Treatment-resistant pigment epithelial detachments can be effectively treated with aflibercept switch therapy. Patients were treated with 3 monthly intravitreal injections and then a Q6 regimen. Mean pigment epithelial detachment height and volume were statistically reduced after the switch at primary endpoint (12 weeks), and best-corrected visual acuity also improved steadily throughout the duration of the study.
Key words: anti-VEGF therapy, pigment epithelial detachment, switch therapy, wet age–related macular degeneration, ranibizumab, aflibercept
Abstract
Purpose:
To analyze the efficacy of aflibercept switch treatment for regression of pigment epithelial detachment (PED) in patients previously treated with ranibizumab.
Methods:
Multicenter, prospective, nonrandomized clinical trial. One eye of patients presenting neovascular age–related macular degeneration with PED of more than 250 μm in height, with persistent fluid, was included. Patients had to have received at least six ranibizumab intravitreal injections during the 12 months before enrollment. Patients were switched from ranibizumab pro re nata to aflibercept (fixed regimen, 3 monthly intravitreal injections, and then Q6). Main outcome measure was change in PED height from baseline to Week 12 after switch. Secondary outcomes were best-corrected visual acuity and PED volume changes.
Results:
Eighty four patients were included. Mean delay between last ranibizumab intravitreal injection and switch was 44.7 days. Mean maximal PED height at baseline visit was 347 μm (±109) and reduced to a mean of 266 μm (±114) at Week 12 (P < 0.001) and 288.2 μm at Week 32 (P < 0.001). Mean PED volume was reduced from 1.3 mm3 to 0.98 mm3 at Week 12 (P < 0.001). Best-corrected visual acuity improved by 3.3 Early Treatment Diabetic Retinopathy Study letters at Week 32 (P = 0.003).
Conclusion:
Aflibercept switch therapy seems to be effective on large PED in patients previously treated with pro re nata ranibizumab.
Age-related macular degeneration (AMD) is one of the leading causes of irreversible blindness in the world. Global estimate of disease prevalence is 8.6%, and it is projected that in the year 2020, 196 million individuals will be affected by any type of AMD, with that number increasing to 288 million individuals by 2040.1 Neovascular or wet AMD (wAMD) has the biggest impact in visual loss. During the last decade, a major breakthrough occurred in the treatment of wAMD: the introduction of anti–vascular endothelial growth factor (anti-VEGF) drug intravitreal injections (IVIs).
The pivotal clinical trials leading the way to FDA approval of ranibizumab (Lucentis) 0.5 mg IVI for wAMD reported visual acuity gains of 15 letters or more in over 30% of subjects in the larger dose group. Visual acuity was 20/40 or better for 40% of subjects (also in the larger dose group) at the 2-year secondary endpoint.2 These trials used a strict monthly IVI treatment protocol. In real-life clinical settings, the challenges in this new age of anti-VEGF treatment are not only gaining visual acuity, but also doing so while being aware that patients' age, accessibility to means of transport, and burden of treatment are important factors that influence compliance.3
Many alternative treatment protocols, such as pro re nata (PRN) or treat and extend, were developed with aims at reducing the number of injections and/or visits to as low as possible while maintaining anatomical and functional improvements.
For some patients, the frequency of IVIs cannot be reduced because of continuous recurrence of exudative signs. Moreover, a precise subset of patients presents a challenge in current clinical practice: large vascularized pigment epithelial detachments (PEDs) with suboptimal response to anti-VEGF treatment.
Pigment epithelial detachment has been shown to be a predictor for vision loss in wAMD.4–6 A post hoc analysis from the HARBOR study showed that large vascularized PEDs persisted in approximately half of the patients receiving ranibizumab IVI 0.5 mg after 2 years of PRN treatment, although there were no statistically significant differences regarding final best-corrected visual acuity (BCVA) when comparing with patients without PED.7 In a study by Freeman et al,8 large PEDs showed little or no response in size after monthly bevacizumab IVI (Avastin).
Reasons for poor response might be local or systemic. Some authors consider that alterations of the vitreoretinal interface might play a role in late AMD,9–11 although there is no clear link between these alterations and the presence of large, vascularized PEDs.12,13 Also, many patients with healthy eyes present with vitreomacular adhesion later in life without any pathological implications.14 As a systemic risk factor, untreated obstructive sleep apnea has been implied in poor response to anti-VEGF.15
Although nonoptimal response to anti-VEGF therapy in wAMD has yet to reach a consensus in definition, the reality is that a tachyphylaxis-like16 effect is observed in some patients presenting large PEDs: sudden decrease in response to anti-VEGF IVI after initial use.
Many therapeutic approaches have been tried for large PEDs: a dosing regimen of 2.0 mg ranibizumab showed efficacy in flattening of PED in a case series and a prospective analysis reported by Chan et al.17,18 Also, in the HARBOR study, a greater reduction of PED height was found for patients receiving the largest dose of ranibizumab (2.0 mg), with a trend for more injections being necessary for PEDs increasing in height.7 Other treatment options have been proposed for unresponsive PEDs, such as intensifying and/or alternating treatment.19,20
If tachyphylaxis plays a role in anti-VEGF treatment resistance, then switching molecules could be considered as a therapeutic option.21
Previous case reports or case series have been published in the last years regarding flattening of PEDs after switch therapy for unresponsive patients with large PEDs previously treated with ranibizumab. Some studies reported impressive anatomical results after switching to bevacizumab IVI,22 and other studies reported similar outcomes after switching to aflibercept (Eylea) IVI.23,24 Visual acuity improvement has also been reported after aflibercept switch therapy in some studies,25 although not all studies have been able to reproduce this finding.26,27
The purpose of this study was to determine prospectively the efficacy of aflibercept (fixed regimen) switch treatment for regression of PED height in patients previously significantly treated with ranibizumab on PRN basis.
Methods
The ARI2 study (registered under number NCT02157077 on clinicaltrials.gov) was a prospective, nonrandomized, single-arm, phase IIIb clinical trial that took place in 10 investigator sites across France, from December 2013 to December 2015. The study was approved by French regulatory agencies (ANSM). International review board approval (CPP Ile de France XI) was obtained prospectively for the whole conduct of this study. All patients gave written informed consent.
Study Population
To be included, patients had to meet the following criteria: age older than 50 years, neovascular AMD with active subfoveal, or juxtafoveal choroidal neovascularization (as evidenced by fluorescein angiography and spectral domain optical coherence tomography [SD-OCT]). Patients had to have been previously treated with ranibizumab IVI for at least 1 year, with a minimum of 6 ranibizumab IVIs having occurred in the 12-month period immediately preceding inclusion (last ranibizumab IVI had to have occurred during the 90-day period preceding study inclusion). Choroidal neovascularization lesions had to have a PED component of more than 250 μm in height (measured by SD-OCT) at its maximal height location, including extrafoveal locations. Pigment epithelial detachment had to be present for at least 2 consecutive visits immediately preceding inclusion in this study. Major exclusion criteria were subfoveal atrophy or scarring, presence of retinal hemorrhage, atrophy or fibrosis constituting more than 50% of lesion component, retinal pigment epithelial rips, other causes of choroidal neovascularization, unclear ocular media, and active ocular inflammation as well as contraindications listed in the product's safety profile.
Study Treatment
Patients were switched to aflibercept 2-mg IVI therapy, receiving three aflibercept IVI at the baseline, Week 4, and Week 8 visits. Patients were treated on a Q6 protocol at the subsequent Weeks 14, 20, and 26 visits. At primary and secondary endpoint evaluation visits (Week 12 and Week 32), no treatment was administered.
Baseline and Follow-up Evaluations
Functional response was evaluated by Early Treatment Diabetic Retinopathy Study (ETDRS) BCVA measurements at every visit. Anatomical response was evaluated by SD-OCT, fluorescein angiography, and indocyanine green angiography (HRA + OCT, Heidelberg Engineering, Heidelberg Germany). Spectral domain optical coherence tomography was performed at all visits according to the following protocol: 4 SD-OCT single line scans in combined depth imaging28 mode with 100 frames averaging at the following locations: 1 horizontal and 1 vertical scan centered at the fovea and 1 horizontal and 1 vertical scan centered at the maximal PED height (maximal PED height was localized by manually searching through PED at the baseline visit and consequently analyzed on follow-up mode for all other visits), as well as a macular volume scan consisting 19 lines covering an area of 20° by 15° centered on the fovea. All centers used the same imaging hardware. Fluorescein angiography, indocyanine green angiography, and fundus photographs were performed at the baseline, Week 12, and Week 32 visits. For these aforementioned visits, additional SD-OCT macular volume scans consisting of 61 scans covering an area of 30° by 25° centered on the fovea in high-resolution setting were obtained. This allowed for semiautomated volume analysis of PED (ReVA Analyzer; ADCIS, Saint Contest, France). Baseline visit was set as initial reference, and subsequent SD-OCT acquisitions were performed in follow-up mode. All imaging was performed before treatment for all concerned visits with surveillance of worsening PED or any adverse events (AEs). Images were reviewed by a single reading center at the end of the study.
Main Outcome Measures
The primary outcome measure was a change in maximal PED height from baseline to 12 weeks after therapeutic switch. Secondary outcome measures were as follows: change in maximal PED height from baseline to 32 weeks, change in central macular thickness, PED volume, and BCVA changes from baseline to 12 weeks and 32 weeks, as well as safety outcome measurements. Information concerning the rate of severe AEs (SAEs) was also collected and analyzed by an independent scientific committee.
Statistical Analysis
Efficacy analyses were performed on an intention-to-treat basis. Best-corrected visual acuity was measured in ETDRS letters but converted to logarithm of the minimum angle of resolution notation for statistical purposes. Qualitative variables were described in percentages, and quantitative variables were described by their mean with SD. Difference of the mean values between baseline and follow-up visits were assessed using paired T tests. P less than 0.05 was considered significant. Analyses were performed with STATA software version 13.0 (StataCorp, TX).
Results
Population
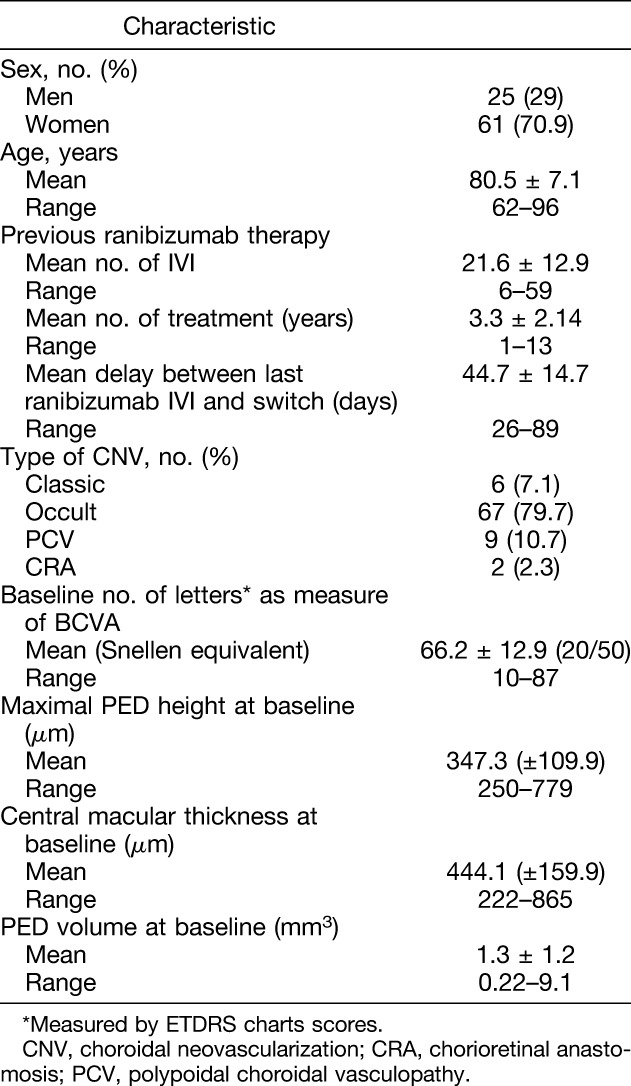
A total of 90 patients were screened for inclusion in this study; four patients were not included because they did not fulfill the inclusion criteria. Two patients withdrew consent before any data other than sex could be collected. Patients who completed at least one complete follow-up visit were analyzed on per intention to treat protocol. Mean age at inclusion was 80.5 years. Mean delay between last ranibizumab IVI and aflibercept switch was 44.7 days. Complete baseline characteristics for included patients are shown in Table 1.
Table 1.
Baseline Characteristics of Patients Included in the ARI2 Study
Vitreous–Retinal Interface Abnormalities
Seven of the 84 patients analyzed presented some kind of vitreomacular interface alteration: 3 patients presented partial posterior vitreous detachment without any traction, and 4 patients presented epiretinal membranes that deformed foveal contour.
Primary and Secondary Outcome Measures
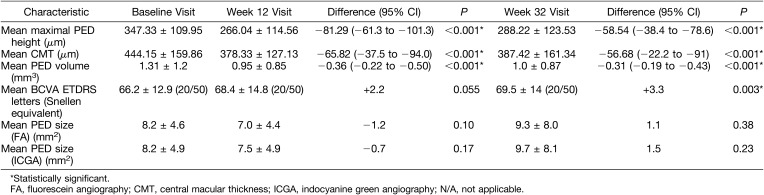
Primary and secondary efficacy results are presented in a summarized manner in Table 2.
Table 2.
Summary of Primary and Secondary Endpoint Measures and Statistical Calculations for the ARI2 Study
Maximal Pigment Epithelial Detachment Height
Mean maximal PED was 347.33 (±109.9) µm at the baseline visit, 266.0 (±114.56) µm at 12 weeks, and 288.22 (±123.5) µm at 32 weeks. Reduction in maximal PED height was statistically significant at both primary and secondary endpoints {mean reduction −81.3 (±92.1) µm (P < 0.001, 95% confidence interval (CI) [−61.3 to −101.2]) and −58.5 (±90.3) µm (P < 0.001, 95% CI [−38.4 to −78.6]), respectively} (Figure 1). The proportion of patients with worsening PEDs at the 32-week visit compared with the primary endpoint was 66.6%.
Fig. 1.
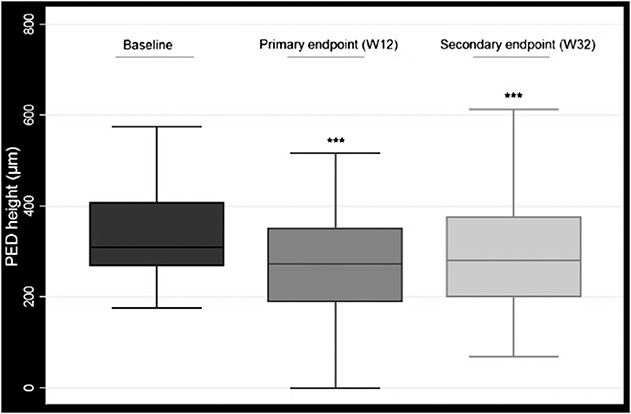
Pigment epithelial detachment (PED) height at baseline, primary endpoint (12 weeks), and secondary endpoint (32 weeks). *** Statistically significant.
Central Macular Thickness
Mean baseline central macular thickness was 444.15 μm (±159.86). Central macular thickness at Week 12 was 378.33 (±127.13) μm, which was a mean reduction of −65.82 μm compared with baseline central macular thickness. At Week 32, mean central macular thickness was 387.42 μm (±161.34), a mean reduction of −56.68 μm compared with baseline values. Both reductions were statistically significant (P < 0.001, 95% CI [−37.5 to −94.0] and P = 0.001, 95% CI [−22.2 to −91.0], respectively) (Figure 2).
Fig. 2.
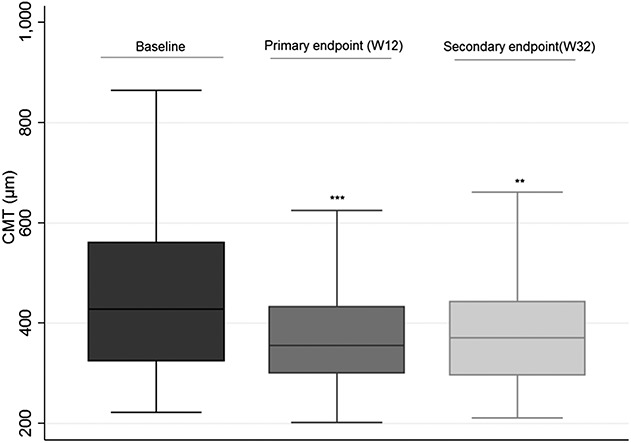
Central macular thickness (CMT) at baseline, primary endpoint (12 weeks), and secondary endpoint (32 weeks). *** Statistically significant.
Pigment Epithelial Detachment Volume
Mean baseline PED volume was 1.31 mm3 (±1.20). Mean PED volume at Week 12 was 0.95 mm3 (±0.85), which represents a mean reduction of −0.36 mm3 from baseline values. This was statistically significant (P < 0.001, 95% CI [−0.22 to −0.50]). At the Week 32 visit, there was a slight increase in PED volume (mean = 1.00 mm3) compared with the Week 12 visit, but statistical significance was maintained when compared with baseline visit {mean reduction of −0.31 mm3 (P = 0.001, 95% CI [−0.19 to −0.43])} (Figure 3).
Fig. 3.
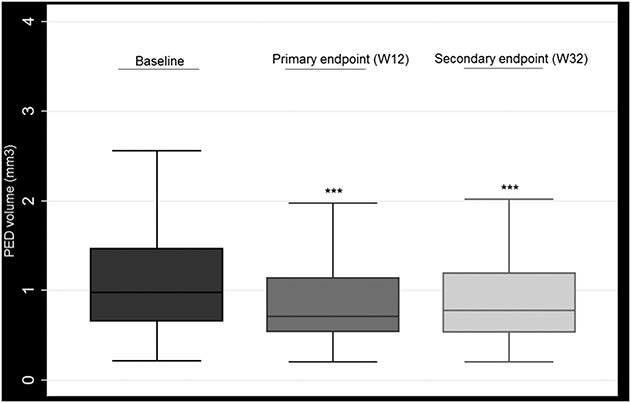
Pigment epithelial detachment (PED) volume at baseline, primary endpoint (12 weeks), and secondary endpoint (32 weeks). *** Statistically significant.
Best-Corrected Visual Acuity
Mean baseline BCVA was 66.2 (±12.9) ETDRS letters (20/50), improving to 68.4 ETDRS letters (±14.8) at the Week 12 visit (20/50). At Week 32, mean BCVA was 69.5 ETDRS letters (±14) (20/50). Mean baseline logarithm of the minimum angle of resolution BCVA equivalent was 0.35 (±0.24) (20/44). At Week 12, mean logarithm of the minimum angle of resolution BCVA equivalent was 0.32 (±0.27) (20/42); this slight improvement was not statistically significant (P = 0.055, 95% CI [−0.00 to −0.6]). At Week 32, mean logarithm of the minimum angle of resolution BCVA was 0.29 (±0.26) (20/40); a difference that when compared with baseline values achieved statistical significance (P = 0.03, 95% CI [−0.02 to −0.10]).
Safety and Adverse Events
Thirteen SAEs were registered during the course of the ARI2 study. Only one (confused state) was probably related to the drug in the study (transient ischemic attack–like symptoms). The patient presenting this SAE discontinued the study after two visits. Three cardiovascular SAEs were also registered, but direct causal relationship was not established.
Discussion
Our study found that aflibercept switch therapy effectively reduces both maximal PED height and overall PED volume at the 12-week and 32-week endpoints. Visual acuity improvements achieved statistical significance at the 32-week endpoint. Pigment epithelial detachment size slightly increased after 32 weeks of follow-up, but statistical significance was maintained.
The mechanisms of suboptimal response are poorly understood. Our studied population did not present a high prevalence of vitreomacular adhesion at inclusion. Suboptimal response might be related to interindividual variations in drug metabolism, tachyphylaxis,21,29 particular traits in the neovascular lesion, or secondary to certain genetic profiles. Increasing evidence suggests the importance of pharmacogenetics in wAMD. In 2012, both Menghini et al and Chen et al30,31 suggested that a polymorphism in the CFH gene might be predictive for wAMD treatment response, but other studies with large cohorts have not confirmed this.32,33 Possibly, a cumulative effect of multiple high-risk alleles determines disease severity and response to treatment.34
Persistent or worsening morphological and/or functional signs despite a correct therapeutic approach (i.e., respect of the loading phase and frequent follow-up visits) constitutes suboptimal response. Partial or insufficient response leads to multiple injections and inability to space treatments. Initiatives to delay treatment are often frustrated by visual acuity loss or anatomical worsening. It is important to state that some of these patients might be late responders or partial responders; thus, continuing therapy is necessary if no AEs are observed and treatment should not be interrupted nor permanently stopped. Therapeutic switch might be considered as an option for patients with suboptimal (or partial) response.35 In our study, patients had an average of 6.5 ranibizumab IVI/year, with large persistent or worsening PEDs. We cannot eliminate the possibility that these patients were undertreated, regarding the persistent vascularized PED. A recent study has shown that PED is a trigger for vision loss,4 highlighting the relevance of our study on the subject of switch therapy for poorly responsive PED.
There have been various previous reports of improvement in PED height after switching anti-VEGF therapy from ranibizumab to aflibercept, with little or no effect on BCVA.25,36–38 In our prospective study, mean BCVA improved steadily throughout the study but did not reach statistical significance up until the secondary endpoint (32 weeks).
Our results are in accordance with other previously published studies that have measured PED volume changes after aflibercept switch therapy.39,40 A slight increase in PED volume and height was noted in our study at the final endpoint compared with primary endpoint values (when compared with baseline values, statistical significance was maintained despite this increase). This could indicate a newly developing tachyphylaxis, as two-thirds of our studied population actually worsened regarding PED height at the secondary endpoint (when compared with the primary endpoint).
We hypothesize that both the nature of these large PEDs and previous “treatment resistance” probably account for the progressive decrease in efficacy of both anti-VEGF molecules in this population. It could be debated that in the future, the inverse scenario could potentially present itself as aflibercept tachyphylaxis or resistance in a particular subset of patients. In that case, switch to ranibizumab or other newer drugs could be an option.
One scientific paper has been published addressing the issue of switch-back for patients with suboptimal response who already switched treatment once with positive short-term results.41
When the methodological design for this study was done, we took into consideration the history of suboptimal response to anti-VEGF therapy characterizing the targeted population. It was decided that treatment was to be modified in two ways: first, molecule switch and second, protocol change. We believe that both mechanisms can account for the results we observed.
Molecule switch has a rationale in tachyphylaxis or tolerance-like scenarios like the one defining our population: Aflibercept differs from ranibizumab in composition and molecular size.
Concerning treatment frequency change, we supposed that although Q8 might be a viable option for most patients, it was not particularly advisable for this “large PED” population. An induction phase consisting of 3 IVIs on a Q4 basis was followed by a Q6 protocol.
Our results prove that this approach results in morphological and functional improvement. Statistically significant visual acuity improvement was achieved at the 32-week endpoint, although the clinical relevance of a three-letter improvement in BCVA might be debated.
One SAE attributable to the aflibercept molecule was reported; this occurred during the loading phase, and not the Q6 phase.
Our study had certain limitations, among which was the lack of a control arm (no patients kept on ranibizumab Q4 and no patients switched to aflibercept Q8). Establishing a trial of that scope is challenging, but it would certainly be very interesting to analyze. One recent publication addresses this issue36 and presents a strictly theoretical model in which patients with ranibizumab-resistant PEDs who switched to aflibercept would have theoretically (assuming a linear evolution of PED regression) regressed to the same degree, had they not been switched.
Concerning the choice of treatment regimen in this study, we cannot conclude whether it was the switching of molecules that drove clinical improvement for this particular population or the change in IVI frequency. The patient population studied had an average of 6.5 ranibizumab IVI/year previous to inclusion (with at least 6 IVI the previous year preceding inclusion), which is consistent with a PRN or treat-and-extend protocol. Mean time from last ranibizumab IVI and aflibercept switch was relatively short (less than 1.5 months [SD 14.7 days]) in the included population.
When switching these patients, they clearly benefited from both a therapeutical molecule change and also intensification in treatment regime (3 monthly IVIs followed by a Q6 protocol). It remains impossible to determine the respective share of molecule change and the protocol change in the positive effects on PED change.
Another important limitation to our study concerns the follow-up period: It would have been optimal to obtain results at 1 year. However, the short-term results are encouraging, for both anatomical and functional improvements.
In conclusion, according to the results of the ARI2 study, switching from ranibizumab with a PRN protocol to aflibercept Q6 regimen is effective in reducing PED volume and height, in patients poorly responding to ranibizumab on PRN basis.
Footnotes
This study was performed through a research grant from the sponsor Bayer (Loos, France). The sponsor had no role in the design and conduct of the study, nor in the writing of this report or in the decision to submit this research.
None of the authors has any conflicting interests to disclose.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e116. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol 2015;38:620–627. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U, Waldstein SM, Deak GG, et al. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology 2015;122:822–832. [DOI] [PubMed] [Google Scholar]
- 5.Pauleikhoff D, Loffert D, Spital G, et al. Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Greaefes Arch Clin Exp Ophthalmol 2002;240:533–538. [DOI] [PubMed] [Google Scholar]
- 6.Mariani A, Deli A, Ambresin A, Mantel I. Characteristics of eyes with secondary loss of visual acuity receiving variable dosing ranibizumab for neovascular age-related macular degeneration. Graefes Arch Clin Ophthalmol 2011;249:1635–1642. [DOI] [PubMed] [Google Scholar]
- 7.Sarraf D, London NJ, Khurana RN, et al. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: post hoc analysis of the HARBOR study. Ophthalmology 2016;123:2213–2224. [DOI] [PubMed] [Google Scholar]
- 8.Freeman WR, Kozak I, Yuson RM, et al. Prognostic implications of pigment epithelial detachment in bevacizumab (avastin)-treated eyes with age-related macular degeneration and choroidal neovascularization. Retina 2011;31:1812–1818. [DOI] [PubMed] [Google Scholar]
- 9.Schulze S, Hoerle S, Mennel S, Kroll P. Vitreomacular traction and exudative age-related macular degeneration. Acta Ophthalmol 2008;86:470–481. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Lee CS, Koh HJ. Posterior vitreomacular adhesion and risk of exudative age-related macular degeneration: paired eye study. Am J Ophthalmol 2009;147:621–626. [DOI] [PubMed] [Google Scholar]
- 11.Maier M, Pfrommer S, Burzer S, et al. Vitreomacular interface and posterior vitreomacular adhesion in exudative age-related macular degeneration (AMD): an OCT-based comparative study. Klin Monbl Augenheilkd 2012;229:1030–1035. [DOI] [PubMed] [Google Scholar]
- 12.El-Hifnawy MA, Ibrahim HA, Gomaa AR, Elmasry MA. The vitreomacular interface in different types of age-related macular degeneration. Int J Ophthalmol 2017;10:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattoussi S, Cougnard-Grégoire A, Delyfer MN, et al. Vitreomacular adhesion and its association with age-related macular degeneration in a population-based setting: the Alienor study. Invest Ophthalmol Vis Sci 2017;58:2180–2186. [DOI] [PubMed] [Google Scholar]
- 14.Palacio AC, Gupta A, Nesmith BL, et al. Vitreomacular adhesion evolution with age in healthy human eyes. Retina 2017;37:118–123. [DOI] [PubMed] [Google Scholar]
- 15.Schaal S, Sherman MP, Nesmith B, Barak Y. Untreated obstructive sleep apnea hinders response to bevacizumab in age-related macular degeneration. Retina 2016;36:791–797. [DOI] [PubMed] [Google Scholar]
- 16.Lehne RA. Individual variation in drug responses. In: Lehne RA, ed. Pharmacology for Nursing Care. 8th ed St. Louis, MO: Elsevier/Saunders; 2013:81. [Google Scholar]
- 17.Chan CK, Abraham P, Sarraf D. High-dose ranibizumab therapy for vascularized pigment epithelial detachment. Eye (Lond) 2012;26:882–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan CK, Abraham P, Sarraf D, et al. Earlier therapeutic effects associated with high dose (2.0 mg) ranibizumab for treatement of vascularized pigment epithelial detachments in age related macular degeneration. Eye (Lond) 2015;29:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart MW, Rosenfled PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab and aflibercept (vascular endothelial growth factor Trap-eye). Retina 2012;32:434–457. [DOI] [PubMed] [Google Scholar]
- 20.Witkin AJ, Rayess N, Garg SJ, et al. Alternating bi-weekly intravitreal ranibizumab and bevacizumab for refractory neovascular age-related macular degeneration with pigment epithelial detachment. Semin Ophthalmol 2015;4:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Yang S, Zhao J, Sun X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des Devel Ther 2016;10:1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasperini JL, Fawzi AA, Khondkaryan A, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol 2012;96:14–20. [DOI] [PubMed] [Google Scholar]
- 23.Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol 2013;156:15–22. [DOI] [PubMed] [Google Scholar]
- 24.Patel KH, Chow CC, Rathod R, et al. Rapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumab. Eye (Lond) 2013;27:663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fassnacht-Riederle H, Becker M, Graf N, Michels S. Effect of aflibercept in insufficient responders to prior anti-VEGF therapy in neovascular AMD. Graefes Arch Clin Exp Ophthalmol 2014;252:1705–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorell MR, Nunes RP, Chen GW, et al. Response to aflibercept after frequent re-treatment with bevacizumab or ranibizumab in eyes with neovascular AMD. Ophthalmic Surg Lasers Imaging Retina 2014;45:526–533. [DOI] [PubMed] [Google Scholar]
- 27.He L, Silva RA, Moshfeghi DM, et al. Aflibercept for the treatment of retinal pigment epithelial detachments. Retina 2016;36:492–498. [DOI] [PubMed] [Google Scholar]
- 28.Barteselli G, Bartsch DU, El-Emam S, et al. Combined depth imaging technique on spectral-domain optical coherence tomography. Am J Ophthalmol 2013;155:727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth facto pharmacotherapy in age related macular degeneration? Ophthalmology 2008;115:2199–2205. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Yu KD, Xu GZ. Association between variant Y402H in age related macular degeneration (AMD) susceptibility gene CFH and treatment response of AMD: a meta analysis. PLoS One 2012;7:e42464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menghini M, Kloeckener-Gruissem B, Fleischhauer J, et al. Impact of loading phase, initial response and CFH genotype on the long-term outcome of treatment for neovascular age-related macular degeneration. PLoS One 2012;7:e42014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagstrom SA, Ying GS, Pauer GJ, et al. ; For the CATT Research Group. Pharmacogenetics for genes associated with age-related macular degeneration in the comparison of AMD Treatments Trials (CATT). Ophthalmology 2013;120:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotery AJ, Gibson J, Cree AJ, et al. For the IVAN Study Group. Pharmacogenetic associations with vascular endothelial growth factor inhibition in participants with neovascular age-related macular degeneration in the IVAN Study. Ophthalmology 2013;120:2637–2643. [DOI] [PubMed] [Google Scholar]
- 34.Fauser S, Lambrou GN. Genetic predictive biomarkers of anti-VEGF treatment response in patients with neovascular age-related macular degeneration. Surv Ophthalmol 2015;60:138–152. [DOI] [PubMed] [Google Scholar]
- 35.Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond) 2015;29:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Massougnes S, Dirani A, Ambresin A, et al. Pigment epithelial detachment response to aflibercept in neovascular age-related macula degeneration refractory to ranibizumab: time course and drug effects. Retina 2016;36:881–888. [DOI] [PubMed] [Google Scholar]
- 37.Major JC, Jr, Wykoff CC, Croft DE, et al. Aflibercept for pigment epithelial detachment for previously treated neovascular age-related macular degeneration. Can J Ophthalmol 2015;50:373–377. [DOI] [PubMed] [Google Scholar]
- 38.Broadhead GK, Hong T, Zhu M, et al. Response of pigment epithelial detachments to intravitreal aflibercept among patients with treatment-resistant neovascular age-related macular degeneration. Retina 2015;35:975–981. [DOI] [PubMed] [Google Scholar]
- 39.Kanesa-Thasan A, Grewal DS, Gill MK, et al. Quantification of change in pigment epithelial detachment volume and morphology after transition to intravitreal Aflibercept in eyes with recalcitrant Neovascular AMD: 18 months results. Ophthalmic Surg Lasers Imaging Retina 2015;46:638–641. [DOI] [PubMed] [Google Scholar]
- 40.Chois CS, Zhang L, Abramoff MD, et al. Evaluating efficacy of aflibercept in refractory exudative age related macular degeneration with OCT segmentation volumetric analysis. Ophthalmic Surg Lasers Imaging Retina 2016;47:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Despreaux R, Cohen SY, Semoun O, et al. Short-term results of switchback from aflibercept to ranibizumab in neovascular age-related macular degeneration in clinical practice. Graefes Arch Clin Exp Ophthalmol 2016;254:639–644. [DOI] [PubMed] [Google Scholar]