Abstract
Introduction:
Experimental evidences from the last 2 decades supports the existence of a special type of neoplastic cell with stem-like features [cancer stem cell (CSC)] and their role in the pathophysiology and therapeutic resistance of breast cancer. However, their clinical value in human breast cancer has not been fully determined.
Materials and Methods:
An immunohistochemistry panel of 10 putative CSC markers (CD34, C-KIT, CD10, SOX-2, OCT 3/4, p63, CD24, CD44, CD133, and ESA/EPCAM) was applied to 74 cases of breast cancer, followed in a Regional Cancer Center of Minas Gerais State, Brazil, from 2004 to 2006. Possible associations between CSC markers and classic variables of clinicopathologic relevance were investigated.
Results:
The most frequently positive CSC markers were CD44, CD24, CD133, and ESA (the others were present in <15% of the cases). Two CSC profiles were defined: CD24−/CD44+ (CSC-1) and CD133+/ESA+ (CSC-2). CSC-1 was significantly associated to patients older than 40 years, tumors of <2.0 cm in diameter, early clinical stages (P<0.05), and increased death risk of 4 times (P=0.03; 95% confidence interval, 1.09-14.41). CSC-2 was related to increased relapse risk of 3.75 times (P=0.04; 95% confidence interval, 1.02-13.69).
Conclusion:
The detection of the most frequently positive CSC markers by immunohistochemistry is of clinicopathologic and prognostic relevance.
Key Words: breast cancer, cancer stem cell, immunohistochemistry
The cancer stem cell (CSC) hypothesis is nowadays a new paradigm of carcinogenesis. These cells can be related with poor therapeutic response and poor prognosis of some malignant neoplasms, for their main features—self-renewal, multilineage differentiation ability, and low proliferative index.1–6 However, it is not possible to identify CSC easily, as these cells are scarce and heterogenously present in the tissues. Besides, surface CSC proteins interact to stromal environmental cells, like fibroblasts, influencing CSC detection and its biological behavior. Sensibility of the performed method to identify these cells depends on the microenvironment around the cell (in vivo or in a cell culture).7–10
In clinical practice, immunohistochemistry (IHC) can detect CSC in paraffin-embedded tissue, considered “in vivo,” as the original microenvironment features are preserved in these specimens. For this reason, IHC may identify CSC in tumors better than another “in vitro” detection methods.3,11–13 However, studies involving isolated putative CSC antibodies have reported controversial results in the literature. To better define CSC immunophenotype in malignant tumors, it is required to perform an IHC panel with several antibodies. Unfortunately, this immunophenotype remained unclear in several studies, even the ones that performed several putative CSC markers.14–16
Human breast cancer was chosen in the current study to investigate putative CSC markers because is the most prevalent and lethal malignant neoplasm worldwide.13,17–19 CSC may be the reason of this frequent unfavorable clinical course and poor therapeutic response of these neoplasms, due to stem-cell feature of self-renewal, preserving the neoplasm and due to poor chemotherapy and radiotherapy responses on quiescent cells, like stem cells.6,15,20
To investigate CSC markers expression in breast cancer and their possible clinicopathologic and prognostic roles in breast cancer, it was performed an IHC panel of putative CSC markers on tissue microarrays (TMA) specimens of breast tumor.
MATERIALS AND METHODS
There were included 74 paraffin-embedded tissues of breast invasive carcinoma samples in this cross-sectional study. These specimens were collected from anatomic pathology reference service of Regional Cancer Center of Poços de Caldas city (UNACON), in Minas Gerais State, Brazil, from 2004 to 2006. The archival medical records were retrieved to collect clinical and therapeutic data. Female patients exclusively followed and treated at this service, who received adjuvant therapy for breast cancer, were included. Patients who received neoadjuvant therapy or had any other concomitant visceral malignant neoplasm were eliminated from this study.
The retrieved clinical data collected from medical records were age, body mass index, smoking habits, fertility (number of pregnancies), familial cancer history, period of follow-up, tumor relapse and time of death, when applicable. The therapeutic data were the information about chemotherapy, radiotherapy, and hormonal therapy.
The original hematoxylin and eosin slides were retrieved by 2 experimented pathologists to reclassify the breast tumors according to current histopathologic criteria21. The reclassified pathologic data were histologic type and differentiation grade of the tumor, tumor size, vascular invasion, breast skin involvement, laterality of breast, tumor multifocality, metastasis to distant organs and to regional lymph nodes, pathologic stage, and molecular subtypes (prognostic and predictive markers of therapeutic response).
Besides, representative areas of the tumors were marked and 2 cores were punched out from donor paraffin blocks to perform TMA. There were included several surgical specimens, as core needle biopsy, mammary quadrant, or radical mastectomy. The core that eventually represented a tumor area smaller than 90% of total was eliminated and a new representative core from the same donor block substituted it.
An IHC panel of 10 antibodies, considered as putative CSC markers, were performed on TMA sections: CD34, C-KIT, CD10, SOX-2, OCT 3/4, p63, CD24, CD44, CD133, and ESA/EPCAM (Abcam, CA). CD24/CD44 (CSC-1) and CD133/ESA (CSC-2) were considered the main CSC profiles in several revised studies, so these 4 putative CSC markers were performed on the same slide, with 2 different stains: 3,3′ diaminobenzidine (Dako) and alkaline phosphatase (Dako) (Fig. 1). The other antibodies were marked only with diaminobenzidine (Fig. 2).
FIGURE 1.
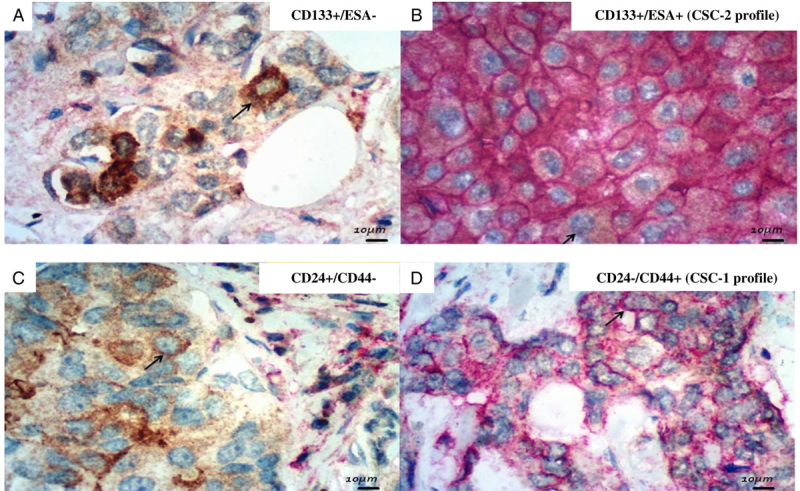
Photomicrographies of double stained putative CSC antibodies (×400, original magnification; immunoperoxidase and DAB). A, CD133: cytoplasm positivity (immunoperoxidase). B, ESA: membrane positivity (DAB); CD 133+/ESA+: CSC-2 profile (black arrow: membrane positivity to DAB and cytoplasm positivity to immunoperoxidase at the same cell). C, CD24: cytoplasm positivity (immunoperoxidase). D, CD24−/CD44+: CSC-1 profile (black arrow: membrane positivity only to DAB). CSC indicates cancer stem cell; DAB, diaminobenzidine.
FIGURE 2.
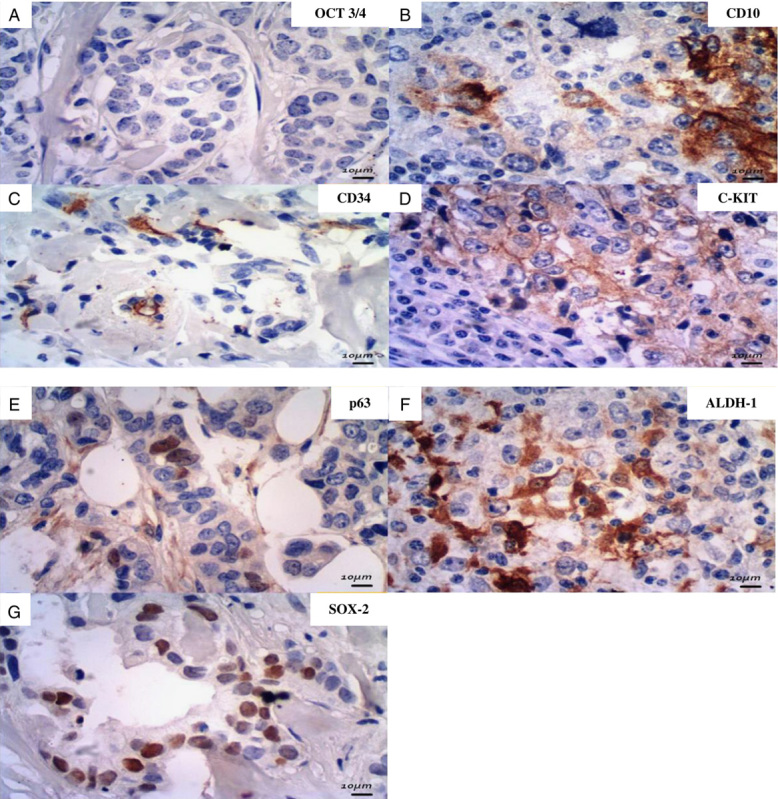
Photomicrographies of simple stained putative CSC antibodies (×400, original magnification; immunoperoxidase). OCT 3/4 (A): no positivity was observed in this study; C-KIT (D) and CD34 (C): membrane positivity; CD10 (B) and ALDH-1 (F): membrane and cytoplasm positivity; p63 (E) and SOX-2 (G): nuclear positivity. CSC indicates cancer stem cell.
Considering that CSC are scarce and heterogenous in the tissues, the immunohistochemical stains were classified as positive if at least 1 cell in the core stained, with any intensity. The 2 pathologists analyzed independently the stains. A positive control of different tissue other than breast, which were strong stained to each antibody, was added to each TMA slide.
Statistical analysis was performed with IBM SPSS Statistics for Windows, version 20.0 (IBM Corporation, Armonk, NY). Descriptive statistics were presented as minimum–maximum for discontinuous variables and as number and percent (%) for nominal variables. To analyze groups of categorical variables (CSC positive markers vs. clinicopathologic, prognostic, and predictive of therapeutic response variables), χ2,“one-way” analysis of variance (ANOVA) and post hoc Tukey test were performed. To evaluate groups of continuous quantitative variables (CSC positive markers vs. clinicopathologic, prognostic, and predictive of therapeutic response variables), student t test was performed. Total number of different positive CSC markers in a same case was compared with the other clinicopathologic variables with Mann-Whitney test. A P<0.05 was considered statistically significant. Linear regression of Spearman was performed to assess possible correlations between the CSC putative markers.
Finally, overall and free of disease survival analysis of Kaplan-Meyer and a proportional risk model (Cox regression) were performed to evaluate the impact of all the variables, with univariate and multivariate analysis.
The present study was approved by the Medical Sciences Faculty of State University of Campinas (CEP-FCM-UNICAMP) Ethical Committee—CEP 1171/2011, on compliance to Helsinki Declaration.
RESULTS
The main clinicopathologic data were resumed on Table 1. In this setting, age group ranged from 28 to 86 year old (average of 57 and median of 58 y old). Body mass index ranged from 13.38 to 39.81 (average of 27.25 and median of 26.04). Number of pregnancies ranged from 0 up to 8 (average of 2.78 and median of 3.0).
TABLE 1.
Resume of Categorical Clinicopathologic and Prognostic/Predictive Variable Descriptive Assessments
In brief, the results of clinicopathologic variables were: menopause (58.9%); tumor size (“T” of the clinical tumor size, nodal status, metastasis (TNM) system, distributed as T1=37.1%, T2=35.7%, T3=7.1%, and T4=20%); regional lymph node status (“N” of the clinical TNM system, N0=44.4%, N1=25.4%, and N2=30.2%); histologic final grade of Nottingham (grade 1=29.2%, grade 2=38.9%, and grade 3=31.9%); and vascular tumor invasion (58.6%), smoking habit (6.85%), familial history of any malignant neoplasm (10.81%), metastasis at the moment of diagnosis of breast cancer (7.58%), histologic type (90.54% of ductal invasive carcinoma), breast skin invasion (absent in 76.39%), and status of surgical margins (negative in 83.8%).
On an alternative molecular classification based on the prognostic/predictive antibodies expression, the majority of the tumors were of luminal subtypes (80.81%), which were treated with hormonal therapy. The majority of patients received adjuvant chemotherapy (94.2%) and radiotherapy (89.6%).
The patients were followed for a median period of 51.52 months, ranging from 1 up to 108 months. The tumors relapsed in 28.57% and the average time period necessary to diagnose the relapse was 37 months. Only 26.67% died during the follow-up (40% during the first year). The main death cause was pulmonary embolism (90%).
Positive results on immunohistochemical reactions of CSC markers were presented on Figures 1 and 2. In the present study, 85.1% of the cases were positive for at least 1 antibody. The putative CSC markers that were positive in <15% of the cases were: CD10 (1.4%), CD34 (1.4%), C-KIT (1.4%), ALDH-1 (6.8%), and SOX-2 (13.5%). OCT 3/4 was consistently negative in all the cases. There were an average of 2 positive antibodies for case, ranging from 0 to up to 4 antibodies for case.
In contrast, the CSC profiles, described in other studies, were positive in up to 70.3% of the cases (CD24−/CD44+ in 52.7% and CD133+/ESA+ in 17.6%). No case expressed both CSC profiles at the same time.
CSC-1 (CD24−/CD44+) was more frequently associated with breast cancer cases arising in patients older than 40 years (P=0.022), as well as isolated CD44+ cancer cells were more frequent in this age group (P=0.048). In contrast, CSC-1 was less frequently associated with advanced tumors, classified as T3/T4 (P=0.039), and with III/IV clinical stages (P=0.032).
There were observed greater frequency of different positive CSC markers in a same case in T3/T4 stages (P=0.004) and in the tumors classified as histologic final grades of Nottingham 2 and 3 (χ2 test; P=0.035). There were unedited observed that the tumors arising during menopause stained for CD133 more frequently than the ones of nonmenopause (P=0.028).
Ratio risks associated with relapse and death were evaluated by univariate and multivariate analysis. The impacts of clinicopathologic variables on relapse and death risk by univariate analysis were resumed on Table 2. In the univariate analysis, CSC-2 (CD133+/ESA+) was associated with a relapse risk of 3.75 times [P=0.045; 95% confidence interval (CI), 1.02-13.69] and CSC-1 (CD24−/CD44+) was related with increased death risk of 4 times (P=0.03; 95% CI, 1.09-14.41). Expression of different CSC markers in a same case increased the death risk (1.9 times, P=0.023; 95% CI, 1.09-3.31). However, these findings were not confirmed in the multivariate analysis.
TABLE 2.
Clinical and Pathologic Variables Significantly Associated to Relapse (A) and to Death (B) on Univariate Analysis (Cox Regression)—P<0.05
Survival charts of Kaplan-Meyer related with relapse and death (respectively, free of disease survival and overall survival) were presented on Figures 3 and 4. CSC-2 (CD133+/ESA+) was associated with diminishing by 40% of free of disease survival, in up to 30 months of follow-up (P=0.045). CSC-1 (CD24−/CD44+) reduced the overall survival by 50%, in up 40 months of follow-up (P=0.036). In the tumors expressing >1 putative CSC antibody, the overall survival similarly reduced by 60%, in up to 40 months of follow-up (P=0.023).
FIGURE 3.
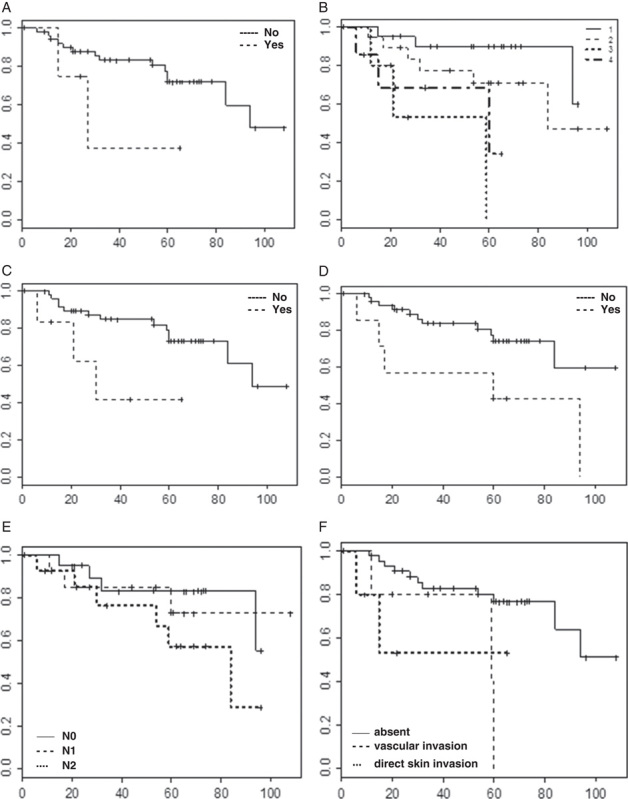
Survival charts related to tumor relapse (free of disease survival) and the effects on it caused by: A, Smoking habit (P=0.006). B, “T” stage (P=0.024). C, CSC-2 profile (P=0.045). D, Familial history for malignant neoplasm (P=0.031). E, “N” stage (P=0.014). F, Breast skin invasion (P=0.039). Column: estimated survival (0.0-1.0); line: time to tumor relapse (months). CSC indicates cancer stem cell.
FIGURE 4.
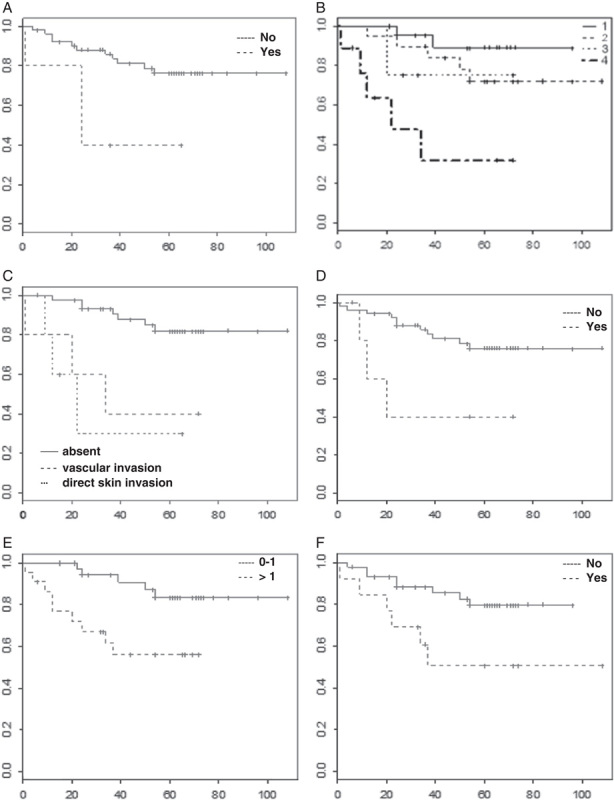
Survival charts related to death (overall survival) and the effects on it caused by: A, Smoking habit (P=0.03). B, “T” stage (P=0.002). C, Breast skin invasion (P=0.006). D, CSC-1 profile (P=0.036). E, Number of different positive CSC antibodies in the same case (P=0.023). F, Tumor multifocality (P=0.031). Column: estimated survival (0.0-1.0); line: time to tumor relapse (months). CSC indicates cancer stem cell.
Sperman regression pointed out valid correlations between isolated CD24 versus CD24−/CD44+ expression (P=0.0018, r=0.35) and isolated CD133 versus CD133+/ESA+ expression (P<0.0001; r=0.08). These correlations were just expected, because isolated antibodies were part of the CSC profiles.
The just known impacts of the other classic clinicopathologic variables on relapse, death risk, and survival were confirmed in this study. Thus, smoking habit was the most impactful variable on relapse risk: on univariate analysis, increased 14.5 times this possibility (P=0.006; 95% CI, 2.11-100), what was confirmed on multivariate analysis, that revealed a 15.6 times greater risk of tumor relapse (P=0.006; 95% CI, 2.1-100.7). Smokers were also under on greater risk to death than nonsmokers, both on univariate analysis (P=0.03; 95% CI, 1.14-15.29, 4.1 times) and multivariate analysis (P=0.004; 95% CI, 1.9-36.4, 8.3 times). Smoking habit impacted both free of disease and overall survival of patients. The former was diminished by 40% in up 30 months of follow-up and the last reduced by 40% in up 25 months of follow-up (P=0.03).
“T” stage was the most impactful variable on death risk. Tumors classified as T3/T4 stages were associated with 12.8 times increased risk of death in the univariate analysis (P=0.002; 95% CI, 2.45-67.15) and with 10.4 times increased risk of relapse in the multivariate analysis (P=0.024; 95% CI, 1.94-55.91). Advanced “T” stages and breast skin invasion were other important variables which negatively influenced similarly free of disease and overall survival.
Other important variable was breast skin invasion. When vascular emboli was observed in the skin, relapse risk increased 4 times in the univariate analysis (P=0.039; 95% CI, 1.07-14.86). When any type of breast skin invasion (vascular or direct) was present, the death risk were 10.5 times greater in the univariate analysis (P=0.006; 95% CI, 1.92-56.58). These results were confirmed in the multivariate analysis: vascular emboli in the skin and direct skin invasion, respectively, increased the death risk by 10.4 times (P=0.006; 95% CI, 1.9-56.6) and by 7.3 times (P=0.007; 95% CI, 1.7-30.9).
Other variables associated with increased relapse risk in the univariate analysis were the “N” stage, familial history of cancer, and tumor volume. Tumor multifocality and tumor volume were the other variables associated with death risk in the univariate analysis. Positive axillary lymph nodes (increased risk of 37.2 times, P=0.0004; 95% CI, 4.9-279.6) and familial history of cancer (increased risk of 13.7 times, P=0.0009; 95% CI, 2.9-64.1) were confirmed in the multivariate analysis as relapse risk associations.
DISCUSSION
This study aimed to characterize immunohistochemical expression of putative CSC antibodies in paraffin-embedded tissue of breast tumors and to discuss their possible clinical and prognostic values. In neoplastic tissue, CSC are very scarce and heterogenously distributed and numerical counting of these cells did not follow a normal distribution. Thus, in the literature, CSC frequency were reported as <2% of the total cell population in neoplastic tissue and the identification of these cells depends on the sampled tumor area.3 For these reasons, CSC counting was categorized simply as positive or negative in this study. So, the cases were considered positive even when an isolated cell was stained.
The sampling of a small area of the tumor in TMA can impair the CSC detection, but minimal invasive procedures are increasing nowadays. So, small surgical specimens in diagnostic routine are frequent. The choice of microarray is consistent with this tendency, as it is an easy procedure to do in the daily routine. Beside to this choice, some studies in the literature considered that TMA can sample satisfactorily the tumors, adding some economic advantages, such the less quantity of antibodies needed on these samples.22,23
In this study, the features of the selected population were in agreement with the epidemiological profiles observed in other studies in the literature, especially the ones involving high socioeconomic levels of population.17–19
The new findings of this study were related mainly with the CSC profiles antibodies (CD24−/CD44+ and CD133+/ESA+). Thus, these profiles were positive in the majority of the cases and were associated with some important clinicopathologic variables and poor prognosis.
In this regard, it was observed that CSC-1 was more frequently positive in early “T” stages (T1/T2) in this study. This finding can be related to another result of this study: CSC-1 was more frequently positive in patients older than 40 years. Even though not confirmed in other studies in the literature, breast cancer is assumed to grow slower in older groups than in younger groups. The cut-off on 40 year old was established in this study for several reasons, mainly for breast cancer epidemiologic features, as that is the recommended age group to begin the screening of this cancer.
So, CSC-1 expression in early stage of breast tumors arising in older patients can explain this unusual decreasing of overall survival in these cases, even if have not been observed associations with other known prognostic factors in breast cancer, as expression of hormonal receptors and overexpression of HER-2/neu oncogen. This finding is unedited in the literature and it was not confirmed in any revised study.6,8,15,26–30,33,38 Immunohistochemical search of this profile can be valid in diagnostic routine, as it seems to be an independent prognostic variable.
Whereas CSC-1 was associated with early “T” stages, isolated CD24+ cells were frequently related with advanced “T” stages (T3/T4) tumors in this study. In other studies, isolated CD24+ cells were associated with earlier breast tumor relapse.31,32 Although, CD24+ cells were supposed to be more prevalent in this present study because of luminal molecular subtypes predominance.15 Moreover, advanced “T” stages are themselves a known worst prognostic factor. Therefore, isolated CD24 cells were not considered an independent factor of bad prognosis.
Another unedited finding of this study was that a greater frequency of different positive CSC markers in a same case was associated to some clinicopathologic factors of advanced tumors (P=0.004), as T3/T4 stages and less differentiated tumors (final histologic grades of Nottingham 2 and 3), reinforcing the association of CSC with clinicopathologic factors of worse prognosis. Thus, isolated positive CSC markers were not related with any prognostic or clinicopathologic relevant variable neither in this study nor in other studies.6,24
However, isolated CD133+ cells were an exception in this study. Interestingly, there were observed that isolated CD133+ cells were more frequently associated with tumors arising in patients in menopause. In breast cancer, this finding opposed the concept that hormones do not influence the CSC, because these cells do not express hormonal receptors.34,35 Actually, hormones could regulate stem-cell phenotype expression.35 In other revised study, the authors found CD133 expression associated to hormonal receptors expression in uterine carcinosarcomas, due to a possible common origin of this tumor from a CD133+ cell, from a mesenchymal müllerian progenitor.36 Moreover, speculation is that CD133 expression can be related to worst therapeutic response in luminal subtypes of breast cancer. Although, additional studies are necessary to investigate this possible association.
Finally, CSC-2 (CD133+/ESA+) was an important factor of bad prognosis in this study, raising the risk of relapse and diminishing free of disease survival, but with no association with any clinicopathologic variable, on compliance to other studies.8,15,26–30,33,37
The other single stained antibodies—OCT 3/4, C-KIT, CD10, CD34, p63, SOX-2, and ALDH-1—were not associated with any clinical or pathologic variables of practical value, confirming other studies findings. For these reasons, they cannot be considered true CSC markers, especially when interpreted in an isolated context.6,24,25
The associations between classical clinicopathologic variables in breast cancer, as smoking habit, breast skin invasion, “T” and “N” stages, with strong relapse and risk of death only were confirmed in this present study and were not new findings.
CONCLUSIONS
There were attested 2 CSC immunohistochemical profiles (CD24−/CD44+ and CD133+/ESA+), respectively, by the negative impact on overall survival and free of disease survival. The absence of associations with known prognostic factors strengthens these CSC profiles as independent prognostic factors in breast cancer in this study.
Some of these putative CSC antibodies were irrefutably associated with important clinicopathologic factors in this study, justifying immunohistochemical search of these antibodies in diagnostic routine.
Thus, CSC-1 were associated with early “T” stages and with patients older than 40 years, a greater number of different positive antibodies in a same case were related with advanced “T” stages and with less differentiated tumors (final histologic grades of Nottingham 2 and 3) and isolated CD133 was related with tumors arising in patients in menopause.
The therapeutic resistance of some of breast cancers is probably caused by these cancer stem cells, which may become an important therapeutic target in the future, to improve survival on breast cancer.
ACKNOWLEDGMENTS
The authors thank specially to Marina França de Resende, MD (A.C. Camargo Cancer Center, São Paulo-SP, Brazil), who performed the IHC technique in the cases and to all this service staff, that supported with the other techniques and materials needed for this study. The authors thank to the statistical service of Medical Sciences Faculty of the State University of Campinas (FCM-UNICAMP, Campinas-SP, Brazil), which helped to analyze the obtained data.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Smalley M, Ashworth A. Stem cells and breast cancer: a field in transit. Nat Rev Cancer. 2003;3:832–844. [DOI] [PubMed] [Google Scholar]
- 2.Shah M, Allegrucci C. Keeping an open mind: highlights and controversies of the breast cancer stem cell theory. Breast Cancer. 2012;26:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klonisch T, Wiechec E, Hombach-Klonisch S. Cancer stem cell markers in common cancers—therapeutic implications. Trends Mol Med. 2008;14:450–460. [DOI] [PubMed] [Google Scholar]
- 4.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves Junior MJ, Schenka AA. Angiogenesis, Positive CD34 Neoplastic Stem Cells and Sinvastatin on a Chemically Induced Mammary Model of Carcinogenesis. Campinas: UNICAMP; 2012. [Google Scholar]
- 6.Velasco-Velázquez MA, Popov VM. The role of breast cancer stem cells in metastasis and therapeutic implications. Am J Pathol. 2011;179:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May CD, Sphyris N, Evans KW, et al. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buess M, Rajski M, Vogel-Durrer BML, et al. Tumor-endothelial interaction links the CD44+/CD24(-) phenotype with poor prognosis in early-stage breast cancer. Neoplasia. 2009;11:987–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zucchi I, Sanzone S, Astigiano S, et al. The properties of a mammary gland cancer stem cell. Proc Natl Acad Sci U S A. 2007;104:10476–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castaño Z, Fillmore CM, Kim CF, et al. The bed and the bugs: interactions between the tumor microenvironment and cancer stem cells. Semin Cancer Biol. 2012;22:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodward WA, Sulman EP. Cancer stem cells: markers or biomarkers? Cancer Metastasis Rev. 2008;27:459–470. [DOI] [PubMed] [Google Scholar]
- 12.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng SQ, Alexandrou AT, Li JJ. Breast cancer stem cells: multiple capacities in tumor metastasis. Cancer Lett. 2014;349:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Nenutil R, Appleyard M, et al. Lack of correlation of stem cell markers in breast cancer stem cells. Br J Cancer. 2014;110:2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CY, Barry-Holson KQ, Allison KH. Breast cancer stem cells: are we ready to go from bench to bedside? Histopathology. 2016;68:119–137. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Shi Q, Wang Z, et al. Clinicopathologic correlation of cancer stem cell markers CD44, CD24, VEGF and HIF-1α in ductal carcinoma in situ and invasive ductal carcinoma of breast: an immunohistochemistry-based pilot study. Pathol Res Pract. 2011;207:505–513. [DOI] [PubMed] [Google Scholar]
- 17.IARC Press. Breast Cancer Screening IARC—International Agency for Cancer Research, WHO—Handbooks of Cancer Prevention (Chapter 1). Lyon: IARC Press; 2002. [Google Scholar]
- 18.Noronha CP, Ferreira JMO, Oliveira JFP, et al. Estimativas 2008: Incidência de Câncer no Brasil, Ministério da Saúde, Secretaria de Atenção à Saúde Instituto Nacional de Câncer Coordenação de Prevenção e Vigilância de Câncer. Rio de Janeiro: INCA Press; 2007. [Google Scholar]
- 19.Benson JR, Jatoi I, Keisch M, et al. Early breast cancer. Lancet. 2009;373:1463–1479. [DOI] [PubMed] [Google Scholar]
- 20.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;17:2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO classification of tumors of the breast. 4 ed. Lyon: IARC, 2012. [Google Scholar]
- 22.Woodward WA, Chen MS, Behbod F, et al. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. [DOI] [PubMed] [Google Scholar]
- 23.Choijamts B, Jimi S, Kondo T, et al. CD133+ cancer stem cell-like cells derived from uterine carcinosarcoma (malignant mixed Müllerian tumor). Stem Cells. 2011;29:1485–1495. [DOI] [PubMed] [Google Scholar]
- 24.Alvarenga AW, Coutinho-Camillo CM, Rodrigues BR, et al. Comparison between manual and automated evaluations of tissue microarray patterns of protein expression. J Histochem Cytochem. 2013;61:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honeth G, Bendahl PO, Ringnér M, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vesuna F, Lisok A, Kimble B, et al. Twist modulates breast cancer stem cells by transcriptional regulation of CD24 expression. Neoplasia. 2010;12:1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigurdsson V, Hilmarsdottir B, Sigmundsdottir H, et al. Endothelial induced EMT in breast epithelial cells with stem cell properties. PLoS One. 2011;6:e23833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang-Verslues WW, Kuo WH, Chang PH, et al. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS One. 2009;4:e8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Tian Y, Yuan X, et al. Enrichment of CD44 in basal-type breast cancer correlates with EMT, cancer stem cell gene profile, and prognosis. Onco Targets Ther. 2016;21:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauter G. Representativity of TMA studies. Methods Mol Biol. 2010;664:27–35. [DOI] [PubMed] [Google Scholar]
- 32.Bernardi MA, Logullo AF, Pasini FS, et al. Prognostic significance of CD24 and claudin-7 immunoexpression in ductal invasive breast cancer. Oncol Rep. 2012;27:28–38. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Li JG, Zheng XY, et al. Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast carcinomas. Chin Med J (Engl). 2009;20:2763–2769. [PubMed] [Google Scholar]
- 34.Piva M, Domenici G, Iriondo O, et al. SOX-2 promotes tamoxifen resistance in breast cancer cells. Embo Mol Med. 2014;6:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finlay-Schultz J, Cittelly DM, Hendricks P, et al. Progesterone downregulation of miR-141 contributes to expansion of stem-like breast cancer cells through maintenance of progesterone receptor and Stat5a. Oncogene. 2015;34:3676–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bane A, Viloria-Petit A, Pinnaduwage D, et al. Clinical–pathologic significance of cancer stem cell marker expression in familial breast cancers. Breast Cancer Res Treat. 2013;140:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harbeck N. Never too late: reducing late breast cancer relapse risk. Curr Med Res Opin. 2008;24:3295–3305. [DOI] [PubMed] [Google Scholar]
- 38.Nakshatri H, Srour EF, Badve S. Breast cancer stem cells and intrinsic subtypes: controversies rage on. Curr Stem Cell Res Ther. 2009;4:50–60. [DOI] [PubMed] [Google Scholar]