Scholar]
- [37].Gøtzsche PC. Why we need a broad perspective on meta-analysis: it may be crucially important for patients. BMJ 2000;321:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Griswold GA, Evans S, Spielman L, Fishman B. Coping strategies of HIV patients with peripheral neuropathy. AIDS Care 2005;17:711–20. [DOI] [PubMed] [Google Scholar]
- [39].Hansen L, Penko J, Guzman D, Bangsberg DR, Miaskowski C, Kushel MB. Aberrant behaviors with prescription opioids and problem drug use history in a community-based cohort of HIV-infected individuals. J Pain Symptom Manage 2011;42:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Harrison AM, McCracken LM, Bogosian A, Moss-Morris R. Towards a better understanding of MS pain: a systematic review of potentially modifiable psychosocial factors. J Psychosom Res 2015;78:12–24. [DOI] [PubMed] [Google Scholar]
- [41].Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 2013;170:834–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hayes SC, Barnes-Holmes D, Wilson KG. Contextual behavioral science: creating a science more adequate to the challenge of the human condition. J Context Behav Sci 2012;1:1–16. [Google Scholar]
- [43].Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley-Blackwell, 2011. [Google Scholar]
- [44].Huggins JL, Bonn-Miller MO, Oser ML, Sorrell JT, Trafton JA. Pain anxiety, acceptance, and outcomes among individuals with HIV and chronic pain: a preliminary investigation. Behav Res Ther 2012;50:72–8. [DOI] [PubMed] [Google Scholar]
- [45].Ioannidis JP, Patsopoulos NA, Rothstein HR. Research methodology: reasons or excuses for avoiding meta-analysis in forest plots. BMJ 2008;336:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jeevanjee S, Penko J, Guzman D, Miaskowski C, Bangsberg DR, Kushel MB. Opioid analgesic misuse is associated with incomplete antiretroviral adherence in a cohort of HIV-infected indigent adults in San Francisco. AIDS Behav 2014;18:1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jiao JM, So E, Jebakumar J, George MC, Simpson DM, Robinson-Papp J. Chronic pain disorders in HIV primary care: clinical characteristics and association with healthcare utilization. PAIN 2016;157:931–7. [DOI] [PubMed] [Google Scholar]
- [48].Keltner JR, Vaida F, Ellis RJ, Moeller-Bertram T, Fitzsimmons C, Duarte NA, Robinson-Papp J, Dworkin RH, Clifford DB, McArthur JC, Simpson DM, Collier AC, Marra CM, Atkinson J, Grant I. Health-related quality of life “well-being” in HIV distal neuropathic pain is more strongly associated with depression severity than with pain intensity. Psychosomatics 2012;53:380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals 2005;14:194–205. [DOI] [PubMed] [Google Scholar]
- [50].Kirkland KE. Psychological factors in HIV-related headaches [doctoral thesis]. Ann Arbor: UMI Company, 2012. [Google Scholar]
- [51].Kirmayer LJ. Beyond the “new cross-cultural psychiatry”: cultural biology, discursive psychology and the ironies of globalization. Transcult Psychiatry 2006;43:126–44. [DOI] [PubMed] [Google Scholar]
- [52].Knowlton AR, Nguyen TQ, Robinson AC, Harrell PT, Mitchell MM. Pain symptoms associated with opioid use among vulnerable persons with HIV: an exploratory study with implications for palliative care and opioid abuse prevention. J Palliat Care 2015;31:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Koeppe J, Armon C, Lyda K, Nielsen C, Johnson S. Ongoing pain despite aggressive opioid pain management among persons with HIV. Clin J Pain 2010;26:190–8. [DOI] [PubMed] [Google Scholar]
- [54].Koeppe J, Lyda K, Johnson S, Armon C. Variables associated with decreasing pain among persons living with human immunodeficiency virus: a longitudinal follow-up study. Clin J Pain 2012;28:32–8. [DOI] [PubMed] [Google Scholar]
- [55].Lagana L, Chen X, Koopman C, Classen C, Kimerling R, Spiegel D. Depressive symptomatology in relation to emotional control and chronic pain in persons who are HIV positive. Rehab Psychol 2002;47:402–14. [Google Scholar]
- [56].Lopez O, Becker J, Dew MA, Caldararo R. Risk modifiers for peripheral sensory neuropathy in HIV infection/AIDS. Eur J Neurol 2004;11:97–102. [DOI] [PubMed] [Google Scholar]
- [57].Lucey BP, Clifford DB, Creighton J, Edwards RR, McArthur JC, Haythornthwaite J. Relationship of depression and catastrophizing to pain, disability, and medication adherence in patients with HIV-associated sensory neuropathy. AIDS Care 2011;23:921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lum PJ, Little S, Botsko M, Hersh D, Thawley RE, Egan JE, Mitty J, Boverman J, Fiellin DA, Collaborative B. Opioid-prescribing practices and provider confidence recognizing opioid analgesic abuse in HIV primary care settings. J Acquir Immune Defic Syndr 2011;56:S91–7. [DOI] [PubMed] [Google Scholar]
- [59].Mahungu TW, Rodger AJ, Johnson MA. HIV as a chronic disease. Clin Med (Lond) 2009;9:125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Malvar J, Vaida F, Sanders CF, Atkinson JH, Bohannon W, Keltner J, Robinson-Papp J, Simpson DM, Marra CM, Clifford DB, Gelman B, Fan J, Grant I, Ellis RJ, Group C. Predictors of new-onset distal neuropathic pain in HIV-infected individuals in the era of combination antiretroviral therapy. PAIN 2015;156:731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mann R, Sadosky A, Schaefer C, Baik R, Parsons B, Nieshoff E, Stacey BR, Tuchman M, Nalamachu S. Burden of HIV-related neuropathic pain in the United States. J Int Assoc Provid AIDS Care 2016;15:114–25. [DOI] [PubMed] [Google Scholar]
- [62].McAuley L, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356:1228–31. [DOI] [PubMed] [Google Scholar]
- [63].McCracken LM, Morley S. The psychological flexibility model: a basis for integration and progress in psychological approaches to chronic pain management. J Pain 2014;15:221–34. [DOI] [PubMed] [Google Scholar]
- [64].McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. PAIN 2003;106:127–33. [DOI] [PubMed] [Google Scholar]
- [65].Merlin JS, Cen L, Praestgaard A, Turner M, Obando A, Alpert C, Woolston S, Casarett D, Kostman J, Gross R, Frank I. Pain and physical and psychological symptoms in ambulatory HIV patients in the current treatment era. J Pain Symptom Manage 2012;43:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Merlin JS, Westfall AO, Chamot E, Saag M, Walcott M, Ritchie C, Kertesz S. Quantitative evaluation of an instrument to identify chronic pain in HIV-infected individuals. AIDS Res Hum Retroviruses 2015;31:623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Merlin JS, Westfall AO, Heath SL, Goodin BR, Stewart JC, Sorge RE, Younger J. Brief report: IL-1beta levels are associated with chronic multisite pain in people living with HIV. J Acquir Immune Defic Syndr 2017;75:e99–e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Merlin JS, Westfall AO, Raper JL, Zinski A, Norton WE, Willig JH, Gross R, Ritchie CS, Saag MS, Mugavero MJ. Pain, mood, and substance abuse in HIV: implications for clinic visit utilization, ART adherence, and virologic failure. J Acquir Immune Defic Syndr 2012;61:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Merlin JS, Zinski A, Norton WE, Ritchie CS, Saag MS, Mugavero MJ, Treisman G, Hooten WM. A conceptual framework for understanding chronic pain in patients with HIV. Pain Pract 2014;14:207–16. [DOI] [PubMed] [Google Scholar]
- [70].Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain 2011;12:1004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Miles MS, Isler MR, Banks BB, Sengupta S, Corbie-Smith G. Silent endurance and profound loneliness: socioemotional suffering in African Americans living with HIV in the rural south. Qual Health Res 2011;21:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mitchell M, Nguyen T, Maragh-Bass A, Isenberg S, Beach M, Knowlton A. Patient-provider engagement and chronic pain in drug-using, primarily African American persons living with HIV/AIDS. AIDS Behav 2017;21:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mitchell MM, Maragh-Bass AC, Nguyen TQ, Isenberg S, Knowlton AR. The role of chronic pain and current substance use in predicting negative social support among disadvantaged persons living with HIV/AIDS. AIDS Care 2016;28:1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mitchell MM, Isenberg SR, Maragh-Bass AC, Knowlton AR. Chronic pain predicting reciprocity of support among vulnerable, predominantly African-American persons living with HIV/AIDS. AIDS Behav 2018;22:2002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Moher D, Liberati A, Tetzlaff J, Altman DG; Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, Ryan E, Yakoushina T, Khan S, Mahboob R, Naseer M, Dorfman D, Sharp V. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol 2004;61:546–51. [DOI] [PubMed] [Google Scholar]
- [77].Nakamoto BK, McMurtray A, Davis J, Valcour V, Watters MR, Shiramizu B, Chow DC, Kallianpur K, Shikuma CM. Incident neuropathy in HIV-infected patients on HAART. AIDS Res Hum Retroviruses 2010;26:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Soc Sci Med 2003;57:13–24. [DOI] [PubMed] [Google Scholar]
- [79].Parker R, Jelsma J, Stein DJ. Pain in amaXhosa women living with HIV/AIDS: a cross-sectional study of ambulant outpatients. BMC Womens Health 2017;17:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: a systematic review. J Int AIDS Soc 2014;17:18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Passik SD, Kirsh KL, Donaghy KB, Portenoy RK. Pain and aberrant drug-related behaviors in medically ill patients with and without histories of substance abuse. Clin J Pain 2006;22:173–81. [DOI] [PubMed] [Google Scholar]
- [82].Patel V. Cultural factors and international epidemiology: depression and public health. Br Med Bull 2001;57:33–45. [DOI] [PubMed] [Google Scholar]
- [83].Patel V, Abas M, Broadhead J, Todd C, Reeler A. Depression in developing countries: lessons from Zimbabwe. BMJ 2001;322:482–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Phillips TJ, Brown M, Ramirez JD, Perkins J, Woldeamanuel YW, Williams ACdC, Orengo C, Bennett DL, Bodi I, Cox S, Maier C, Krumova EK, Rice AS. Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: a cross-sectional deep profiling study. PAIN 2014;155:1846–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS One 2010;5:e14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pierson HM. The role of acceptance in cognitive behavioral treatment for chronic pain in an HIV-positive community sample [doctoral thesis]. Ann Arbor: UMI Company, 2009. [Google Scholar]
- [87].Pike A, Hearn L, Williams ACDC. Effectiveness of psychological interventions for chronic pain on health care use and work absence: systematic review and meta-analysis. PAIN 2016;157:777–85. [DOI] [PubMed] [Google Scholar]
- [88].Pillay P, Wadley AL, Cherry CL, Karstaedt AS, Kamerman PR. Psychological factors associated with painful versus non-painful HIV-associated sensory neuropathy. AIDS Behav 2018;22:1584–95. [DOI] [PubMed] [Google Scholar]
- [89].Robbins NM, Chaiklang K, Supparatpinyo K. Undertreatment of pain in HIV plus adults in Thailand. J Pain Symptom Manage 2013;45:1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Robbins NM, Chaiklang K, Supparatpinyo K. Better antiretroviral central nervous system penetration is not associated with reduced chronic pain in people living with human immunodeficiency virus. Antiinfect Agents 2016;14:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rosenfeld B, Breitbart W, McDonald MV, Passik SD, Thaler H, Portenoy RK. Pain in ambulatory AIDS patients. II: impact of pain on psychological functioning and quality of life. PAIN 1996;68:323–8. [DOI] [PubMed] [Google Scholar]
- [92].Safo S, Blank AE, Cunningham C, Quinlivan EB, Lincoln T, Blackstock OJ. Pain is associated with missed clinic visits among HIV-positive women. AIDS Behav 2017;21:1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sandoval R, Roddey T, Giordano TP, Mitchell K, Kelley C. Pain, sleep disturbances, and functional limitations in people living with HIV/AIDS-associated distal sensory peripheral neuropathy. J Int Assoc Provid AIDS Care 2014;13:328–34. [DOI] [PubMed] [Google Scholar]
- [95].Saylor D, Nakigozi G, Nakasujja N, Robertson K, Gray RH, Wawer MJ, Sacktor N. Peripheral neuropathy in HIV-infected and uninfected patients in Rakai, Uganda. Neurology 2017;89:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schifitto G, McDermott M, McArthur J, Marder K, Sacktor N, Epstein L, Kieburtz K. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology 2002;58:1764–8. [DOI] [PubMed] [Google Scholar]
- [97].Schifitto G, McDermott M, McArthur J, Marder K, Sacktor N, McClernon D, Conant K, Cohen B, Epstein L, Kieburtz K. Markers of immune activation and viral load in HIV-associated sensory neuropathy. Neurology 2005;64:842–8. [DOI] [PubMed] [Google Scholar]
- [98].Shacham E, Rosenburg N, Onen NF, Donovan MF, Overton ET. Persistent HIV-related stigma among an outpatient US clinic population. Int J STD AIDS 2015;26:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sherr L, Nagra N, Kulubya G, Catalan J, Clucas C, Harding R. HIV infection associated post-traumatic stress disorder and post-traumatic growth–a systematic review. Psychol Health Med 2011;16:612–29. [DOI] [PubMed] [Google Scholar]
- [100].Silver NC, Dunlap WP. Averaging correlation coefficients: should Fisher's z transformation be used? J App Psychol 1987;72:146. [Google Scholar]
- [101].Simmonds MJ, Novy D, Sandoval R. The differential influence of pain and fatigue on physical performance and health status in ambulatory patients with human immunodeficiency virus. Clin J Pain 2005;21:200–6. [DOI] [PubMed] [Google Scholar]
- [102].Simms RW, Zerbini CAF, Ferrante N, Anthony J, Felson DT, Craven DE. Fibromyalgia syndrome in patients infected with human immunodeficiency virus. Am J Med 1992;92:368–74. [DOI] [PubMed] [Google Scholar]
- [103].Simpson DM, Rice AS, Emir B, Landen J, Semel D, Chew ML, Sporn J. A randomized, double-blind, placebo-controlled trial and open-label extension study to evaluate the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with human immunodeficiency virus neuropathy. PAIN 2014;155:1943–54. [DOI] [PubMed] [Google Scholar]
- [104].Singer EJ, Kim J, Fahy-Chandon B, Datt A, Tourtellotte W. Headache in ambulatory HIV-1-infected men enrolled in a longitudinal study. Neurology 1996;47:487–94. [DOI] [PubMed] [Google Scholar]
- [105].Singer EJ, Zorilla C, Fahy-Chandon B, Chi S, Syndulko K, Tourtellotte WW. Painful symptoms reported by ambulatory HIV-infected men in a longitudinal study. PAIN 1993;54:15–19. [DOI] [PubMed] [Google Scholar]
- [106].Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004;8:119–32. [DOI] [PubMed] [Google Scholar]
- [107].Smith MY, Egert J, Winkel G, Jacobsen J. The impact of PTSD on pain experience in persons with HIV/AIDS. PAIN 2002;98:9–17. [DOI] [PubMed] [Google Scholar]
- [108].Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? J Int AIDS Soc 2013;16:18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- [110].Surratt HL, Kurtz SP, Levi-Minzi MA, Cicero TJ, Tsuyuki K, O'Grady CL. Pain treatment and antiretroviral medication adherence among vulnerable HIV-positive patients. AIDS Patient Care STDS 2015;29:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Teti M, Bowleg L, Lloyd L. “Pain on top of pain, hurtness on top of hurtness”: social discrimination, psychological well-being, and sexual risk among women living with HIV/AIDS. Int J Sex Health 2010;22:205–18. [Google Scholar]
- [112].Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, Orrell C, Altice FL, Bangsberg DR, Bartlett JG. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012;156:817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Trafton JA, Sorrell JT, Holodniy M, Pierson H, Link P, Combs A, Israelski D. Outcomes associated with a cognitive-behavioral chronic pain management program implemented in three public HIV primary care clinics. J Behav Health Serv Res 2012;39:158–73. [DOI] [PubMed] [Google Scholar]
- [114].Tsui JI, Cheng DM, Coleman SM, Blokhina E, Bridden C, Krupitsky E, Samet JH. Pain is associated with heroin use over time in HIV-infected Russian drinkers. Addiction 2013;108:1779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tsui JI, Cheng DM, Coleman SM, Lira MC, Blokhina E, Bridden C, Krupitsky E, Samet JH. Pain is associated with risky drinking over time among HIV-infected persons in St. Petersburg, Russia. Drug Alcohol Depend 2014;144:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tsui JI, Cheng DM, Libman H, Bridden C, Samet J. Hepatitis C virus infection is associated with painful symptoms in HIV-infected adults. AIDS Care 2012;24:820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tsui JI, Cheng DM, Quinn E, Bridden C, Merlin JS, Saitz R, Samet JH. Pain and mortality risk in a cohort of HIV-infected persons with alcohol use disorders. AIDS Behav 2016;20:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Uebelacker LA, Weisberg RB, Herman DS, Bailey GL, Pinkston-Camp MM, Garnaat SL, Stein MD. Pilot randomized trial of collaborative behavioral treatment for chronic pain and depression in persons living with HIV/AIDS. AIDS Behav 2016;20:1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Uebelacker LA, Weisberg RB, Herman DS, Bailey GL, Pinkston-Camp MM, Stein MD. Chronic pain in HIV-infected patients: relationship to depression, substance use, and mental health and pain treatment. Pain Med 2015;16:1870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].van Sighem A, Gras L, Reiss P, Brinkman K, de Wolf F. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 2010;24:1527–35. [DOI] [PubMed] [Google Scholar]
- [121].Wadley AL, Mitchell D, Kamerman PR. Resilience does not explain the dissociation between chronic pain and physical activity in South Africans living with HIV. PeerJ 2016;4:e2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wadley AL, Pincus T, Evangeli M. A preliminary analysis of the association between perceived stigma and HIV-related pain. Unpublished. Accessed September 27, 2017. 10.1101/194191. [DOI] [PMC free article] [PubMed]
- [123].Ware NC, Wyatt MA, Tugenberg T. Social relationships, stigma and adherence to antiretroviral therapy for HIV/AIDS. AIDS Care 2006;18:904–10. [DOI] [PubMed] [Google Scholar]
- [124].Williams A, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012;11:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Williams ACdC. Defeating the stigma of chronic pain. PAIN 2016;157:1581–2. [DOI] [PubMed] [Google Scholar]
- [126].Williams ACdC. What can evolutionary theory tell us about chronic pain? PAIN 2016;157:788–90. [DOI] [PubMed] [Google Scholar]
- [127].Williams ACdC, Craig KD. Updating the definition of pain. PAIN 2016;157:2420–3. [DOI] [PubMed] [Google Scholar]
- [128].Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The multicentre WHO/ADAMHA field trials. Br J Psychiatry 1991;159:645–53. [DOI] [PubMed] [Google Scholar]
- [129].Woldeamanuel YW, Kamerman PR, Veliotes DG, Phillips TJ, Asboe D, Boffito M, Rice AS. Development, validation, and field-testing of an instrument for clinical assessment of HIV-associated neuropathy and neuropathic pain in resource-restricted and large population study settings. PLoS One 2016;11:e0164994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].World Health Organization. Global health observatory data. Available at: http://www.who.int/gho/hiv/en/. Accessed March 19, 2018.
- [131].Zelman DC, Dukes E, Brandenburg N, Bostrom A, Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. PAIN 2005;115:29–36. [DOI] [PubMed] [Google Scholar]
- [132].Zukoski AP, Thorburn S. Experiences of stigma and discrimination among adults living with HIV in a low HIV-prevalence context: a qualitative analysis. AIDS Patient Care STDS 2009;23:267–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.